Abstract
A comparative study was completed to determine the influence of various environmental stimuli on the transcription of three different fimbrial operons in Escherichia coli and to determine the role of the histone-like protein H-NS in this environmental regulation. The fimbrial operons studied included the pap operon, which encodes pyelonephritis-associated pili (P pili), the daa operon, which encodes F1845 fimbriae, and the fan operon, which encodes K99 fimbriae. Using lacZYA transcriptional fusions within each of the fimbrial operons, we tested temperature, osmolarity, carbon source, rich medium, oxygen levels, pH, amino acids, solid medium, and iron concentration for their effects on fimbrial gene expression. Low temperature, high osmolarity, glucose as a carbon source, and rich medium repressed transcription of all three operons. High iron did not alter transcription of any of the operons tested, whereas the remaining stimuli had effects on individual operons. For the pap and daa operons, introduction of the hns651 mutation relieved the repression, either fully or partially, due to low temperature, glucose as a carbon source, rich medium, and high osmolarity. Taken together, these data indicate that there are common environmental cues that regulate fimbrial transcription in E. coli and that H-NS is an important environmental regulator for fimbrial transcription in response to several stimuli.
Bacteria are able to sense a variety of environmental stimuli, such as temperature, pH, osmolarity, oxygen levels, carbon source, and concentrations of various ions and compounds (34, 36), and then use this information to regulate gene expression based on their surroundings. This is particularly true among bacterial pathogens, in which the expression of virulence factors is often regulated in response to the environment. Presumably, the bacterium uses these environmental cues to determine whether it is within a host and then regulate virulence gene expression accordingly so as to more efficiently utilize its resources.
Expression of fimbriae is an important virulence trait for many strains of pathogenic Escherichia coli. The expression of fimbriae facilitates the attachment of bacteria to host tissue and is one of the initial steps in colonization. In this work, we completed a comparative study to determine the influence of various environmental stimuli on the transcription of three different fimbrial operons to ascertain if there are common environmental cues that control fimbrial gene expression in E. coli. The fimbrial operons studied include the pap operon, which encodes pyelonephritis-associated pili (P pili), the daa operon, which encodes F1845 fimbriae, and the fan operon, which encodes K99 fimbriae. P pili are associated with E. coli that cause upper urinary tract infections (42, 44). F1845 fimbriae are expressed by a diffusely adherent strain of E. coli that was isolated from an infant with persistent diarrhea (7). K99 fimbriae are associated with E. coli strains that cause diarrheal disease in calves and lambs (23). In addition to environmental regulation, the pap and daa operons are also controlled by a phase variation mechanism in which individual bacteria within a given population can alternate between two states of expression: phase ON, in which they are expressing fimbriae, and phase OFF, in which they are not expressing fimbriae (33, 55). Phase variation in both of these operons is controlled at the transcriptional level by the formation of specific DNA methylation patterns (8, 55). Formation of these patterns relies on the global regulators deoxyadenosine methylase (Dam) and leucine-responsive regulatory protein (Lrp) as well as operon-specific proteins (8, 12, 43, 55). Transcription in the fan operon is not known to be subject to phase variation but is controlled by the global regulator Lrp (12).
We tested a variety of environmental stimuli (temperature, osmolarity, rich medium, carbon source, oxygen levels, pH, amino acids, solid substrate, and iron concentration) for their effects on fimbrial transcription. We provide evidence that some of these environmental cues (low temperature, high osmolarity, glucose as a carbon source, and rich medium) repress fimbrial transcription of all three operons characterized in this study.
Another component of this study was to determine the role of the protein H-NS in controlling the transcription of these operons in response to the environmental cues tested. H-NS is a histone-like nucleoid-structuring protein that binds and compacts DNA (1, 52, 58). It has been found to control a number of different environmentally controlled genes in E. coli and other gram-negative enteric bacteria (1).
H-NS has been shown to control the expression of several fimbriae expressed by E. coli, including Pap, type I, CFA/I, and 987P fimbriae (17, 25, 27, 29, 45, 57). In this study, we wanted to determine if H-NS controls the fan and daa operons, thus expanding the number of fimbrial operons that are controlled by this regulator. Additionally, we wanted to determine if H-NS controls transcription in the pap, daa, and fan operons in response to a variety of environmental cues, supporting the hypothesis that H-NS serves as a global regulator of fimbrial gene expression in E. coli. Here we provide evidence that H-NS controls transcription of the daa and pap operons in response to multiple environmental cues. For the fan operon, the effect of the hns651 mutation on fan transcription could not be quantitatively determined, as the strain used was susceptible to secondary mutations.
