Supplemental Digital Content is Available in the Text.
The combination of depressive symptoms and sleep disturbance was positively associated with chronic pain, but there were no additive interactions in community-dwelling older adults.
Keywords: Depressive symptom, Sleep disturbance, Chronic pain, Severe chronic pain, Additive interaction
Abstract
Introduction:
There is limited evidence regarding whether depressive symptoms and sleep disturbance are independently or synergistically associated with chronic pain.
Objectives:
We investigated the independent and combined associations of depressive symptoms and sleep disturbance with chronic pain and its severity (and the additive interactions) in community-dwelling older adults.
Methods:
This cross-sectional study analyzed the data of 1374 individuals who were 65 to 75 year old, not in need of long-term care, and completed questionnaires assessing sociodemographic factors, depressive symptoms, sleep disturbance, and chronic pain. The severity of chronic pain was assessed based on pain intensity, pain distribution, and pain type. The participants' status of depressive symptoms and sleep disturbance were categorized in the following 4 groups: neither condition, depressive symptoms alone, sleep disturbance alone, and both conditions.
Results:
Among the 1374 participants, 849 (61.8%) had chronic pain. The multivariable-adjusted odds ratios and 95% confidence intervals of the presence of chronic pain in those with depressive symptoms alone, sleep disturbance alone, and both conditions were 1.40 (0.97–2.03), 1.98 (1.41–2.78), and 2.12 (1.39–2.23), respectively, compared with the neither-condition group. Similar associations were observed for severe chronic pain. However, there were no significant additive interactions. In addition, only sleep disturbance was significantly associated with chronic pain, after adjusting for depressive symptoms.
Conclusions:
Our analyses did not reveal a synergistic effect of depressive symptoms and sleep disturbance on chronic pain and its severity, suggesting that most of the effects of depressive symptoms on chronic pain may be mediated by sleep disturbance.
1. Introduction
Chronic pain is highly prevalent, and it is a major reason that people seek medical care.30 A Global Burden of Disease Study published in 2017 reported that chronic pain is one of the most prominent causes affecting the number of years lived with disability and disability-adjusted life years.14 The comorbidity of chronic pain and psychological distress has also been linked to increased health care utilization and costs,28 and understanding the relationship between chronic pain and psychological distress thus has essential implications for public health and the economy.
It is widely known that depression and sleep problems often coexist in individuals with chronic pain.20,21,25,33 These reports suggested the possibility of depression–pain3 and sleep–pain12 bidirectional relationships. Although a bidirectional relationship has been observed between depressive symptoms (or sleep disturbance) and pain, compelling evidence from 2 studies suggested that the presence of depressive symptoms or sleep disturbance was a factor that exacerbated the participants' pain, rather than vice versa.12,32
Studies of individuals with type 2 diabetes mellitus have reported that sleep quality combined with anxiety or depressive symptoms has an additive effect on the quality of life.10,39 However, existing studies of individuals with chronic pain have examined depressive symptoms or sleep disturbance separately,21,33 and no studies have examined their combined effects on chronic pain. Considering that depressive symptoms, sleep disturbance, and chronic pain share common underlying neurobiological mechanisms,3,12,36,40 we hypothesized that a population of individuals with both depressive symptoms and sleep disturbance is more likely to have severe types of chronic pain due to the combined effects, and that depressive symptoms and sleep disturbance may have one or more additive effects on chronic pain and its severity. If one or more additive effects appear when these 2 types of psychological distress are combined, the demands for the assessment of and the need for interventions for depressive symptoms and sleep disturbance will increase from a clinical and public health perspective.
We conducted this study to investigate the effects of additive interactions between depressive symptoms and sleep disturbance on chronic pain and its severity, and we explored the independent and combined associations of depressive symptoms and sleep disturbance with chronic pain and its severity in community-dwelling older adults.
2. Participants and methods
2.1. Study population
The population-based Itoshima Frail Study was conducted in 2017 in the city of Itoshima, Japan, as an investigation of modifiable lifestyle and social factors. Our study included individuals aged 65 to 75 years who were not certified as requiring nursing care by the Japan's National Long-term Care Insurance System. Of approximately 10,000 older adults, we randomly selected 5000 according to their residential area, sex, and age. A set of study information sheets and questionnaires was mailed to these participants, inviting them to community centers for further assessments. Of the 5000 individuals contacted, 1589 submitted the questionnaires. We excluded the 215 individuals with missing psychological data (n = 198) and pain-related data (n = 17) for the present cross-sectional analyses. This study was approved by the Institutional Review Board of Kyushu University, Japan. None of the participants received compensation for their participation, and they each provided written informed consent to have their data used and published.
