Abstract
The aim of the present study was to perform clinical, biochemical, and radiological evaluation of the efficacy of mesenchymal stem cells derived from Wharton jelly (WJ) present within the human umbilical cord in the treatment of knee osteoarthritis. Between 2018 and 2019, 10 patients with knee osteoarthritis for whom the conservative treatment was not beneficial were included in the study. Patients were clinically, radiologically, and biochemically evaluated before treatment initiation. Thereafter, the patients were intra-articularly injected using a solution containing 1 × 108 WJ-derived MSCs. Evaluations were performed on day 21 (V1) and 42 (V2) and month 3 (V3), 6 (V4), and 12 (V5) after the procedure. At 1-year post-injection, visual analogue scale, Western Ontario and McMaster Universities Osteoarthritis Index, and Lequesne scores of patients were lower than those observed during the initial evaluation, whereas the mean 36-Item Short Form Health Survey score was higher. Cartilage thicknesses were found to be increased in all regions except in the medial femur, medial posterior femur, lateral posterior femur, and lateral posterior tibia regions in magnetic resonance imaging. A significant increase was observed in tumor necrosis factor-alpha, interleukin-1β, adiponectin, resistin, and interleukin-6 levels compared with pre-injection values. The leptin levels at 6-month and 1-year controls were lower than the pre-injection levels, and the decrease observed at 6 months was significant. In patients with knee osteoarthritis, intra-articular WJ-derived MSC injection causes significant pain reduction, satisfactory functional improvement, and increased patient satisfaction following a 1-year follow-up. These clinical improvements were supported by magnetic resonance images, along with changes in adiponectin and leptin levels in synovial fluid.
Level of evidence: IV.
Keywords: Gonarthrosis, knee injection, stem cell
1. Introduction
Osteoarthritis (OA) the most common joint disease is defined as a chronic degenerative disease of articular cartilage and is the most common cause of injuries among adults.[1] The disease frequency has increased 2 to 3 times in recent years with the increase in life expectancy, and it is expected to increase further in the future.[2] Although the etiology of OA is multifactorial, conventionally associated with overloading due to mechanical factors, trauma, and obesity.[3] Moreover, genetic and local inflammatory factors reportedly have a negative impact on the pathophysiology of OA. Obesity can cause impairments in the joint cartilage via overloading the joint as well as by initiating the inflammatory process with adipokine release by adipocytes.[4] Chondrocytes, synoviocytes, osteoblasts, stromal cells, macrophages, and immune cells are also known as sources of adipokine release, although adipocytes are the primary source.[5] In this regard, adipokines, such as leptin, resistin, adiponectin, tumor necrosis factor-alpha (TNF-α), and interleukins (IL)-1β, (IL-1β), and IL-6, present in the synovial fluid contribute to the pathogenesis of OA with synovial inflammation, matrix metalloproteinase production, cartilage damage, bone remodeling, and inflammatory and anti-inflammatory effects.[5] Adipokines present their effects on the joint by regulation of the metabolic balance within the joint realized by regulating the cytokine release; however, the mechanism of action has not been completely elucidated.[6]
The knowledge that OA is a chronic progressive disease and that conventional pharmacological treatments do not prevent disease progression has led clinicians to seek for alternative treatment modalities.[7] With the recent advances in cell therapy, the use of mesenchymal stromal cells (MSCs) as a potential treatment agent in the treatment of OA has been initiated.[8] MSCs are easily derived from tissues, such as bone marrow, adipose tissue, synovial membrane, umbilical cord, and Wharton jelly (WJ).[9] WJ is the mucous ligament in the umbilical cord that contains myofibroblast-like stromal cells, collagen fibers, proteoglycan, and hyaluronic acid and is used as a stem cell source owing to these properties. MSCs can be surgically and intra-articularly employed in the treatment of OA.[8] Studies have shown that an intra-articular injection is an effective method.[10]
Magnetic resonance imaging (MRI) is a noninvasive and reliable method used to evaluate cartilage volume and thickness as well as changes in cartilage structure.[11] Quantitative T2*mapping is a proven method to evaluate the water content and collagen sequence of cartilage tissues, wherein an increased value indicates that collagen fiber integrity is impaired and water content of the cartilage structure is reduced.[12]
This study aimed to clinically, biochemically, and radiologically evaluate the efficacy and usability of MSCs derived from WJ present within the human umbilical cord in the treatment of knee OA.
2. Materials and Methods
A total of 10 patients diagnosed with OA according to the criteria of the American College of Rheumatology between 2018 and 2019 and without diabetes mellitus, bleeding diathesis, infection, human immunodeficiency virus infection, hepatitis, malignancy, immunosuppression, and lumbar pathological disorders were included in the study. These patients were mandatorily administered conventional treatment for 6 months before the procedure. Patients who did not benefit from conservative treatment were included in the next stage of the study. After obtaining informed consent from these patients, the initial clinical, biochemical, and radiological evaluations (V0) were performed, following which the treatment using WJ-derived MSCs (WJ-MSCs) was initiated. Patients were instructed to visit for control evaluations on day 21 (V1) and 42 (V2) as well as on month 3 (V3), 6 (V4), and 12 (V5) post-procedure, that is, they were evaluated on 5 different times.
