Abstract
Purpose
Young age at diagnosis has been considered a poor prognostic factor. However, considering young age itself as an independent poor prognostic factor for all breast cancers is unwarranted. We analyzed the different prognostic effects of age as a prognostic factor according to molecular subtype.
Methods
We retrieved data from 1,819 patients with primary breast cancer at the breast cancer center between 2007 and 2012. We classified each molecular subtype in 3 age cohorts (<40, 40–50, and >50 years). The associations of age and molecular subtypes with relapse-free survival (RFS) and disease-specific survival (DSS) were assessed.
Results
Patients aged <40 years showed a poor histologic grade, hormone receptor negative expression than older patients, and had a higher proportion of triple-negative breast cancer (TNBC) (P < 0.001). This was thought to have led to a significantly shorter RFS than that of older patients (P < 0.001). In the subgroup analysis according to molecular subtypes, the poorer RFS was observed only in patients aged <40 years with luminal type breast cancer (P < 0.001). Age was an independent prognostic factor of RFS in luminal-type breast cancer (P = 0.001). However, no difference in RFS between age groups was found for patients with other subtypes (human epidermal growth factor receptor 2 overexpression, TNBC). No significant effect between age groups was found in DSS for patients with all molecular subtypes.
Conclusion
Age at diagnosis of breast cancer affected prognosis differently according to molecular subtype. Age itself is not an independent prognostic factor. Age of <40 years showed a limited worse prognostic impact of recurrence in luminal type breast cancer only.
Keywords: Age, Breast neoplasms, Immunohistochemistry, Prognosis
INTRODUCTION
There have been many studies on the effects of age on breast cancer. Typically, young age at diagnosis has been considered a poor prognostic factor associated with poor differentiation, high proliferation index, high incidence of lymphovascular invasion (LVI), and lower estrogen receptor (ER) expression [1,2]. However, it may be unwarranted to consider young age an independent poor prognostic factor of all breast cancers.
This assumption is unwarranted because not only biologic but molecular factors also influence the prognosis of breast cancer. Additionally, the age-specific incidence of breast cancer differs by race, and the definition of ‘young age’ has not been determined. It has been estimated that less than 6.0% are diagnosed under the age of 40 years in the United States, and different incidence rates according to race and ethnicity in the United States have been shown [3,4,5]. The incidence rate of young breast cancer is significantly higher in Asia, especially in China and Korea [6,7]. The definition of ‘young age,’ defined by various authors as patients younger than 30, 35, 40, 45, or even 50 years, is currently debated [1,8,9,10]. Accordingly, the young age cutoffs vary between 30 and 50 years old. Young breast cancer patients show a higher proportion of triple-negative breast cancer (TNBC), which is considered an aggressive molecular subtype, and these patients are often found with higher stages of cancer [11]. These trends are likely to affect evaluations of the prognosis of young breast cancer patients.
According to the 2013 annual report of the Korean central cancer registry in Korea, breast cancers developing before the age of 40 years comprised 10.3% of all breast cancers, which is much higher than the proportion reported in western countries [3,4,7]. This finding is of great clinical importance for young breast cancer patients in Korea.
We hypothesized that young age itself would not be an independent prognostic factor for all breast cancers and that because young breast cancer is associated with worse clinicopathologic features and more aggressive subtypes such as TNBC, it seems to be frequently related to inferior outcomes. Accordingly, we aimed to analyze the different effects of age as a prognostic factor according to molecular subtypes.
METHODS
Patient cohort
In total, 1,819 patients who were diagnosed with and treated for invasive breast cancer at the breast cancer center between 2007 and 2012 were included in our study. This study was approved by the Institutional Review Board of College of Medicine, The Catholic University of Korea (No. KC 15RISI0805). This study was considered of minimum risk, so patient consent was not required. Data from a prospective cohort of patients with invasive breast cancer who underwent surgical treatment and adjuvant treatment were reviewed retrospectively from the hospital’s breast cancer center database and the patient’s medical records as a single-center trial.
The inclusion criteria for this study were as follows: female breast cancer patients pathologically diagnosed with invasive breast cancer; patients with unilateral breast cancer who had no distant metastasis at initial diagnosis; and patients with complete ER, progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) data. The exclusion criteria were the presence of noninvasive carcinoma (e.g., ductal carcinoma in situ); distant metastasis at diagnosis, neoadjuvant treatment; and any other malignancy.
This study classified each molecular subtype into 3 age cohorts including those aged <40, 40–50, and >50 years. Patients underwent surgical treatment and received adjuvant chemotherapy, endocrine therapy, and/or radiation therapy according to the institutional breast cancer treatment protocol by 2 physicians.
Immunohistochemistry and cancer staging
Immunohistochemistry (IHC) staining for ER (SP1, prediluted; Roche Science, Mannheim, Germany), PR (1E2, prediluted; Roche), HER2 (4B5, prediluted; Roche), and Ki-67 (MIB-1, prediluted; Roche) was performed on whole tissue section slides using BenchMark ULTRA (Ventana Medical Systems, Tucson, AZ, USA). Positive ER and PR status were defined as an Allred score ≥3 or nuclear staining ≥10%. IHC or fluorescence in situ hybridization (FISH) was performed to evaluate HER2 status. Positive HER2 status was defined as an IHC score of 3+ or 2+ with confirmed HER2 gene amplification by FISH. The amplification ratio was defined as the HER2 gene locus copy number relative to the chromosome 17 centromere copy number, and an amplification ratio of ≥2.0 was considered positive. Ki-67 was classified by cutoff points according to the expressing cell ratio (<14% and ≥14%). A single pathologist interpreted all IHC results.
