Abstract
Purpose
Although breast cancer is known to show a left predominance, the clinical characteristics and causes underlying this finding remain unclear. In addition, no related studies on breast cancer laterality have been conducted in patients with breast cancer in Korea. Therefore, we aimed to analyze differences in breast cancer laterality and the associated clinicopathological characteristics and prognosis among Korean patients with breast cancer.
Methods
We conducted a retrospective analysis using large-scale data on clinicopathological factors and prognosis differences related to breast cancer laterality from the Korean Breast Cancer Society Registration system. The left-to-right ratio (LRR) of breast cancer was calculated through binomial distribution, and factors related to breast cancer laterality were identified through logistic regression analysis. In addition, the differences in the survival rates for left and right breast cancers were analyzed using the Kaplan-Meier method and Cox proportional hazards model.
Results
In 171,500 patients, the LRR was 1.031 (95% confidence interval, 1.022–1.041; P < 0.001). Multivariate analysis showed that the ratio of left breast cancer was related to age, body mass index (BMI), location, and human epidermal growth factor receptor 2 (HER2) status. The survival rate of patients with left and right breast cancers showed no significant difference.
Conclusion
A large-scale analysis revealed a left predominance in breast cancer laterality in Korean patients. Over time, this predominance gradually decreased. Age, BMI, location, and HER2 status affected breast cancer laterality. However, while left breast cancer showed relatively aggressive characteristics, it was not associated with a difference in the survival rate.
Keywords: Epidemiology, Survival, Unilateral breast neoplasms
INTRODUCTION
Although anatomically paired organs in the human body may show symmetry in their appearance, they differ slightly in shape, size, position, and vasculature, resulting in asymmetry. In addition to this anatomical asymmetry, the frequency of cancer in paired organs may vary on both sides, despite the shared causes that contribute to cancer, such as genetic and environmental factors [1]. Breast cancer is one such cancer and is known to show a left predominance in laterality. The incidence of left breast cancer is approximately 7%–26% higher than that of right breast cancer [1,2,3,4].
Previous studies have proposed several hypotheses to explain the factors underlying laterality, such as breast size [5,6,7], handedness [4,8], and lactation [9]. However, the results obtained for these factors can be significantly influenced by aspects such as the errors related to various proportional differences in breast substance when measuring breast size or biases in surveys.
As for breast cancer laterality, the difference between the incidence of left to right breast cancer is not significant, and the clinical significance of this difference for the treatment process is unclear [10]. Therefore, large-scale studies are required to determine breast cancer laterality [1,3,11,12]. Nevertheless, the clinical characteristics related to breast cancer laterality differ across studies, and the factors that cause laterality remain controversial. These differences among studies may be attributable to the lack of significant differences in the clinical characteristics of left and right breast cancer, indicating the increased influence of other variables such as study period and race. In addition, no related studies have analyzed the laterality of patients with breast cancer in Korea. Therefore, we analyzed the differences in breast cancer laterality and the associated clinical characteristics in Korean patients with breast cancer and compared the findings with those of previous studies.
METHODS
This study was approved by the Institutional Review Board of Daejeon St. Mary’s Hospital (No. DC21ZASI0075) and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent from patients was not required in this study.
Data collection
This study used data approved by the Korean Breast Cancer Society Registration system (KBCR). The KBCR has been described in detail in previous literature, and it includes data accumulated by breast surgeons who voluntarily registered breast cancer cases at training hospitals nationwide since 1996 [13]. The causes and dates of death were linked to the Korean Central Cancer Registration Data of the Ministry of Health and Welfare with the National Statistical Office. Under the Personal Information Protection Act, complete death data updated until 2014 were used (Korean Central Cancer Registry Ministry of Health and Welfare, Korea. Korean National Statistical Office with complete death statistics).
Patients and clinicopathological variables
In this study, a retrospective analysis of the differences in the related clinicopathological factors as well as the prognoses of Korean patients with left and right breast cancer was conducted using large-scale data from the KBCR. In total, 201,635 patients underwent surgery for breast cancer between 1981 and 2021. We analyzed the data for 171,500 patients after excluding cases with bilateral breast cancer (976 patients) and those in which the laterality of breast cancer could not be confirmed (29,159 patients). The clinical characteristics of the patients included the year of operation, age, T and N stages, body mass index (BMI), tumor multiplicity and location, histologic and nuclear grade, estrogen or progesterone receptor (ER or PR) status, human epidermal growth factor receptor 2 (HER2) status, Ki-67 status, and treatment variables such as breast and axillary surgery, chemotherapy, radiotherapy, and hormonal therapy. We used year of operation, age, BMI, and Ki-67 index as continuous variables for linear regression analysis. Breast cancer location was classified as one of the quadrants or central. Cases of multicentricity or the whole breast were excluded and expressed as missing values in the location.
