Abstract
Hepatocellular carcinoma is one of the leading causes of cancer mortality globally, and its incidence is increasing. Immune checkpoint therapy has revolutionized the treatment of hepatocellular carcinoma over the past few years. However, only a limited proportion of patients with hepatocellular carcinoma respond to immunotherapy. Despite the significant breakthroughs, the molecular mechanisms that drive immune responses and evasion are largely unresolved. Predicting tumor response and resistance to immune checkpoint inhibitors is a significant challenge. In this review, we focus on the current research progress of immune checkpoint inhibitors in hepatocellular carcinoma. Importantly, this review highlights the underlying mechanisms of resistance to immune checkpoint inhibitors and summarizes potential strategies to overcome the resistance to immune checkpoint inhibitors in hepatocellular carcinoma.
Keywords: hepatocellular carcinoma, resistance, immune checkpoint inhibitors, mechanism, combination therapy
Introduction
With an estimated 906,000 new cases and 830,000 deaths in 2020, primary liver cancer is the sixth most often diagnosed cancer and the third leading cause of cancer mortality globally. Hepatocellular carcinoma (HCC) accounts for 75%–85% of all primary liver cancer cases. The overwhelming number of patients diagnosed with HCC is in advanced stages, and just a tiny number are candidates for potentially effective treatment (1). The multikinase inhibitors sorafenib and lenvatinib have been the systematic treatment of choice for individuals with advanced HCC until recently. In recent years, the rapid development of immune checkpoint inhibitors (ICIs), particularly those targeting cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), programmed death-1 (PD-1), and programmed death ligand-1 (PD-L1), has altered therapeutic alternatives for solid malignancies. In HCC, ICIs have evolved as potentially promising anticancer therapeutic approaches, and several ICIs have been approved for the treatment of HCC, resulting in a paradigm shift in the management of HCC. In particular, the combination of atezolizumab and bevacizumab has emerged as the preferred systemic treatment option for patients with advanced or inoperable HCC in 2021 (2–5). Although ICIs offer new hope to patients with advanced HCC, ICI alone, such as nivolumab and pembrolizumab, is only moderately effective in patients with HCC. The findings of the current trial show significant heterogeneity in the extent to which tumors respond to these ICIs. In a phase 3 trial (CheckMate 459), nivolumab monotherapy did not significantly enhance overall survival (OS) in the first-line setting for advanced HCC patients compared with sorafenib. The negative results suggest that nivolumab monotherapy might be a first-line systemic therapy option for patients who are not candidates for tyrosine kinase inhibitors or antiangiogenic agents (6). Similarly, second-line pembrolizumab treatment did not significantly improve OS and progression-free survival (PFS) compared with sorafenib in a phase 3 trial (KEYNOTE-240) (7).
Potential predictors and biomarkers of ICIs in HCC with the ability to explain the heterogeneity would be beneficial for optimizing patient selection in the clinical setting. Although immunohistochemistry for PD-L1 is utilized to identify a variety of cancers that are most likely to respond to anti-PD-1/PD-L1 therapy, including bladder cancer, breast cancer, non-small cell lung cancer, head and neck cancer, gastric cancer, esophageal and esophagogastric junction cancer, cervical cancer, and vulvar cancer, its predictive value for HCC response is questionable (8). Multiple cancers with a high tumor mutational burden (TMB), such as bone cancer, melanoma, breast cancer, head and neck cancer, prostate cancer, and testicular cancer, are more likely to respond to ICIs. However, its ability to predict HCC response is debatable (9, 10). Furthermore, high microsatellite instability (MSI-H) or deficient mismatch repair (dMMR) may predict immunotherapy response in advanced solid tumors, including bone cancer, breast cancer, cervical cancer, colon cancer, esophageal and esophagogastric junction cancer, gastric cancer, head and neck cancer, extrahepatic cholangiocarcinoma, gallbladder cancer, intrahepatic cholangiocarcinoma, pancreatic adenocarcinoma, penile cancer, epithelial ovarian cancer/fallopian tube cancer/primary peritoneal cancer, prostate cancer, rectal cancer, small bowel adenocarcinoma, testicular cancer, thyroid carcinoma, uterine neoplasms, and vulvar cancer (11, 12). Dostarlimab-gxly (an anti-PD-1 antibody) can be considered in patients with recurrent or advanced HCC carrying MSI-H/dMMR (13, 14).
Although ICIs have revolutionized the management of HCC, the treatment outcomes may be unpredictable and inconsistent. A considerable proportion of patients do not respond to ICIs or develop resistance to them. Determining which patients can benefit from immunotherapy is one of the significant challenges. Combination therapy strategies may improve the efficacy of ICIs (15). This review sheds light on the mechanisms behind immunotherapy resistance in HCC and provides prospective options for overcoming ICI resistance.
Immune checkpoint inhibitors in hepatocellular carcinoma
Cancer cells block T-cell activation and produce immune checkpoint proteins on T cells, making immune activation more challenging. When CTLA-4 and PD-1 bind to their ligands, T-cell activity is reduced, and antitumor immunity is further blocked. As a result, CTLA-4, PD-1, or PD-L1 inhibitors may be effective in cancer immunotherapies ( Table 1 ) (16). CTLA-4 acts as a negative modulator of T-cell effector activity, making it an appealing target for cancer treatment. In the CTLA-4 pathway, exocytosis of CTLA-4 from intracellular vesicles to the T-cell surface is activated by binding signals of T-cell receptor (TCR)-major histocompatibility complex (MHC) and CD28-B7. CLTA-4 can bind to B7 with a higher binding affinity than CD28, resulting in the decreased activity of T cells. CTLA-4 inhibitors can interfere with the interaction of CTLA-4 on T cells with B7 ligands on antigen-presenting cells (APCs), suppressing regulatory T cell (Treg)-related immunosuppression and promoting the function of T-cell effector, resulting in an immune response. The T-cell effector function is suppressed by the interaction between PD-1 on T cells and its ligand PD-L1 on APCs. The PD-1/PD-L1 pathway is a negative modulator of immune responses and a crucial route for tumor immune escape. PD-1 inhibitors specifically target PD-1 and block the interaction between PD-L1 and PD-1. Inhibitors that target PD-L1 prevent PD-L1 from binding to PD-1. PD-1/PD-L1 inhibitors disrupt the feedback loop between T cells and tumor cells in the tumor microenvironment (TME), restoring T-cell effector function and increasing antitumor efficacy (17).
Table 1.
Selected trials of immune checkpoint inhibitors in advanced hepatocellular carcinoma.
| Trial Name | Phase | Intervention | Target | Setting |
|---|---|---|---|---|
| NCT02576509 | Phase III | Nivolumab | PD-1 | Advanced HCC |
| NCT02702401 | Phase III | Pembrolizumab | PD-1 | Participants with previously systemically treated advanced HCC |
| NCT03434379 | Phase III | Bevacizumab + Atezolizumab | VEGF; PD-L1 | Untreated locally advanced or metastatic HCC |
| NCT04039607 | Phase III | Nivolumab + Ipilimumab | PD-1; CTLA-4 | Advanced HCC |
| NCT03764293 | Phase III | Camrelizumab + Apatinib | PD-1; VEGF | First-line therapy in patients with locally advanced or metastatic and unresectable HCC |
| NCT03755791 | Phase III | Atezolizumab + Cabozantinib | VEGF; PD-L1 | Advanced HCC who have not received previous systemic anticancer therapy |
| NCT03298451 | Phase III | Durvalumab + Tremelimumab | PD-1; CTLA-4 | Advanced HCC |
| NCT03713593 | Phase III | Lenvatinib + Pembrolizumab | VEGF; PD-1 | First-line therapy in participants with advanced HCC |
| NCT03794440 | Phase II/III | Sintilimab + IBI308 | PD-1; VEGF | Advanced HCC |
| NCT03412773 | Phase III | Tislelizumab | PD-1 | Unresectable HCC |
| NCT04102098 | Phase III | Bevacizumab + Atezolizumab | VEGF; PD-L1 | Adjuvant therapy in patients with HCC at high risk of recurrence after surgical resection or ablation |
| NCT04665609 | Phase III | Thermal Ablation+Anlotinib+TQB2450 | VEGF; PD-L1 | Advanced HCC |
| NCT04167293 | Phase II/III | Radiation + Sintilimab | PD-1 | HCC |
| NCT04709380 | Phase III | Radiotherapy + Toripalimab | PD-1 | Advanced HCC with portal vein/hepatic vein tumor thrombosis |
| NCT04523493 | Phase III | Toripalimab + Lenvatinib | PD-1; VEGF | Advanced HCC |
| NCT04803994 | Phase III | Bevacizumab + Atezolizumab | VEGF; PD-L1 | Intermediate-stage HCC |
| NCT03867084 | Phase III | Pembrolizumab | PD-1 | Adjuvant therapy in participants with HCC and complete radiological response after surgical resection or local ablation |
| NCT04246177 | Phase III | Lenvatinib + Pembrolizumab + TACE | VEGF; PD-1 | Incurable/non-metastatic HCC |
| NCT03062358 | Phase III | Pembrolizumab | PD-1 | Previously treated advanced HCC |
| NCT04044651 | Phase III | Lenvatinib + Nivolumab | VEGF; PD-1 | Advanced HCC with hepatitis B virus infection |
| NCT03605706 | Phase III | Camrelizumab + FOLFOX4 | PD-1 | Advanced HCC who have never received prior systemic treatment |
| NCT04465734 | Phase III | HLX10 + HLX04 | PD-1; VEGF | First-line treatment in patients with locally advanced or metastatic HCC |
| NCT03859128 | Phase II/III | Toripalimab | PD-1 | Adjuvant therapy in HCC after radical resection |
| NCT05250843 | Phase II/III | TACE/HAIC + Lenvatinib + Sintilimab | VEGF; PD-1 | Neoadjuvant therapy for intermediate-stage HCC |
HAIC, hepatic arterial infusion of FOLFOX-based chemotherapy; TACE, transarterial chemoembolization; HCC, hepatocellular carcinoma.