2.1. Isolation and culture of WJ-MSCs
The umbilical cord tissue from a healthy donor was collected and transferred to the current Good Manufacturing Practice (cGMP) Laboratory at affiliated university. All donation, cell isolation, and culture protocols were approved by the Health of Ministry of our country and adhered to cGMP standards of Europe.
Briefly, 10 cm umbilical cord tissue was longitudinally cut, followed by washing with phosphate buffered saline, and WJ tissue present between the perivascular structure of cord tissue was isolated and diced into cubes for explant culture. The sections of WJ tissue were placed into culture flasks containing alpha-Modified Eagle Medium (Biological Industries, Kibbutz Beit HaEmek, Israel) with 1% antibiotic (penicillin/streptomycin) and 10% allogeneic human serum and were incubated at 37°C and 5% CO2. At the end of primary culture, when the cells reached 80% to 90% confluence, the cells were washed with phosphate buffered saline, detached with trypsinethylenediaminetetraacetic acid and 1 to 1.4 × 106 cells/per flask were subcultured using the same culture media and under the same conditions. The WJ-MSCs cultured in the second passage were cryopreserved.
At 5 days before intra-articular transplantation, WJ-MSCs were thawed and re-plated. WJ-MSCs were washed with saline and 100-µm strainers were used before suspending WH-MSCs into the glass bottles. Routine quality control assessments including immunophenotypical characterization using flow cytometry, microbiological monitoring, endotoxin testing, and telomerase enzyme activity analysis were conducted before releasing each batch of WJ-MSCs from the cGMP laboratory.
The cell count of WJ-MSCs was 100.13 ± 0.34 in 5 mL of physiological isotonic solution per glass bottle. WJ-MSCs were transported to the clinical team for intra-articular transplantation at 4°C.
2.2. Intra-articular transplantation of WJ-MSCs
After the relevant knees of patients were sterilized, a 20-gauge needle was used for injecting into the knees. First, 2 cc of synovial fluid was aspirated, followed by an intra-articular injection of a solution containing 1 × 108 WJ-MSCs into the joint. Their movements were restricted for 1 week, and a cold compress was applied. After 1 week, all activities of daily living were allowed.
2.3. Clinical evaluation
All patients were clinically evaluated in all controls. The visual analogue scale (VAS) a subjective and commonly used method was employed to determine the changes in the pain level. Functional changes were evaluated using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), which facilitates simultaneous assessment in terms of pain, stiffness and function in knee OA, and the Lequesne Index, which is a personal questionnaire that evaluates pain and functional disability. The 36-Item Short Form Health Survey (SF-36) was used to assess the changes in the quality of life of the patients.
2.4. MRI evaluation
All participants were instructed to relax for 15 minutes prior to the examination for ensuring that the cartilage is at resting condition. MRI was performed using a 1.5T scanner (Magnetom Aera; Siemens, Erlangen, Germany) and a 16-channel knee coil. Imaging sequence parameters were as follows: fat-saturated (FS) sagittal proton density-weighted turbo spin-echo (TSE) [repetition time msec/echo time ms: 2500/40, slice thickness: 3 mm, field of view (FOV): 160 mm, matrix: 240 × 320]; FS coronal proton density-weighted TSE (repetition time msec/echo time msec: 2500/40, slice thickness: 3 mm, FOV: 160 mm, matrix: 240 × 320), sagittal T1-weighted spin-echo (repetition time msec/echo time ms: 590/14, slice thickness: 3 mm, FOV: 160 mm, matrix: 512 × 640), coronal T1-weighted spin-echo (repetition time msec/echo time msec: 590/14, slice thickness: 3 mm, FOV: 160 mm, matrix: 512 × 640) and FS axial proton density-weighted TSE (repetition time ms/echo time ms: 3200/33, slice thickness: 3 mm, FOV: 160 mm, matrix: 448 × 640). T2*mapping used a sagittal multi-echo spin-echo sequence (slice thickness: 3 mm; slice space: 3.6 mm; five echo/repetition times of 4.2, 11.3, 18.5, 25.6 and 32.7/810 ms; flip angle: 60°; FOV: 160 mm; matrix: 256 × 256).
Analysis of T2*cartilage mapping and cartilage thickness was performed by two experts in analysis of musculoskeletal MRI (14 years of experience) in consensus on a multimodal workstation (Syngo MR D13 Numaris/4 software; Siemens Medical Solutions, Erlangen, Germany).
Quantitative cartilage analysis was performed according to the MRI Osteoarthritis Knee Score (MOAKS) cartilage segmentation. The knee was divided into 14 articular subregions as follows: the patella medial and patella lateral; the medial trochlea (MT), lateral trochleam, medial central femur, lateral central femur (LCF), medial posterior femur, and lateral posterior femur (LPF); and the medial anterior tibia, lateral anterior tibia, medial central tibia (MCT), lateral central tibia, medial posterior tibia (MPT), and lateral posterior tibia (Figs. 1 and 2).