Statistical analysis
The distribution of clinicopathological factors was compared between left and right breast cancers using the chi-square test to determine the difference between left and right breast cancers in relation to each factor. In addition, the ratio of left breast cancer to right breast cancer (left-to-right ratio, LRR) for each factor was analyzed by assessing the binomial distribution, and the 95% confidence interval (CI) was obtained by approximating the binomial distribution to the normal distribution. To determine the changes in the LRR pattern in relation to an increase or decrease in the continuous variables, the correlation between continuous variables and LRR was expressed as the slope of the change in LRR according to changes in variables by using linear regression analysis. The influence of the variables was defined by the coefficient of determination (R2). The year of operation was analyzed only to assess the pattern of change in the LRR. Univariate logistic regression analysis was used to identify factors associated with breast cancer laterality. Next, a multivariate analysis of factors with significant values was performed to identify factors that could affect breast cancer laterality. The univariate and multivariate analyses were performed except for treatment variables. In addition, using the Kaplan-Meier method and Cox proportional hazards model, the breast cancer-specific survival (BCSS) and overall survival (OS) rates in left and right breast cancer were compared and analyzed. All statistical analyses were performed using IBM SPSS Statistics ver. 26.0 (IBM Corp., Armonk, NY, USA), and P-values of <0.05 were considered statistically significant.
RESULTS
Distribution of clinicopathological factors in left and right breast cancer
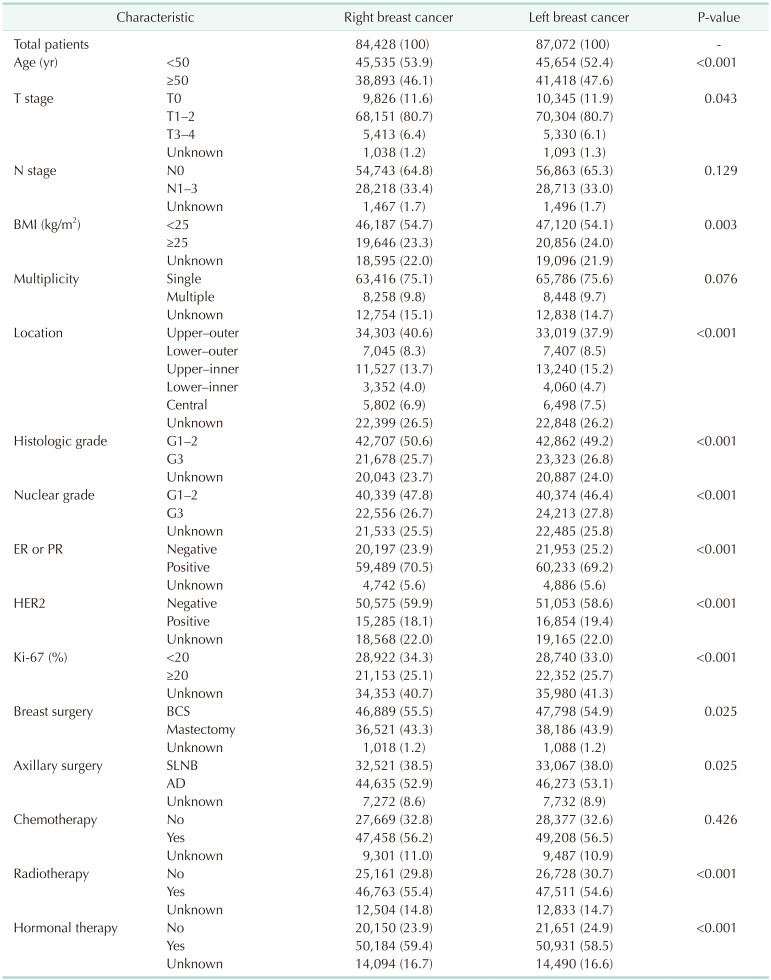
The ratio for each factor differed by up to 2.7% between left and right breast cancers. Factors with a significantly higher proportion for left breast cancer were age of ≥50 years, T0 stage, BMI of ≥25 kg/m2, lower-outer/upper-inner/lower-inner quadrant and central location, histologic grade 3, nuclear grade 3, ER or PR negative, HER2 positive, Ki-67 of ≥20%, mastectomy, axillary dissection, no radiotherapy, and no hormonal therapy (Table 1).