2.2. Measurement of depressive symptoms and sleep disturbance
Psychological distress was assessed using the 6-item Kessler Psychological Distress Questionnaire (K6).16 The K6 measures feeling nervous, hopeless, restless, depressed, or worthless or feeling that everything is an effort (over the last 30 days) on a 5-point scale (0–4), and the total score (range 0–24 points) has been used to indicate mood and anxiety disorders and severe mental disorders. The K6 has demonstrated good internal consistency (α = 0.86)11 and is validated to screen for depressive and anxious symptoms, with a K6 score ≥5 points indicating moderate psychological distress.26,31 Its screening performance for mood and anxiety disorders in a Japanese community sample was similar, with a K6 score ≥5 points showing 100% sensitivity and 68.7% specificity and a Center for Epidemiologic Studies–Depression scale (CES-D)27 score ≥16 points showing 100% sensitivity and 69.4% specificity, indicating that the K6 is an acceptable alternative to the CES-D as an assessment tool for epidemiological studies.29 In this study, a K6 score ≥5 points was thus considered to reflect depressive symptoms.
Sleep disturbance was measured using the Pittsburgh Sleep Quality Index (PSQI), a self-administered questionnaire for assessing subjective sleep quality during the previous month.6 The PSQI consists of 7 components: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication, and daytime functional impairment. We rated the PSQI on a 4-point scale from 0 to 3, and an overall score is calculated (range 0–21 points); the higher the overall score, the lower the subjective quality of sleep. The PSQI demonstrated good internal consistency based on Cronbach alpha.23 The reliability and validity of the Japanese version of the PSQI have been confirmed,9 and sleep disturbance was defined in this study as a total PSQI score ≥6 points.
The participants' status of depressive symptoms and sleep disturbance were defined as depressive symptoms (−) and sleep disturbance (−) for neither condition, depressive symptoms (+) and sleep disturbance (−) for depressive symptoms alone, depressive symptoms (−) and sleep disturbance (+) for sleep disturbance alone, and depressive symptoms (+) and sleep disturbance (+) for both conditions.
2.3. Assessment of chronic pain conditions
Chronic pain was assessed using questions ascertaining the respondent's pain lasting ≥3 months in the previous 12-month period.18 Response options were “yes” and “no.” When answering “yes,” the respondent was asked to indicate the affected musculoskeletal sites (total of 8 areas: neck, shoulders, elbows, wrists or hands, hips, knees, feet, and low back) on a body diagram.18 We assessed the severity of chronic pain subgroups based on the aspects of pain intensity, pain distribution, and pain type. We used a subjective Numerical Rating Scale (NRS) for the assessment of the intensity of current pain, and based on the participants' ratings, we classified the individual participants' chronic pain into mild-to-moderate (NRS ≤ 6) and severe (NRS ≥ 7) pain groups based on the pain intensity.34
Chronic widespread pain (CWP) was initially defined as pain present in both the left and right sides of the body as well as above and below the waist, including the axial skeleton, according to the 1990 American College of Rheumatology (ACR) criteria for fibromyalgia.38 However, because of a lack of detailed information, we modified this definition by omitting the condition of bilateral presentation; CWP was thus defined as pain at all of the following body sites: the axial skeleton (neck or low back), upper extremity (shoulders, elbows, or wrists or hands), and lower extremity (hips, knees, or feet). If participants did not meet this definition, they were categorized in the non-CWP group.
We assessed the participants' pain types by using the painDETECT questionnaire (PD-Q).13 The PD-Q was developed as a self-administered psychometric questionnaire to identify the type of pain (nociceptive and neuropathic pain). The Japanese version of the PD-Q has established validity and reliability.22 It comprises 3 items evaluating characteristics of the gradation of pain, the pain course pattern, and radiating pain, which contribute to an aggregate score (range 0–38 points). In this study, to compare purely nociceptive pain (PD-Q scores ≤12) with the neuropathic component, we combined the positive components (PD-Q score ≥19) and the unclear components (PD-Q score 13–18) with the neuropathic pain, which is referred to as neuropathic-like pain (NeP). If participants did not meet this definition, they were categorized as non-NeP.
2.4. Potential confounding factors
Considering the biopsychosocial model of chronic pain,8 we investigated the following variables. A questionnaire collected the following sociodemographic characteristics: age, sex, educational level (<10 or ≥10 years), employment status (employed or unemployed), subjective economic status (low or high), comorbidities (yes or no), current smoking (yes or no), current drinking (yes or no), regular exercise (≥3 times/week or not), living alone (yes or no), number of communications with someone (eg, family, relatives, neighborhood, and friends) per month (none or ≥1 person), and experiences of bereavement (yes or no). Comorbidities were confirmed by asking the participants whether they had any of the following currently being treated: osteoporosis, hypertension, hyperlipidemia, diabetes mellitus, stroke, and cardiovascular disease.
