Abstract
Background & Aims
Population-level trends and factors associated with HBV-related decompensated cirrhosis (DC), hepatocellular carcinoma (HCC), and liver-related mortality are crucial to evaluate the impacts of therapeutic interventions.
Methods
Trends in HBV-DC and -HCC diagnoses and liver-related mortality in New South Wales, Australia, were determined through linkage of HBV notifications (1993-2017) to hospital admissions (2001-2018), mortality (1993-2018), and cancer registry (1994-2014) databases. Late HBV notification was defined as notification at or within 2 years of a DC or HCC diagnosis. Cox proportional-hazards regression and multivariable logistic regression analyses were performed to evaluate associated factors.
Results
Among 60,660 people with a HBV notification, 1,276 (2.0%) DC and 1,087 (1.8%) HCC diagnoses, and 1,219 (2.0%) liver-related deaths were documented. Since the early 2000s, the number of DC and HCC diagnoses increased; however, age-standardised incidence decreased from 2.64 and 1.95 in 2003 to 1.14 and 1.09 per 1,000 person-years in 2017, respectively. Similarly, age-standardised liver mortality decreased from 2.60 in 2003 to 1.14 per 1,000 person-years in 2017. Among people with DC and HCC diagnoses, late HBV notification declined from 41% and 40% between 2001-2009 to 29% and 25% in 2010-2018, respectively. Predictors of DC diagnosis included older age (birth <1944, adjusted hazard ratio [aHR] 2.06, 95% CI 1.57–2.69), alcohol use disorder (aHR 4.82, 95% CI 3.96–5.87) and HCV co-infection (aHR 1.88, 95% CI 1.53–2.31). Predictors of HCC diagnosis included older age (birth <1944, aHR 3.94, 95% CI 2.91–5.32) and male sex (aHR 3.79, 95% CI 3.05–4.71).
Conclusion
In an era of improved antiviral therapies, the risk of HBV-related liver morbidity and mortality has declined. HCV co-infection and alcohol use disorder are key modifiable risk factors associated with the burden of HBV.
Lay summary
Rising hepatitis B-related morbidity and mortality is a major public health concern. However, the development of highly effective medicines against hepatitis B virus (HBV) has brought renewed optimism for its elimination by 2030. This study shows a steady decline in HBV-related liver morbidity and mortality in New South Wales, Australia. Moreover, late hepatitis notification has also declined, allowing individuals with HBV to have access to timely antiviral treatment. Despite this, hepatitis C co-infection and alcohol use disorder are key modifiable risk factors associated with HBV disease burden. To attain the desired benefits from highly effective antiviral treatment, managing comorbidities, including hepatitis C and high alcohol use, must improve among individuals with hepatitis B.
Keywords: DC, HCC, hepatitis B, late HBV notification, liver disease, liver mortality, population-level, record linkage, risk factors, cause of death
Abbreviations: APDC, Admitted Patient Data Collection; AUD, alcohol use disorder; CCI, Charlson comorbidity index; DC, decompensated cirrhosis; ESLD, end-stage liver disease; HCC, hepatocellular carcinoma; LHD, local health district; NCIMS, Notifiable Conditions Information Management System; NHR, National HIV Registry; NSW, New South Wales; NSWCR, NSW Cancer Registry; PBS, Pharmaceutical Benefits Scheme; RBDM, Registry of Births, Deaths and Marriages; WHO, World Health Organization
Graphical abstract
Highlights
-
•
The World Health Organization has set a 65% HBV mortality reduction target by 2030.
-
•
Since the early 2000s, diagnoses of decompensated cirrhosis and HCC increased, but age-standardised incidence rates decreased.
-
•
Age-standardised liver mortality rates decreased from 2.64 in 2003 to 0.97 per 1,000 person-years in 2017.
-
•
Late HBV notification declined from 41% and 40% during 2001-2009 to 28% and 26% in 2010-2018, respectively.
-
•
Hepatitis C co-infection and alcohol-use disorder are key modifiable risk factors associated with HBV disease burden.
Introduction
Worldwide, chronic hepatitis B (CHB) is a significant public health threat.1,2 The World Health Organization (WHO) estimated that around 296 million people were living with CHB in 2019, with an estimated 820,000 deaths attributed to HBV-associated end-stage liver disease (ESLD),3 most notably decompensated cirrhosis (DC) and hepatocellular carcinoma (HCC).1,3,4 Given the availability of highly effective antiviral regimens,5 the WHO has defined a set of targets for eliminating HBV as a public health threat by 2030, including a 65% reduction in mortality.2 While enhanced HBV diagnosis and timely linkage to care are required to achieve the WHO elimination targets, in many countries, including Australia, HBV diagnosis and treatment uptake remain suboptimal.6 Further, the impact of the existing HBV programs is unknown in most settings, given limited capacity for evaluation of HBV liver disease burden at the population level.
Over their lifetimes, 15%–40% of people with CHB develop cirrhosis, liver failure, or HCC, and 15–25% are at risk of dying secondary to HBV-related ESLD.7 The 5-year survival rate is 14-45% after DC 8 and 18-20% after HCC.9 The prolonged subclinical period and suboptimal screening can lead to late HBV diagnosis resulting in ESLD. Although potent and highly effective nucleos(t)ide analogue (NA) therapy reduces the risk of liver disease progression,10 late hepatitis notification leads to a potential missed opportunity, limiting the chances of preventing hepatic carcinogenesis or fibrosis.[11], [12], [13] Moreover, other factors including alcohol use disorder (AUD), ageing, suboptimal treatment uptake, and HBV/HCV co-infection contribute to higher risk of liver morbidity and mortality.14 In Australia, the epidemiology of HBV is complex, with a high prevalence among migrants from high- or intermediate HBV prevalence countries and Aboriginal or Torres Strait Islander peoples.15,16 Over the past two decades, remarkable strides have been made in increasing HBV diagnosis, vaccination and treatment uptake 17 and understanding the epidemiology, risk factors, and molecular profiles of HBV-related DC and HCC.18 However, enhanced HBV diagnosis and treatment uptake are needed if Australia is to achieve the WHO 2030 elimination targets.6
Australia is among the few countries with established national surveillance systems and mandatory HBV notification, thus providing an opportunity to evaluate HBV-related liver disease burden by linking notifications with hospitalisation and mortality databases.11,19 This study aimed to evaluate trends and factors associated with HBV-related DC and HCC diagnoses, along with trends in liver-related mortality. Further, we assessed trends and factors associated with late notifications among people with an HBV notification in New South Wales (NSW), Australia.
Patients and methods
Study setting, data sources and record linkages
This study utilises routinely collected data from NSW, Australia. As of March 2018, the total NSW population was 7.95 million, and about 65% of the population lived in Sydney.20 In 2018, an estimated 226,566 people were living with HBV in Australia, with 30% in NSW.21
Under the Public Health Act 1991, mandatory notification for all individuals with positive HBV and HCV serology tests is required in NSW.22 This record is maintained at the NSW Notifiable Conditions Information Management System (NCIMS). NSW residents with an HBV notification (notifiable HBV cases require detection of HBV surface antigen or HBV DNA) and DC and/or HCC diagnosis or a history of AUD were identified through the NSW Admitted Patient Data Collection (APDC) for DC and AUD diagnoses, and APDC and NSW Cancer Registry (NSWCR) for HCC. APDC covers all inpatient admissions from public and private hospitals since 2001. Each hospitalisation record includes demographic, administrative and diagnostic information coded at discharge according to the ICD-10. People with HIV co-infection were identified through the National HIV Registry (NHR), receiving mandatory HIV notifications since 1985. Data on mortality was extracted from the Registry of Births, Deaths and Marriages (RBDM), which holds the information on all deaths registered in NSW since 1993.22 People who received HBV treatment were identified through the Pharmaceutical Benefits Scheme (PBS) since 2010.