We defined the molecular subtypes as follows [12]: luminal A: all ER and/or PR positive, HER2 negative, Ki-67 low (<14%) cases; luminal B: all ER and/or PR positive, HER2 negative/positive, any Ki-67 cases; HER2 overexpression: ER and PR absent, HER2 positive cases; and TNBC: ER and PR absent, HER2 negative cases.
All cancers were staged according to the eighth edition of the American Joint Committee on Cancer staging manual.
Follow-up and endpoint
Patients were assessed for disease recurrence according to standard clinical practice. A history and physical examination were performed every 6 months for 5 years and annually thereafter. The primary endpoint of this study was relapse-free survival (RFS), and the secondary endpoint was disease-specific survival (DSS). RFS was the time between diagnosis and the first evidence of locoregional or distant recurrence. DSS was the time between diagnosis and death as a result of recurrence. The follow-up deadline of this study was December 31, 2021.
Statistical analysis
Clinical and pathological features were calculated and compared using the chi-square test and Fisher exact test. The cumulative RFS and DSS durations and probabilities were estimated using Kaplan-Meier analysis. Survival curves were compared using a log-rank test between survival rates. A multivariate analysis was performed using Cox proportional hazards model. All variables were described with a hazard ratio and 95% confidence interval. All statistical tests were two-sided, and a P-value of <0.05 was considered statistically significant. The statistical analyses were performed with PASW Statistics ver. 18.0 (IBM Corp., Armonk, NY, USA).
RESULTS
Patient characteristics
Of the 1,819 patients, 1,205 were included in the analysis. Six hundred fourteen patients whose IHC data and follow-up data were missing from our database and who met the exclusion criteria were excluded.
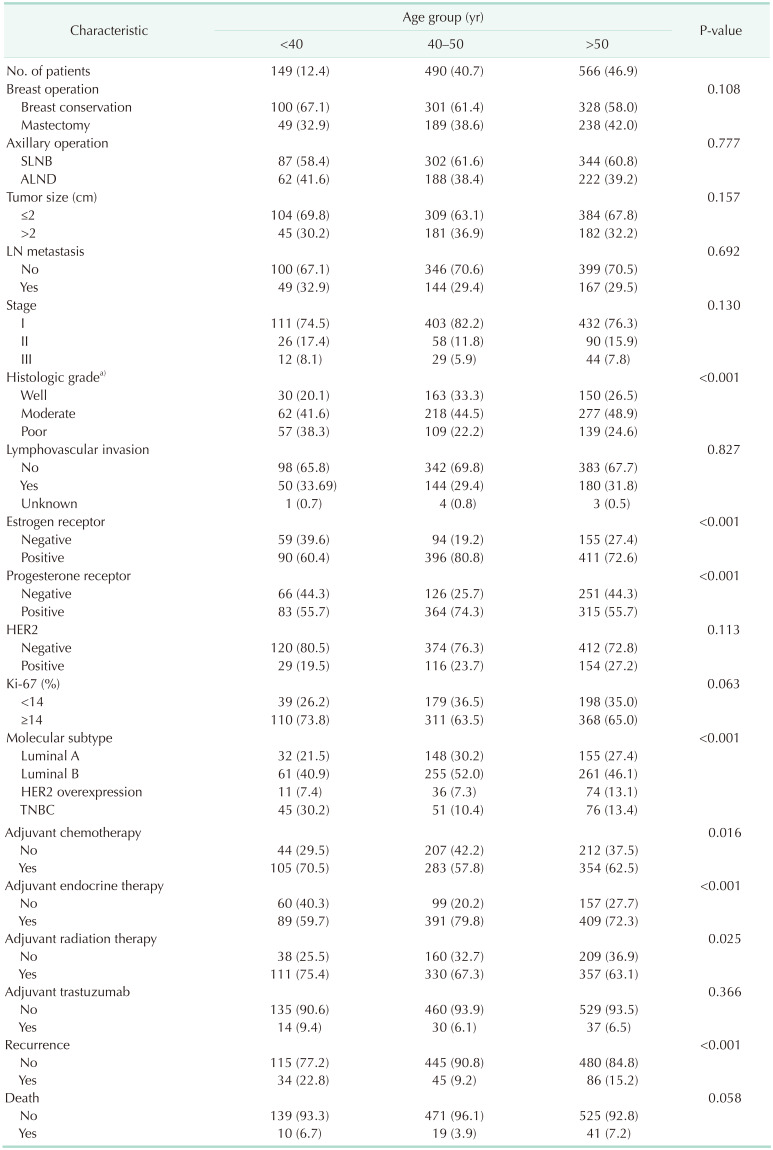
The clinicopathologic characteristics of patients according to the 3 age cohorts of those aged <40, 40–50, and >50 years are shown in Table 1. The mean patient age was 50.89 ± 10.57 years, and the mean follow-up was 108.01 ± 37.71 months. Additionally, 149 (12.4%) were younger than 40 years, 490 (40.7%) were between 40 and 50 years, and 566 (46.9%) were older than 50 years. The group of younger patients (aged <40 years) showed a greater association with poor prognostic factors, such as poor histologic grade, ER and PR negative expression than older patients (groups aged 40–50 years and >50 years). This is thought to have led to the impact on poor prognosis. The groups aged 40–50 years and >50 years had similar clinicopathologic characteristics including lymph node metastasis, pathologic stage, and histologic grade (Table 1).