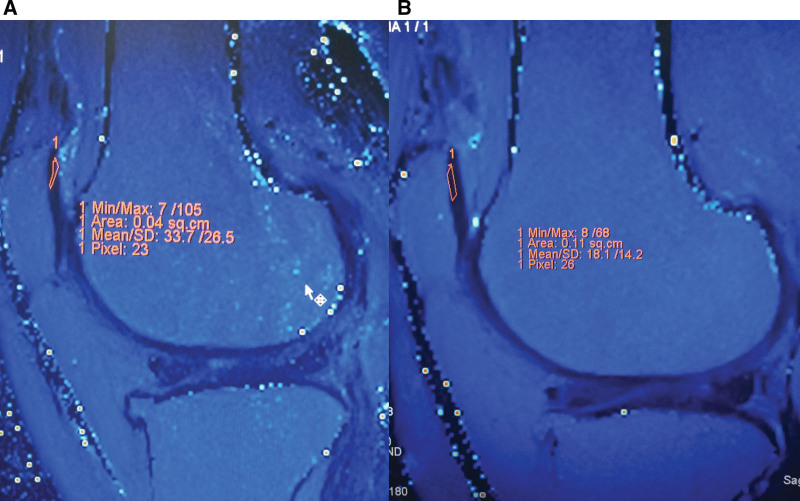
Figure 1.
Sixty-one year old female. T2 cartilage mapping magnetic resonance imaging of the knee. The mean values of T2 are measured before (A) and after treatment (B) at lateral patellar cartilage. The mean T2 value was decreased from 33.7 to 18.1 ms at this site.
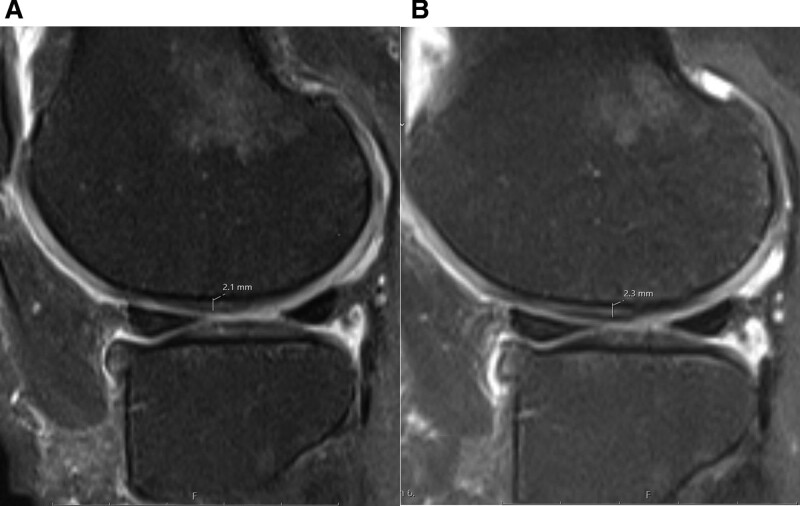
Figure 2.
Sixty-one year old female. Sagittal proton density weighted fat-suppressed knee magnetic resonance imaging before (A) and after (B) treatment. The measurements of cartilage thickness at lateral central femoral are seen. The cartilage thickness increased from 2.1 to 2.3 mm at this site.
Cartilage thickness measurements were performed on FS axial proton density-weighted TSE images for patellar cartilage and FS sagittal proton density-weighted TSE images for femoral and tibial cartilages.
2.5. Biochemical analysis
The samples collected from the synovial liquid of the patients were stored at − 80°C until use for the biochemical tests. The samples were thawed at room temperature and TNF-α, IL-6, IL-1β, adiponectin, resistin, and leptin levels were analyzed using enzyme-linked immunosorbent assay method with commercial kits (Shanghai Sunred Biological Technology Co. Ltd, Shanghai, China), according to the manufacturer’s instructions.
2.6. Statistical analysis
Data were analyzed using SPSS software for Windows 20.0 (Statistical Product and Service Solutions, Inc., Chicago, IL). The normality of the data was determined using Shapiro–Wilk test. Friedman and dependent samples t tests were used for the analysis of the non-normal repetitive measurements. The statistical significance level was set at P < .05.
3. Results
3.1. WJ-MSCs
The released WJ-MSCs were negative for pathogenic microorganisms and exhibited endotoxin levels of <0.5 EU/mL and telomerase enzyme activity of 0.44. The mean cell viability was 93.81% ± 0.98% (n = 10).
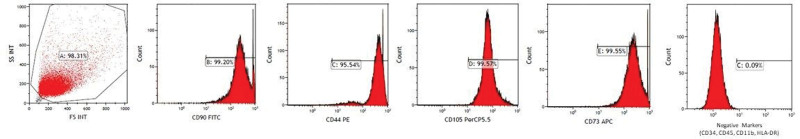
Flow cytometry results showed that the mean negative marker expression (CD14, CD45, CD11b, and HLA-DR) was 0.3% ± 0.2%; CD105 expression was 98.38% ± 2.12%; CD73 expression, 98.56% ± 0.46%; CD90 expression, 99.81% ± 0.14% and CD44 expression, and 94.19% ± 1.13% for the released WJ-MSCs (Fig. 3).
Figure 3.
Representative flow cytometry histograms of WJ-MSCs. The positive expression of CD, CD90, CD44, CD105, and CD73 and negative expression of CD14, CD45, CD11b, and HLA-DR are shown. WJ-MSCs = Wharton jelly-mesenchimal stem cells.