2.5. Statistical analyses
Descriptive data are presented as the mean (±SD) for continuous variables and as the frequency (percentage) for categorical variables according to the status of depressive symptoms and sleep disturbance. We calculated the internal consistency based on Cronbach alpha for each of the K6 and PSQI questionnaires. We performed the Dunnett test for group differences with the neither condition as the reference group. We used a direct method to calculate the age-adjusted and sex-adjusted prevalence of chronic pain and subgroups (pain intensity, pain distribution, and pain type) according to the participants' depressive symptoms and sleep disturbance status. We performed multivariable-adjusted binomial logistic regression analyses to investigate the association between the combination of depressive symptoms and sleep disturbance and chronic pain. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated, and the neither-condition group was regarded as the reference group.
The multivariable model was adjusted for age, sex, education level, employment status, subjective economic status, comorbidities, current smoking, current drinking, regular exercise, living alone, number of communications with someone per month, and experiences of bereavement. We also used a multinomial logistic regression model to examine the association between the combination of depressive symptoms and sleep disturbance and chronic pain subgroups (pain intensity, pain distribution, and pain type). We examined multiplicative interactions by using a series of logistic regression models with a product term, and we determined the relative excess risk due to interaction (RERI) to investigate the presence of interactions on an additive scale of whether the combination of depressive symptoms and sleep disturbance poses a greater risk than the sum of their independent effects. The RERI was calculated as the difference between the expected value based on the addition of the ORs of 2 separate risk factors and the observed value in both conditions, where RERI values (95% CI) > 0 indicate a synergistic effect with depressive symptoms and sleep disturbance.2
Another multivariate analysis was conducted in a separate model with depressive symptoms and sleep disturbance as confounders with each other to determine whether these factors influence the association with chronic pain. In addition, we calculated the ORs and 95% CIs of the presence of chronic pain with sleep disturbance alone against depressive symptoms alone as a reference group to directly compare the effects of depressive symptoms alone and sleep disturbance alone on chronic pain. The data were processed using SAS software ver. 9.4 (SAS Institute, Cary, NC). The statistical significance was defined as a 2-tailed P-value <0.05.
3. Results
Overall, 1374 participants with a mean age of 70.7 years (men: n = 704, 51.2%; women: n = 670, 48.8%) were included in the analyses (Table 1). The prevalence of depressive symptoms alone was 11.1% (n = 152), that of sleep disturbance alone was 15.1% (n = 208), and that of both conditions was 10.0% (n = 138). Cronbach alpha values for the K6 and PSQI were 0.83 and 0.74, respectively. Table 1 summarizes the characteristics of the participants according to the status of depressive symptoms and sleep disturbance. In the both-condition group, the frequencies of women, educational level <10 years, low subjective economic status, and comorbidities were significantly higher. By contrast, the frequencies of employed and regular exercise were significantly lower in the both-condition group compared with the neither-condition group.
Table 1.
Characteristics of the study participants according to the status of depressive symptoms and sleep disturbance.
| Overall | Neither condition (n = 876) | Depressive symptoms alone (n = 152) | Sleep disturbance alone (n = 208) | Both conditions (n = 138) | |
|---|---|---|---|---|---|
| Age, y, mean (SD) | 70.7 (3.0) | 70.5 (3.0) | 70.8 (3.1) | 71.2 (2.9)* | 71.2 (3.1) |
| Women, % | 48.8 | 46.4 | 52.6 | 50.0 | 58.0* |
| Educational level, <10 y, % | 13.9 | 11.0 | 18.4* | 19.2* | 19.6* |
| Employment status, employed, % | 35.9 | 38.1 | 35.5 | 33.2 | 26.8* |
| Subjective economic status, low, % | 51.8 | 46.4 | 64.2† | 55.1* | 67.7† |
| Comorbidities % | 69.1 | 66.8 | 74.3 | 70.2 | 76.1* |
| Osteoporosis, % | 7.9 | 7.0 | 10.5 | 9.1 | 9.4 |
| Hypertension, % | 42.4 | 42.2 | 40.8 | 45.2 | 41.3 |
| Hyperlipidemia, % | 30.4 | 29.2 | 30.3 | 29.8 | 38.4* |
| Diabetes mellitus, % | 16.2 | 15.9 | 15.8 | 16.8 | 18.1 |
| Stroke, % | 4.7 | 3.4 | 7.9* | 6.7* | 5.8 |
| Cardiovascular disease, % | 11.4 | 9.9 | 11.2 | 14.4 | 16.7* |
| Current smoking, % | 9.9 | 9.9 | 12.5 | 8.2 | 9.5 |
| Current drinking, % | 51.1 | 53.0 | 48.7 | 48.1 | 45.7 |
| Regular exercise, ≥3 times/wk, % | 65.1 | 66.6 | 65.1 | 66.0 | 54.7* |
| Living alone, % | 10.3 | 9.7 | 12.6 | 12.0 | 8.7 |
| No. of communications with someone per month, none, % | 17.3 | 15.8 | 23.7* | 15.4 | 22.5 |
| Experiences of bereavement, % | 46.7 | 45.4 | 43.4 | 49.5 | 54.4 |
P < 0.05.