Patients with advanced HCC receiving 3 mg/kg nivolumab (an anti-PD-1 antibody) exhibited an objective response rate of 20% (95% CI: 15–26) in a phase I/II trial (CheckMate-040), contributing to its approval as the first second-line systemic treatment for HCC. In this trial, 25% (12/48) of patients had grade 3/4 treatment-related adverse events. Treatment-related serious adverse events occurred in 6% (3/50) of patients, including adrenal insufficiency, liver disorder, and pemphigoid (2).
Pembrolizumab (an anti-PD-1 antibody) also received accelerated approval as a second-line treatment after reporting an objective response in 17% (18/104) of advanced HCC patients in a phase II trial (KEYNOTE-224). In this trial, 28% (29/104) of patients had grade 3/4 treatment-related adverse events, mainly including increased aspartate aminotransferase (7%), fatigue (4%), increased alanine aminotransferase (4%), hyperbilirubinemia (2%), and adrenal insufficiency (2%) (3). In an Asian subgroup analysis of the KEYNOTE-240 trial, the pembrolizumab group had a median PFS of 2.8 months (95% CI: 2.6–4.1), whereas the placebo group had a median OS of 1.4 months (95% CI: 1.4–2.4) [hazard ratio (HR): 0.48; 95% CI: 0.32–0.70] after a median follow-up of 13.8 months for the pembrolizumab group and 8.3 months for the placebo group. The median OS was 13.8 months (95% CI: 10.1–16.9) and 8.3 months (95% CI: 6.3–11.8) (HR: 0.55; 95% CI: 0.37–0.80) for pembrolizumab and placebo, respectively. In this trial, 58.9% (63/107) and 48.0% (24/50) of patients in the pembrolizumab and placebo groups experienced treatment-related adverse events, respectively. Grade 3–5 treatment-related adverse events occurred in 13.1% (14/107) and 4.0% (2/50) of patients in the pembrolizumab and placebo groups (18).
The phase I/II trial (CheckMate 040) was the first to exhibit the efficacy of a CTLA-4 inhibitor (ipilimumab) in combination with a PD-1 inhibitor (nivolumab) in patients with advanced HCC. Patients receiving ipilimumab (3 mg/kg) plus nivolumab (1 mg/kg) every 3 weeks (four doses) followed by nivolumab 240 mg every 2 weeks achieved an investigator-assessed objective response rate of 32%. One grade 5 treatment-related adverse event of pneumonitis occurred (4). Furthermore, the IMbrave150 trial demonstrated that the combination of atezolizumab (an anti-PD-L1 antibody) and bevacizumab [an anti- vascular endothelial growth factor A (anti- VEGFA) antibody] was superior to sorafenib in the treatment of unresectable HCC. Patients in the atezolizumab plus bevacizumab group had significantly better 12-month OS than that in the sorafenib group (67.2% vs. 54.6%). Median PFS in the atezolizumab plus bevacizumab group was 6.8 months (95% CI: 5.7–8.3), whereas it was only 4.3 months in the sorafenib group (95% CI: 4.0–5.6). In this trial, 56.5% (186/329) of patients who received atezolizumab plus bevacizumab and 55.1% (86/156) of patients who received sorafenib had grade 3/4 adverse events (5).
In the COSMIC-312 trial (NCT03755791), the median PFS of cabozantinib plus atezolizumab and sorafenib monotherapy was 6.8 months (99% CI: 5.6–8.3) and 4.2 months (99% CI: 2.8–7.0) HR: 0.63, 99% CI: 0.44–0.91, p = 0.0012) for patients with advanced HCC; the median OS was 15.4 months (96% CI: 13.7–17.7) and 15.5 months (12.1–not estimable), respectively. Treatment-related adverse events occurred in 93% (399/429) of 429 patients in the cabozantinib plus atezolizumab group and 95% (178/188) of patients in the sorafenib group. Furthermore, 18% (78/433) of patients in the cabozantinib plus atezolizumab group and 8% (16/200) of patients in the sorafenib group experienced serious treatment-related adverse events (19). The HIMALAYA study showed that the OS for patients with unresectable HCC was dramatically improved with tremelimumab plus durvalumab compared to sorafenib HR: 0.78; 96% CI: 0.65–0.92; p = 0.0035). Moreover, 25.8%, 12.9%, and 36.9% of patients experienced grade 3/4 treatment-related adverse events in the tremelimumab plus durvalumab, durvalumab, and sorafenib groups, respectively (20). After a median follow-up of 10.0 months, the median PFS of sintilimab (a PD-1 inhibitor) plus IBI305 (a bevacizumab biosimilar) was 4.6 months (95% CI: 4.1–5.7) and sorafenib was 2.8 months (95% CI: 2.7–3.2) for patients with unresectable HCC in the ORIENT32 study (NCT03794440). In this study, 32% (123/384) of patients in the sintilimab plus IBI305 group and 19% (36/189) of patients in the sorafenib group experienced serious adverse events (21). Therefore, the paradigm for treating HCC has shifted as a result of the development of ICIs. ICIs are becoming more and more significant in the management of HCC.
Underlying mechanisms of resistance to immune checkpoint inhibitors in hepatocellular carcinoma
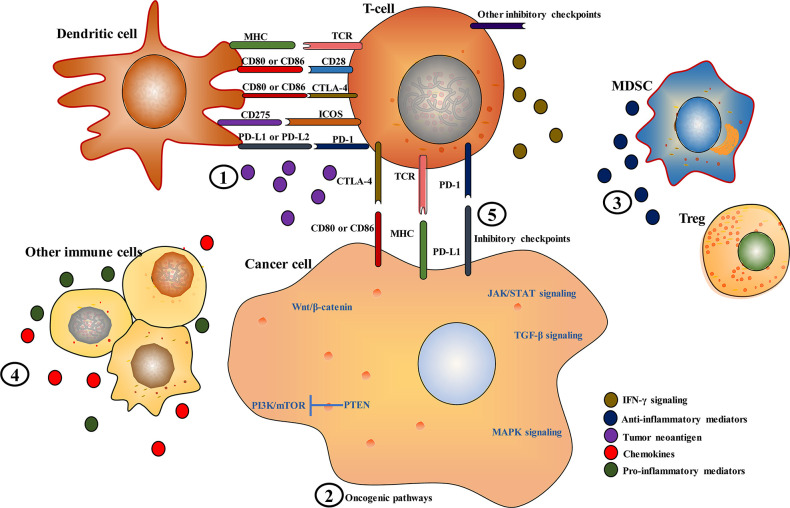
A proportion of patients with HCC benefit from ICIs. However, primary (intrinsic) and acquired resistance are the primary clinical barriers to improving the outcome of patients with advanced HCC (9). Primary resistance occurs when there is no initial response to immunotherapy. Acquired resistance often develops disease progression after an initial response to immunotherapy. Mechanisms of primary and acquired resistance to immunotherapy somehow overlap (22). Primary resistance is primarily associated with the innate inability of the immune system to initiate an efficient immune response. Changes in epigenetic or translational processes may lead to adaptive changes in tumor cells and the TME, resulting in immunotherapy resistance. Acquired resistance to ICIs may occur in cancer cells that experienced clonal evolution and genetic alterations. Both tumor intrinsic and extrinsic mechanisms may cause primary or acquired resistance to ICIs. Understanding the underlying molecular mechanisms behind ICI resistance is necessary to optimize current treatment strategies or develop innovative ones. Intratumor heterogeneity is a significant contributor to the fatal result of cancer, treatment failure, and drug resistance. Intratumor heterogeneity offers a variety of genetic and epigenetic materials for Darwinian evolution. The evolving mutational landscape may affect immune surveillance and response to ICIs (23, 24). In tumors, the cancer immunity cycle involves cancer cell antigen release, cancer antigen presentation, priming and activation, trafficking of T cells to tumors, infiltration of T cells into tumors, recognition of cancer cells by T cells, and killing of cancer cells. Immunotherapy resistance is closely associated with the alterations in the cancer immunity cycle (25, 26). Therefore, decreased neoantigen expression, impaired antigen recognition, ineffective antigen presentation, insufficient priming and activation of tumor-specific T cells, poor trafficking of the activated effector T cells to tumors, decreased infiltration of the activated effector T cells into tumors, insufficient cancer cell recognition by T cells, inadequate expansion of T cells or lack of costimulation, the presence of T-cell inhibitory factors or other T-cell inhibitory immune cells in the TME can cause resistance to immunotherapy in tumors. Here, mechanisms that contribute to the resistance of ICIs in HCC are roughly summarized ( Figure 1 ).