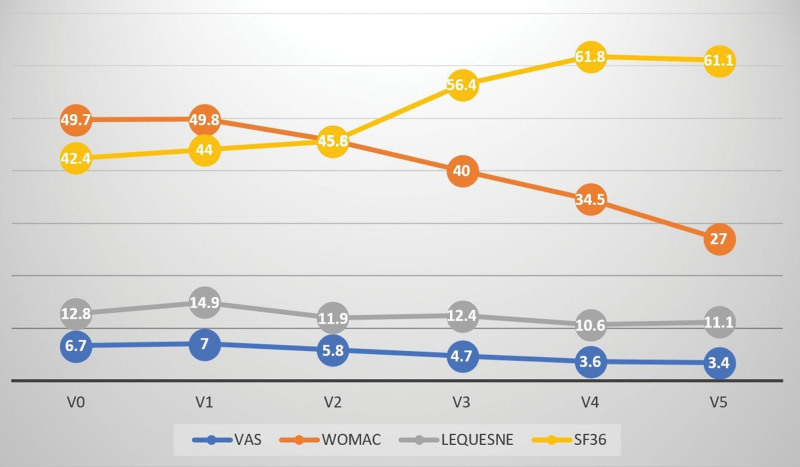
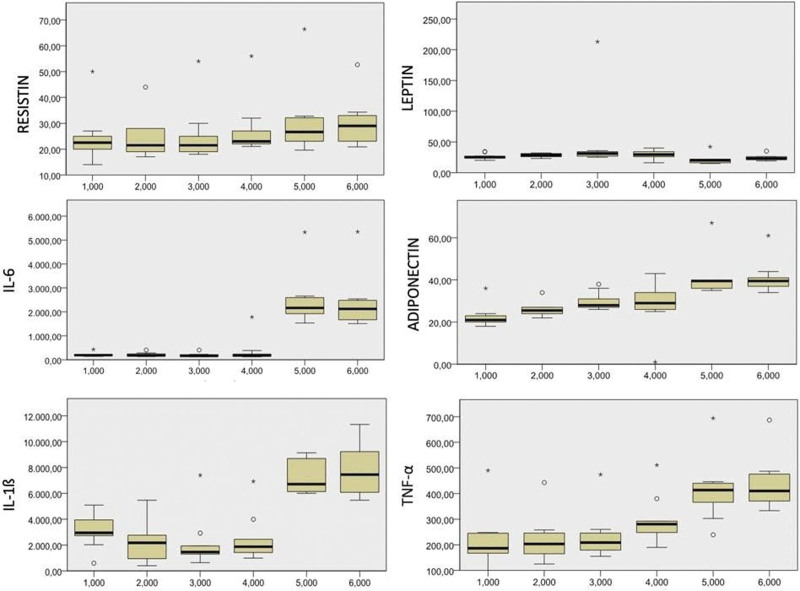
Table 1 presents the age–sex distribution and mean height, weight, and body mass index of the participants. Clinical evaluation of the patients at 1-year post-injection revealed that VAS, WOMAC and Lequesne scores were lower than those observed at the pre-injection evaluation, whereas the mean SF-36 score was higher. The decrease in VAS and WOMAC scores along with the increase in SF-36 values were significant (Table 2; Fig. 4). T2*cartilage mapping and cartilage thickness were measured for 14 different regions in the MRI results obtained pre-injection and at 1-year post-injection. The cartilage thickness increased in the medial central femur, MPS, LPF, and lateral posterior tibia regions. A significant increase was observed in the MT, LCF, MAT, MCT, and MPT regions. In T2*mapping, a decrease in nine regions and an increase in five regions were noted, with only the decrease in the LPF region being significant (Table 3). Compared with pre-injection values, an increasing trend for the markers except leptin was observed. The increase in TNF-α, IL-1β, IL-6, adiponectin, and resistin levels was significant compared with pre-injection values. The leptin levels measured at 6-month and 1-year controls were lower than that observed at pre-injection evaluation, with the decrease observed at 6 months being significant (Table 4; Fig. 5).
Table 1.
Patient demographics.
| Age (yr) | 58.2 ± 10.0 |
| Gender | |
| Female | 7 |
| Male | 3 |
| Height (cm) | 159.0 ± 4.7 |
| Weight (kg) | 83.0 ± 7.4 |
| BMI (kg/m2) | 32.7 ± 4.4 |
BMI = body mass index.
Table 2.
Friedman test results for the difference between the clinical and functional scoring results over time.
| V0 (n = 10) | V1 (n = 10) | V2 (n = 10) | V3 (n = 10) | V4 (n = 10) | V5 (n = 10) | P | |
|---|---|---|---|---|---|---|---|
| VAS | 6.7 ± 2.24 | 7.0 ± 1.9 | 5.8 ± 3.8 | 4.7 ± 3.5 | 3.6 ± 2.8* | 3.4 ± 3.3* | .000 |
| WOMAC | 49.7 ± 19.4 | 49.8 ± 28.1 | 45.8 ± 32.8 | 40.0 ± 29.7 | 34.5 ± 24.6* | 27.0 ± 22.9* | .007 |
| Leqeusne | 12.8 ± 3.56 | 14.9 ± 5.1 | 11.9 ± 7.2 | 12.4 ± 6.8 | 10, ± 6.7 | 11.1 ± 7.01 | .239 |
| SF-36 | 42.4 ± 20.6 | 44.0 ± 22.3 | 45.6 ± 28.3 | 56.4 ± 29.6 | 61.8 ± 26.6* | 61.1 ± 29.9* | .032 |
SF-36 = 36-Item Short Form Health Survey, VAS = visual analog scale, WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
P < 0.05 to values that are significantly different from the initial (V0) values.