Table 1. Clinicopathological characteristics of right and left breast cancers.
Values are presented as number (%).
BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; BCS, breast-conserving surgery; SLNB, sentinel lymph node biopsy; AD, axillary dissection.
Correlation of left and right breast cancer ratio with continuous variables
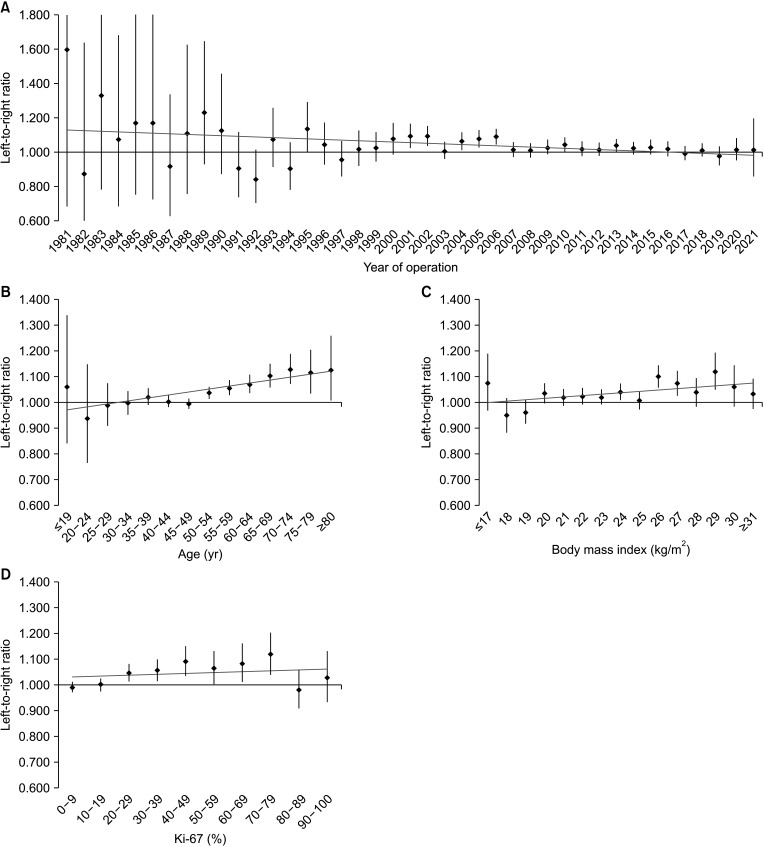
In a linear regression analysis of continuous variables, the regression coefficient of the year of operation showed a negative correlation with LRR (–0.004; R2 = 0.125; 95% CI, –0.007 to –0.001; P = 0.024). Age (0.012; R2 = 0.678; 95% CI, 0.007–0.017; P < 0.001) and BMI (0.005; R2 = 0.271; 95% CI, <0.001 to 0.011; P = 0.046), showed a positive correlation with LRR, and the Ki-67 index showed no correlation with LRR (0.004; R2 = 0.057; 95% CI, –0.008–0.015; P = 0.506) (Fig. 1).
Fig. 1. Linear regression models of the unilateral breast cancer laterality with continuous variables, such as (A) year of operation, (B) age, (C) body mass index, and (D) Ki-67. The black dots and lines indicate the ratios of left breast cancers to right breast cancers and their 95% confidence intervals. The slopes of the gray lines indicate the correlation between breast cancer laterality and the continuous variables.