Table 1. Clinicopathologic characteristics of patients according to age at diagnosis (n = 1,205).
Values are presented as number (%).
SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; LN, lymph node; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
a)Nottingham histologic score system.
The distribution of molecular subtypes according to age showed statistical significance (P < 0.001) (Table 1). The incidence of TNBC was higher, whereas the incidence of luminal type breast cancer was lower, in the group aged <40 years compared with the other age groups.
The proportion of patients who received adjuvant endocrine therapy was greater in the 40–50-year and >50-year age groups than in the <40-year age group (P < 0.001). This could be attributed to the different incidence rates of the luminal breast cancer subtypes. The proportion of patients who received adjuvant chemotherapy and radiation therapy was greater in the <40-year age group than in the 40–50-year and >50-year age groups (P = 0.016 and P < 0.001, respectively). Although it was not significantly different, breast conservation was performed more frequently in the <40-year age group (P = 0.108).
The recurrence rate was statistically significantly higher in those under 40 years of age, but there was no statistical difference in mortality.
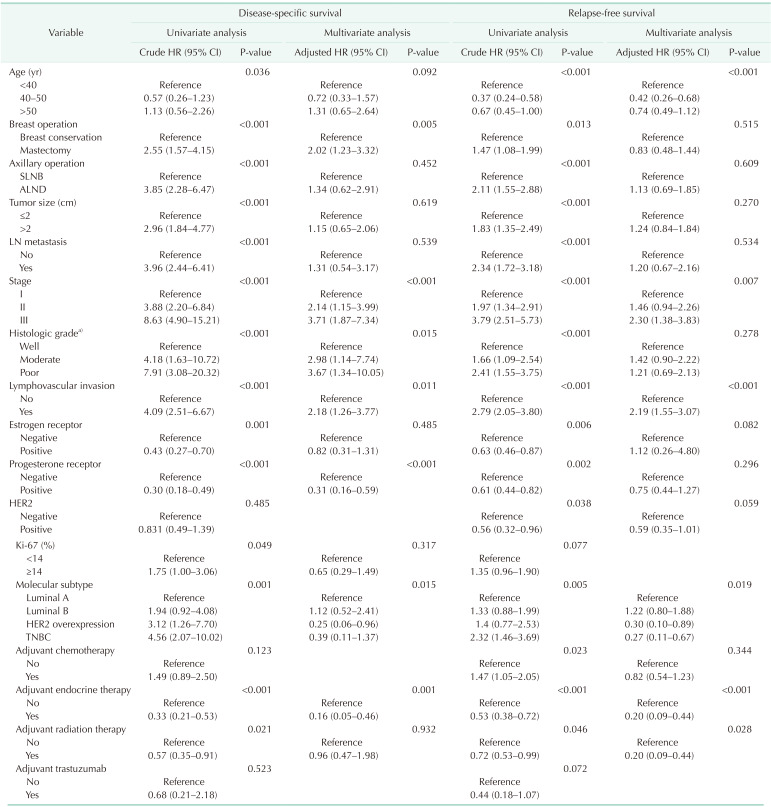
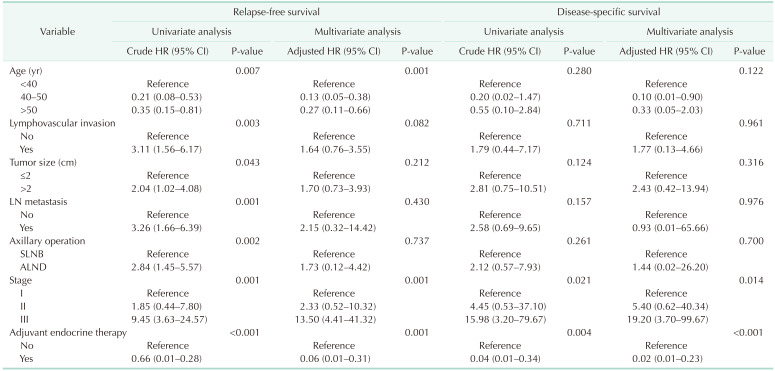
Univariate and multivariate survival analysis with Cox proportional hazard model showed that pathologic stage, LVI, molecular subtype, and adjuvant endocrine therapy were the only independent prognostic factors of recurrence and survival for all patients (Table 2). Age was associated with recurrence in the multivariate analysis only, but was not found to be an independent predictor of survival in breast cancer patients.
Table 2. Univariate and multivariate Cox proportional hazards regression analysis in all patients.
HR, hazard ratio; CI, confidence interval; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; LN, lymph node; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
a)Nottingham histologic score system.