Figure 1.
Key mechanisms of immune checkpoint inhibitor resistance in hepatocellular carcinoma. (1) Inadequate, absence or alterations in the presentation or processing of tumor neoantigens; (2) alterations in oncogenic pathways, such as phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR), mitogen-activated protein kinase (MAPK), Janus kinase/signal transducer and activator of transcription (JAK/STAT), Wnt/β-Catenin, and transforming growth factor beta (TGF-β) signaling pathways; (3 and 4) polarization toward an immunosuppressive microenvironment by reducing pro-inflammatory mediators and increasing anti-inflammatory mediators; (5) other novel immune checkpoint molecules, such as T-cell immunoglobulin and ITIM domain (TIGIT), lymphocyte activation gene-3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), B7 homolog 3 protein (B7-H3), B and T lymphocyte attenuator (BTLA), V-domain immunoglobulin suppressor of T cell activation (VISTA), and inducible T-cell costimulatory (ICOS).
Tumor intrinsic mechanisms are closely associated with tumor cell alterations that affect neoantigen expression, antigen presentation, and expression of immune co-inhibitory signals, resulting in defective antigen recognition and decreased T-cell recruitment and activity in tumors. In HCC, alterations in several oncogenic pathways, such as Wnt/β-Catenin, mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR), and transforming growth factor beta (TGF-β) pathways, can affect T-cell recruitment and function.
Activation of tumor-intrinsic β-catenin signaling leads to T-cell exclusion and resistance to ICI therapy (27). Immune classification investigations established an immune exclusion class characterized by Wnt/CTNNB1 mutations that accounts for about 30% of HCCs (28, 29). Strong associations between T-cell exclusion and CTNNB1 mutations in HCC revealed that β-catenin activation might contribute to immune evasion and immunotherapy resistance (29, 30). In HCC cells, activation of β-catenin hampers the recruitment of CD103+ dendritic cells (DCs) and antigen-specific T cells, resulting in a diminished antitumor immune response. Chemokines, such as C-C Motif Chemokine Ligand 5 (CCL5), C-X-C Motif Chemokine Ligand 1 (CXCL1), and Chemokine (C-C motif) ligand 20 (CCL20), were significantly decreased in CTNNB1-mutant tumors. CCL5 overexpression in β-catenin-driven HCC cells enhanced the recruitment of antigen-specific CD8+ T cells and CD103+ DCs and restored immune surveillance. Numerous studies have exhibited that an aberrant WNT/β-catenin pathway is closely associated with resistance to anti-PD-(L)1 therapy in HCC (28, 31, 32). According to a recent study, two of three HCC patients with CTNNB1 mutations did not respond to anti-PD-1 therapy (31). In another study, none of 10 HCC patients with CTNNB1 or AXIN1 alterations responded to the anti-PD-(L)1 agent, while 53% (9/17) of patients without WNT/β-catenin pathway alterations attained durable stable disease as the best response, indicating that aberrant WNT/β-catenin pathway alterations may enhance immune evasion and resistance to anti-PD-(L)1 therapy (28). Suppression of β‐catenin signaling in HCC may improve antitumor T-cell activation, resulting in the production of CD8+ effector T cells, promoting their penetration into the TME, and reducing CD8+ T-cell exhaustion after an initial response to anti‐PD‐1 treatment (33). Moreover, the Wnt/β-catenin signaling pathway activation causes M2 macrophage polarization in HCC cells, and Wnt ligands produced by macrophages further trigger the Wnt/β-catenin signaling pathway (34).
TGF-β signaling is involved in the regulation of immune cells, such as myeloid-derived suppressor cells (MDSCs), Dendritic cells (DCs), natural killer (NK) cells, and tumor-associated macrophages (TAMs), and plays a critical role in the proliferation, development, and differentiation of immune cells. TGF-β stimulates SMAD Family Member 2/3 (SMAD2/3) and interacts with interleukin (IL)-21 to enhance the maturation of CD4+ T cells into Th17 cells, which produce IL-17 and contribute to the development of HCC (35). TGF-β acts as a suppressor of the Th1 and Th2 lineages by suppressing T-bet and Sox4 expression, respectively, and facilitating the transformation of Th1 cells into Th2 cells (36). TGF-β collaborates with Activating Transcription Factor 1 (ATF1) to decrease interferon (IFN)-γ expression in CD8+ cytotoxic T cells, impairing its anticancer efficacy (37). TGF-β induces the development of M2-type macrophages, which inhibit the CD8+ T cell, NK cell, and DC activity and enhance the CD4+ Treg activity. TGF-β stimulates PD-1 expression in antigen-specific T cells via the SMAD3-dependent pathway (38). Elevated TGF-β signaling may lead to T-cell exhaustion through activation of PD-1 signaling, and TGF-β signaling suppression may improve antitumor immunity in HCC. The TGF-β signaling may block immunotherapy with anti-PD-(L)1 antibody via increasing PD-L1 expression. The TGF-β signaling may stimulate Treg expansion and disrupt immunotherapy with anti-CTLA-4 antibodies. In HCC, immune checkpoint molecules are modulated by TGF-β1-mediated epithelial–mesenchymal transition (EMT) (39). The TGF-β signature in HCC may be a promising indicator for recognizing the “exhausted” immune signature (40).
MAPK-RAP1A signaling is closely associated with tumor-infiltrating immune cells (TICs) in HCC (41). In HCC cells, the epidermal growth factor receptor (EGFR)‐P38 MAPK axis enhanced the aerobic glycolysis in HCC cells, which may stimulate tumor immunosuppression. The EGFR‐P38 MAPK axis can enhance PD‐L1 expression through miR‐675‐5p and suppress HLA‐I (HLA‐ABC) expression via glycolysis‐related enzyme hexokinase 2 (HK2) in HCC (42). IFN-γ enhanced CD274 transcriptional activity, and MAPK signaling elevated the stability of CD274 mRNA. Suppression of the MAPK pathway blocked the EGF and IFN-induced overexpression of PD-L1 in HCC cells (43). RAF proto-oncogene serine/threonine-protein kinase (RAF) dimers and ERK signaling activation induce immunosuppression through MAPK/Nuclear factor kappa B (NF-κB)-dependent PD-L1 expression in HCC (44).
Phosphatase and tensin homolog (PTEN) loss can lead to PI3K signaling pathway activation, resulting in decreased T-cell infiltration and increased immunosuppression (45). PTEN-deficient tumors may display impaired stimulation of the type I IFN and NF-κB pathways, contributing to tumor progression owing to the immunosuppressive TME (46). Endoplasmic reticulum (ER)-stressed HCC cells produce exosomes to increase PD-L1 expression in macrophages, which further suppress T-cell activity via an exosome miR-23a/PTEN/AKT pathway (47).
IFN-γ interacts with IFN-γ receptors (IFNGRs) and activates Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling pathway, which further promotes an IFN-stimulated gene (ISG) transcriptional pathway and modulates the immune response. Abnormalities in IFN-γ receptors or IFN-γ pathway-related genes such as JAK1, JAK2, IFNGR1, and IFNGR2 may hamper tumor immune response and cause ICI resistance (48). IFN-γ-induced elevation of B7-H1 expression through the JAK/STAT1 pathway is responsible for adaptive immune resistance in HCC (49). PD-L1 expression induced by IFN-γ can be mediated by the IFN-γ/JAK/STAT1/IFN regulatory factor 1 (IRF1) pathway in HCC cells. MYC (MYC proto-oncogene, bHLH transcription factor) suppression increased STAT1 expression, a crucial component of the IFN-γ signaling pathway, increasing PD-L1 expression induced by IFN-γ in HCC cells (50). The aberrant activation of the IL-10/JAK1/STAT5/Foxp3 pathway may facilitate the accumulation of Tregs in the TME (51).