The proportion of left and right breast cancers
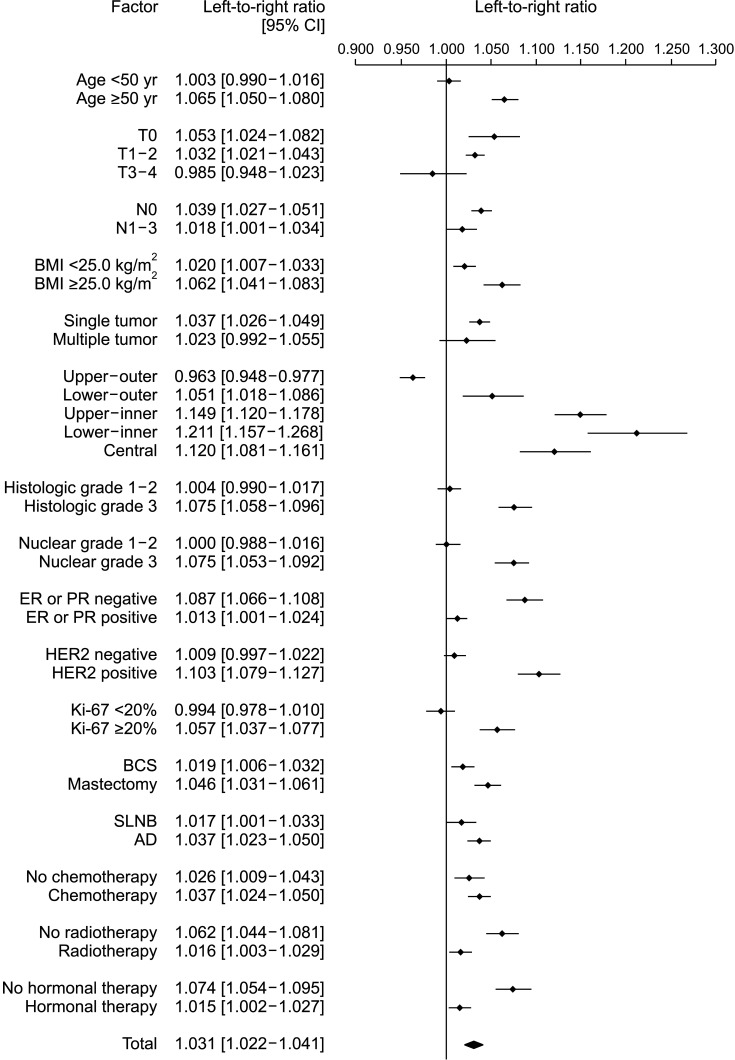
The LRR in 171,500 patients was 1.031 (95% CI, 1.022–1.041; P < 0.001), with left breast cancer being significantly more frequent than right breast cancer. The proportion of left breast cancer in each factor was usually greater than right breast cancer, while right breast cancer was significantly more frequent than left breast cancer only in the upper-outer quadrant (LRR, 0.963; 95% CI, 0.948–0.977; P < 0.001). In particular, the LRR value increased sequentially in the lower-outer, central, upper-inner, and lower-inner quadrants, with reference to the upper-outer quadrant (Fig. 2).
Fig. 2. Unilateral breast cancer laterality distributions according to the factors. CI, confidence interval; BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; BCS, breast-conserving surgery; SLNB, sentinel lymph node biopsy; AD, axillary dissection.
Factors associated with breast cancer laterality
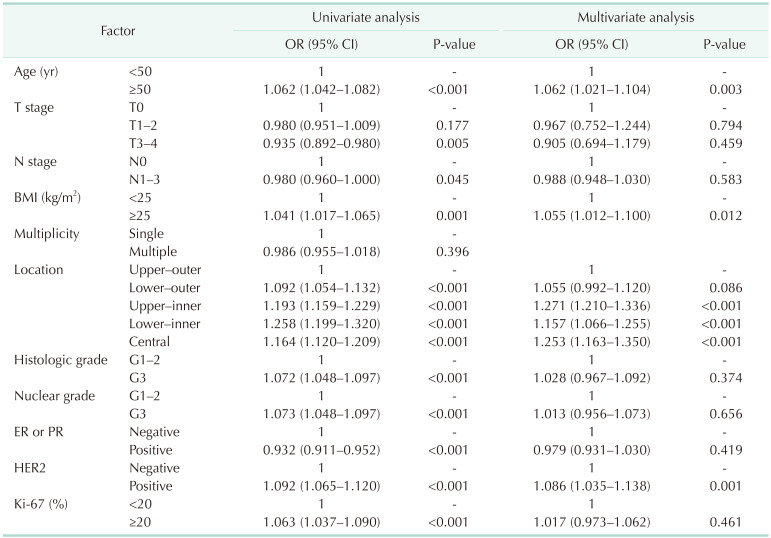
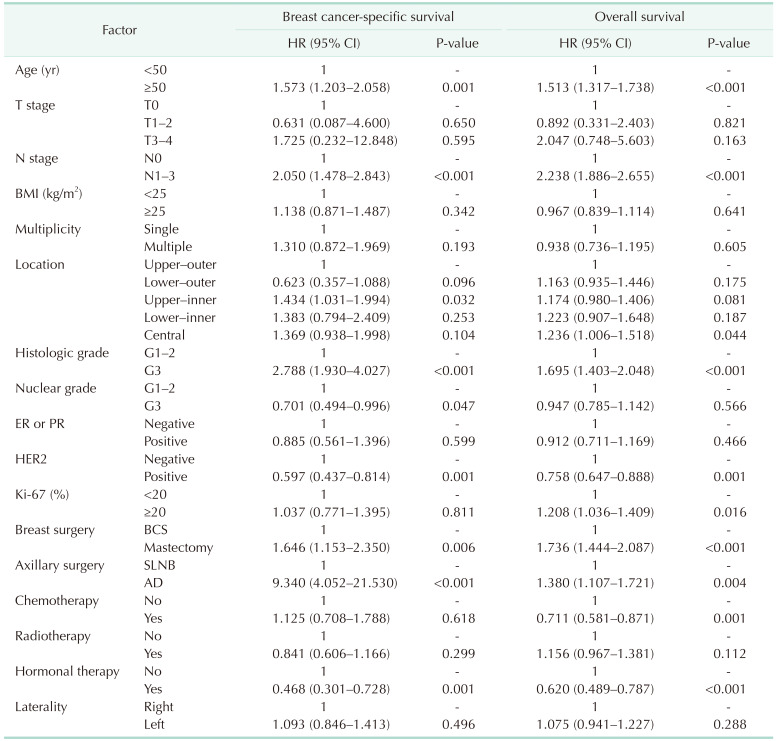
In the univariate analysis, the factors showing a greater ratio for left breast cancer than the reference category were age, BMI, location, histologic grade, nuclear grade, and HER2 status, and the factors with a greater ratio for right breast cancer were T stage, N stage, and ER or PR positive. In multivariate analysis, when the factors were adjusted, the factors showing