P < 0.001 vs neither-condition group.
Among the 1374 participants, 849 individuals (61.8%) had chronic pain, 90 (6.6%) individuals had severe pain, 128 (9.3%) individuals had CWP, and 119 (8.7%) individuals had NeP. Figure 1 depicts the age-adjusted and sex-adjusted prevalence of chronic pain, pain intensity, pain distribution, and pain type compared with the neither-condition group. The prevalence of chronic pain was significantly higher in the depressive symptoms alone, sleep disturbance alone, and both-condition groups compared with the neither-condition group. The results for each chronic pain subgroups showed a generally similar trend, but the prevalence of non-CWP and NeP in the depressive symptoms alone group was not significantly different compared with that of the neither-condition group.
Figure 1.
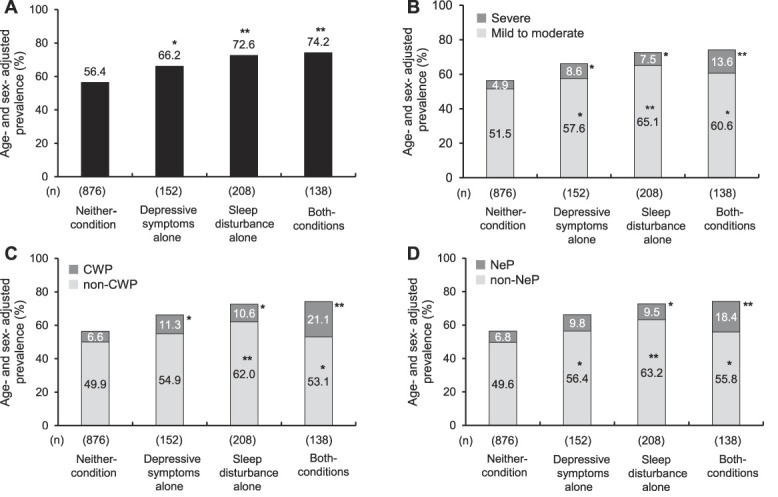
Age-adjusted and sex-adjusted prevalence of chronic pain (A), pain intensity (B), pain distribution (C), and pain type (D) according to the status of depressive symptoms and sleep disturbance. *P < 0.05, **P < 0.001 vs Neither-condition group. CWP, chronic widespread pain; NeP, neuropathic-like pain.
Table 2 provides the ORs and 95% CIs of the presence of chronic pain according to the status of depressive symptoms and sleep disturbance. Age-adjusted and sex-adjusted ORs for chronic pain were significantly higher for depressive symptoms alone, sleep disturbance alone, and both conditions compared with the neither-condition group. These associations did not materially change after adjusting for potential confounders, except for depressive symptoms alone group.
Table 2.
The odds ratios and 95% confidence intervals of the presence of chronic pain according to the status of depressive symptoms and sleep disturbance.
| Neither condition | Depressive symptoms alone | Sleep disturbance alone | Both conditions | |
|---|---|---|---|---|
| No. of events | 497 | 101 | 149 | 102 |
| Age-adjusted and sex-adjusted | 1.00 (ref.) | 1.52 (1.06–2.19) | 1.99 (1.43–2.77) | 2.22 (1.48–3.32) |
| P | 0.024 | <0.001 | <0.001 | |
| Multivariable-adjusted | 1.00 (ref.) | 1.40 (0.97–2.03) | 1.98 (1.41–2.78) | 2.12 (1.39–3.23) |
| P | 0.075 | <0.001 | <0.001 |
The data are n-values or odds ratio (95% confidence interval) values that were computed by using binomial logistic regression analysis. The multivariable model was adjusted for age, sex, education level, employment status, subjective economic status, comorbidities, current smoking, current drinking, regular exercise, living alone, number of communications with someone per month, and experiences of bereavement.