Other tumor cell-intrinsic mechanisms are closely associated with the expression of co-inhibitory signals, inadequate antigen expression, and impaired antigen processing and presentation. Tumor-specific neoantigens are the repertoires of peptides that bind to the MHC to form a stable complex for T-cell recognition, thereby eliciting anticancer T-cell responses. Tumor cells may evade immune surveillance through insufficient antigen and genetic and epigenetic changes in antigens (52). The β2-microglobulin (B2M) is a component of MHC Class I molecules, and homozygous B2M deficiency causes inadequate antigen presentation, hindering the recognition of CD8+ T cells (8). The function of the MHC to present neoantigens was impeded by loss of heterozygosity (LOH) of human leukocyte antigen (HLA) alleles, which was detected in 17% of multifocal HCC and associated with immune escape (53).
Tumor extrinsic mechanisms include the status of the host immune homeostasis and the features of the TME, which may affect T-cell priming and activation, antigen recognition, immune cell recruitment and activity, and co-inhibitory or costimulatory signals. The TME contains TAMs, MDSCs, and Tregs that exert immunosuppressive effects. These immunosuppressive immune cells interact with immunosuppressive cytokines, such as Indoleamine 2,3-dioxygenase (IDO), TGF-β, adenosine, IL-10, and CD73, to decrease antigen presentation and cytotoxic T-cell activity (54). Various immune checkpoint molecules such as PD-1, lymphocyte activation gene-3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), CTLA-4, T-cell immunoglobulin and ITIM domain (TIGIT), and V-domain immunoglobulin suppressor of T-cell activation (VISTA) exist within the TME. Immune cells and APCs in the TME may induce the expression of these immune checkpoint molecules, promoting T-cell exhaustion and hence decreasing the response to ICIs (55).
Tumor-associated neutrophils (TANs) cause CD8 T-cell apoptosis through the tumor necrosis factor (TNF)-α pathway, contributing to the immunosuppressive response. TANs and granulocytic myeloid-derived suppressor cells (G-MDSCs) inhibit the proliferation, activation, and antitumor activity of CD8 T cells. Moreover, TANs can inhibit the proliferation of T cells by releasing arginase 1 (ARG1) and regulating PD-L1/PD-1 signaling and induce an immunosuppressive response (56, 57).
Tregs can block immunological responses and sustain homeostasis and self-tolerance. Furthermore, Tregs can decrease T-cell proliferation and cytokine secretion and suppress autoimmunity (58). Treg enrichment was found in primary HCC, resulting in an immunosuppressive setting, while Treg exclusion and CD8+ T cell enrichment were detected in early-recurrent HCC (59). Infiltrating Tregs and exhausted CD8+ T cells are clonally enriched in HCC. Layilin (LAYN) is a signature gene for tumor-specific Tregs and exhausted CD8+ T cells, and LAYN overexpression in CD8+ T cells suppressed the production of IFN-γ, suggesting that LAYN had an inhibitory effect on CD8+ T cells. T cells in the TME are prone to exhaustion or Treg suppression, blocking CD8 cells from inducing T cell-mediated tumor cell killing (60).
The primary mechanisms by which MDSCs suppress immune responses in the TME include encouraging Treg differentiation and expansion, blocking DC, NK, and macrophage polarization to the M2 phenotype, depriving essential amino acids of T cells, and causing oxidative stress, indicating that MDSCs participate in multiple immunosuppressive pathways and are viable immunotherapeutic targets for HCC (61). MDSCs aggregate in the TME and act as pro-inflammatory mediators, inhibiting T‐cell activities and contributing to immune evasion. Triggered MDSCs produce cytokines and enzymes that suppress NK cells and T cells and stimulate Tregs. Hepatic receptor-interacting protein kinase 3 (RIP3) defect stimulates chemokine (CXC motif) ligand 1 (CXCL1)/chemokine (CXC motif) receptor 2 (CXCR2)–induced MDSC recruitment and decreases IFN+ CD8+ T-cell infiltration, thereby facilitating immune escape and HCC progression (62). MDSCs suppress hepatic NK cell function through the TGF-β pathway and promote Treg expansion (40).
TAMs typically recruit monocytes from the periphery through the action of chemokines and subsequently deposit them in tumor tissue. Tissue-resident macrophages move to hypoxic or necrotic regions of tumors where they are stimulated to undergo TAM transformation. TAMs evolved from monocytes into functional macrophages and acquired a range of immunosuppressive functions to maintain the TME. TAMs produce cytokines and chemokines, which stimulate tumor growth and suppress antitumor immunity (63, 64). M2 TAMs facilitate HCC progression via producing protumor and proangiogenic factors and inhibiting tumor-infiltrating T cell activation, while M1 TAMs secrete IL1b, reactive oxygen species (ROS), and other pro-inflammatory cytokines to inhibit tumor progression. TAMs are the predominant immunosuppressive component in the TME of HCC (65). Receptor-interacting protein kinase 3 (RIPK3) deficiency accelerated TAM polarization toward an M2 phenotype and facilitated the immunosuppressive function of TAMs (66).
Immunosuppressive cells, such as TAMs, TANs, MDSCs, tumor-associated DCs (tDCs), and Tregs, are important contributors to immune resistance and can also affect the therapeutic potential of ICIs (67, 68). Numerous mechanisms of immunotherapeutic resistance have been identified and well-described, and new ones are constantly being found. To aid in therapeutic decision-making, further knowledge of resistance mechanisms is required.
Potential strategies to overcome immune checkpoint inhibitor resistance in hepatocellular carcinoma
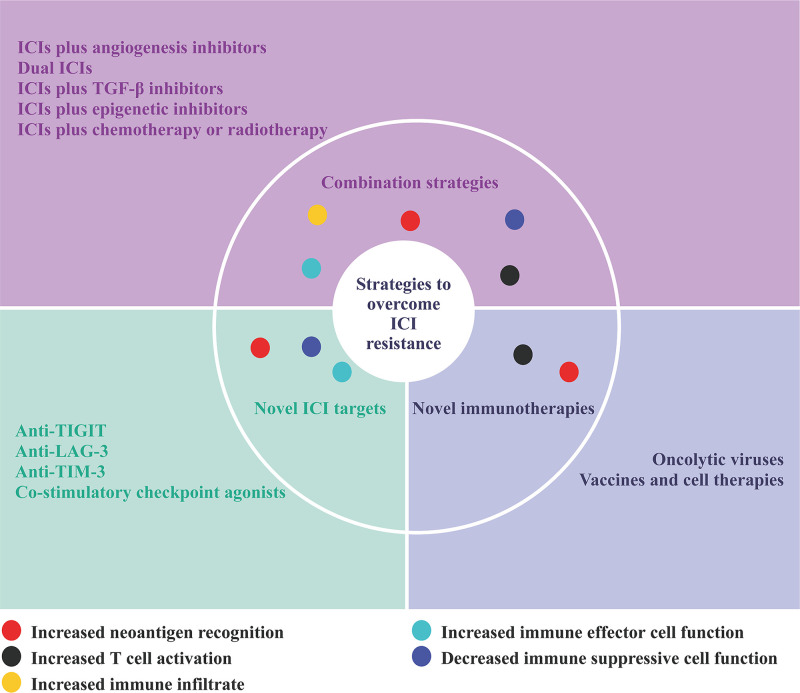
As the mechanisms of ICI resistance are understood, several therapeutic strategies have been developed to overcome ICI resistance. Current efforts to overcome ICI resistance for advanced HCC mainly focus on the following aspects: combination strategies involving ICIs, novel ICI targets, and novel immunotherapies ( Figure 2 ) (69).
Figure 2.
Strategies to overcome ICI resistance.