Data linkages occurred at 2 stages. First, HBV and HCV notifications were linked internally to identify people with HBV/HCV co-infection. Additionally, using demographic details (including full name, sex, date of birth, and address), probabilistic linkages of records between the NCIMS, APDC, NSWCR, and RBDM were undertaken by the NSW Centre for Health Record Linkage,22 which has deterministically linked NCIMS with NHR, using 2x2 name codes. Subsequently, the Australian Institute of Health and Welfare has probabilistically linked NCIMS with PBS records, using the aforementioned demographic details.23
Study population and period
The study population included people with an HBV notification in NSW, Australia, during 1993-2017. Linked data were extracted for the following periods: NCIMS (1 January 1993 - 31 December 2017); APDC (1 July 2001 - 30 June 2018); NSWCR (1 January 1993 - 31 December 2014); RBDM (1 January 1993 - 30 June 2018); NHR (1 January 1985- 31 December 2017); and PBS (1 April 2010 - 31 December 2018). Administrative data sources were extracted for different durations of time, depending on available periods for linkage. Hence, the study time period was restricted (2001-2018) to optimise data coverage.
Study outcomes
The primary outcomes of interest were HBV-related DC and HCC diagnoses and liver-related mortality. DC diagnosis was inferred using hospital discharge diagnosis code (ICD-10); primary or secondary diagnosis fields with following codes: DC ascites (R18.0), bleeding oesophageal varices (I85.0, I98.3, and I98.21), chronic hepatic failure (including hepatic encephalopathy; K72.1, K72.9), alcoholic hepatic failure (K70.4), or hepatorenal syndrome (K76.7).11,19,24 Similarly, a hospital discharge diagnosis code was used to define HCC diagnosis, coded in either the principal or secondary diagnosis fields of a linked inpatient hospital record (C22.0) or identified through NSWCR records.11,19,24 Hereafter, first-time DC and HCC hospitalisations or HCC diagnosis via cancer registry are referred to as DC and HCC diagnosis. Liver-related mortality was defined by any death following a DC and/or HCC diagnosis.
The secondary outcome was late HBV notification, defined by an HBV notification after, at the time or within 2 years before DC or HCC diagnosis.12
Exclusion criteria
Exclusion criteria were applied as follows: records where the data of HBV notification occurred after censoring (post-mortem HBV notifications); HBV notification without a Medicare (universal healthcare) number and duplicate HBV notifications; records where the date of HCC diagnosis or death was prior to 1 January 2001. Additionally, small cells (cell count with values <5) were not included in this analysis.
Statistical analysis
The linked dataset was cleaned, edited, and prepared for this study in the STATA software version 14.2 (StataCorp, College Station, Texas). Descriptive statistics including medians and interquartile ranges were calculated for continuous variables where the data was normally distributed, and frequencies with percentages were calculated for all categorical variables. Temporal trends in HBV-DC, HCC, and liver-related mortality were evaluated, including numbers and age-standardised incidence rates between 2001 to 2018. We calculated the rates where the denominator included all people with a HBV notification in NSW; people entered the cohort from the date of HBV notification and observation time (for evaluation of DC and HCC) started from 2001, when both hospital admissions and cancer registry data were available. To reduce the potential for individuals to have the outcome of interest (DC and HCC) at study entry, a sensitivity analysis with an observation period of a 5-year look back window was adjusted to start from 2005. HCC diagnoses were obtained using hospital admission and cancer registry data; however, the cancer registry data was only available up to 2014. To evaluate whether the HCC diagnosis data source biased our results, an additional sensitivity analysis was carried out with only diagnoses through hospitalisation data available for the entire study period. Age-standardised rates (per 1,000 person-years) and corresponding 95% CIs were calculated assuming a Poisson distribution. Among people with a DC diagnosis, numbers and age-standardised incidence rates were evaluated overall and stratified by HBV/HCV co-infection notifications. Smoothing was carried out to create the impression of trends by ensuring that any fluctuation to a high or low value is amplified and the point-to-point distortion/variability is muted. Smooth trends were observed after averaging 4 values on each side and using a second-order smoothing polynomial. Standardisation was performed using the Australian Standard Population in 2013 (by 5-year age-grouping).
Unadjusted and adjusted Cox proportional-hazards regression analyses were performed to evaluate factors associated with DC and HCC diagnoses. In unadjusted analysis, variables that did not violate the assumption of proportionality were considered for inclusion in the adjusted analysis. Covariates included age (birth cohort ≤1944, 1945-1964, and ≥1965), sex, country of birth, ethnicity, history of AUD, HBV-HCV/HIV co-infection, and local health district (LHD) of residence at the time of notification. Comorbidity burden was assessed using the Charlson comorbidity index (CCI).25,26 The CCI was categorised from 0 to 3+ groups based on its score, where higher scores indicate a worse health condition.26 The codes for moderate or severe liver morbidity were excluded from the CCI to avoid overlapping with the main variables that were included separately. Finally, HBV treatment trends among people with an HBV notification were calculated from 2010 to 2017. Given HBV treatment data was not available prior to 2010, HBV notifications were included in this analysis only for the 2009-2017 period. In all time-to-event analyses, time at risk started on HBV notification date and ended on date of DC or HCC diagnosis, death, or last follow-up (30 June 2018), whichever occurred first (depending on the outcome of interest in each analysis).
To assess the number of people with a late HBV notification, people with a DC or HCC diagnosis were stratified into 5 categories according to the time between their HBV notification and DC or HCC diagnosis 12; people with an HBV notification a) after or at the time of DC or HCC diagnosis; b) less than 1 year before; c) between 1 and 2 years before; d) between 2 and 5 years before and; e) more than 5 years before. The proportion of those with a late HBV notification (categories a, b and c above) was calculated overall and during the 2001-2009 and 2010-2018 time periods. Following unadjusted analyses, multivariable logistic regression evaluated factors associated with late HBV notification, considering factors significant at the 0.20 level in the unadjusted models. Covariates included age, sex, country of birth, ethnicity, history of AUD, HBV-HCV/HIV co-infection, and LHD of residence at the time of DC or HCC diagnosis.19 Further, the calendar period of DC and HCC diagnosis was hypothesised to be associated with late HBV notification, assuming late notification was more likely to occur in the early 2010s, compared to later periods. In all analyses, LHD of residence at the time of HBV notification was defined by the 2011 NSW boundaries, including 8 LHDs covering metropolitan regions and 7 covering rural and regional locations.27
AUD is a standard term used to define continued drinking despite adverse mental and physical consequences. Liver-related consequences of alcohol use are not included in the definition of AUD.28 AUD was defined according to hospitalisation with any of the following principal or additional ICD-10 codes: alcohol abuse counselling and surveillance (Z71.4), alcoholic cardiomyopathy (I42.6), alcohol-induced pseudo-Cushing's syndrome (E24.4), alcoholic myopathy (G72.1), alcoholic polyneuropathy (G62.1), alcohol rehabilitation (Z50.2), degeneration of nervous system due to alcohol (G31.2) or mental and behavioural disorders due to alcohol (F10).28
Results
Study participants
Between 1993 and 2017, there were 60,660 individuals with an HBV notification in NSW. The median year of birth was 1966 (interquartile range 1956-1977). Overall, 55% were male, 21% were born in Australia, 5% identified as Aboriginal and/or Torres Strait Islander, 4% had a history of AUD, 7% had HCV co-infection, and only 1% had HIV co-infection. During 2001–2018, there were 1,276 (2.0%) with a DC and 1,087 (1.8%) with an HCC diagnosis (Table 1, Table 2).
Table 1.