Five-year relapse-free survival and 5-year disease-specific survival in all patients according to molecular subtype
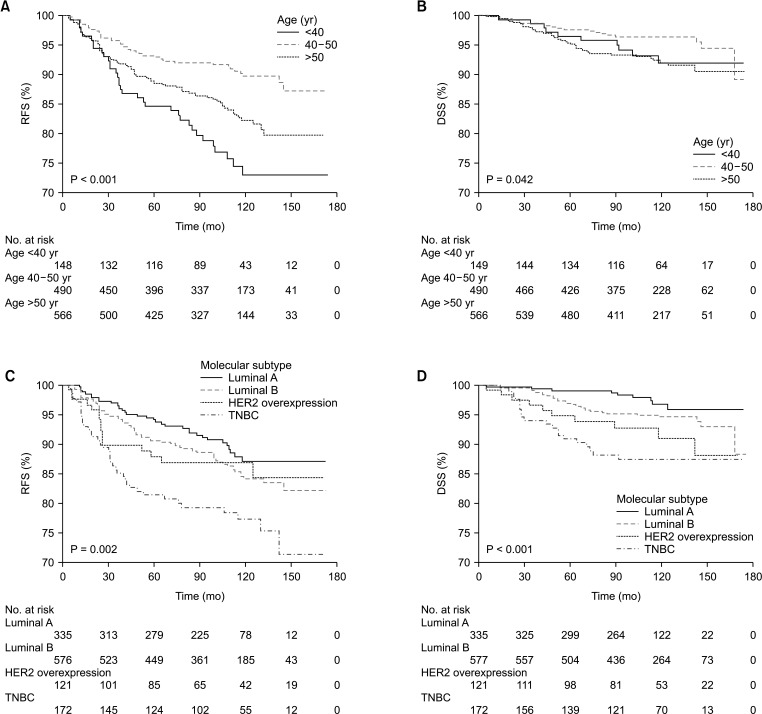
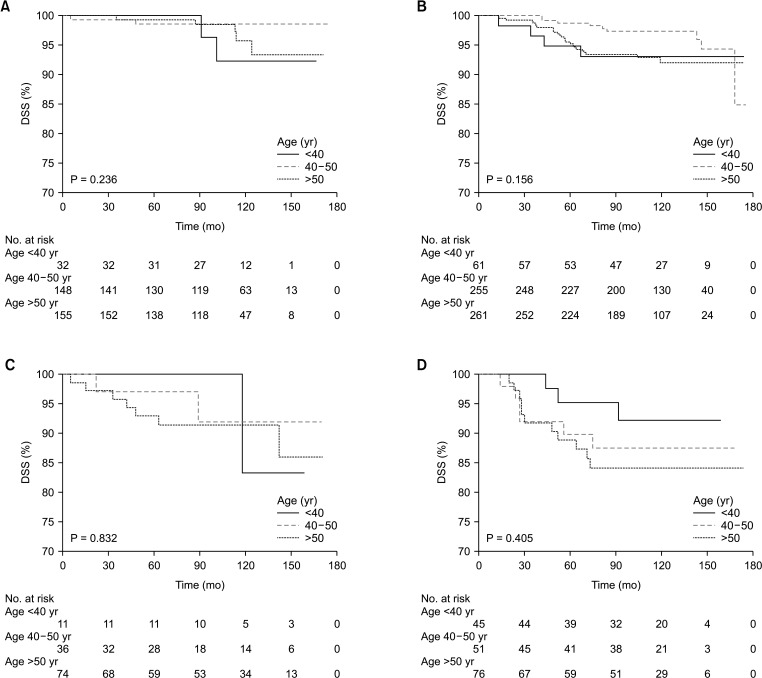
When classified by age, worse 5-year RFS (P < 0.001) and 5-year DSS (P = 0.042) were observed in younger ages than in older ages (Fig. 1). The 5-year RFS of those <40, 40–50, and >50 years were 84.7%, 93.2%, and 88.5%, respectively. The 5-year DSS of those <40, 40–50, and >50 years were 96.5%, 97.7%, and 95.2%, respectively. When classified by molecular subtype, as expected, a significant difference in 5-year RFS (P = 0.002) and 5-year DSS (P < 0.001) according to molecular subtype was observed (Fig. 1). The 5-year RFS of luminal A, luminal B, HER2 overexpression, and TNBC were 94.1%, 90.5%, 87.9%, and 81.4%, respectively. The 5-year DSS of luminal A, luminal B, HER2 overexpression, and TNBC were 99.1%, 96.7%, 94.8%, and 90.9%, respectively.