a significantly greater ratio for left breast cancer were age (odds ratio [OR], 1.062; P = 0.003), BMI (OR, 1.055; P = 0.012), location (upper-inner [OR, 1.271; P < 0.001], lower-inner [OR, 1.157; P < 0.001], central [OR, 1.253; P < 0.001]), and HER2 status (OR, 1.086; P = 0.001) (Table 2).
Table 2. Associated factors of breast cancer laterality.
OR, odds ratio; CI, confidence interval; BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Prognosis
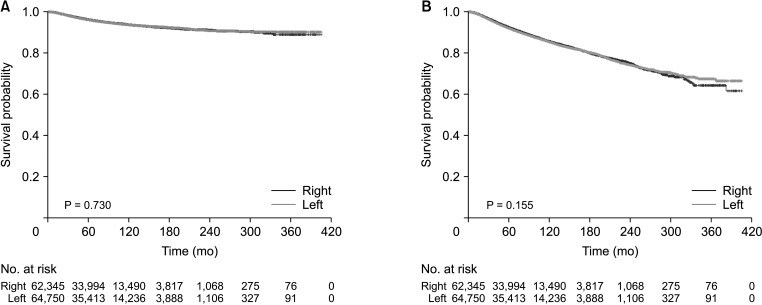
The median observation period for all patients was 67 months (range, 0–405 months). The Kaplan-Meier method showed no significant difference in the 5-year survival rates of left and right breast cancer, with BCSS values of 96.0% and 96.1% (P = 0.730) and OS values of 91.9% and 92.3% (P = 0.155), respectively (Fig. 3). Both BCSS (hazard ratio [HR], 1.093; P = 0.496) and OS (HR, 1.075; P = 0.288) showed no difference in relation to breast cancer laterality when the effects of each factor were adjusted and compared using the Cox proportional hazards ratio (Table 3).
Fig. 3. (A) Breast cancer-specific survival and (B) overall survival curves of left and right breast cancer groups.
Table 3. Prognosis of breast cancer laterality with adjusted factors.
HR, hazard ratio; CI, confidence interval; BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; BCS, breast-conserving surgery; SLNB, sentinel lymph node biopsy; AD, axillary dissection.