Demographic characteristics among people with an HBV notification (1993-2017), by decompensated cirrhosis diagnosis status (2000-2018).
| Characteristics (n, %) | All HBV |
No DC 2001-2018 |
DC 2001-2018 |
Period of DC |
|
|---|---|---|---|---|---|
| 2001-2009 |
2010-2018 |
||||
| n = 60,660 | n = 59,384 | n = 1,276 | n = 608 | n = 668 | |
| Year of birth, median (IQR)∗ | 1966 (56-77) | 1967 (57-77) | 1954 (45-62) | 1951 (41-60) | 1956 (47-65) |
| Birth cohort, n (%)∗ | |||||
| ≤1944 | 4,908 (8) | 4,589 (8) | 319 (25) | 199 (33) | 120 (18) |
| 1945-1964 | 22,583 (37) | 21,882 (37) | 701 (55) | 320 (53) | 381 (57) |
| ≥1965 | 33,164 (55) | 32,908 (55) | 256 (20) | 89 (14) | 167 (25) |
| Male sex∗∗ | 33,367 (55) | 32,391 (55) | 976 (77) | 471 (78) | 505 (76) |
| Aboriginal and Torres Strait Islander∗∗∗ | 2,118 (5) | 2,003 (5) | 115 (9) | 50 (8) | 65 (10) |
| Place of birth∗∗∗∗ | |||||
| Australia | 8,666 (21) | 8,252 (21) | 414 (33) | 185 (31) | 229 (34) |
| Americas, Europe, New Zealand | 4,057 (10) | 3,837 (10) | 220 (17) | 116 (19) | 104 (16) |
| Africa | 1,360 (3) | 1,331 (3) | 29 (2) | 11 (2) | 18 (3) |
| East Asia | 11,661 (28) | 11,431 (28) | 230 (18) | 114 (19) | 116 (17) |
| Oceania and Southeast Asia | 12,914 (31) | 12,605 (31) | 309 (24) | 146 (24) | 163 (25) |
| Western Asia | 2,606 (6) | 2,600 (6) | 66 (5) | 31 (5) | 31 (5) |
| Co-infection | |||||
| With HCV | 3,943 (7) | 3,649 (6) | 294 (23) | 122 (20) | 172 (26) |
| With HIV | 394 (1) | 364 (1) | 30 (2) | 19 (3) | 11 (2) |
| LHD at the time of HBV notification∗∗∗∗∗ | |||||
| Metropolitan NSW | 6,049 (10) | 5,832 (10) | 217 (17) | 107 (18) | 110 (17) |
| Outer metropolitan NSW | 27,773 (46) | 26,198 (46) | 575 (45) | 263 (43) | 312 (47) |
| Rural NSW | 25,961 (43) | 25,489 (44) | 472 (37) | 237 (39) | 235 (36) |
| History of alcohol use disorder diagnosis | 2,295 (4) | 1,911 (3) | 384 (30) | 169 (28) | 215 (32) |
DC, decompensated cirrhosis; HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; LHD, local health district; NSW, New South Wales.
5 had missing information.
387 had missing information.
17,722 had missing information.
19,336 had missing information.
877 had missing information.
Table 2.
Demographic characteristics among people with an HBV notification (1993-2017), by hepatocellular carcinoma diagnosis status (2000-2018).
| Characteristics (n, %) | All HBV |
No HCC 2001-2018 |
HCC 2001-2018 |
Period of HCC |
|
|---|---|---|---|---|---|
| 2001-2009 |
2010-2018 |
||||
| n = 60,660 | n = 59,573 | n = 1,087 | n = 481 | n = 606 | |
| Year of birth, median (IQR)∗ | 1966 (56-77) | 1967 (57-77) | 1951 (42-59) | 1947 (37-56) | 1954 (46-61) |
| Birth cohort∗ | |||||
| ≤1944 | 4,908 (8) | 4,572 (8) | 336 (31) | 211 (44) | 125 (21) |
| 1945-1964 | 22,583 (37) | 21,965 (37) | 618 (57) | 231 (48) | 387 (64) |
| ≥1965 | 33,164 (55) | 33,032 (55) | 132 (12) | 38 (8) | 94 (15) |
| Male sex∗∗ | 33,367 (55) | 32,475 (55) | 892 (82) | 393 (82) | 499 (83) |
| Aboriginal and Torres Strait Islander∗∗∗ | 2,118 (5) | 2,092 (5) | 26 (2) | 9 (2) | 17 (3) |
| Place of birth∗∗∗∗ | |||||
| Australia | 8,666 (21) | 8,549 (21) | 117 (11) | 37 (8) | 80 (13) |
| Americas, Europe, New Zealand | 4,057 (10) | 3,908 (10) | 149 (14) | 76 (16) | 73 (12) |
| Africa | 1,360 (3) | 1,328 (3) | 32 (3) | 12 (3) | 20 (3) |
| East Asia | 11,661 (28) | 11,303 (28) | 358 (33) | 169 (35) | 189 (31) |
| Oceania and Southeast Asia | 12,914 (31) | 12,545 (31) | 369 (34) | 156 (33) | 213 (35) |
| Western Asia | 2,606 (6) | 2,612 (6) | 54 (5) | 28 (6) | 26 (4) |
| Co-infection | |||||
| With HCV | 3,943 (7) | 3,815 (6) | 128 (12) | 43 (9) | 85 (14) |
| With HIV | 394 (1) | 381 (1) | 13 (1) | 6 (1) | 7 (1) |
| LHD at the time of HBV notification∗∗∗∗∗ | |||||
| Metropolitan NSW | 6,049 (10) | 5,957 (10) | 92 (9) | 39 (8) | 53 (9) |
| Outer metropolitan NSW | 27,773 (46) | 27,253 (46) | 520 (48) | 227 (47) | 293 (49) |
| Rural NSW | 25,961 (43) | 25,496 (43) | 465 (43) | 212 (44) | 253 (42) |
| History of alcohol use disorder diagnosis | 2,295 (4) | 2,148 (4) | 111 (10) | 36 (7) | 75 (12) |
HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IQR, interquartile range; LHD, local health district; NSW, New South Wales.
5 had missing information.
387 had missing information.
17,722 had missing information.
19,336 had missing information.
877 had missing information.
The number of people with an HBV notification who developed DC increased from 608 in 2001-2009 to 668 in 2010-2018. Compared to people without DC, individuals with DC were older (birth before 1945, 25% vs. 8% or during 1945-1964, 55% vs. 37%), more likely born in Australia (33% vs. 21%), and male (77% vs. 55%). Further, higher proportions had HBV/HCV co-infection (23% vs. 6%), history of AUD (30% vs. 3%), and lived in metropolitan areas at HBV notification (17% vs. 10%) (Table 1).
The number of people with an HBV notification who developed HCC increased from 481 in 2001-2009 to 606 in 2010-2018. Compared to people without HCC, individuals with HCC were older (birth before 1945, 31% vs. 8% or during 1945-1964, 57% vs. 37%) and more likely male (82% vs. 55%). A higher proportion of people with HCC were born overseas, predominantly in East Asia (33% vs. 28%) and Oceania/Southeast Asia (34% vs. 31%). Further, people with HCC were more likely to have HBV/HCV co-infection (12% vs. 6%), and a history of AUD (10% vs. 4%) (Table 2).
Temporal trends in DC and HCC diagnoses and liver-related mortality
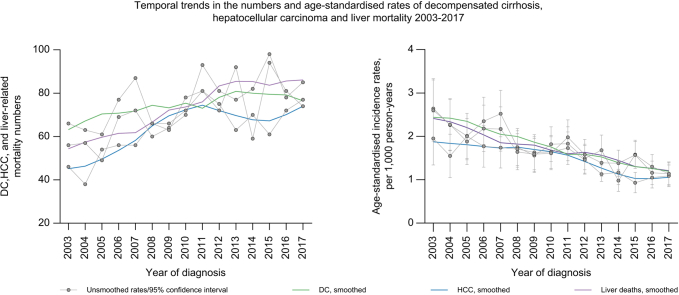
Between 2003 and 2017, there was an increase in the number of annual DC diagnoses (66 to 74); however, age-standardised incidence declined from 2.64 (95% CI 1.95–3.33) to 1.14 (95% CI 0.86–1.41) per 1,000 person-years. In this period, HCC diagnosis numbers increased from 46 to 77, and age-standardised incidence declined from 1.95 (95% CI 1.34–2.56) to 1.09 (95% CI 0.84–1.34) per 1,000 person-years. Similarly, liver-related deaths increased from 56 in 2003 to 85 in 2017. However, the age-standardised incidence of liver mortality decreased from 2.60 (95% CI 1.89–3.30) in 2003 to 1.14 (95% CI 0.89–1.39) per 1,000 person-years in 2017 (Fig. 1). Additional sensitivity analysis (presented as Fig. S1) shows similar trends and rates for a 5-year look back window. Trends in HCC diagnoses based on hospitalisation data only, available for the entire study period, were similar to those based on both hospitalisation and cancer registry data (combined HCC diagnosis, Fig. S2).