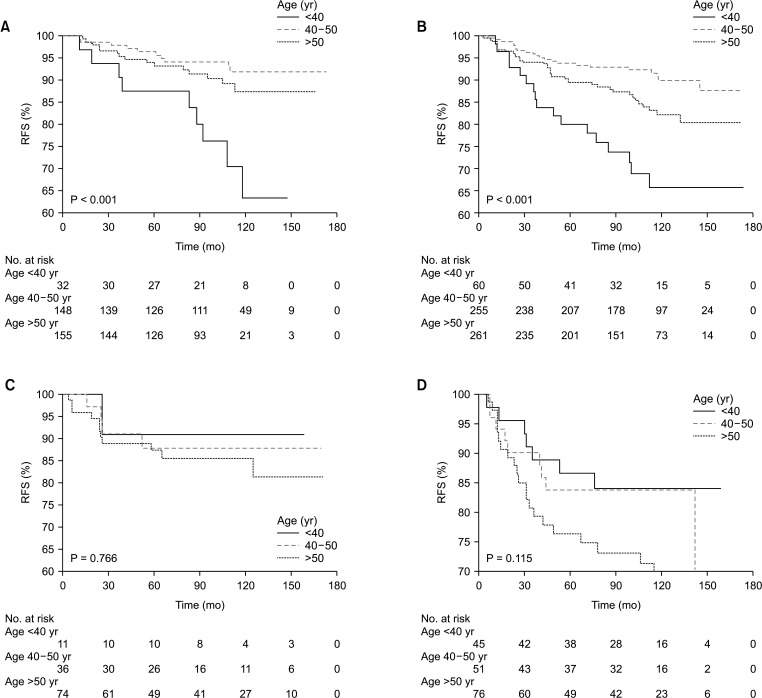
Fig. 1. Kaplan-Meier analysis of relapse-free survival (RFS) and disease-specific survival (DSS) in all breast cancer patients. (A) RFS according to age at diagnosis, (B) DSS according to age at diagnosis, (C) RFS according to molecular subtypes, and (D) DSS according to molecular subtypes. HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer.
Prognostic impact of age at diagnosis according to molecular subtype
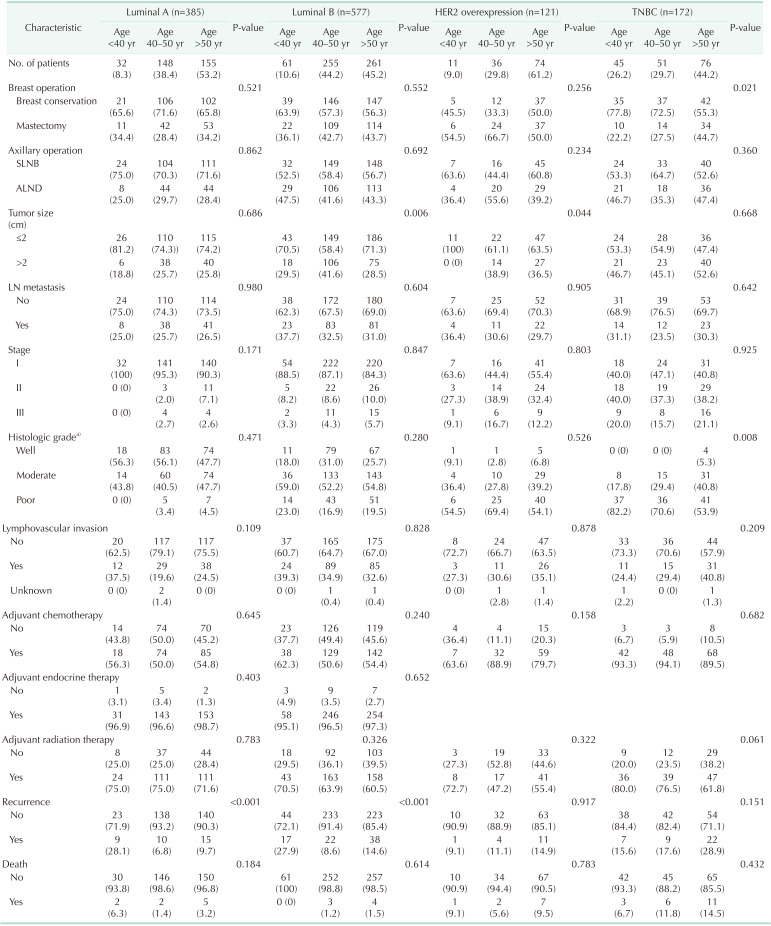
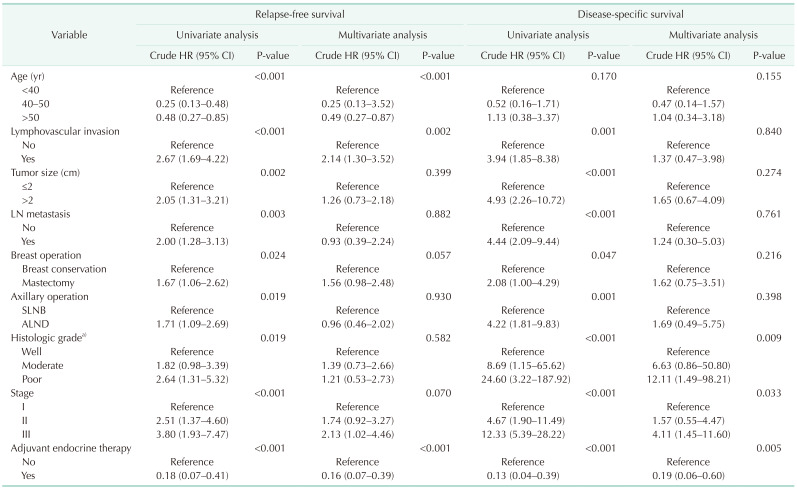
The distribution of age according to molecular subtypes is shown in Table 3. There were no clinicopathologic differences between those aged <40, 40–50, and >50 years in each molecular subtype. The <40-year age group presented only high rates of breast conservation (P = 0.021) and poor histologic grade (P = 0.008) in TNBC. Only the luminal A (P < 0.001) and B (P < 0.001) breast cancer showed a high recurrence rate in the group under 40 years of age (Table 3). Only younger patients (<40-year age group) with luminal A and B breast cancer had a worse RFS than older patients (Fig. 2A, B). The 5-year RFS rate was 87.5% for patients in the <40-year age group, 96.5% for patients in the 40–50-year age group, and 93.2% for patients in the >50-year age group for luminal A breast cancer. The 5-year RFS rate was 80.1% for patients in the <40-year age group, 93.9% for patients in the 40–50-year age group, and 89.5% for patients in the >50-year age group for luminal B breast cancer. The univariate and multivariate analysis also revealed that age of <40 years were independent prognostic factors of recurrence in luminal A and B breast cancer (Table 4, 5).