DISCUSSION
This study analyzed the ratio of left to right breast cancers in Korean patients with breast cancer. Although the study period and race in our study differed from those in previous studies, the results showing the greater frequency of left breast cancer were not different. The percentage of left breast cancer was 3.1% higher than that of right breast cancer, and the difference was statistically significant, with the predominance of left breast cancer gradually decreasing over time. In addition, when LRR was analyzed by dividing each factor into subgroups for each category, the frequency of left breast cancer was high in most categories. In particular, the ratio of left breast cancer was relatively higher in the categories with a poor prognosis. In analyses based on the location of breast cancer, the LRR value was the highest in the lower-inner quadrant, and only the upper-outer quadrant showed a higher ratio of right breast cancer than left breast cancer. For each factor shown in the univariate analysis, the relative difference in the ratio of left and right breast cancers based on the reference category can be considered to be related to laterality. Most of them showed significant differences. However, factors with no significant differences in the multivariate analysis could be assumed to have a greater effect on other factors. Therefore, in the multivariate analysis, the factors affecting breast cancer laterality were age, BMI, location, and HER2 status. In particular, in each reference category for age, location, and HER2 status, LRR was right predominance or not significant. Among them, the location with the highest OR value was the factor that most affected breast cancer laterality.
The analysis of continuous variables confirmed that LRR increased most significantly with age, and most previous studies have reported similar results [1,11,14]. The difference in the rate of development of left and right breast cancers seems to gradually increase as the effects of other factors related to breast cancer accumulate over time due to the fundamental differences in left and right breast cancer.
The developmental and molecular biological differences between the left and right breasts are thought to be fundamental key factors in breast cancer laterality. The related research results may explain this study’s findings more objectively. For example, Wilting and Hagedorn [15] reported that growth factors and genes involved in the occurrence of breast cancer showed left predominance. Thus, breast cancer laterality may be influenced by these characteristics.
Regarding breast cancer laterality, the diseases related to developmental factors include Poland syndrome and polythelia. Poland syndrome is a disease characterized by unilateral underdevelopment of a breast or pectoralis muscle, and it occurs more frequently on the right side [16,17]. Polythelia is a congenital condition involving extra nipples and related tissues at birth, which occurs more frequently on the left [18]. Although these are rare diseases, their developmental effects are thought to reflect the left-sided laterality in breast growth and development and even the occurrence of breast cancer.
In this study, the left-sided laterality of HER2 positive was greater than that of HER2 negative. HER2 is a growth factor receptor that dimerizes with other ErbB receptors, which are overexpressed and cause breast cancer [15]. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is a ligand of the EGF family, which includes HER2. It has a strong influence on the expression of breast cancer cells by acting mainly on ErbB1 and ErbB4 [19], and it shows a left predominance in the expression of embryonic myotomes [15,20]. Therefore, HER2 can be assumed to be related to breast cancer laterality.
This study showed a somewhat weak but significant linear relationship between BMI and left-sided breast cancer laterality. Obesity causes insulin resistance and thus activates insulin-like growth factor 1 (IGF-1), which increases the risk of breast cancer [21,22]. In addition, some studies have shown that IGF-1 is involved in the occurrence of HB-EGF in vitro [23,24]. Moreover, while several reports have described obesity and breast cancer, few studies have analyzed the effects of BMI on breast cancer laterality. In an analysis of breast cancer laterality that included BMI, BMI did not affect breast cancer laterality [25]. However, since the previous study was a small-scale study compared to the present study, this finding is significant since it implies that the effects of BMI on breast cancer laterality can be observed in a large-scale study.
The location of breast cancer had the most significant influence on breast cancer laterality. The upper-outer quadrant showed the highest right-sided laterality, and the lower-inner quadrant showed the highest left-sided laterality. In addition, the difference in laterality between the inner and outer sides was more significant than that between the upper and lower sides. Several studies on the association of breast cancer with laterality and location showed left-sided laterality in other quadrants, except for the upper-outer quadrant [3,11]. Breast cancer laterality is thought to be greatly affected by developmental or anatomical effects, which can be considered the cause of the consistent left predominance of breast cancer incidence in similar previous studies targeting different groups in terms of period and race. Although, to our knowledge, no reports have considered this factor, the differences in the laterality of each quadrant are thought to be a result of a combination of effects, such as molecules associated with the signaling pathway involved in breast growth and development in the mammary ridge [26,27] and breast tissue distribution or density [28].
When comparing the differences in characteristics between left and right breast cancer, many factors were associated with a poor prognosis in patients with left breast cancer. However, these factors only showed slightly statistically significant differences, so there was no difference between the survival rates for left and right breast cancer. Previous studies have shown no difference in survival rates to the extent where the laterality of breast cancer would influence treatment [12,29,30].