Fig. 1.
Temporal trends in DC and HCC diagnoses, and liver-related mortality and age-standardised incidence rates in 2003-2017, among people with an HBV notification in NSW, 1993-2017.
∗2003-2017 was only chosen for visual display of data – the study period was 2001-2018. DC, decompensated cirrhosis; HCC, hepatocellular carcinoma; NSW, New South Wales.
Trends in DC diagnosis, stratified by HBV/HCV co-infection notification
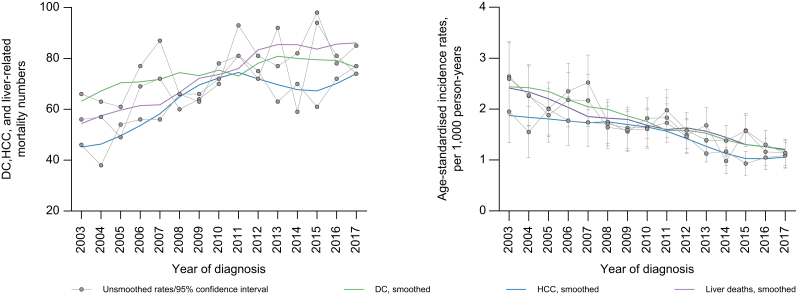
Between 2003 and 2017, DC diagnoses and age-standardised incidence declined from 58 to 54 and 2.52 (95% CI 1.82–3.23) to 0.88 (95% CI 0.63–1.13) per 1,000 person-years, respectively, among people with HBV mono-infection. However, the number and age-standardised incidence of DC among people with HBV/HCV co-infection increased from 6 to 20 and 4.02 (95% CI 0.53–7.51) to 6.14 (95% CI 4.31–7.96) per 1,000 person-years, respectively (Fig. 2).
Fig. 2.
Trends in DC diagnosis numbers and rates, overall and by HBV/HCV co-infection in 2003-2017, among people with an HBV notification in NSW, 1993-2017.
∗2003-2017 was only chosen for visual display of data – the study period was 2001-2018. DC, decompensated cirrhosis; NSW, New South Wales.
Trends in antiviral treatment during 2010-2017
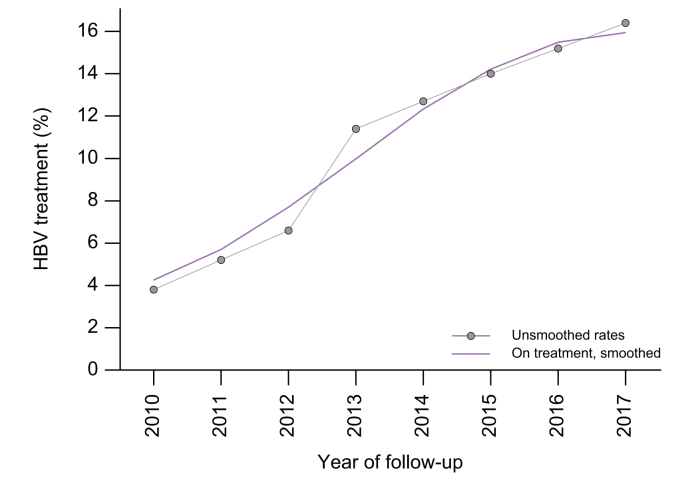
Between 2010 and 2017, the percentage of individuals on HBV treatment per calendar year increased from 3.8% to around 16.4% (Fig. 3).
Fig. 3.
Trends in antiviral treatment during 2010-2017 among people with an HBV notification (2009-2017), n = 17,340.
Time to DC diagnosis after an HBV notification
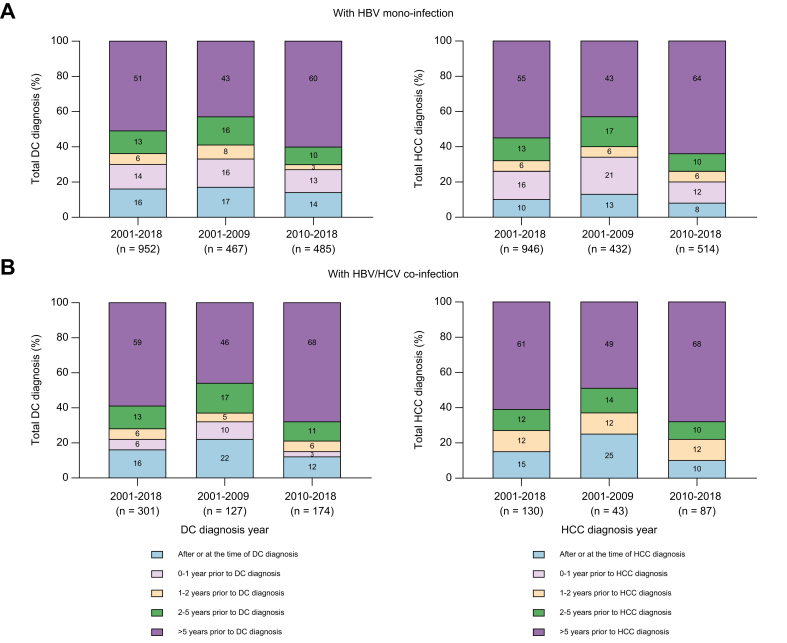
Overall, among 1,276 people with a DC diagnosis, 33% (n = 427) had a late HBV notification, declining from 41% (244 of 594) in 2001-2009 to 29% (192 of 659) in 2010-2018. Compared to people with an HBV mono-infection notification, those with HBV/HCV co-infection had a slightly larger decline in the proportion with late HBV notification (41% and 37% in 2001-2009 vs. 30% and 21% in 2010-2018, respectively) (Fig. 4). Among 379 people with a history of AUD and DC diagnosis, 31% (n = 118) had a late HBV notification, declining from 39% (64 of 165) in 2001-2009 to 25% (54 of 214) in 2010-2018 (Table S1). In adjusted analysis, late HBV notification was associated with older age at the time of HBV notification (≥47 years, median) (adjusted odds ratio [aOR] 2.91, 95% CI 1.97–4.31), Aboriginal and Torres Strait Islander ethnicity (aOR 0.41, 95% CI 0.17–0.94) and period of DC diagnosis (≥2014, aOR 0.58, 95% CI 0.41–0.83) (Table 3).
Fig. 4.
Time from HBV notification to decompensated cirrhosis or hepatocellular carcinoma diagnosis.
Among (A) people with an HBV mono-infection notification (n = 952 for DC and n = 946 for HCC), and (B) people with an HBV/HCV co-infection notification (n = 301 for DC and n = 130 for HCC). ∗(B) (for HCC) combined 2 categories, small cells (<5). DC, decompensated cirrhosis; HCC, hepatocellular carcinoma.
Table 3.