Table 3. Clinicopathologic characteristics of molecular subtypes according to age at diagnosis.
Values are presented as number (%).
SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection; LN, lymph node.
a)Nottingham histologic score system.
Fig. 2. Kaplan-Meier analysis of relapse-free survival (RFS) according to age at diagnosis in each molecular subtype. (A) Luminal A breast cancer, (B) luminal B breast cancer, (C) human epidermal growth factor receptor 2 overexpression breast cancer, and (D) triple-negative breast cancer.
Table 4. Univariate and multivariate Cox proportional hazards regression analysis in luminal A breast cancer.
HR, hazard ratio; CI, confidence interval; LN, lymph node; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
Table 5. Univariate and multivariate Cox proportional hazards regression analysis in luminal B breast cancer.
HR, hazard ratio; CI, confidence interval; LN, lymph node; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
a)Nottingham histologic score system.
However, no difference in RFS between age groups was found for patients with other subtypes, i.e., HER2 overexpression, and TNBC (Fig. 2C, D). Furthermore, no significant effect between age groups was found in DSS for patients with all molecular subtypes (Fig. 3).
Fig. 3. Kaplan-Meier analysis of disease-specific survival (DSS) according to age at diagnosis in each molecular subtype. (A) Luminal A breast cancer, (B) luminal B breast cancer, (C) human epidermal growth factor receptor 2 overexpression breast cancer, and (D) triple-negative breast cancer.
DISCUSSION
In this study, we showed that age at diagnosis of breast cancer had different effects on prognosis according to molecular subtype. Age had only a limited prognostic role in recurrence in both luminal A and B breast cancer.
It was estimated that 12.4% of breast cancer patients were diagnosed at <40 years of age, 40.7% were diagnosed between 40 and 50 years of age, and 46.9% were diagnosed at >50 years of age in our study; thus, those under 50 years of age accounted for more than half of all patients, similar to the findings described by previous Asian studies [13,14]. In western countries, young (<40 years) patients represent approximately 17.8%, 40- to 50-year-old patients represent 14.0%, and >50-year-old patients constitute 68.2%; thus, patients older than 50 years account for more than half of the cases, which is in contrast to Asian patients [15,16]. As such, the incidence rate of breast cancer according to age differs between Asian and western countries. The effect of age as a prognostic factor may differ due to the different age distribution between Asian and western countries.
Our study suggested that the clinicopathological characteristics and distribution of molecular subtypes according to age were different. Patients aged <40 years were associated with poor prognostic factors, such as poor histologic grade, ER and PR negative expression, and more than twice as many in the <40-year age group as in the >50-year age group had TNBC (30.2% and 13.4%, respectively). As a result, the <40-year age group showed a poorer RFS than the 40–50-year age group and >50-year age group in the Kaplan-Meier analysis of all patients. However, there was no significant difference in DSS by age. The 40–50-year and >50-year age groups had similar clinicopathological characteristics and prognoses. The finding of poor RFS in young breast cancer patients was similar to the results of previous studies [17], but the finding regarding DSS in young breast cancer patients did not correspond with the results of past studies [15]. Some studies have found that the prognosis of young patients did not differ from that of older patients, as was found in our study [18,19,20,21]. This is likely due to differences in race and distribution of age.
This study focused on the different effects of age according to molecular subtype. We hypothesized that young age was not a poor prognostic factor for all molecular subtypes of breast cancer. Based on our findings, young age was a poor prognostic factor in luminal A and B breast cancer only, and the other molecular subtypes such as HER2 overexpression, and TNBC were not affected by age. Consistent with a recent study, this proved that age has a subtype-specific prognostic impact [5,11].
This study showed a poor RFS with young age (<40 years) in luminal breast cancer. This result is in line with data from Liedtke et al. [16], who observed a clear prognostic effect of age in luminal breast cancer. Our finding of a lack of different prognoses according to age in TNBC was not consistent with the results of Liedtke et al. [16]. They investigated 4,467 breast cancer patients from 40 publicly available datasets and included incomplete information about adjuvant treatment and heterogeneous treatment modalities. Therefore, their results differ from ours, which included homogenous patients given standard clinical treatment.