Combination strategies
Immune checkpoint inhibitors plus angiogenesis inhibitors
Numerous immunosuppressive mechanisms involved in the recurrence of HCC are regulated by VEGF or/and immune checkpoints. The combination of ICIs and antiangiogenic agents has been investigated in several clinical studies, and the results showed that dual blockade of ICIs and VEGF can improve anticancer immunity. The combination of ICIs and antiangiogenic agents may exert synergistic therapeutic effects through multiple mechanisms, such as vascular normalization, antitumor immune cell subset activation, and inhibition of tumor-promoting immune cells. Anti-PD-1/PD-L1 treatments may enhance recruitment or activation of DCs, effector CD8+ T cells, and NK cells and induce an antitumor M1 macrophage phenotype. Anti-VEGF treatments may decrease MDSC and Treg infiltration and activity and diminish the polarization of M2 macrophages. Suppression of DC maturation, inadequate antigen presentation, suppression of T-cell responses via upregulating PD-L1, PD-L2, and immunosuppressive molecules (IL-6, IDO-1, and IL-10), initiation of Tregs, and accumulation of MDSCs are some of the mechanisms by which VEGF has a significant impact on cancer immunity (69). In a phase III trial (IMbrave150), atezolizumab (PD-L1 antibody) plus bevacizumab (antiangiogenic agent) significantly improved overall and PFS compared to sorafenib, thus becoming the new standard of first-line treatment for advanced HCC (5, 70). Other combinations of ICIs and antiangiogenic agents, including durvalumab (an anti-PD-L1 agent) plus bevacizumab (EMERALD-1), pembrolizumab (an anti-PD-1 agent) plus lenvatinib (LEAP-012; LEAP-002), regorafenib plus nivolumab (an anti-PD-1 agent) (RENOTACE), atezolizumab (an anti-PD-L1 agent) plus cabozantinib (COSMIC-312) (19), camrelizumab (an anti-PD-1 agent) plus apatinib (NCT03764293), CS1003 (an anti-PD-1 agent) plus lenvatinib (NCT04194775), sintilimab (an anti-PD-1 agent) plus IBI305 (ORIENT-32) (21) and atezolizumab plus lenvatinib or sorafenib (IMbrave251), are under clinical investigation in HCC (69).
Dual immune checkpoint inhibitors
Dual ICIs are another promising strategy in HCC. The CheckMate 040 study presented the efficacy and safety of nivolumab (an anti-PD-1 inhibitor) in combination with ipilimumab (a CTLA-4 inhibitor) in advanced HCC. The U.S. Food and Drug Administration (FDA) has approved this strategy for the treatment of patients with advanced HCC (4). Other combinations of dual ICIs, including durvalumab (an anti-PD-L1 agent) plus tremelimumab (a CTLA-4 inhibitor) (EMERALD-2; HIMALAYA), are under clinical investigation in HCC (69). Other dual ICI strategies, including an anti-PD-1/PD-L1 agent plus an anti-TIM-3/anti-LAG-3/anti-TIGIT agent, are currently in clinical trials. Agonists targeting costimulatory pathways such as OX40 (CD134)/OX40L (CD252), CD40/CD40L, Glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR)/GITR ligand (GITRL), CD27/CD70, inducible T-cell costimulatory (ICOS)/ICOS ligand (ICOSL), 4-1BB (CD137, tumor necrosis factor receptor superfamily 9)/4-1BB ligand (4-1BBL) can stimulate T-cell activity and activate antitumor immunity. These combination strategies showed great potential in preclinical studies, and more research on patients with advanced solid tumors is warranted. Numerous clinical trials that combine costimulatory molecule agonists and α-PD-1/PD-L1 are ongoing in advanced solid tumors (71).
Immune checkpoint inhibitors plus TGF-β inhibitors
TGF-β, an immunosuppressive cytokine, inhibits effector T-cell activity and infiltration and increases Treg activity. Preclinical studies have indicated that inhibiting TGF-β signaling makes tumor cells more susceptible to ICIs. Targeting the TGF-β pathway with galunisertib (an anti-TGF-β agent) can increase the antitumor activity of anti-PD-(L)1 agents in a preclinical study (72). In a phase II study (NCT02423343), galunisertib (LY2157299) was evaluated with nivolumab in patients with advanced refractory solid tumors, including HCC. In addition, the TGF-β signature may serve as a potential biomarker for individualized immunotherapy in patients with HCC (40).
Immune checkpoint inhibitors plus epigenetic inhibitors
Epigenetic modulation may alleviate resistance to ICIs in HCC tumors with low immunogenicity and inadequate antigen presentation. DNA methyltransferase inhibitor therapy reverses MHC class I epigenetic inhibition and promotes antigen presentation, immunogenicity, and tumor immune targeting. In a humanized mouse model of immune deficiency, histone deacetylase 8 (HDAC8) inhibition elevated global and enhancer acetylation of H3K27, allowing HCC cells to generate T cell-trafficking chemokines and alleviating T-cell exclusion. HDAC8 inhibition enhanced tumor-infiltrating CD8+ T cells, improving the efficacy of the anti-PD-L1 agent in HCC (73). HDAC6 inhibits the pathogenicity of IL-17-producing helper T (TH 17) cells and the antitumor immune function via modulating forkhead box protein O1 (FoxO1). HDAC6-depleted T cells induce PD-1-PD-L1 expression to generate a powerful synergistic impact that makes advanced HCC more susceptible to ICIs (74). SGI-110 (a DNA methyltransferase inhibitor) inhibits both DNA methyltransferases and the PRC2 (polycomb repressive complex 2) complex in HCC. It can stimulate endogenous retrovirus (ERV) to activate the immune response pathway of cancer cells, offering a foundation for the combination therapy of DNA methyltransferase inhibitors and ICIs (75).
Immune checkpoint inhibitors plus chemotherapy or radiotherapy
Radiotherapy or chemotherapy can not only kill cancer cells but also modulate immunity. Numerous preclinical studies have demonstrated that the combination of radiotherapy or chemotherapy and ICIs is promising for multiple cancers, and several related clinical trials are currently underway. These combination strategies may improve the microenvironment for immune cells to interact with the tumor antigenic environment. However, more clinical evidence is needed to confirm whether radiotherapy or chemotherapy can enhance the immunotherapy effect of ICIs in HCC (76).
Novel immune checkpoint inhibitor targets
Targeting alternative immune checkpoints is one strategy for overcoming ICI resistance. Immune checkpoints such as TIGIT, LAG-3, TIM-3, B7 homolog 3 protein (B7-H3), B and T lymphocyte attenuator (BTLA), VISTA, and ICOS are viable and attractive targets for solid tumor treatment. Relevant clinical studies are now underway ( Table 2 ) (77, 78).
Table 2.
Novel immune checkpoint inhibitors in HCC.
| Drug | Target | NCT number | Phase | Settings | Treatment arms |
|---|---|---|---|---|---|
| Relatlimab | LAG-3 | NCT04658147 | I | Hepatocellular carcinoma | Nivolumab; Nivolumab + Relatlimab |
| SRF388 | LAG-3 | NCT04374877 | I | Advanced solid tumor | SRF388; SRF388 + Pembrolizumab |
| Relatlimab | LAG-3 | NCT04567615 | II | Liver cancer | Nivolumab; Nivolumab + Relatlimab |
| INCAGN02385 | LAG-3 | NCT03538028 | I | Advanced solid tumor | INCAGN02385 |
| Relatlimab | LAG-3 | NCT02465060 | II | Advanced solid tumor | Relatlimab |
| Pavunalimab | CTLA-4 and LAG-3 | NCT03849469 | I | Advanced solid tumor | Pavunalimab; Pavunalimab + Pembrolizumab |
| Cobolimab | TIM-3 | NCT03680508 | II | Liver cancer | Dostarlimab + Cobolimab |
| INCAGN02390 | TIM-3 | NCT03652077 | I | Advanced solid tumor | INCAGN02390 |
| KY1044 | ICOS | NCT03829501 | I and II | Advanced solid tumor | KY1044; KY1044 + Atezolizumab |
LAG-3 (CD223), a membrane receptor, is mainly expressed on activated T cells and NK cells. When LAG-3 binds with MHC class II, it suppresses the activity of T cells. Blocking the interaction between LAG-3 and MCH II with LAG-3 inhibitors induces immune activation and antitumor activity. In a preclinical study, LAG-3 and PD-1 can be coexpressed on T cells, and LAG-3 and PD-1/PD-L1 may synergistically modulate T-cell activity to facilitate immune escape, suggesting that the combination of anti-PD-(L)1 antibody and anti-LAG-3 antibody may be a feasible strategy, especially for patients who are resistant to anti-PD-(L)1 inhibitor therapy (79). In the phase III RELATIVITY-047 (NCT03470922) study, the median PFS of patients with advanced melanoma treated with relatlimab (an anti-LAG-3 antibody) plus nivolumab (an anti-PD-1 antibody) was substantially longer than that of patients treated with nivolumab with a median follow-up duration of 13.2 months [10.1 months vs. 4.6 months; HR: 0.75 (95% CI: 0.62–0.92); p = 0.006]. Grade 3/4 treatment-related adverse events were reported more commonly in the relatlimab-nivolumab group than in the nivolumab group (18.9% vs. 9.7%) (80). A phase I trial (NCT04658147) is currently recruiting patients to evaluate the feasibility and efficacy of nivolumab with or without relatlimab in the treatment of HCC. A phase II trial (NCT04567615) is currently enrolling patients to assess the safety and efficacy of nivolumab plus relatlimab in treating advanced HCC patients. Pavunalimab (XmAb22841) is a bispecific antibody that targets CTLA-4 and LAG-3 and may contribute to an improved antitumor immune response. A phase I multiple-dose study (NCT03849469) is currently enrolling patients to assess the safety and tolerability of pavunalimab monotherapy and in combination with pembrolizumab in patients with advanced solid tumors. Moreover, preclinical studies demonstrate that IBI323, a bispecific antibody against PD-L1/LAG-3, improves tumor-specific immunity, suggesting that dual-blockade bispecific antibodies targeting PD-L1 and LAG-3 may represent a viable treatment option (81).