Figure 4.
Gradual changes in clinical and functional scoring results.
Table 3.
Paired t test results of difference between magnetic resonance imaging results of first and last controls.
| n | Cartilage thickness | SD | P | T2* | SD | P | |||
|---|---|---|---|---|---|---|---|---|---|
| V0 | V5 | V0 | V5 | ||||||
| PM | 9 | 2,4678 | , 2722 | , 01236 | , 312 | 22.3667 | 21.9889 | 3,04622 | , 720 |
| PL | 9 | , 3000 | , 3089 | , 01833 | , 184 | 21,5111 | 19,8333 | 3,84538 | , 227 |
| MT | 9 | , 1722 | , 1967 | , 02920 | , 036 | 21.1444 | 21.8556 | 4,49067 | , 647 |
| MCF | 3 | , 2167 | , 2167 | , 01000 | 1000 | 18.6667 | 18.9333 | 10,68145 | , 969 |
| MPF | 9 | , 4300 | , 2411 | , 58171 | , 359 | 24,0667 | 18,7444 | 7,26993 | , 059 |
| LT | 9 | , 1978 | , 2000 | , 01563 | , 681 | 21,8111 | 20.9667 | 2.38281 | , 319 |
| LCF | 8 | , 1575 | , 1662 | , 00835 | , 021 | 22.9375 | 20,0500 | 7,15491 | , 291 |
| LPF | 8 | , 2312 | , 2275 | , 01996 | , 612 | 25,8778 | 17,9222 | 8,74873 | , 026 |
| MAT | 6 | , 1400 | , 1567 | , 01033 | , 011 | 20,0667 | 22,1000 | 3.19854 | , 180 |
| MCT | 6 | , 1367 | , 1450 | , 00753 | , 042 | 21,5000 | 20.4500 | 6,95751 | , 727 |
| MPT | 8 | , 1450 | , 1575 | , 01488 | , 049 | 19,0250 | 21.1125 | 7,74623 | , 471 |
| LAT | 6 | , 1450 | , 1550 | , 02000 | , 275 | 20,3000 | 19.3333 | 5,57231 | , 689 |
| LCT | 8 | , 1863 | , 1925 | , 00916 | , 095 | 21,6125 | 21.8250 | 4,72937 | , 902 |
| LPT | 9 | , 1644 | , 1644 | , 01225 | 1000 | 22,6333 | 22,0667 | 7,49767 | , 826 |
Magnetic resonance imaging results of cartilage thickness and T2* mapping by regions.
LAT = lateral anterior tibia, LCF = lateral central femur, LCT = lateral central tibia, LPF = lateral posterior femur, LPT = lateral posterior tibia, LT = lateral trochlea, MAT = medial anterior tibia, MCF = medial central femur, MCT = medial central tibia, MPF = medial posterior femur, MPT = medial posterior tibia, MT = medial trochlea, PL = patella lateral, PM = patella medial.
*P < 0.05 results were considered statistically significant.
Table 4.
Friedman test results of the difference between adipocytokine levels over time.
| TNF-α (ng/L) | IL-1 β (pg/mL) | Adiponectin (mg/L) | IL-6 (ng/L) | Resistin (ng/mL) | Leptin (ng/mL) | |
|---|---|---|---|---|---|---|
| V0 | 215.2 ± 106.4 | 3041.2 ± 1234.8 | 22.5 ± 5.1 | 214.7 ± 79.4 | 24.6 ± 9.7 | 26.3 ± 4.6 |
| V1 | 215.2 ± 100.9 | 2167.5 ± 1704.1 | 25.4 ± 1.8 | 198.8 ± 89.2 | 23.2 ± 8.8 | 28.6 ± 2.1 |
| V2 | 232.5 ± 91.6 | 2090.8 ± 1958.5 | 29.8 ± 4.0 | 190.2 ± 81.7 | 25.1 ± 10.7 | 29.7 ± 3.7 |
| V3 | 292.1 ± 93.4* | 2455.7 ± 1787.3 | 28.7 ± 11.6 * | 353.4 ± 506.7 | 27.3 ± 10.6 | 29.8 ± 6.9 |
| V4 | 412.7 ± 118.4* | 7210.2 ± 1250.4* | 41.1 ± 9.4* | 2461.6 ± 1065.1* | 30.3 ± 13.4* | 21.1 ± 7.8* |
| V5 | 433.3 ± 102.2* | 7923.2 ± 1952.6* | 40.9 ± 7.6* | 2361.5 ± 1117.8* | 29.9 ± 9.2* | 23.9 ± 4.6 |
| P | .000 | .000 | .000 | .000 | .001 | .003 |
Refers to values that are significantly different from the initial (V0) values.
IL = interleukin, TNF = tumor necrosis factor.
Figure 5.
Gradual change in adipocytokine levels.
No allergic or adverse reaction to WJ-MSCs was reported on the day of transplantation; however, 3 patients showed mild effusions that may be a possible reaction to the high number of cells.
4. Discussion
Intra-articular WJ-MSC administration leads to an improvement in clinical and radiological parameters along with significant changes in adipokine levels observed in the synovial fluid of patients with knee OA.