Logistic regressionanalysis, evaluating factors associated with late HBV notification among people with decompensated cirrhosis (n = 668) and hepatocellular carcinoma (n = 606).
| Characteristic, n % | Late HBV notification (DC), n = 187 | aOR | 95% CI | p value | Late HBV notification (HCC), n = 152 | aOR | 95% CI | p value |
|---|---|---|---|---|---|---|---|---|
| Age at HBV notification, by median | ||||||||
| <47 years | 57 (17) | 1.00 | 34 (11) | 1.00 | ||||
| ≥47 years | 130 (38) | 2.91 | 1.97–4.31 | <0.001 | 118 (39) | 4.98 | 3.25, 7.63 | <0.001 |
| Sex | ||||||||
| Female | 47 (29) | 24 (23) | ||||||
| Male | 140 (28) | - | - | - | 128 (26) | - | - | - |
| Aboriginal and Torres Strait Islander∗ | ||||||||
| No | 178 (30) | 1.00 | - | - | - | - | ||
| Yes | 8 (12) | 0.41 | 0.17–0.94 | 0.037 | ||||
| Country of birth | ||||||||
| Australia | 57 (25) | 1.00 | 20 (25) | |||||
| Americas/Europe, New Zealand | 27 (26) | 0.56 | 0.31–1.01 | 0.054 | 19 (26) | - | - | - |
| Africa | 7 (35) | 1.64 | 0.57–4.74 | 0.356 | ||||
| East Asia | 35 (30) | 0.68 | 0.38–1.22 | 0.200 | 50 (26) | - | - | - |
| Oceania and Southeast Asia | 50 (31) | 0.75 | 0.44–1.27 | 0.289 | 51 (24) | - | - | - |
| Western Asia | 9 (26) | 0.57 | 0.23–1.38 | 0.216 | 6 (23) | - | - | - |
| Co-infection with HCV | ||||||||
| No | 150 (30) | 1.00 | 133 (26) | |||||
| Yes | 37 (21) | 0.82 | 0.50–1.34 | 0.437 | 19 (22) | - | - | - |
| LHD of residence at the time of HBV notification | ||||||||
| Rural NSW | 27 (25) | 14 (26) | ||||||
| Outer metro NSW | 88 (28) | 72 (25) | - | - | - | |||
| Metro NSW | 69 (29) | 65 (26) | - | - | - | |||
| History of alcohol use disorder | ||||||||
| No | 132 (29) | 132 (25) | ||||||
| Yes | 55 (26) | 20 (27) | - | - | - | |||
| Period of DC/HCC diagnosis | ||||||||
| ≤2013 | 108 (33) | 1.00 | 82 (28) | 1.00 | ||||
| ≥2014 | 79 (23) | 0.58 | 0.41–0.83 | 0.003 | 70 (22) | 0.68 | 0.46, 1.01 | 0.060 |
Levels of significance: p <0.05 (multivariable logistic regression analysis).
aOR, adjusted odds ratio; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LHD, local health district.
Not shown, small n (<5).
Time to HCC diagnosis after an HBV notification
Overall, among 1,087 people with an HCC diagnosis, 31% (n = 339) had a late HBV notification, declining from 40% (188 of 475) in 2001-2009 to 25% (151 of 601) in 2010-2018. People with an HBV mono-infection notification and those with HBV/HCV co-infection had a similar decline in the proportion of late HBV notification (40% and 37% in 2001-2009 vs. 26% and 22% in 2010-2018, respectively) (Fig. 4). In adjusted analysis, late HBV notification among those with an HCC diagnosis was associated with older age at the time of HBV notification (≥50 years, median) (aOR 4.98, 95% CI 3.25, 7.63) (Table 3).
Factors associated with DC diagnosis during 2009-2018
In adjusted analysis, DC diagnosis was associated with older age (birth before 1944) (adjusted hazard ratio [aHR] 2.06, 95% CI 1.57–2.69), history of AUD (aHR 4.82, 95% CI 3.96–5.87), HCV co-infection (aHR 1.88, 95% CI 1.53–2.31), and male sex (aHR 1.93, 95% CI 1.60–2.31). Moreover, CCI groups 1-2 and 3+ were significantly associated with a higher risk of DC diagnosis (aHR 3.74, 95% CI 3.04–4.60 and 10.29, 95% CI 8.46–12.51), respectively (Table 4). The association between DC diagnosis and HCV co-infection was driven by the LHD of residence at the time of HBV notification (aHR 2.42, 95% CI 1.73–3.40 for outer metro LHD residents and aHR 1.74, 95% CI 1.17–2.59 for people living in metro NSW) (Table S2).
Table 4.
Cox proportional-hazards regression analysis, evaluating factors associated with decompensated cirrhosis and hepatocellular carcinoma diagnosis (2009-2018) among people with an HBV notification (1993-2017), n = 60,660.
| Characteristic, n % | DC |
HCC |
||||||
|---|---|---|---|---|---|---|---|---|
| n = 668∗ | aHR | 95% CI | p value | n = 606∗ | aHR | 95% CI | p value | |
| Birth cohort | ||||||||
| Born ≥1965 | 167 (1) | 1.00 | 94 (<1) | 1.00 | ||||
| Born 1945-1964 | 381 (2) | 2.07 | 1.70–2.52 | <0.001 | 386 (2) | 3.69 | 2.90–4.68 | <0.001 |
| Born ≤1944 | 120 (2) | 2.06 | 1.57–2.69 | <0.001 | 125 (3) | 3.94 | 2.91–5.32 | <0.001 |
| Sex | ||||||||
| Female | 161 (1) | 1.00 | 104(<1) | 1.00 | ||||
| Male | 505 (2) | 1.94 | 1.60–2.31 | <0.001 | 498 (2) | 3.79 | 3.05–4.71 | <0.001 |
| Aboriginal and Torres Strait Islander | ||||||||
| No | 599 (1) | 1.00 | 576 (1) | 1.00 | ||||
| Yes | 65 (3) | 0.88 | 0.66–1.18 | 0.417 | 17 (1) | 0.78 | 0.46–1.32 | 0.362 |
| Country of birth | ||||||||
| Australia | 229 (3) | 1.00 | 80 (1) | 1.00 | ||||
| Americas, Europe, New Zealand | 104 (3) | 1.00 | 0.77–1.29 | 0.995 | 73 (2) | 1.60 | 1.12–2.27 | 0.008 |
| Africa | 18 (1) | 1.18 | 0.72–1.93 | 0.505 | 20 (1) | 3.12 | 1.85–5.26 | <0.001 |
| East Asia | 116 (1) | 1.14 | 0.87–1.50 | 0.331 | 188 (2) | 3.73 | 2.70–5.15 | <0.001 |
| Oceania and Southeast Asia | 163 (1) | 1.11 | 0.87–1.42 | 0.361 | 213 (2) | 3.05 | 2.24–4.15 | <0.001 |
| Western Asia | 35 (1) | 0.87 | 0.59–1.29 | 0.510 | 26 (1) | 1.48 | 0.93–2.37 | 0.096 |
| Co-infection with HCV | ||||||||
| No | 494 (1) | 1.00 | 518 (1) | 1.00 | ||||
| Yes | 174 (4) | 1.88 | 1.53–2.31 | <0.001 | 87 (2) | 2.06 | 1.59–2.66 | <0.001 |
| LHD of residence at HBV | ||||||||
| Rural NSW | 110 (2) | 1.00 | 53 (1) | 1.00 | ||||
| Outer metro NSW | 312 (1) | 1.22 | 0.97–1.55 | 0.092 | 293 (1) | 1.26 | 0.91–1.75 | 0.152 |
| Metro NSW | 235 (1) | 0.99 | 0.77–1.27 | 0.981 | 252 (1) | 1.13 | 0.81–1.57 | 0.442 |
| History of alcohol use disorder | ||||||||
| No | 453 (1) | 1.00 | 530 (1) | 1.00 | ||||
| Yes | 215 (9) | 4.82 | 3.96–5.87 | <0.001 | 75 (3) | 2.18 | 1.66–2.86 | <0.001 |
| †Charlson comorbidity index | ||||||||
| 0 | 133 (1) | 1.00 | 292 (1) | 1.00 | ||||
| 1-2 | 219 (5) | 3.74 | 3.04–4.60 | <0.001 | 99 (2) | 1.73 | 1.37–2.20 | <0.001 |
| 3+ | 316 (9) | 10.29 | 8.46–12.51 | <0.001 | 210 (7) | 6.23 | 5.11–7.59 | <0.001 |
Levels of significance: p <0.05 (cox proportional-hazards regression analyses).
aHR, adjusted hazard ratio; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LHD, local health district; NSW, New South Wales.
Missing values were included in all adjusted models.
Charlson comorbidity index score indicates degree of health; higher scores indicate worse health condition.