Similar to our results, Sheridan et al. [22] suggested that the effect of age varies by subtype and that age of <40 years is not associated with an inferior RFS and overall survival (OS) in TNBC. Kim et al. [23] also concluded that the prognostic significance of young age differs by molecular subtype and that age of <35 years was not correlated with a poor prognosis in patients with TNBC. However, because each of these studies, including our own, used different definitions of young age, and as young breast cancer patients with TNBC receive more aggressive treatment than those with other molecular subtypes in Korea, this may have influenced the results. More recently, Partridge et al. [5], who analyzed the National Comprehensive Cancer Network database enrolled breast cancer patients, found that the effect of age on the prognosis of breast cancer is different according to molecular subtype, and age of <40 years has a particular prognostic role with luminal breast cancer. Although their study evaluated a large data set, the patients from a multicenter cohort had heterogenetic characteristics and could have received inconsistent treatment. There may be a lack of pathologic and follow-up data thereby creating a bias in these results.
More recent studies suggest a negative prognostic impact on recurrence at the young age of the luminal subtype, as Sheridan et al. [22] have previously described that age of <40 years was an independent predictor of RFS and OS for the luminal subtype. Kim et al. [24] investigated the association of young age breast cancer patients with locoregional recurrence according to molecular subtype with positive lymph nodes who received curative surgery and adjuvant chemotherapy. A total of 574 patients were reviewed retrospectively, and patients aged <40 years were independent factors for a lower LRRFS (locoregional recurrence-free survival rate) in both luminal A and luminal B type breast cancer. They noticed the negative effect of young age on LRRFS only in luminal subtypes. In contrast, young age did not have a significant impact on nonluminal subtypes [24]. Lian et al. [25] analyzed a large cohort of cases obtained from 2,125 Chinese women and described the association between age group and 5-year disease-free survival (DFS), 5-year distant metastasis-free survival (DMFS), or 5-year breast cancer special-survival (BCSS) by molecular subtype. Results showed significantly worse 5-year DFS, 5-year DMFS, and 5-year BCSS in younger patients with luminal A subtype, and younger women with luminal B (HER2 negative) showed worse 5-year DFS and 5-year DMFS than the 41–50-year age group. As our data shows, in line with previous studies [6,17,24,25], young age might be considered as a poor prognostic factor of recurrence in luminal subtype breast cancer.
In our opinion, all young patients with breast cancer do not have aggressive biologic characteristics, and biology differs according to molecular subtype. Therefore, as more young patients with hormone receptor-positive subtypes such as luminal A and B breast cancer experience menstruation recovery and are exposed to high levels of estrogen for longer periods after completing 5-year endocrine therapy, there might be a negative effect on their clinical outcomes. As a result, for younger age patients with hormone receptor-positive breast cancer, a longer duration of endocrine therapy might be beneficial.
This study has some limitations. First, there is a limitation in that it is unknown whether the results of our study are generalizable to other patient cohorts of different races or ethnicities, such as Caucasian, Hispanic, or African American. So, further studies including different patient cohorts are needed. Second, this study did not consider the effects of chemo-induced amenorrhea in the 40–50-year age group. Third, it could be considered a limitation that we did not investigate menopausal status over time in all patient cohorts. Additionally, because genetic tests including breast cancer type 1 (BRCA1) and BRCA2 are not routinely obtained in our institution, the impact of genetic mutation may have been inadvertently ruled out.
Nevertheless, there are many strengths. The characteristics of the patient population were homogenous because the study included patients from a single institution, the analysis of pathologic results was conducted by a single pathologist, and the selection of adjuvant treatment was performed according to the institutional breast cancer treatment protocol, and it was thus possible to minimize selection bias. The study drew conclusions from the results of a considerably long follow-up period. Finally, as the study included a large number of patients, these results may represent the characteristics of Asian patients, who differ from western patients.
In summary, breast cancer does not have age-specific biology. Because there is a different distribution of molecular subtypes according to age, breast cancer diagnosed at a young age could be considered aggressive. Age at diagnosis of breast cancer had different roles in prognosis according to molecular subtype. Age itself is not an independent prognostic factor. Age of <40 years showed a limited worse prognostic impact of recurrence in luminal A and B breast cancer only, with these patients being exposed to high levels of estrogen for longer periods even after completing 5-year endocrine therapy.
Footnotes
Fund/Grant Support: None.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: NHK, YHE, SHC.
- Formal Analysis, Methodology: YHE.
- Investigation: HWB.
- Project Administration: YHE, SHC.
- Writing – Original Draft: NHK, YHE.
- Writing – Review & Editing: All authors.