A limitation of this study is the retrospective nature of data analysis, which may have resulted in selection bias due to missing data for some factors. In addition, because of the Personal Information Protection Act, the survival rate was analyzed only with death data until 2014, and the findings do not reflect recent data. The results of this study may differ from the characteristics of laterality in the entire case because the percentage of unknown variables in some significant factors was more than 20%, and the unknown variables were excluded during analysis. However, a univariate analysis was performed on the cases without unknown variables, analyzed by the multivariate analysis; the result was similar to those of this study, at least in terms of the significant factors. Therefore, it is considered that the significant factors in the entire case probably show similar results. Although previous studies included some analyses of Asian patients, these cohorts were as small as a few hundred patients, and analysis of heterogeneous races was also performed in large-scale patient groups, such as the SEER (Surveillance, Epidemiology, and End Results) data. Therefore, we conducted a large-scale single-race analysis.
In this study, breast cancer laterality tended to show a left predominance, which was confirmed in Korean patients with breast cancer. Despite the subclinical results, as in most previous studies, these consistent results regarding the left predominance of breast cancer beyond temporal and regional differences are presumed to underlie the developmental and molecular biological effects. The factors affecting left-sided breast cancer laterality are also thought to result from this cause. Further research is needed on the relationship between breast cancer laterality and the developmental and molecular biological factors.
Footnotes
Fund/Grant Support: This article was supported by the Korean Breast Cancer Society.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization, Methodology, Project Administration: BKK, WYS.
- Formal Analysis: BKK.
- Investigation: All authors.
- Writing – Original Draft: BKK.
- Writing – Review & Editing: All authors.
References
- 1.Roychoudhuri R, Putcha V, Møller H. Cancer and laterality: a study of the five major paired organs (UK) Cancer Causes Control. 2006;17:655–662. doi: 10.1007/s10552-005-0615-9. [DOI] [PubMed] [Google Scholar]
- 2.Busk T, Clemmesen J. The frequencies of left- and right-sided breast cancer. Br J Cancer. 1947;1:345–351. doi: 10.1038/bjc.1947.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perkins CI, Hotes J, Kohler BA, Howe HL. Association between breast cancer laterality and tumor location, United States, 1994-1998. Cancer Causes Control. 2004;15:637–645. doi: 10.1023/B:CACO.0000036171.44162.5f. [DOI] [PubMed] [Google Scholar]
- 4.Senie RT, Rosen PP, Lesser ML, Snyder RE, Schottenfeld D, Duthie K. Epidemiology of breast carcinoma II: factors related to the predominance of left-sided disease. Cancer. 1980;46:1705–1713. doi: 10.1002/1097-0142(19801001)46:7<1705::aid-cncr2820460734>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh CC, Trichopoulos D. Breast size, handedness and breast cancer risk. Eur J Cancer. 1991;27:131–135. doi: 10.1016/0277-5379(91)90469-t. [DOI] [PubMed] [Google Scholar]
- 6.Garfinkel L, Craig L, Seidman H. An appraisal of left and right breast cancer. J Natl Cancer Inst. 1959;23:617–631. [PubMed] [Google Scholar]
- 7.Loughry CW, Sheffer DB, Price TE, Einsporn RL, Bartfai RG, Morek WM, et al. Breast volume measurement of 598 women using biostereometric analysis. Ann Plast Surg. 1989;22:380–385. doi: 10.1097/00000637-198905000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kramer MA, Albrecht S, Miller RA. Handedness and the laterality of breast cancer in women. Nurs Res. 1985;34:333–337. [PubMed] [Google Scholar]
- 9.Ing R, Petrakis NL, Ho JH. Unilateral breast-feeding and breast cancer. Lancet. 1977;2:124–127. doi: 10.1016/s0140-6736(77)90131-3. [DOI] [PubMed] [Google Scholar]
- 10.Kamby C, Andersen J, Ejlertsen B, Birkler NE, Rytter L, Zedeler K, et al. Pattern of spread and progression in relation to the characteristics of the primary tumour in human breast cancer. Acta Oncol. 1991;30:301–308. doi: 10.3109/02841869109092375. [DOI] [PubMed] [Google Scholar]