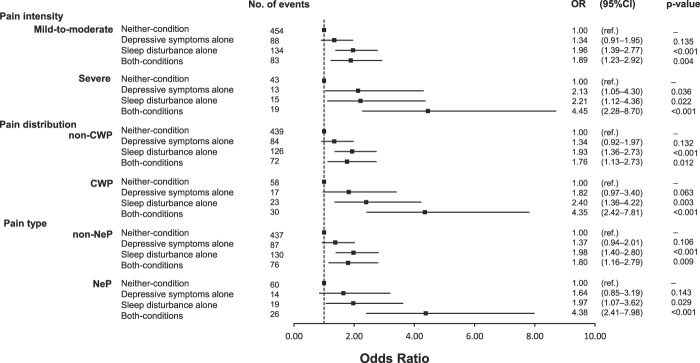
In the same way, the ORs and 95% CIs by chronic pain subgroups according to the status of depressive symptoms and sleep disturbance are shown in Figure 2. The multivariable-adjusted ORs for individuals in the sleep disturbance alone and both conditions had greater ORs of having severe, CWP, and NeP than the neither-condition group. Compared with the participants in the neither-condition group, those in the depressive symptoms alone group had a greater OR of severe pain but not CWP and NeP.
Figure 2.
Forest plot depicting multivariable-adjusted ORs and 95% CIs by chronic pain subgroups (pain intensity, pain distribution, and pain type) according to the status of depressive symptoms and sleep disturbance. ORs and 95% CIs were computed by a multinomial logistic regression analysis. The multivariable model was adjusted for age, sex, education level, employment status, subjective economic status, comorbidities, current smoking, current drinking, regular exercise, living alone, number of communications with someone per month, and experiences of bereavement. CI, confidence interval; CWP, chronic widespread pain; NeP, neuropathic-like pain; OR, odds ratio.
All multiplicative interactions between depressive symptoms and sleep disturbance on chronic pain and its severity had no significant associations. When the RERI was calculated by using chronic pain and its severity (ie, severe pain, CWP, and NeP), there were no significant additive interactions (chronic pain: RERI = −0.26, 95% CI = −1.41 to 0.89; severe pain: RERI = 1.11, 95% CI = −2.36 to 4.58; CWP: RERI = 1.13, 95% CI = −1.44 to 4.10; and NeP: RERI = 1.77, 95% CI = −0.95 to 4.84).
The results of our analyses of depressive symptoms and sleep disturbance in the separate models demonstrated that sleep disturbance remained significantly associated with chronic pain after the adjustment for depressive symptoms. By contrast, depressive symptoms were no longer significantly associated with chronic pain after the adjustment for sleep disturbance (Suppl. Table S1, available at http://links.lww.com/PR9/A169). In addition, the results of a direct comparison of the effects of depressive symptoms alone and sleep disturbance alone on chronic pain showed that the OR of the presence of chronic pain tend to be higher in the sleep disturbance alone group compared with the depressive symptoms alone group after adjustment for confounding factors (Suppl. Table S2, available at http://links.lww.com/PR9/A169).
4. Discussion
Our findings demonstrated that the combination of depressive symptoms and sleep disturbance was significantly associated with higher ORs for the presence of chronic pain compared with the neither-condition group in community-dwelling older adults, independently of the confounding factors. Similar associations were observed for severe chronic pain (severe pain, CWP, and NeP). However, we did not observe a synergistic effect of depressive symptoms and sleep disturbance on chronic pain and its severity. In addition, the sleep–pain association was stronger than the depression–pain association in this study.
Several research groups have reported that depressive symptoms predicted the development of chronic pain,7,21 severe chronic pain,17,32 CWP,19 and NeP.24 On the other hand, we detected no association between depressive symptoms and CWP or NeP. This discrepancy in findings between the earlier study and the present investigation may be attributed to the fact that the definitions of CWP and NeP differ from those of previous studies and did not exclude sleep disturbance from the depressive symptoms. Our present results demonstrated a strong association when depressive symptoms were combined with sleep disturbance compared with the neither-condition group.
A 2013 review published in The Journal of Pain summarized the longitudinal data on the association between sleep and pain.12 The review revealed that a key trend emerging from population-based longitudinal studies was that sleep disturbance reliably predicts new incidents and exacerbations of chronic pain (both regional and widespread). Longitudinal studies also supported the notion that sleep disturbance was a more reliable predictor of pain than pain is of sleep disturbance.12 Although the earlier studies support our present observations, our study adds the new finding that even after adjusting for confounding factors (including social factors), sleep disturbance alone was associated with chronic pain and its severity, and the association was much more potent when depressive symptoms were combined.