TIM-3 acts as a negative modulator of T lymphocytes. When it interacts with its ligands, it can induce T-cell exhaustion and the production of MDSCs in the TME and promote tumor growth. TIM-3 inhibition lowers MDSCs, enhances T-cell proliferation and cytokine secretion, and improves antitumor efficacy. The presence of cytokines such as IL-4, TGF-β, and IL-6 in the TME induces the expression of TIM-3 in HCC cells. Tumor cell-intrinsic TIM-3 stimulates NF-κB phosphorylation, which increases IL-6 production and STAT3 phosphorylation (82). TIM-3 facilitates the development of HCC via stimulating TGF-β-mediated alternative macrophage activation, suggesting that TIM-3 interference may have significant therapeutic implications for HCC (83). Several TIM-3 inhibitors are being evaluated in clinical trials, including LY3321367, Sym023, TSR-022, and BGB-A425. Moreover, clinical trials evaluating the safety, tolerability, and efficacy of TIM-3/PD-(L)1 bispecific antibodies such as RO7121661 and LY3415244 are ongoing (84).
TIGIT, an immune checkpoint, is mainly expressed on T cells and NK cells. By interacting with ligands with its ligands CD155, PVRL3, and CD112, TIGIT blocks NK cell-mediated tumor killing, activates immunosuppressive DCs, decreases CD8 T-cell initiation and differentiation, and inhibits CD8 T cell-mediated tumor killing, thus causing immunosuppression. Several anti-TIGIT agents have been investigated in clinical trials. TIGIT inhibitors such as ociperlimab, tiragolumab, and BMS-986207 may be effective against solid tumors alone or with a PD-(L)1 inhibitor. Moreover, bispecific antibodies against PD-(L)1/TIGIT, such as HLX301 and AZD2936, have significant therapeutic potential in the future (85).
Several checkpoint molecules show positive immunoregulatory activities in the setting of cancer. Immune costimulator (ICOS) is a costimulatory molecule secreted on T cells, which stimulates CD8+ T-cell and Treg activity. ICOS agonist monoclonal antibodies such as JTX-2011, feladilimab, and BMS-986226 are being explored as single agents or with anti-PD-(L)1/anti-CTLA-4 therapies (86). In addition, glucocorticoid-induced TNFR-related gene (GITR) or OX40, belonging to the TNF receptor superfamily, is being evaluated as stimulatory factors. GITR is mainly expressed on effector T cells, Tregs, and NK cells, where it interacts with its ligand GITRL to perform positive immunoregulatory effects. OX40 can promote T-cell expansion and modulate T helper activation (87). Clinical trials for GITR agonist antibodies (BMS-986156 and TRX518) and OX40 agonist antibodies (MEDI6469 and PF-04518600) are undergoing in solid tumors.
Novel immunotherapies
Developing novel strategies for ICI resistance management is attracting growing interest. A growing body of evidence demonstrated the significance of oncolytic viruses, gut and tumor microbiome, vaccines, and cell therapies in tumor immunity. For example, oncolytic viruses destroy tumor cells through a variety of mechanisms, including inducing cytotoxicity, lysis, and activation of antitumor immune responses. Oncolytic viruses have immune-stimulating effects and can transform non-inflammatory microenvironments into inflammatory microenvironments, and their combination with ICI strategies may improve therapeutic outcomes (88). A phase I/II study (NCT02509507) evaluating talimogene laherparepvec (a modified oncolytic virus) plus pembrolizumab in patients with HCC or other solid tumors with liver metastases is ongoing. Based on a phase II study (NCT00554372), JX-594 (an oncolytic and immunotherapeutic vaccinia virus) can kill HCC cells through viral oncolysis and immunity, and it shows promise as a treatment strategy for advanced HCC, and further clinical studies are warranted (89). A better understanding of the underlying mechanisms of these factors is a prerequisite for the application of these emerging strategies.
Prospects and conclusions
ICIs have revolutionized advanced HCC treatment. However, ICIs can elicit a durable antitumor response in only a subset of HCC, and several mechanisms can lead to primary or acquired resistance to ICIs. Anti-PD-(L)1 ICIs plus anti-VEGFA agents provide patients with a favorable objective response rate. The approval of atezolizumab plus bevacizumab is a new milestone in treating advanced HCC. Treatment strategies after the resistance to anti-PD-(L)1 ICIs in combination with anti-VEGFA agents remain to be determined. Whether patients who once benefited from atezolizumab plus bevacizumab might respond to other ICI-based combination strategies remains unclear. Identification of patients at risk of ICI resistance and timely change of treatment strategy are currently feasible options to overcome ICI resistance. The high burden of single-nucleotide variants and small insertion and deletion can induce increased neoantigen abundance and enhanced mutant binding specificity. Tumor cells with inherently low TMB are more likely to be deficient in neoantigens and are more susceptible to ICI resistance. Decreased production of MHC-I and β2-microglobulin can affect how tumor antigens are processed or presented and cause ICI resistance. Additionally, epigenetic alterations can affect how antigens are processed and presented, alter how cytokines are produced, and result in ICI resistance. Moreover, the abnormal IFN-γ pathway is a significant factor in ICI resistance. Developing combinatorial biomarkers to predict response to ICIs is an urgent need. In addition to the currently widely used immunotherapy markers (PD-L1 expression, TMB, and MMR/MSI), tumor-intrinsic biomarkers (neoantigens, WNT/β-catenin, DNA damage pathways, IFN signaling mutations, AT-rich interactive domain-containing protein 1A (ARID1A) mutation, PTEN loss) and immune-specific biomarkers (T cells, IFN-γ signature, Teff/Treg ratio, CD103+ DCs, tumor lysis syndrome (TLS), B-cell signature, CXCL13, M1 and M2 cells) are worthy of further exploration. Biomarker-based treatments make treatment more precise, and identifying the molecular alterations that cause ICI resistance may provide a new standard of therapy.
Further research is needed to elucidate the mechanisms of resistance to ICIs in patients with HCC. In particular, the influence of different immune cell subsets and signaling pathways on ICI treatment response requires further explanation. In addition, the relationship between gene signatures, immune classes and specific mutations, and treatment response or resistance needs to be further explored. Closer integration of cancer immunotherapy with fields including cancer biology, computational biology, and epigenetics will contribute to a better understanding of the mechanisms of ICI resistance. Further advancement of ICIs is required in combination with other therapies such as targeted therapy, chemotherapy, radiotherapy, or other immunotherapies such as novel ICIs, and chimeric antigen receptor T cells. It is also worth exploring how to select the appropriate combination therapy more accurately. The future of ICIs is promising, and they still have the potential to further improve outcomes in HCC.