Factors associated with HCC diagnosis during 2009-2018
In adjusted analysis, HCC diagnosis was associated with older age (birth before 1944) (aHR 3.94, 95% CI 2.91–5.32), male sex (aHR 3.79, 95% CI 3.05–4.71), history of AUD (aHR 2.18, 95% CI 1.66–2.86), and HCV co-infection (aHR 2.06, 95% CI 1.59–2.66). Moreover, CCI groups 1-2 and 3+ were associated with a significantly higher risk of HCC diagnosis (aHR 1.73, 95% CI 1.37–2.20 and 6.23, 95% CI 5.11–7.59), respectively. Further, compared to people born in Australia, those born in Africa (aHR 3.12, 95% CI 1.85–5.26), East Asia (aHR 3.73, 95% CI 2.70–5.15), and Oceania & Southeast Asia (aHR 3.05, 95% CI 2.24–4.15) had a higher risk of an HCC diagnosis (Table 4).
Discussion
Our study demonstrated important trends in DC and HCC diagnosis, and liver-related mortality among people with an HBV notification in NSW, Australia. Between 2003 and 2017, the number of first hospitalisation for DC and HCC, and liver-related deaths increased. In contrast, age-standardised incidence rates for these events declined. An increase in the population-level burden of advanced HBV-related liver disease, in the context of increasing therapeutic intervention uptake and declining individual-level risk, is explained by an expanding and ageing HBV-infected population. Improved HBV therapies (entecavir or tenofovir) were introduced from the mid-2000s, with progressively increasing treatment initiation rates.29,30 Further potential advanced liver disease risk reductions were limited by late HBV diagnosis, HCV co-infection, and AUD. Thus, enhanced HBV screening, HCV cure, improved AUD management, and increases in HBV therapeutic uptake all have the potential to reduce the burden of advanced HBV-related liver disease.31,32
Suboptimal HBV screening and CHB latency that can span decades has limited opportunities to prevent progression to ESLD.14 Overall, during this period, 33% and 31% of HBV-related DC and HCC cases were diagnosed late (from 2 years prior to after these events), respectively, consistent with the recent estimates of under-diagnosis in the overall Australian HBV-infected population (31-40%).12,15 Encouragingly, the proportion of late diagnosis has declined over time; continued population-level screening and surveillance efforts, including consideration of one-off universal HBV testing, are required.33
Achieving higher HBV therapeutic coverage among the eligible population is clearly a goal to address continued disease burden. Even in patients with DC, appropriate antiviral therapy can ameliorate hepatic dysfunction.34,35 Although antiviral therapy also improves survival following HCC,36,37 prognosis remains poor.36,38 Thus, the primary focus for reducing advanced liver disease burden and mortality relies heavily on preventing disease progression.39 Recent systematic reviews reinforce the favourable response of timely antiviral therapy by demonstrating an estimated 30% to 75% reduction in HCC risk.34,35
In Australia, the number of individuals on HBV therapy has increased since the mid-2000s 29; however, considerable gaps remain in the HBV cascade of care, given that modelling estimates that two-thirds of eligible individuals are not receiving treatment.40 Our study does, however, show a steady increase in treatment uptake over time. Enhancing HBV antiviral therapy coverage and increasing screening are clear priorities within the Australian Hepatitis B Strategy.41 Broadened antiviral therapy prescription through general practitioners has commenced but should be accelerated. Similarly, improved HBV screening strategies facilitated by primary care electronic health systems need to be implemented, including pop-up reminders of high-risk groups, particularly based on country of birth.33
Although later study period (2010-2018) was associated with reduced individual risk of HBV-related DC and HCC, several associated factors persisted: older age, male sex, history of AUD, and HCV co-infection. These factors have a known association with fibrosis progression or advanced liver disease,42,43 but importantly HCV co-infection is modifiable. Most HCV infections (more than 80%) are diagnosed in Australia, and since 2016, an unrestricted HCV direct-acting antiviral therapy program has facilitated high treatment uptake.44 However, those with HBV/HCV co-infection appear to have had relatively lower uptake.45 Therefore, a particular emphasis should be placed on HCV cure in this population, given their higher risk of advanced liver disease. Further efforts are also required to improve AUD management, including cross-discipline education and training for practitioners involved in HBV management.
Interestingly, most cases of HBV-related DC were observed among individuals born in Australia, given a higher rate of AUD and HCV co-infection among them.46 In contrast, most HBV-related HCC diagnoses were among people born overseas, predominantly in the Asia-Pacific region. It is unclear why the non-Australian country of birth is a common risk factor for developing HBV-related HCC; literature suggests a higher prevalence of IFNλ3 gene polymorphisms among Asians that may be associated with fibrosis progression.47,48 Moreover, the prevalence of oncogenic HBV genotype (genotype C) in Asian populations has a potential relationship with HCC development.49
Older age was associated with late HBV notification among people with DC and HCC, congruent with other Australian and Canadian studies.12,50 This raises considerable concern of a "missed opportunity" for this older cohort to have had access to effective and timely antiviral therapy. In contrast, after accounting for other factors in another Canadian study, people in the older birth cohort did not show an association with late diagnosis.13 Nonetheless, there remains a need to further reduce the late diagnosis of hepatitis.12,13
There are several limitations to our study. First, HBV notifications in NSW are predominantly based on evidence of chronic infection as defined by HBV serology. However, the number of individuals with active viral replication could not be evaluated. Second, we relied on hospitalisation coding data to diagnose DC and HCC, which could introduce misclassification (over-diagnosis) or missed cases (under-diagnosis). A Canadian validation study of health administrative data vs. a tertiary care hepatology clinic demonstrated 90% sensitivity and 88% specificity for the diagnosis of DC and 78% sensitivity and 99% specificity for diagnosing HCC through administrative data.51 Similarly, an NSW linkage study demonstrated high (around 90%) HCC diagnostic concordance between hospitalisation data and the NSWCR. However, sensitivity analysis for validating DC events through hospital admission records has not been undertaken in NSW.12,19,24 Third, in NSW, administrative data sources were available for different periods of time. Hence, to improve the accuracy of trends in DC and HCC diagnosis numbers and age-standardised rates and to minimise biased estimations of time to DC or HCC diagnosis, the study time period was restricted. We also undertook a sensitivity analysis evaluating trends in hospitalisation-based HCC diagnoses (excluding cancer registry cases) which were available for the total study period, with similar findings. Fourth, to study the real-world mortality among individuals with HBV notifications in NSW, liver-related mortality was based on deaths following hospital admissions for DC or HCC and thus excludes liver-related deaths which occurred without a previous hospitalisation and may include some deaths unrelated to liver disease.24 Information on cause-specific mortality from death certificates will be available in the future, but there are also limitations with cause of death ascertainment. Fifth, although compensated cirrhosis is a major risk factor for both DC and HCC, ICD-10 coded hospitalisation data does not have a code for compensated cirrhosis. Sixth, data on Aboriginal ethnicity and the place of birth was unavailable for many individuals. In the analyses of factors associated with DC and HCC diagnoses, missing information of each variable were identified and included in the unadjusted and adjusted models. Lastly, using administrative data to define AUD has clear limitations, given the low sensitivity of administrative data (68% sensitivity and 97% specificity for the diagnosis of heavy alcohol intake).52
In conclusion, this population-level study provides evidence for declining risk of HBV-related DC, HCC, and liver-related mortality, suggesting an impact of highly effective antiviral therapy from the mid-2000s. The proportion of HBV-related DC and HCC cases with late hepatitis notification has also declined. Further strategies to reduce HBV disease burden include enhanced HBV screening and earlier diagnosis, increased HBV treatment coverage for eligible individuals, and addressing modifiable co-factors such as HCV co-infection and AUD.
Financial support
The Kirby Institute is funded by the Australian Government Department of Health, under the agreement ID number 2-D3X513. This publication is part of the Bloodborne viruses and sexually transmissible infections Research, Strategic Interventions and Evaluation (BRISE) program, funded by the New South Wales Ministry of Health. JG is supported by an NHMRC of Australia Program Grant (1053206), project grants (632630, 1049857), a NHMRC Practitioner Fellowship, a Sydney West Translational Cancer Research Centre grant funded by the Cancer Institute New South Wales, and the Robert W. Storr Bequest to the Sydney Medical Foundation, University of Sydney. GD is supported by an NHMRC of Australia Program Grant (1150078) and Investigator Fellowship (2008276). MM is supported by an NHMRC Early Career Fellowship.