Several mechanisms are postulated as possible pathways linking depression, sleep, and chronic pain. Changes in central processes such as the descending pain inhibitory system, the stress system, the corticothalamic network, and neuroinflammation were reported to be able to affect pain sensitivity.3,12,36,39 A 2016 review of neurobiological factors involved in the interactions among depression, sleep disruption, and chronic pain showed comparable changes in the levels of serotonin, proinflammatory cytokines, brain-derived neurotrophic factor, and other transmitters in these disorders.4 The results of our present analyses demonstrated that individuals with both depressive symptoms and sleep disturbance are at high risk of developing chronic pain and its severity compared with the neither-condition group, but no synergistic effect of these risk factors was observed. This suggests that (1) much of the shared risk associated with depressive symptoms and sleep disturbance may be accounted for by characteristics that are intrinsic to depressive symptoms and to sleep disturbance, and (2) the risk is less dependent on the interaction between these psychological states.
Our study indicates that compared with depressive symptoms alone, sleep disturbance alone seems to have a more significant effect on chronic pain and its severity. Several mediation analyses have examined the relationships among sleep disturbance, depressive symptoms, and chronic pain. A systematic review of studies on the mediation of sleep and pain showed that the association between sleep disturbance and the onset of chronic pain seems to be partially mediated by depressive symptoms.36 However, an independent effect of sleep disturbance with chronic pain remains. Several studies have shown that the association between insomnia symptoms and chronic pain remained even after controlling for depression,35,37 suggesting that sleep and pain may be modulated beyond the effects of depression.
This study's results also showed that sleep disturbance remained significantly associated with chronic pain after the adjustment for depressive symptoms, but that depressive symptoms were no longer a significant association after the adjustment for sleep disturbance. We thus propose that (1) depressive symptoms are part of the symptoms manifested through sleep disturbance, and (2) most of the effects of depressive symptoms on chronic pain are mediated by sleep disturbance. Generaal et al.15 obtained evidence that an increase in depressive symptoms over time contributes to the link between insomnia and chronic pain and that sleep quality (but not sleep quantity) and difficulties initiating or maintaining sleep could worsen depressive symptoms over time.
The strengths of our study are as follows. Our analysis' results demonstrated that the combination of depressive symptoms and sleep disturbance was associated with chronic pain and its severity (severe pain, CWP, and NeP), independently of social factors such as living alone, the number of communications with someone per month, and experiences of bereavement. We then examined the effects of depressive symptoms alone and sleep disturbance alone on chronic pain and its severity separately. We were able to identify the consequences of pure depressive symptoms and sleep disturbance that may have been masked in previous studies. However, when interpreting the results of this study, some limitations need to be considered. First, the cross-sectional design does not allow conclusions about the direction of causality of these associations. Second, we used a reliable and valid K6 questionnaire, but it did not constitute clinical diagnoses of depressive symptoms. Future research should use clinical measures of sleep quality and depression or anxiety to examine their effects on chronic pain and its severity. Third, this study could not examine the association of the severity of depressive symptoms or sleep disturbance with chronic pain. Since this study was conducted in general community residents, there were few subjects with severe depressive symptoms or sleep disturbance (K6 ≥ 13 points = 1.0%, PSQI ≥ 9 points = 10.4%). We performed a sensitivity analysis with a more severe cutoff value for sleep disturbances (PSQI scores ≥ 9 points), and the direction of our conclusions remained the same. On the other hand, when the cutoff value for K6 was ≥13 points, we could not perform a sensitivity analysis because of poor statistical power. Fourth, the results may be underestimated because the individuals excluded from the study (n = 215) had a significantly higher prevalence of chronic pain compared with the individuals included in the analysis (61.8% vs 73.0%). Fifth, we could not rule out residual confounding effects such as trauma, emotional, physical, and sexual abuse.1 In addition, pain catastrophizing may be a potential confounding factor explaining the association between sleep disturbance and chronic pain.5 Future studies should examine the effects of catastrophic thoughts. Finally, the generalizability of our findings to other age groups and populations may be limited because the results of this study were obtained in an older population in a Japanese community. Studies with different age groups and ethnicities are needed to confirm the applicability of the present results to other populations.