OA is a degenerative joint disease that causes progressive damage to the joint cartilage, thereby leading to limited joint motion.[13] Procedures including surgical resurrection, microfracture, and mosaicplasty in addition to the use of anti-inflammatory drugs and lumbar materials are among the current treatment modalities for OA; however, all these procedures result in only a temporary regression in symptoms.[14] Despite the advances in treatment methods, disease progression cannot be prevented.[15] However, the use of cell-based novel treatment methods has been initiated for the suppression of disease progression, and these methods have been yielding promising results. Accordingly, MSCs have frequently been used owing to their chondrogenic potential and ease of availability.[16] MSCs used for treatment purpose can be easily obtained from tissues such as bone marrow, adipose tissue, synovial membrane, umbilical cord and WJ.[9] MSCs showed different characteristics when isolated from different regions. Several studies have attempted to use umbilical cord-derived cells, such as umbilical cord-derived MSCs and cord blood-derived MSCs, for OA treatment.[17] The stem cells derived from the umbilical cord can be obtained from the subendothelial part of the cord, its perivascular part, WJ, tissues surrounding the cord and the cord itself, and the obtained cells can stimulate chondrogenic differentiation.[18] Moreover, these stem cells have several advantages such as a high differentiation potential, low immunogenicity, non-tumorigenicity, and availability as a ready-to-use product as well as being unaffected by age and weight of the individual.[16,19] We decided to use stem cells originating from the WJ in the present study owing to the abovementioned advantages proven in the clinical settings. Although this method reportedly has a low potential for side effects, with the most frequent complaints being joint pain, swelling, and movement limitation, 3 patients in our study reported moderate joint swelling and increased synovial fluid, which was then drained via a joint puncture in these patients.[20] Our results showed that WJ-MSCs can be safely used in OA treatment. MSCs can be applied in various ways for knee OA in the clinical setting. If simultaneous surgical procedures are required, they are commonly used in the surgical field over the scaffold or via intra-articular injection.[20,21] Therefore, the evaluation of the effectiveness, safety, and ease of application of the administration routes showed that the intra-articular administration was a suitable method.[10,20]
Clinical, radiological, and biochemical evaluations are often performed to evaluate the efficacy and benefit of the product used in clinical studies. Changes in functional results, pain levels, and patient satisfaction are generally used for clinical evaluation. To that end, we decided to use VAS, WOMAC, Lequesne, and SF-36, which have previously been evaluated and are among the most commonly used methods. The previous clinical MSC studies have shown that decreased scores in functionality evaluation scales (such as WOMAC and Lequesne), decreased scores in pain level evaluation scales (such as VAS), and increased scores in patient satisfaction evaluation scales (such as SF-36) indicating that patient has clinically benefited from the treatment.[19,21] In the present study, a significant decrease was observed in WOMAC and VAS scores, and a non-significant decrease was noted in the Lequesne scores, which were the tools used for clinical evaluation. Moreover, a significant increase was noted in the SF-36 scores, which was used to evaluate patient satisfaction. These findings proved that the intra-articular injection of WJ-MSCs was clinically useful in our patients.
In recent studies, T2*mapping method and changes in cartilage thickness have been used as a valid and applicable method for demonstrating cartilage damage and changes in cartilage structure in the early stages of disease.[22] The increment in T2*values and decrement in cartilage thickness indicate the severity of cartilage damage.[23,24] Patients with inflammatory arthritis show increased T2*values compared with normal healthy individuals.[25] Although numerous evaluation methods have been used for the quantitative evaluation using MRI, MOAKS is one of the most commonly used methods.[26] MOAKS is an advantageous method because it allows a detailed evaluation by dividing the knee joint into 14 different regions.[24] In our study, we preferred to use MOAKS because it allows detailed cartilage evaluation. Moreover, both cartilage thickness and T2*values were measured in our patients; thus, two different evaluation methods were used. Our results revealed that compared with pre-injection values, T2*values post-injection decreased in 9 of 14 regions measured. Further analysis showed that only the decrease in the LPF region was significant. Regarding changes in cartilage thickness, an increase was observed in 10 of 14 regions, and this increase was significant in MT, LCF, MAT, MCT, and MPT regions.
The relationship between adipokines and OA is multifactorial.[2] The functional evaluation showed that adipokines regulate the metabolic balance in the joint and provide these effects via cytokines. Adiponectin exhibits an anti-inflammatory effect as well as a protective effect against degeneration on cartilage tissue. Moreover, it has the following effects on bone metabolism: inhibition of osteoclastic activity and enhancement of osteoblastic activity.[2] Recently, leptin has frequently been studied in patients with OA and has been shown to induce chondrocyte proliferation and differentiation.[27] Leptin levels reportedly increase in the synovial fluid of patients with OA.[28] Therefore, an increase in leptin levels in OA patients is responsible for chondrocyte hypertrophy, degeneration, and diffuse ossification. Resistin plays a role in inflammation-related activities; however, some researchers have claimed that its level increases in the synovial fluid of patients with OA. Further, few researchers have reported that resistin shows the abovementioned effect by the stimulation of bone remodeling via regulating osteoblast and osteoclast differentiation; therefore, further studies involving OA and resistin are warranted.[29] In our study, adiponectin and resistin levels in synovial fluid significantly increased, particularly in month 6 and 12 post-injection, whereas leptin levels significantly decreased. These findings are consistent with those in the literature that the degenerative process is suppressed during these periods and a cartilage protective environment is formed.