Authors' contributions
SHBU, MA, and GD contributed to study conception and design, data acquisition and analysis, interpretation of findings, and drafting of the manuscript; and MM, MD, GM, JA, BH, and JG contributed to data acquisition and analysis and interpretation of findings. HV contributed to the revised manuscript and re-analysis.
Data availability statement
This publication involved information collected by population-based health administration registries. Data used for this research cannot be deposited on servers other than those approved by ethics committees. This publication has used highly sensitive health information through linkage of several administrative datasets. De-identified linked information has been provided to the research team under strict privacy regulations. Except in the form of conclusions drawn from the data, researchers do not have permission to disclose any data to any person other than those authorized for the research project.
Ethics statement
This publication involved information already collected by population-based health administration registries; therefore, people have not been 'recruited' for the purposes of this research. Ethics approvals for the study were granted by the New South Wales Population & Health Services Research Ethics Committee, Cancer Institute New South Wales (reference number HREC/13/CIPHS/63), the Australian Institute of Health and Welfare (reference number EO2014/3/114) and the Aboriginal Health and Medical Research Council of New South Wales (reference number 1215/6).
Conflict of interest
ML has received research support from Merck, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen-Cilag, Gilead Sciences, and ViiV HealthCare. ML has received consultancy and workshop fees from Gilead Sciences. ML has received Data Safety Monitoring Board Committee fees from Sirtex Pty Ltd. JG is on the speaker’s bureau for Gilead Sciences, Merck, Janssen, Roche, and Pharmaxis. JG is a member of advisory board for Gilead Sciences, Merck, Janssen, Bristol-Myers Squibb, AbbVie, Roche, GlaxoSmithKline, Pharmaxis and Pfizer. JG has received travel support from Gilead Sciences, Merck, Bristol-Myers Squibb, AbbVie, and Roche. GD has received research support from Gilead Sciences, Merck, and AbbVie. Other authors have no commercial relationships that might pose a conflict of interest in connection with this manuscript. GM has received research support from Gilead Sciences and AbbVie.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
The authors would like to acknowledge the New South Wales Ministry of Health for the provision of HBV notifications, hospital admissions, death data and the Australian Institute for Health and Welfare for the provision of Pharmaceutical Benefits Scheme data. We also thank the ethics committees of New South Wales Population and Health Services Research, AIHW, New South Wales Ministry of Health, and the Aboriginal Health and Medical Research Council of New South Wales for their approval of this publication.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100552.
Supplementary data
The following is the supplementary data to this article:
References
- 1.World Health Organization . World Health Organization; Geneva: 2017. Global hepatitis report, 2017.https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/pdf [Internet] [cited 2021 Oct 17]. Available from: [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva: 2016. Global health sector strategy on viral hepatitis 2016–2021.https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ [Internet] [cited 2021 Oct 17]. Available from: [Google Scholar]
- 3.World Health Organization . World Health Organization; Geneva: 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Accountability for the global health sector strategies 2016–2021: actions for impact.https://www.who.int/publications/i/item/9789240027077 [cited 2021 Oct 17]. Available from: [Google Scholar]
- 4.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Lok A.S., McMahon B.J., Brown R.S., Jr., Wong J.B., Ahmed A.T., Farah W., et al. Antiviral therapy for chronic hepatitis B viral infection in adults: a systematic review and meta-analysis. Hepatology (Baltimore, Md) 2016;63(1):284–306. doi: 10.1002/hep.28280. [DOI] [PubMed] [Google Scholar]
- 6.Howell J., Pedrana A., Cowie B.C., Doyle J., Getahun A., Ward J., et al. Aiming for the elimination of viral hepatitis in Australia, New Zealand, and the Pacific Islands and Territories: where are we now and barriers to meeting World Health Organization targets by 2030. J Gastroenterol Hepatol. 2019;34(1):40–48. doi: 10.1111/jgh.14457. [DOI] [PubMed] [Google Scholar]
- 7.Bertuccio P., Turati F., Carioli G., Rodriguez T., La Vecchia C., Malvezzi M., et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol. 2017;67(2):302–309. doi: 10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Poordad F.F. Presentation and complications associated with cirrhosis of the liver. Curr Med Res Opin. 2015;31(5):925–937. doi: 10.1185/03007995.2015.1021905. [DOI] [PubMed] [Google Scholar]
- 9.Pascual S., Miralles C., Bernabé J.M., Irurzun J., Planells M. Surveillance and diagnosis of hepatocellular carcinoma: a systematic review. World J Clin cases. 2019;7(16):2269–2286. doi: 10.12998/wjcc.v7.i16.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cholongitas E., Papatheodoridis G.V., Goulis J., Vlachogiannakos J., Karatapanis S., Ketikoglou J., et al. The impact of newer nucleos(t)ide analogues on patients with hepatitis B decompensated cirrhosis. Ann Gastroenterol. 2015;28(1):109–117. [PMC free article] [PubMed] [Google Scholar]
- 11.Waziry R., Grebely J., Amin J., Alavi M., Hajarizadeh B., George J., et al. Trends in hepatocellular carcinoma among people with HBV or HCV notification in Australia (2000-2014) J Hepatol. 2016;65(6):1086–1093. doi: 10.1016/j.jhep.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Alavi M., Law M.G., Grebely J., Amin J., Hajarizadeh B., George J., et al. Time to decompensated cirrhosis and hepatocellular carcinoma after an HBV or HCV notification: a population-based study. J Hepatol. 2016;65(5):879–887. doi: 10.1016/j.jhep.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Samji H., Yu A., Kuo M., Alavi M., Woods R., Alvarez M., et al. Late hepatitis B and C diagnosis in relation to disease decompensation and hepatocellular carcinoma development. J Hepatol. 2017;67(5):909–917. doi: 10.1016/j.jhep.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Papatheodoridis G.V., Chan H.L., Hansen B.E., Janssen H.L., Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62(4):956–967. doi: 10.1016/j.jhep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 15.MacLachlan J.H., Allard N., Towell V., Cowie B.C. The burden of chronic hepatitis B virus infection in Australia, 2011. Aust N Z J Public Health. 2013;37(5):416–422. doi: 10.1111/1753-6405.12049. [DOI] [PubMed] [Google Scholar]
- 16.MacLachlan J.H., Cowie B.C. Cultural and linguistic diversity of people living with chronic hepatitis B in 2011-2016: changing migration, shifting epidemiology. Aust N Z J Public Health. 2018;42(5):441–443. doi: 10.1111/1753-6405.12826. [DOI] [PubMed] [Google Scholar]
- 17.Xiao Y., Howell J., van Gemert C., Thompson A.J., Seaman C.P., McCulloch K., et al. Enhancing the hepatitis B care cascade in Australia: a cost-effectiveness model. J Viral Hepat. 2020;27(5):526–536. doi: 10.1111/jvh.13252. [DOI] [PubMed] [Google Scholar]
- 18.Robotin M.C., Masgoret X., Porwal M., Goldsbury D., Khoo C., George J. Using a chronic hepatitis B Registry to support population-level liver cancer prevention in Sydney, Australia. Clin Epidemiol. 2018;10:41–49. doi: 10.2147/CLEP.S146275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alavi M., Grebely J., Hajarizadeh B., Amin J., Larney S., Law M.G., et al. Mortality trends among people with hepatitis B and C: a population-based linkage study, 1993-2012. BMC Infect Dis. 2018;18(1):215. doi: 10.1186/s12879-018-3110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Australian Bureau of Statistics . Australian Bureau of Statistics; Canberra (ACT): 2021. National, state and territory population.https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population/mar-2021 [Internet] [cited 2021 Oct 17]. Available from: [Google Scholar]