In summary, our study demonstrated independent and combined associations of depressive symptoms and sleep disturbance with chronic pain and its severity in community-dwelling older adults. However, a synergistic effect of depressive symptoms and sleep disturbance on chronic pain and its severity was not observed. We suggest that depressive symptoms are part of the symptoms manifested through sleep disturbance and that most of the effects on chronic pain are mediated by sleep disturbance. It is thus reasonable to speculate that (1) the assessment of sleep disturbance (which may be a more important driving factor compared with depressive symptoms, particularly for CWP and NeP) is very important in efforts to combat individuals' chronic pain, and (2) combined interventions for sleep disturbance and depressive symptoms will become increasingly important to reduce the burden of chronic pain in community-dwelling older adults.
Disclosures
The authors have no conflict of interest to declare.
This study was supported in part by a grant from the Japan Agency for Medical Research and Development (no. JP17942839); by Grants-in-Aid for Scientific Research (B) (nos. JP 20H04016 and JP20H04030) and (C) (nos. JP20K102692, JP20K11446, and JP20K12510) and (Research Activity Start-up) (no. JP19K24259) from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by Itoshima City (2021-0032), and by Asanohi Orthopaedic Clinic (2020-0528). None of the funding sources had any role in the study design, data analysis, data interpretation, writing of the manuscript, or decision on the submission of this manuscript.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A169.
Acknowledgements
The authors acknowledge the Itoshima City office for their support of the participant recruitment.
Author contributions: T. Saito and H. Kishimoto were involved in the study design and conceptualization. T. Saito, T. Chen, H. Yatsugi, T. Chu, X. Liu, and H. Kishimoto were involved in the measurements and data set creation. T. Saito and H. Kishimoto were involved in the statistical analyses and interpretation of the results. T. Saito, T. Chen, H. Yatsugi, T. Chu, X. Liu, and H. Kishimoto were involved in the drafting and revising of the article. All authors have reviewed the article and approved its final version.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Takafumi Saito, Email: t.saito@rhs-u.ac.jp.
Tao Chen, Email: chentwhy@tongji.edu.cn.
Harukaze Yatsugi, Email: haru19920424@gmail.com.
Tianshu Chu, Email: chutianshu_japan@yahoo.co.jp.
Xin Liu, Email: liuxinjp1992@gmail.com.
References
- [1].Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, Cuneo JG. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med 2014;76:2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575–79. [DOI] [PubMed] [Google Scholar]
- [3].Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med 2003;163:2433–45. [DOI] [PubMed] [Google Scholar]
- [4].Boakye PA, Olechowski C, Rashiq S, Verrier MJ, Kerr B, Witmans M, Baker G, Joyce A, Dick BD. A critical review of neurobiological factors involved in the interactions between chronic pain, depression, and sleep disruption. Clin J Pain 2016;32:327–36. [DOI] [PubMed] [Google Scholar]
- [5].Buenaver LF, Quartana PJ, Grace EG, Sarlani E, Simango M, Edwards RR, Haythornthwaite JA, Smith MT. Evidence for indirect effects of pain catastrophizing on clinical pain among myofascial temporomandibular disorder participants: the mediating role of sleep disturbance. PAIN 2012;153:1159–66. [DOI] [PubMed] [Google Scholar]
- [6].Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- [7].Chou KL. Reciprocal relationship between pain and depression in older adults: evidence from the English Longitudinal Study of Ageing. J Affect Disord 2007;102:115–23. [DOI] [PubMed] [Google Scholar]
- [8].Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet 2021;397:2082–97. [DOI] [PubMed] [Google Scholar]
- [9].Doi Y, Minowa M, Uchiyama M, Okawa M, Kim K, Shibui K, Kamei Y. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res 2000;97:165–72. [DOI] [PubMed] [Google Scholar]
- [10].Dong D, Lou P, Wang J, Zhang P, Sun J, Chang G, Xu C. Interaction of sleep quality and anxiety on quality of life in individuals with type 2 diabetes mellitus. Health Qual Life Outcomes 2020;18:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ferro MA. The psychometric properties of the Kessler Psychological Distress Scale (K6) in an epidemiological sample of Canadian youth. Can J Psychiatry 2019;64:647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain 2013;14:1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–20. [DOI] [PubMed] [Google Scholar]
- [14].GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Generaal E, Vogelzangs N, Penninx BWJH, Dekker J. Insomnia, sleep duration, depressive symptoms, and the onset of chronic multisite musculoskeletal pain. Sleep 2017;40:40. [DOI] [PubMed] [Google Scholar]