Although IL-6, IL-1β, and TNF-α are pro-inflammatory cytokines, their levels increase in the synovial fluid of patients with OA. Therefore, an increased amount of these cytokines in the synovial fluid of untreated patients is considered to indicate disease progression.[5] The cytokines demonstrate these effects by increasing osteoclastic activity and suppressing the osteoblastic activity.[5] A study compared the effects of stem cells derived from adipose tissue, bone marrow, and WJ on osteogenic and chondrogenic potential and on inflammatory, anti-inflammatory, and growth factor release and found that the pro-inflammatory release caused by WJ-MSCs is high.[30] In our study, a significant increase was observed in TNF-α,IL-6, and IL-1β levels, which are pro-inflammatory cytokines, in months 6 and 12. We believe that this effect was caused by the high inflammatory effect of WJ-MSCs.
There are some limitations to the present study. These include the limited number of participants, short follow-up period, absence of a control group, and absence of histopathological parameter analysis. These limitations can be overcome by performing longer follow-up including a larger patient population, introducing a control group, and performing histopathological evaluations.
5. Conclusion
In patients with knee OA, the intra-articular administration of WJ-MSCs resulted in significant pain reduction, satisfactory functional improvement, and increased patient satisfaction within a 1-year follow-up period. These clinical improvements are supported by the radiological improvements detected using MRI and changes in adiponectin and leptin levels in the synovial fluid.
Author contributions
AEG, IK, AG and MBY conceived and designed the study. ZFK and SD performed the radiological evaluation. ZBG prepared the stem cells. ED and MBY performed the biochemical assay. AEG analised the statistical data. All authors contributed to writing of the manuscript.
Conceptualization: Ali Eray Günay, Ibrahim Karaman.
Data curation: Ali Eray Günay, Ibrahim Karaman.
Formal analysis: Ali Eray Günay.
Funding acquisition: Ali Eray Günay, Ibrahim Karaman.
Investigation: Ali Eray Günay, Ibrahim Karaman, Ahmet Guney, Eren Demirpolat, Zeynep Burcin Gonen, Mukerrem Betul Yerer.
Methodology: Ali Eray Günay, Ibrahim Karaman, Ahmet Guney, Zehra Filiz Karaman, Eren Demirpolat, Zeynep Burcin Gonen, Serap Dogan, Mukerrem Betul Yerer.
Project administration: Eren Demirpolat, Zeynep Burcin Gonen, Serap Dogan, Mukerrem Betul Yerer.
Resources: Zeynep Burcin Gonen.
Software: Zehra Filiz Karaman, Serap Dogan.
Supervision: Ibrahim Karaman, Zehra Filiz Karaman.
Writing – original draft: Ali Eray Günay, Ibrahim Karaman, Ahmet Guney, Zehra Filiz Karaman, Eren Demirpolat, Zeynep Burcin Gonen, Serap Dogan, Mukerrem Betul Yerer.
Writing – review & editing: Ali Eray Günay, Ibrahim Karaman, Ahmet Guney, Zehra Filiz Karaman, Eren Demirpolat, Zeynep Burcin Gonen, Serap Dogan, Mukerrem Betul Yerer.
Abbreviations:
- cGMP =
- current good manufacturing practice
- FOV =
- field of view
- FS =
- fat-saturated
- IL =
- interleukin
- LCF =
- lateral central femur
- LCF =
- lateral posterior femur
- MAT =
- medial anterior tibia
- MCF =
- medial central femur
- MCT =
- medial central tibia
- MOAKS =
- MRI Osteoarthritis Knee Score
- MSC =
- mesenchimal stem cells
- MT =
- medial trochlea
- OA =
- osteoarthritis
- SF-36 =
- 36-Item Short Form Health Survey
- TNF =
- tumor necrosis factor
- TSE =
- turbo spin-echo
- VAS =
- visual analog scale
- WJ =
- Wharton jelly
- WOMAC =
- Western Ontario and McMaster Universities Osteoarthritis Index
Clinical Trials Register Number: NCT04313894.
This study was funded by Erciyes University Council of Scientific Investigations (Project Code TCD-2017-6783).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study protocol was approved by the local ethics committee (approval date 28.08.2015; number. 2015/397).
All participants gave informed consent.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Günay AE, Karaman I, Guney A, Filiz Karaman Z, Demirpolat E, Gonen ZB, Dogan S, Betul Yerer M. Assessment of clinical, biochemical, and radiological outcomes following intra-articular injection of Wharton jelly-derived mesenchymal stromal cells in patients with knee osteoarthritis: A prospective clinical study. Medicine 2022;101:37(e30628).
Contributor Information
Ibrahim Karaman, Email: dr.fkaraman@gmail.com.
Ahmet Guney, Email: dr.aguney@gmail.com.
Zehra Filiz Karaman, Email: dr.fkaraman@gmail.com.
Eren Demirpolat, Email: erendemirpolat@yahoo.com.
Zeynep Burcin Gonen, Email: zeynepburcin@erciyes.edu.tr.
Serap Dogan, Email: serapdogan@hotmail.com.
Mukerrem Betul Yerer, Email: eczbetul@yahoo.com.