- 21.The Kirby Institute . The Kirby Institute; NSW (AU): 2016. Hepatitis B and C in Australia annual surveillance report supplement 2016.https://kirby.unsw.edu.au/sites/default/files/kirby/report/SERP_HepBandC-Annual-Surveillance-Report-Supp-2016.pdf [Internet] [cited 2021 Oct 17]. Available from: [Google Scholar]
- 22.The centre for health record linkage (CHeReL) 2021. https://www.cherel.org.au/ Available from: [Google Scholar]
- 23.Australian Institute of Health and Welfare . Australian Institute of Health and Welfare; Canbera (ACT): 2021. Data linkage.https://www.aihw.gov.au/our-services/data-linkage [Internet] [cited 2021 Oct 17]. Available from: [Google Scholar]
- 24.Alavi M., Law M.G., Valerio H., Grebely J., Amin J., Hajarizadeh B., et al. Declining hepatitis C virus-related liver disease burden in the direct-acting antiviral therapy era in New South Wales, Australia. J Hepatol. 2019;71(2):281–288. doi: 10.1016/j.jhep.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Quan H., Li B., Couris C.M., Fushimi K., Graham P., Hider P., et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 27.NSW Health . NSW Health; NSW (Au): 2018. Local health districts and specialty networks.https://www.health.nsw.gov.au/lhd/pages/default.aspx [Internet] [cited 2021 Oct 17]. Available from: [Google Scholar]
- 28.Friedmann P.D. Alcohol use in adults. New Engl J Med. 2013;368(17):1655–1656. doi: 10.1056/NEJMc1302445. [DOI] [PubMed] [Google Scholar]
- 29.MacLachlan J., Smith C., Towell V., Cowie B. Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM); Darlinghurst: 2020. Viral hepatitis mapping project: national report 2018–19. [Google Scholar]
- 30.Matthews G., Robotin M. Australasian Society for HIV Medicine and Cancer Council NSW; Sydney: 2008. All you wanted to know about hepatitis B: a guide for primary care providers. [Google Scholar]
- 31.Mathurin P., Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015;62(1 Suppl):S38–S46. doi: 10.1016/j.jhep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pimpin L., Cortez-Pinto H., Negro F., Corbould E., Lazarus J.V., Webber L., et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–735. doi: 10.1016/j.jhep.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Allard N.L., MacLachlan J.H., Tran L., Yussf N., Cowie B.C. Time for universal hepatitis B screening for Australian adults. Med J Aust. 2021;215(3):103–105.e1. doi: 10.5694/mja2.51114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papatheodoridis G.V., Lampertico P., Manolakopoulos S., Lok A. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53(2):348–356. doi: 10.1016/j.jhep.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 35.Govan L., Wu O., Xin Y., Hutchinson S.J., Hawkins N. Comparative effectiveness of antiviral treatment for hepatitis B: a systematic review and Bayesian network meta-analysis. Eur J Gastroenterol Hepatol. 2015;27(8):882–894. doi: 10.1097/MEG.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 36.Thein H.H., Walter S.R., Gidding H.F., Amin J., Law M.G., George J., et al. Survival after diagnosis of hepatocellular carcinoma and potential impact of treatment in a hepatitis B or C infected cohort. Hepatol Res Off J Jpn Soc Hepatol. 2012;42(12):1175–1186. doi: 10.1111/j.1872-034X.2012.01037.x. [DOI] [PubMed] [Google Scholar]
- 37.Papatheodoridis G.V., Idilman R., Dalekos G.N., Buti M., Chi H., van Boemmel F., et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology (Baltimore, Md) 2017;66(5):1444–1453. doi: 10.1002/hep.29320. [DOI] [PubMed] [Google Scholar]
- 38.Zhong J.H., Ke Y., Gong W.F., Xiang B.D., Ma L., Ye X.P., et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–340. doi: 10.1097/SLA.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 39.Singal A.G., Pillai A., Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4) doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCulloch K., Romero N., MacLachlan J., Allard N., Cowie B. Modeling progress toward elimination of hepatitis B in Australia. Hepatology (Baltimore, Md) 2020;71(4):1170–1181. doi: 10.1002/hep.30899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Department of Health Australian Government . Canbera (ACT); 2018. Third national hepatitis B strategy.https://www1.health.gov.au/internet/main/publishing.nsf/Content/ohp-bbvs-1/$File/Hep-B-Third-Nat-Strategy-2018-22.pdf [Internet] [cited 2021 Oct 17]. Available from: [Google Scholar]
- 42.Wong G.L. Prediction of fibrosis progression in chronic viral hepatitis. Clin Mol Hepatol. 2014;20(3):228–236. doi: 10.3350/cmh.2014.20.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y.J., Xu M.Y., Lu L.G. Clinical advances in fibrosis progression of chronic hepatitis B and C. J Clin Translational Hepatol. 2014;2(4):222–227. doi: 10.14218/JCTH.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hajarizadeh B., Grebely J., Matthews G.V., Martinello M., Dore G.J. Uptake of direct-acting antiviral treatment for chronic hepatitis C in Australia. J viral Hepat. 2018;25(6):640–648. doi: 10.1111/jvh.12852. [DOI] [PubMed] [Google Scholar]
- 45.Valerio H., Alavi M., Law M., Tillakeratne S., Amin J., Janjua N.Z., et al. High hepatitis C treatment uptake among people with recent drug dependence in New South Wales, Australia. J Hepatol. 2021;74(2):293–302. doi: 10.1016/j.jhep.2020.08.038. [DOI] [PubMed] [Google Scholar]
- 46.Valery P.C., McPhail S., Stuart K.A., Hartel G., Clark P.J., O'Beirne J., et al. Changing prevalence of aetiological factors and comorbidities among Australians hospitalised for cirrhosis. Intern Med J. 2021;51(5):691–698. doi: 10.1111/imj.14809. [DOI] [PubMed] [Google Scholar]
- 47.Eslam M., Hashem A.M., Leung R., Romero-Gomez M., Berg T., Dore G.J., et al. Interferon-λ rs12979860 genotype and liver fibrosis in viral and non-viral chronic liver disease. Nat Commun. 2015;6:6422. doi: 10.1038/ncomms7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eslam M., Ahlenstiel G., George J. Interferon lambda and liver fibrosis. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res. 2019;39(10):627–635. doi: 10.1089/jir.2018.0175. [DOI] [PubMed] [Google Scholar]
- 49.Wong G.L., Chan H.L., Yiu K.K., Lai J.W., Chan V.K., Cheung K.K., et al. Meta-analysis: the association of hepatitis B virus genotypes and hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37(5):517–526. doi: 10.1111/apt.12207. [DOI] [PubMed] [Google Scholar]
- 50.Lapointe-Shaw L., Chung H., Holder L., Kwong J.C., Sander B., Austin P.C., et al. Diagnosis of chronic hepatitis B pericomplication: risk factors and trends over time. Hepatology (Baltimore, Md) 2021;73(6):2141–2154. doi: 10.1002/hep.31557. [DOI] [PubMed] [Google Scholar]
- 51.Lapointe-Shaw L., Georgie F., Carlone D., Cerocchi O., Chung H., Dewit Y., et al. Identifying cirrhosis, decompensated cirrhosis and hepatocellular carcinoma in health administrative data: a validation study. PloS one. 2018;13(8) doi: 10.1371/journal.pone.0201120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim H.M., Smith E.G., Stano C.M., Ganoczy D., Zivin K., Walters H., et al. Validation of key behaviourally based mental health diagnoses in administrative data: suicide attempt, alcohol abuse, illicit drug abuse and tobacco use. BMC Health Serv Res. 2012;12:18. doi: 10.1186/1472-6963-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This publication involved information collected by population-based health administration registries. Data used for this research cannot be deposited on servers other than those approved by ethics committees. This publication has used highly sensitive health information through linkage of several administrative datasets. De-identified linked information has been provided to the research team under strict privacy regulations. Except in the form of conclusions drawn from the data, researchers do not have permission to disclose any data to any person other than those authorized for the research project.