References
- 1.Axelrod D, Smith J, Kornreich D, Grinstead E, Singh B, Cangiarella J, et al. Breast cancer in young women. J Am Coll Surg. 2008;206:1193–1203. doi: 10.1016/j.jamcollsurg.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 2.Peng R, Wang S, Shi Y, Liu D, Teng X, Qin T, et al. Patients 35 years old or younger with operable breast cancer are more at risk for relapse and survival: a retrospective matched case-control study. Breast. 2011;20:568–573. doi: 10.1016/j.breast.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual report to the Nation on the Status Of Cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeSantis C, Howlader N, Cronin KA, Jemal A. Breast cancer incidence rates in U.S. women are no longer declining. Cancer Epidemiol Biomarkers Prev. 2011;20:733–739. doi: 10.1158/1055-9965.EPI-11-0061. [DOI] [PubMed] [Google Scholar]
- 5.Partridge AH, Hughes ME, Warner ET, Ottesen RA, Wong YN, Edge SB, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34:3308–3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 6.Tang LC, Jin X, Yang HY, He M, Chang H, Shao ZM, et al. Luminal B subtype: a key factor for the worse prognosis of young breast cancer patients in China. BMC Cancer. 2015;15:201. doi: 10.1186/s12885-015-1207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Min SY, Kim Z, Hur MH, Yoon CS, Park EH, Jung KW, et al. The basic facts of Korean breast cancer in 2013: results of a nationwide survey and breast cancer registry database. J Breast Cancer. 2016;19:1–7. doi: 10.4048/jbc.2016.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beadle BM, Woodward WA, Buchholz TA. The impact of age on outcome in early-stage breast cancer. Semin Radiat Oncol. 2011;21:26–34. doi: 10.1016/j.semradonc.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wray CJ, Phatak UR, Robinson EK, Wiatek RL, Rieber AG, Gonzalez A, et al. The effect of age on race-related breast cancer survival disparities. Ann Surg Oncol. 2013;20:2541–2547. doi: 10.1245/s10434-013-2913-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou P, Recht A. Young age and outcome for women with early-stage invasive breast carcinoma. Cancer. 2004;101:1264–1274. doi: 10.1002/cncr.20523. [DOI] [PubMed] [Google Scholar]
- 11.Ribnikar D, Ribeiro JM, Pinto D, Sousa B, Pinto AC, Gomes E, et al. Breast cancer under age 40: a different approach. Curr Treat Options Oncol. 2015;16:16. doi: 10.1007/s11864-015-0334-8. [DOI] [PubMed] [Google Scholar]
- 12.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei JT, Huang WH, Du CW, Qiu SQ, Wei XL, Liu J, et al. Clinicopathological features and prognostic factors of young breast cancers in Eastern Guangdong of China. Sci Rep. 2014;4:5360. doi: 10.1038/srep05360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia KS, Du WB, Sankaranarayanan R, Sankila R, Wang H, Lee J, et al. Do younger female breast cancer patients have a poorer prognosis? Results from a population-based survival analysis. Int J Cancer. 2004;108:761–765. doi: 10.1002/ijc.11632. [DOI] [PubMed] [Google Scholar]
- 15.Brandt J, Garne JP, Tengrup I, Manjer J. Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol. 2015;13:33. doi: 10.1186/s12957-014-0429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liedtke C, Rody A, Gluz O, Baumann K, Beyer D, Kohls EB, et al. The prognostic impact of age in different molecular subtypes of breast cancer. Breast Cancer Res Treat. 2015;152:667–673. doi: 10.1007/s10549-015-3491-3. [DOI] [PubMed] [Google Scholar]
- 17.Gajdos C, Tartter PI, Bleiweiss IJ, Bodian C, Brower ST. Stage 0 to stage III breast cancer in young women. J Am Coll Surg. 2000;190:523–529. doi: 10.1016/s1072-7515(00)00257-x. [DOI] [PubMed] [Google Scholar]
- 18.Lee MK, Varzi LA, Chung DU, Cao MA, Gornbein J, Apple SK, et al. The effect of young age in hormone receptor positive breast cancer. Biomed Res Int. 2015;2015:325715. doi: 10.1155/2015/325715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins EO, Deal AM, Anders CK, Prat A, Perou CM, Carey LA, et al. Age-specific changes in intrinsic breast cancer subtypes: a focus on older women. Oncologist. 2014;19:1076–1083. doi: 10.1634/theoncologist.2014-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ring A, Sestak I, Baum M, Howell A, Buzdar A, Dowsett M, et al. Influence of comorbidities and age on risk of death without recurrence: a retrospective analysis of the arimidex, tamoxifen alone or in combination trial. J Clin Oncol. 2011;29:4266–4272. doi: 10.1200/JCO.2011.35.5545. [DOI] [PubMed] [Google Scholar]
- 21.Muss HB, Woolf S, Berry D, Cirrincione C, Weiss RB, Budman D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan W, Scott T, Caroline S, Yvonne Z, Vanessa B, David V, et al. Breast cancer in young women: have the prognostic implications of breast cancer subtypes changed over time? Breast Cancer Res Treat. 2014;147:617–629. doi: 10.1007/s10549-014-3125-1. [DOI] [PubMed] [Google Scholar]
- 23.Kim EK, Noh WC, Han W, Noh DY. Prognostic significance of young age (<35 years) by subtype based on ER, PR, and HER2 status in breast cancer: a nationwide registry-based study. World J Surg. 2011;35:1244–1253. doi: 10.1007/s00268-011-1071-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim SW, Chun M, Han S, Jung YS, Choi JH, Kang SY, et al. Young age is associated with increased locoregional recurrence in node-positive breast cancer with luminal subtypes. Cancer Res Treat. 2017;49:484–493. doi: 10.4143/crt.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lian W, Fu F, Lin Y, Lu M, Chen B, Yang P, et al. The impact of young age for prognosis by subtype in women with early breast cancer. Sci Rep. 2017;7:11625. doi: 10.1038/s41598-017-10414-x. [DOI] [PMC free article] [PubMed] [Google Scholar]