- 11.Sughrue T, Brody JP. Breast tumor laterality in the United States depends upon the country of birth, but not race. PLoS One. 2014;9:e103313. doi: 10.1371/journal.pone.0103313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rutter CE, Chagpar AB, Evans SB. Breast cancer laterality does not influence survival in a large modern cohort: implications for radiation-related cardiac mortality. Int J Radiat Oncol Biol Phys. 2014;90:329–334. doi: 10.1016/j.ijrobp.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Kang SY, Lee SB, Kim YS, Kim Z, Kim HY, Kim HJ, et al. Breast cancer statistics in Korea, 2018. J Breast Cancer. 2021;24:123–137. doi: 10.4048/jbc.2021.24.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekbom A, Adami HO, Trichopoulos D, Lambe M, Hsieh CC, Pontén J. Epidemiologic correlates of breast cancer laterality (Sweden) Cancer Causes Control. 1994;5:510–516. doi: 10.1007/BF01831378. [DOI] [PubMed] [Google Scholar]
- 15.Wilting J, Hagedorn M. Left-right asymmetry in embryonic development and breast cancer: common molecular determinants? Curr Med Chem. 2011;18:5519–5527. doi: 10.2174/092986711798347252. [DOI] [PubMed] [Google Scholar]
- 16.Caouette-Laberge L, Borsuk D. Congenital anomalies of the breast. Semin Plast Surg. 2013;27:36–41. doi: 10.1055/s-0033-1343995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romanini MV, Calevo MG, Puliti A, Vaccari C, Valle M, Senes F, et al. Poland syndrome: a proposed classification system and perspectives on diagnosis and treatment. Semin Pediatr Surg. 2018;27:189–199. doi: 10.1053/j.sempedsurg.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt H. Supernumerary nipples: prevalence, size, sex and side predilection: a prospective clinical study. Eur J Pediatr. 1998;157:821–823. doi: 10.1007/s004310050944. [DOI] [PubMed] [Google Scholar]
- 19.Yotsumoto F, Oki E, Tokunaga E, Maehara Y, Kuroki M, Miyamoto S. HB-EGF orchestrates the complex signals involved in triple-negative and trastuzumab-resistant breast cancer. Int J Cancer. 2010;127:2707–2717. doi: 10.1002/ijc.25472. [DOI] [PubMed] [Google Scholar]
- 20.Golding JP, Tsoni S, Dixon M, Yee KT, Partridge TA, Beauchamp JR, et al. Heparin-binding EGF-like growth factor shows transient left-right asymmetrical expression in mouse myotome pairs. Gene Expr Patterns. 2004;5:3–9. doi: 10.1016/j.modgep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Engin A. Obesity-associated breast cancer: analysis of risk factors. Adv Exp Med Biol. 2017;960:571–606. doi: 10.1007/978-3-319-48382-5_25. [DOI] [PubMed] [Google Scholar]
- 22.Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem. 2000;275:22583–22589. doi: 10.1074/jbc.M002915200. [DOI] [PubMed] [Google Scholar]
- 24.Mulligan C, Rochford J, Denyer G, Stephens R, Yeo G, Freeman T, et al. Microarray analysis of insulin and insulin-like growth factor-1 (IGF-1) receptor signaling reveals the selective up-regulation of the mitogen heparin-binding EGF-like growth factor by IGF-1. J Biol Chem. 2002;277:42480–42487. doi: 10.1074/jbc.M206206200. [DOI] [PubMed] [Google Scholar]
- 25.Amer MH. Genetic factors and breast cancer laterality. Cancer Manag Res. 2014;6:191–203. doi: 10.2147/CMAR.S60006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veltmaat JM, Ramsdell AF, Sterneck E. Positional variations in mammary gland development and cancer. J Mammary Gland Biol Neoplasia. 2013;18:179–188. doi: 10.1007/s10911-013-9287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robichaux JP, Hallett RM, Fuseler JW, Hassell JA, Ramsdell AF. Mammary glands exhibit molecular laterality and undergo left-right asymmetric ductal epithelial growth in MMTV-cNeu mice. Oncogene. 2015;34:2003–2010. doi: 10.1038/onc.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scutt D, Lancaster GA, Manning JT. Breast asymmetry and predisposition to breast cancer. Breast Cancer Res. 2006;8:R14. doi: 10.1186/bcr1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melnik Y, Slater PE, Steinitz R, Davies AM. Breast cancer in Israel: laterality and survival. J Cancer Res Clin Oncol. 1979;95:291–293. doi: 10.1007/BF00410651. [DOI] [PubMed] [Google Scholar]
- 30.Zeeneldin AA, Ramadan M, Elmashad N, Fakhr I, Diaa A, Mosaad E. Breast cancer laterality among Egyptian patients and its association with treatments and survival. J Egypt Natl Canc Inst. 2013;25:199–207. doi: 10.1016/j.jnci.2013.09.003. [DOI] [PubMed] [Google Scholar]