Author contributions
QX and CZ conceived of and designed the work. PZ, YW, WM drafted, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This present study was supported in part by the National Natural Science Foundation of China (grant numbers: 81660755), and the Science and Technology Project of Shenzhen of China (grant numbers: JCYJ2021032414261403 and JCYJ20190808162605484).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (2017) 389(10088):2492–502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol (2018) 19(7):940–52. doi: 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 4. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol (2020) 6(11):e204564. doi: 10.1001/jamaoncol.2020.4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 6. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol (2022) 23(1):77–90. doi: 10.1016/S1470-2045(21)00604-5 [DOI] [PubMed] [Google Scholar]
- 7. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J Clin Oncol (2020) 38(3):193–202. doi: 10.1200/JCO.19.01307 [DOI] [PubMed] [Google Scholar]
- 8. Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, et al. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J (2019) 17:661–74. doi: 10.1016/j.csbj.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inarrairaegui M, Melero I, Sangro B. Immunotherapy of hepatocellular carcinoma: Facts and hopes. Clin Cancer Res (2018) 24(7):1518–24. doi: 10.1158/1078-0432.CCR-17-0289 [DOI] [PubMed] [Google Scholar]
- 10. Rizzo A, Ricci AD. PD-L1, TMB, and other potential predictors of response to immunotherapy for hepatocellular carcinoma: how can they assist drug clinical trials? Expert Opin Investig Drugs (2022) 31(4):415–23. doi: 10.1080/13543784.2021.1972969 [DOI] [PubMed] [Google Scholar]
- 11. Lee V, Murphy A, Le DT, Diaz LA, Jr. Mismatch repair deficiency and response to immune checkpoint blockade. Oncologist (2016) 21(10):1200–11. doi: 10.1634/theoncologist.2016-0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao P, Li L, Jiang X, Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol (2019) 12(1):54. doi: 10.1186/s13045-019-0738-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berton D, Banerjee SN, Curigliano G, Cresta S, Arkenau H-T, Abdeddaim C, et al. Antitumor activity of dostarlimab in patients with mismatch repair-deficient/microsatellite instability–high tumors: A combined analysis of two cohorts in the GARNET study. J Clin Oncol (2021) 39(15_suppl):2564–64. doi: 10.1200/JCO.2021.39.15_suppl.2564 [DOI] [Google Scholar]
- 14. Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-Instability-High advanced colorectal cancer. N Engl J Med (2020) 383(23):2207–18. doi: 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 15. Barrueto L, Caminero F, Cash L, Makris C, Lamichhane P, Deshmukh RR. Resistance to checkpoint inhibition in cancer immunotherapy. Transl Oncol (2020) 13(3):100738. doi: 10.1016/j.tranon.2019.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Doorn DJ, Takkenberg RB, Klumpen HJ. Immune checkpoint inhibitors in hepatocellular carcinoma: An overview. Pharm (Basel) (2020) 14(1):3. doi: 10.3390/ph14010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Russell BL, Sooklal SA, Malindisa ST, Daka LJ, Ntwasa M. The tumor microenvironment factors that promote resistance to immune checkpoint blockade therapy. Front Oncol (2021) 11:641428. doi: 10.3389/fonc.2021.641428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kudo M, Lim HY, Cheng AL, Chao Y, Yau T, Ogasawara S, et al. Pembrolizumab as second-line therapy for advanced hepatocellular carcinoma: A subgroup analysis of Asian patients in the phase 3 KEYNOTE-240 trial. Liver Cancer (2021) 10(3):275–84. doi: 10.1159/000515553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol (2022) 23(8):995–1008. doi: 10.1016/S1470-2045(22)00326-6 [DOI] [PubMed] [Google Scholar]
- 20. Abou-Alfa GK, Chan SL, Kudo M, Lau G, Kelley RK, Furuse J, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol (2022) 40(4_suppl):379. doi: 10.1200/JCO.2022.40.4_suppl.379 [DOI] [Google Scholar]
- 21. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol (2021) 22(7):977–90. doi: 10.1016/S1470-2045(21)00252-7 [DOI] [PubMed] [Google Scholar]
- 22. Ramos RN, Piaggio E, Romano E. Mechanisms of resistance to immune checkpoint antibodies. Handb Exp Pharmacol (2018) 249:109–28. doi: 10.1007/164_2017_11 [DOI] [PubMed] [Google Scholar]
- 23. McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell (2015) 27(1):15–26. doi: 10.1016/j.ccell.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 24. McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell (2017) 168(4):613–28. doi: 10.1016/j.cell.2017.01.018 [DOI] [PubMed] [Google Scholar]
- 25. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity (2013) 39(1):1–10. doi: 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 26. Pio R, Ajona D, Ortiz-Espinosa S, Mantovani A, Lambris JD. Complementing the cancer-immunity cycle. Front Immunol (2019) 10:774. doi: 10.3389/fimmu.2019.00774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature (2015) 523(7559):231–5. doi: 10.1038/nature14404 [DOI] [PubMed] [Google Scholar]
- 28. Harding JJ, Nandakumar S, Armenia J, Khalil DN, Albano M, Ly M, et al. Prospective genotyping of hepatocellular carcinoma: Clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res (2019) 25(7):2116–26. doi: 10.1158/1078-0432.CCR-18-2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology (2017) 153(3):812–26. doi: 10.1053/j.gastro.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pinyol R, Sia D, Llovet JM. Immune exclusion-Wnt/CTNNB1 class predicts resistance to immunotherapies in HCC. Clin Cancer Res (2019) 25(7):2021–3. doi: 10.1158/1078-0432.CCR-18-3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruiz de Galarreta M, Bresnahan E, Molina-Sanchez P, Lindblad KE, Maier B, Sia D, et al. Beta-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov (2019) 9(8):1124–41. doi: 10.1158/2159-8290.CD-19-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwee SA, Tiirikainen M. Beta-catenin activation and immunotherapy resistance in hepatocellular carcinoma: mechanisms and biomarkers. Hepatoma Res (2021) 7:8. doi: 10.20517/2394-5079.2020.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuda A, Ishiguro K, Yan IK, Patel T. Extracellular vesicle-based therapeutic targeting of beta-catenin to modulate anticancer immune responses in hepatocellular cancer. Hepatol Commun (2019) 3(4):525–41. doi: 10.1002/hep4.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang K, Qiu X, Zhao Y, Wang H, Chen L. The wnt/beta-catenin signaling pathway in the tumor microenvironment of hepatocellular carcinoma. Cancer Biol Med (2021) 19(3):305. doi: 10.20892/j.issn.2095-3941.2021.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gonzalez-Sanchez E, Vaquero J, Fernandez-Barrena MG, Lasarte JJ, Avila MA, Sarobe P, et al. The TGF-beta pathway: A pharmacological target in hepatocellular carcinoma? Cancers (Basel) (2021) 13(13):3248. doi: 10.3390/cancers13133248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol (2010) 10(8):554–67. doi: 10.1038/nri2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. David CJ, Massague J. Contextual determinants of TGFbeta action in development, immunity and cancer. Nat Rev Mol Cell Biol (2018) 19(7):419–35. doi: 10.1038/s41580-018-0007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Park BV, Freeman ZT, Ghasemzadeh A, Chattergoon MA, Rutebemberwa A, Steigner J, et al. TGFbeta1-mediated SMAD3 enhances PD-1 expression on antigen-specific T cells in cancer. Cancer Discov (2016) 6(12):1366–81. doi: 10.1158/2159-8290.CD-15-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shrestha R, Bridle KR, Crawford DHG, Jayachandran A. Immune checkpoint molecules are regulated by transforming growth factor (TGF)-beta1-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma. Int J Med Sci (2021) 18(12):2466–79. doi: 10.7150/ijms.54239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen J, Gingold JA, Su X. Immunomodulatory TGF-beta signaling in hepatocellular carcinoma. Trends Mol Med (2019) 25(11):1010–23. doi: 10.1016/j.molmed.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 41. Li H, Han G, Li X, Li B, Wu B, Jin H, et al. MAPK-RAP1A signaling enriched in hepatocellular carcinoma is associated with favorable tumor-infiltrating immune cells and clinical prognosis. Front Oncol (2021) 11:649980. doi: 10.3389/fonc.2021.649980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu Z, Ning F, Cai Y, Sheng H, Zheng R, Yin X, et al. The EGFR-P38 MAPK axis up-regulates PD-L1 through miR-675-5p and down-regulates HLA-ABC via hexokinase-2 in hepatocellular carcinoma cells. Cancer Commun (Lond) (2021) 41(1):62–78. doi: 10.1002/cac2.12117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xing S, Chen S, Yang X, Huang W. Role of MAPK activity in PD-L1 expression in hepatocellular carcinoma cells. J BUON (2020) 25(4):1875–82. [PubMed] [Google Scholar]