- [16].Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SLT, Walters EE, Zaslavsky AM. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 2002;32:959–76. [DOI] [PubMed] [Google Scholar]
- [17].Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain 2011;12:964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kuorinka I, Jonsson B, Kilbom A, Vinterberg H, Biering-Sørensen F, Andersson G, Jørgensen K. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon 1987;18:233–37. [DOI] [PubMed] [Google Scholar]
- [19].Larsson B, Björk J, Börsbo B, Gerdle B. A systematic review of risk factors associated with transitioning from regional musculoskeletal pain to chronic widespread pain. Eur J Pain 2012;16:1084–93. [DOI] [PubMed] [Google Scholar]
- [20].Magni G, Marchetti M, Moreschi C, Merskey H, Luchini SR. Chronic musculoskeletal pain and depressive symptoms in the National Health and Nutrition Examination. I. Epidemiologic follow-up study. PAIN 1993;53:163–68. [DOI] [PubMed] [Google Scholar]
- [21].Magni G, Moreschi C, Rigatti-Luchini S, Merskey H. Prospective study on the relationship between depressive symptoms and chronic musculoskeletal pain. PAIN 1994;56:289–97. [DOI] [PubMed] [Google Scholar]
- [22].Matsubayashi Y, Takeshita K, Sumitani M, Oshima Y, Tonosu J, Kato S, Ohya J, Oichi T, Okamoto N, Tanaka S. Validity and reliability of the Japanese version of the painDETECT questionnaire: a multicenter observational study. PLoS One 2013;8:e68013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev 2016;25:52–73. [DOI] [PubMed] [Google Scholar]
- [24].Ohayon MM, Stingl JC. Prevalence and comorbidity of chronic pain in the German general population. J Psychiatr Res 2012;46:444–50. [DOI] [PubMed] [Google Scholar]
- [25].Ohayon MM. Relationship between chronic painful physical condition and insomnia. J Psychiatr Res 2005;39:151–59. [DOI] [PubMed] [Google Scholar]
- [26].Prochaska JJ, Sung HY, Max W, Shi Y, Ong M. Validity study of the K6 scale as a measure of moderate mental distress based on mental health treatment need and utilization. Int J Methods Psychiatr Res 2012;21:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- [28].Rayner L, Hotopf M, Petkova H, Matcham F, Simpson A, McCracken LM. Depression in patients with chronic pain attending a specialised pain treatment centre: prevalence and impact on health care costs. PAIN 2016;157:1472–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sakurai K, Nishi A, Kondo K, Yanagida K, Kawakami N. Screening performance of K6/K10 and other screening instruments for mood and anxiety disorders in Japan. Psychiatry Clin Neurosci 2011;65:434–41. [DOI] [PubMed] [Google Scholar]
- [30].St Sauver JL, Warner DO, Yawn BP, Jacobson DJ, McGree ME, Pankratz JJ, Melton LJ, III, Roger VL, Ebbert JO, Rocca WA. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc 2013;88:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Staples LG, Dear BF, Gandy M, Fogliati V, Fogliati R, Karin E, Nielssen O, Titov N. Psychometric properties and clinical utility of brief measures of depression, anxiety, and general distress: the PHQ-2, GAD-2, and K-6. Gen Hosp Psychiatry 2019;56:13–8. [DOI] [PubMed] [Google Scholar]
- [32].Stubbs B, Vancampfort D, Veronese N, Thompson T, Fornaro M, Schofield P, Solmi M, Mugisha J, Carvalho AF, Koyanagi A. Depression and pain: primary data and meta-analysis among 237 952 people across 47 low- and middle-income countries. Psychol Med 2017;47:2906–17. [DOI] [PubMed] [Google Scholar]
- [33].Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep 2007;30:213–18. [DOI] [PubMed] [Google Scholar]
- [34].Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand'homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). PAIN 2019;160:19–27. [DOI] [PubMed] [Google Scholar]
- [35].Vgontzas A, Cui L, Merikangas KR. Are sleep difficulties associated with migraine attributable to anxiety and depression? Headache 2008;48:1451–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Whibley D, Alkandari N, Kristensen K, Barnish M, Rzewuska M, Druce KL, Tang NKY. Sleep and pain: a systematic review of studies of mediation. Clin J Pain 2019;35:544–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wilson KG, Eriksson MY, D'Eon JL, Mikail SF, Emery PC. Major depression and insomnia in chronic pain. Clin J Pain 2002;18:77–83. [DOI] [PubMed] [Google Scholar]
- [38].Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- [39].Zhang P, Lou P, Chang G, Chen P, Zhang L, Li T, Qiao C. Combined effects of sleep quality and depression on quality of life in patients with type 2 diabetes. BMC Fam Pract 2016;17:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zis P, Daskalaki A, Bountouni I, Sykioti P, Varrassi G, Paladini A. Depression and chronic pain in the elderly: links and management challenges. Clin Interv Aging 2017;12:709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A169.