References
- [1].Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol. 2006;18:147–56. [DOI] [PubMed] [Google Scholar]
- [2].Nguyen US, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–26. [DOI] [PubMed] [Google Scholar]
- [4].Yamauchi T, Iwabu M, Okada-Iwabu M, Kadowaki T. Adiponectin receptors: a review of their structure, function and how they work. Best Pract Res Clin Endocrinol Metab. 2014;28:15–23. [DOI] [PubMed] [Google Scholar]
- [5].Xie C, Chen Q. Adipokines: new therapeutic target for osteoarthritis? Curr Rheumatol Rep. 2019;21:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Calvet J, Orellana C, Gratacós J, et al. Synovial fluid adipokines are associated with clinical severity in knee osteoarthritis: a cross-sectional study in female patients with joint effusion. Arthritis Res Ther. 2016;18:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lohmander LS, Roos EM. Clinical update: treating osteoarthritis. Lancet. 2007;370:2082–4. [DOI] [PubMed] [Google Scholar]
- [8].Jo CH, Lee YG, Shin WH, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254–66. [DOI] [PubMed] [Google Scholar]
- [9].McIntyre JA, Jones IA, Danilkovich A, Vangsness CT, Jr. The placenta: applications in orthopaedic sports medicine. Am J Sports Med. 2018;46:234–47. [DOI] [PubMed] [Google Scholar]
- [10].Jo CH, Chai JW, Jeong EC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med. 2017;45:2774–83. [DOI] [PubMed] [Google Scholar]
- [11].Waldenmeier L, Evers C, Uder M, et al. Using cartilage MRI T2-mapping to analyze early cartilage degeneration in the knee joint of young professional soccer players. Cartilage. 2019;10:288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Apprich S, Welsch GH, Mamisch TC, et al. Detection of degenerative cartilage disease: comparison of high-resolution morphological MR and quantitative T2 mapping at 3.0 Tesla. Osteoarthritis Cartilage. 2010;18:1211–7. [DOI] [PubMed] [Google Scholar]
- [13].Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. [DOI] [PubMed] [Google Scholar]
- [15].Martel-Pelletier J, Wildi LM, Pelletier JP. Future therapeutics for osteoarthritis. Bone. 2012;51:297–311. [DOI] [PubMed] [Google Scholar]
- [16].Dilogo IH, Canintika AF, Hanitya AL, Pawitan JA, Liem IK, Pandelaki J. Umbilical cord-derived mesenchymal stem cells for treating osteoarthritis of the knee: a single-arm, open-label study. Eur J Orthop Surg Traumatol. 2020;30:799–807. [DOI] [PubMed] [Google Scholar]
- [17].Matas J, Orrego M, Amenabar D, et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2019;8:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li X, Duan L, Liang Y, Zhu W, Xiong J, Wang D. Human umbilical cord blood-derived mesenchymal stem cells contribute to chondrogenesis in coculture with chondrocytes. Biomed Res Int. 2016;2016:3827057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Song JS, Hong KT, Kim NM, et al. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: two-year follow-up. Regen Ther. 2020;14:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8:504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Song JS, Hong KT, Kim NM, Park HS, Choi NH. Human umbilical cord blood-derived mesenchymal stem cell implantation for osteoarthritis of the knee. Arch Orthop Trauma Surg. 2020;140:503–9. [DOI] [PubMed] [Google Scholar]
- [22].Hesper T, Hosalkar HS, Bittersohl D, et al. T2* mapping for articular cartilage assessment: principles, current applications, and future prospects. Skeletal Radiol. 2014;43:1429–45. [DOI] [PubMed] [Google Scholar]
- [23].Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76–86. [DOI] [PubMed] [Google Scholar]
- [24].Kim YS, Choi YJ, Lee SW, et al. Assessment of clinical and MRI outcomes after mesenchymal stem cell implantation in patients with knee osteoarthritis: a prospective study. Osteoarthritis Cartilage. 2016;24:237–45. [DOI] [PubMed] [Google Scholar]
- [25].Tsushima H, Okazaki K, Takayama Y, et al. Evaluation of cartilage degradation in arthritis using T1ρ magnetic resonance imaging mapping. Rheumatol Int. 2012;32:2867–75. [DOI] [PubMed] [Google Scholar]
- [26].Runhaar J, Schiphof D, van Meer B, Reijman M, Bierma-Zeinstra SM, Oei EH. How to define subregional osteoarthritis progression using semi-quantitative MRI osteoarthritis knee score (MOAKS). Osteoarthritis Cartilage. 2014;22:1533–6. [DOI] [PubMed] [Google Scholar]
- [27].Wang L, Shao YY, Ballock RT. Leptin antagonizes peroxisome proliferator-activated receptor-γ signaling in growth plate chondrocytes. PPAR Res. 2012;2012:756198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ben-Eliezer M, Phillip M, Gat-Yablonski G. Leptin regulates chondrogenic differentiation in ATDC5 cell-line through JAK/STAT and MAPK pathways. Endocrine. 2007;32:235–44. [DOI] [PubMed] [Google Scholar]
- [29].Lee JH, Ort T, Ma K, et al. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthritis Cartilage. 2009;17:613–20. [DOI] [PubMed] [Google Scholar]
- [30].Amable PR, Teixeira MV, Carias RB, Granjeiro JM, Borojevic R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res Ther. 2014;5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]