- 44. Chen Y, Liu YC, Sung YC, Ramjiawan RR, Lin TT, Chang CC, et al. Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci Rep (2017) 7:44123. doi: 10.1038/srep44123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin Z, Huang L, Li SL, Gu J, Cui X, Zhou Y. PTEN loss correlates with T cell exclusion across human cancers. BMC Cancer (2021) 21(1):429. doi: 10.1186/s12885-021-08114-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vidotto T, Melo CM, Castelli E, Koti M, Dos Reis RB, Squire JA. Emerging role of PTEN loss in evasion of the immune response to tumours. Br J Cancer (2020) 122(12):1732–43. doi: 10.1038/s41416-020-0834-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu J, Fan L, Yu H, Zhang J, He Y, Feng D, et al. Endoplasmic reticulum stress causes liver cancer cells to release exosomal miR-23a-3p and up-regulate programmed death ligand 1 expression in macrophages. Hepatology (2019) 70(1):241–58. doi: 10.1002/hep.30607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sangro B, Sarobe P, Hervas-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol (2021) 18(8):525–43. doi: 10.1038/s41575-021-00438-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li N, Wang J, Zhang N, Zhuang M, Zong Z, Zou J, et al. Cross-talk between TNF-alpha and IFN-gamma signaling in induction of B7-H1 expression in hepatocellular carcinoma cells. Cancer Immunol Immunother (2018) 67(2):271–83. doi: 10.1007/s00262-017-2086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zou J, Zhuang M, Yu X, Li N, Mao R, Wang Z, et al. MYC inhibition increases PD-L1 expression induced by IFN-gamma in hepatocellular carcinoma cells. Mol Immunol (2018) 101:203–9. doi: 10.1016/j.molimm.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 51. Zhang S, Gan X, Qiu J, Ju Z, Gao J, Zhou J, et al. IL-10 derived from hepatocarcinoma cells improves human induced regulatory T cells function via JAK1/STAT5 pathway in tumor microenvironment. Mol Immunol (2021) 133:163–72. doi: 10.1016/j.molimm.2021.02.014 [DOI] [PubMed] [Google Scholar]
- 52. Hazini A, Fisher K, Seymour L. Deregulation of HLA-I in cancer and its central importance for immunotherapy. J Immunother Cancer (2021) 9(8):e002899. doi: 10.1136/jitc-2021-002899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dong LQ, Peng LH, Ma LJ, Liu DB, Zhang S, Luo SZ, et al. Heterogeneous immunogenomic features and distinct escape mechanisms in multifocal hepatocellular carcinoma. J Hepatol (2020) 72(5):896–908. doi: 10.1016/j.jhep.2019.12.014 [DOI] [PubMed] [Google Scholar]
- 54. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell (2017) 168(4):707–23. doi: 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pisibon C, Ouertani A, Bertolotto C, Ballotti R, Cheli Y. Immune checkpoints in cancers: From signaling to the clinic. Cancers (Basel) (2021) 13(18):4573. doi: 10.3390/cancers13184573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol (2019) 9:1146. doi: 10.3389/fonc.2019.01146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arvanitakis K, Mitroulis I, Germanidis G. Tumor-associated neutrophils in hepatocellular carcinoma pathogenesis, prognosis, and therapy. Cancers (Basel) (2021) 13(12):2899. doi: 10.3390/cancers13122899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kondelkova K, Vokurkova D, Krejsek J, Borska L, Fiala Z, Ctirad A. Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Med (Hradec Kralove) (2010) 53(2):73–7. doi: 10.14712/18059694.2016.63 [DOI] [PubMed] [Google Scholar]
- 59. Sun Y, Wu L, Zhong Y, Zhou K, Hou Y, Wang Z, et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell (2021) 184(2):404–21 e16. doi: 10.1016/j.cell.2020.11.041 [DOI] [PubMed] [Google Scholar]
- 60. Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell (2017) 169(7):1342–56 e16. doi: 10.1016/j.cell.2017.05.035 [DOI] [PubMed] [Google Scholar]
- 61. Consonni FM, Porta C, Marino A, Pandolfo C, Mola S, Bleve A, et al. Myeloid-derived suppressor cells: Ductile targets in disease. Front Immunol (2019) 10:949. doi: 10.3389/fimmu.2019.00949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li YM, Liu ZY, Wang JC, Yu JM, Li ZC, Yang HJ, et al. Receptor-interacting protein kinase 3 deficiency recruits myeloid-derived suppressor cells to hepatocellular carcinoma through the chemokine (C-X-C motif) ligand 1-chemokine (C-X-C motif) receptor 2 axis. Hepatology (2019) 70(5):1564–81. doi: 10.1002/hep.30676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang Q, Guo N, Zhou Y, Chen J, Wei Q, Han M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta Pharm Sin B (2020) 10(11):2156–70. doi: 10.1016/j.apsb.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Petty AJ, Owen DH, Yang Y, Huang X. Targeting tumor-associated macrophages in cancer immunotherapy. Cancers (Basel) (2021) 13(21):5318. doi: 10.3390/cancers13215318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sung PS. Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma. Clin Mol Hepatol (2021). doi: 10.3350/cmh.2021.0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wu L, Zhang X, Zheng L, Zhao H, Yan G, Zhang Q, et al. RIPK3 orchestrates fatty acid metabolism in tumor-associated macrophages and hepatocarcinogenesis. Cancer Immunol Res (2020) 8(5):710–21. doi: 10.1158/2326-6066.CIR-19-0261 [DOI] [PubMed] [Google Scholar]
- 67. Tie Y, Tang F, Wei YQ, Wei XW. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol (2022) 15(1):61. doi: 10.1186/s13045-022-01282-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lu C, Rong D, Zhang B, Zheng W, Wang X, Chen Z, et al. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer (2019) 18(1):130. doi: 10.1186/s12943-019-1047-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol (2022) 19(3):151–72. doi: 10.1038/s41571-021-00573-2 [DOI] [PubMed] [Google Scholar]
- 70. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol (2021) 76(4):862–73. doi: 10.1016/j.jhep.2021.11.030 [DOI] [PubMed] [Google Scholar]
- 71. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer (2022) 21(1):28. doi: 10.1186/s12943-021-01489-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, et al. Targeting the TGFbeta pathway with galunisertib, a TGFbetaRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer (2018) 6(1):47. doi: 10.1186/s40425-018-0356-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang W, Feng Y, Zhou J, Cheung OK, Cao J, Wang J, et al. A selective HDAC8 inhibitor potentiates antitumor immunity and efficacy of immune checkpoint blockade in hepatocellular carcinoma. Sci Transl Med (2021) 13(588):eaaz6804. doi: 10.1126/scitranslmed.aaz6804 [DOI] [PubMed] [Google Scholar]
- 74. Qiu W, Wang B, Gao Y, Tian Y, Tian M, Chen Y, et al. Targeting histone deacetylase 6 reprograms interleukin-17-Producing helper T cell pathogenicity and facilitates immunotherapies for hepatocellular carcinoma. Hepatology (2020) 71(6):1967–87. doi: 10.1002/hep.30960 [DOI] [PubMed] [Google Scholar]
- 75. Liu M, Zhang L, Li H, Hinoue T, Zhou W, Ohtani H, et al. Integrative epigenetic analysis reveals therapeutic targets to the DNA methyltransferase inhibitor guadecitabine (SGI-110) in hepatocellular carcinoma. Hepatology (2018) 68(4):1412–28. doi: 10.1002/hep.30091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Choi C, Yoo GS, Cho WK, Park HC. Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J Gastroenterol (2019) 25(20):2416–29. doi: 10.3748/wjg.v25.i20.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer (2019) 18(1):155. doi: 10.1186/s12943-019-1091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Leone P, Solimando AG, Fasano R, Argentiero A, Malerba E, Buonavoglia A, et al. The evolving role of immune checkpoint inhibitors in hepatocellular carcinoma treatment. Vaccines (Basel) (2021) 9(5):532. doi: 10.3390/vaccines9050532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res (2012) 72(4):917–27. doi: 10.1158/0008-5472.CAN-11-1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutierrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med (2022) 386(1):24–34. doi: 10.1056/NEJMoa2109970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jiang H, Ni H, Zhang P, Guo X, Wu M, Shen H, et al. PD-L1/LAG-3 bispecific antibody enhances tumor-specific immunity. Oncoimmunology (2021) 10(1):1943180. doi: 10.1080/2162402X.2021.1943180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang H, Song Y, Yang H, Liu Z, Gao L, Liang X, et al. Tumor cell-intrinsic Tim-3 promotes liver cancer via NF-kappaB/IL-6/STAT3 axis. Oncogene (2018) 37(18):2456–68. doi: 10.1038/s41388-018-0140-4 [DOI] [PubMed] [Google Scholar]
- 83. Yan W, Liu X, Ma H, Zhang H, Song X, Gao L, et al. Tim-3 fosters HCC development by enhancing TGF-beta-mediated alternative activation of macrophages. Gut (2015) 64(10):1593–604. doi: 10.1136/gutjnl-2014-307671 [DOI] [PubMed] [Google Scholar]
- 84. Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol (2020) 20(3):173–85. doi: 10.1038/s41577-019-0224-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Zheng Q, Xu J, Gu X, Wu F, Deng J, Cai X, et al. Immune checkpoint targeting TIGIT in hepatocellular carcinoma. Am J Transl Res (2020) 12(7):3212–24. [PMC free article] [PubMed] [Google Scholar]
- 86. Amatore F, Gorvel L, Olive D. Role of inducible Co-stimulator (ICOS) in cancer immunotherapy. Expert Opin Biol Ther (2020) 20(2):141–50. doi: 10.1080/14712598.2020.1693540 [DOI] [PubMed] [Google Scholar]
- 87. Kumar P, Arbieva ZH, Maienschein-Cline M, Ganesh BB, Ramasamy S, Prabhakar BS. Induction of antigen-independent proliferation of regulatory T-cells by TNF superfamily ligands OX40L and GITRL. Methods Mol Biol (2021) 2248:63–71. doi: 10.1007/978-1-0716-1130-2_4 [DOI] [PubMed] [Google Scholar]
- 88. Chon HJ, Lee WS, Yang H, Kong SJ, Lee NK, Moon ES, et al. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade. Clin Cancer Res (2019) 25(5):1612–23. doi: 10.1158/1078-0432.CCR-18-1932 [DOI] [PubMed] [Google Scholar]
- 89. Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med (2013) 19(3):329–36. doi: 10.1038/nm.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]