MATERIALS AND METHODS
Strains and media.
The strains, plasmids, and bacteriophages used in this study are described in Table 1. Luria-Bertani (LB) broth, tryptone broth (TB), M9 minimal (M9) broth, and M9 agar were prepared as described previously (37, 50). Antibiotics, when used, were at final concentrations of 25 μg ml−1 (kanamycin) and 25 μg ml−1 (tetracycline). M9 agar-based media contained the chromogenic substrate 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) at a final concentration of 40 μg ml−1.
TABLE 1.
Bacterial strains and bacteriophages used in this study
| Strain or bacteriophage | Description | Reference(s) or source |
|---|---|---|
| E. coli strains | ||
| MC4100 | F−araD139 Δ(lacIPOZYA-argF)U169 rpsL thi-1 | 14 |
| NH757 | B178 hns651 tyrTβ::Tn10 | 19 |
| CA8445-1 | relA1 rpsL136 spoT1 thi-1 Δcrp-45 | 48 |
| DL812 | MC4100 λMW01 lysogen (fanABC′-lacZYA) | 53 |
| DL1530 | MC4100 λ366 lysogen (daa-lacZYA) | 55 |
| DL1504 | MC4100 λ354 lysogen (papBA-lacZYA) | 11 |
| DL1947 | DL1504 hns651 | 54 |
| DL3087 | DL1504 Δcrp-45 zhd-3083::Tn10 | D. A. Low |
| CWZ263 | DL1530 hns651 | This work |
| CWZ369 | DL812 Δcrp-45 zhd-3083::Tn10 | This work |
| CWZ370 | DL1530 Δcrp-45 zhd-3083::Tn10 | This work |
| Bacteriophages | ||
| P1L4 | Virulent phage P1 | D. A. Low |
| λ354 | pap-lacZYA fusion phage | 11 |
| λ366 | daa-lacZYA fusion phage | 55 |
| λMW01 | fan-lacZYA fusion phage | 12, 53 |
Construction of mutant strains.
P1 transduction was used to introduce the hns651 mutation (19) into strains DL812 and DL1530 (Table 1). The preparation of P1 lysates and P1 transductions were carried out as described previously (50). hns651 transductants with a pink colony phenotype were selected on MacConkey medium containing tetracycline and salicin as described elsewhere (19). The hns651 mutation is an IS1 insertion in the 13th codon of the hns gene (N. P. Higgins, personal communication). Based on Western blot analysis, no H-NS protein was detected in strains containing the hns651 mutation (57).
Similarly, P1 transduction was used to introduce the Δcrp-45 mutation (48) into strains DL812 and DL1530 to create CWZ369 and CWZ370, respectively (Table 1). The Δcrp-45 mutation is a deletion mutation in the cyclic AMP (cAMP) receptor protein (CRP) (48). A P1 lysate was grown on strain DL3087, which contains a Tn10 insertion linked to the Δcrp-45 mutation (Table 1). Δcrp-45 transductants with a white colony phenotype were selected on MacConkey medium containing tetracycline and maltose.
Growth conditions.
For standard growth conditions, the bacteria were cultured in 10 ml of M9 glyc (M9 minimal liquid medium containing 2.45 μM ferric citrate, 30 μM thiamine, 100 μM calcium chloride, 1 mM magnesium sulfate, and 0.2% glycerol as a carbon source, pH 7) in a 37°C shaking water bath in a 50-ml Erlenmeyer flask. To test the effect of low temperature on fimbrial transcription, bacteria were grown at 18 to 20°C in a shaking water bath in M9 glyc. The effect of low oxygen levels was assessed by growing standing cultures in M9 glyc at 37°C. The effect of growth on a solid substrate was measured by plating cells on M9 glyc agar medium that did not contain X-Gal. The bacteria were collected by rinsing the plate with 10 ml of M9 salts at approximately the same time as the liquid M9 glyc culture was harvested. Bacteria were cultured in LB broth at 37°C in a shaking water bath to determine the effect of rich medium.
To measure the effects of the other stimuli, M9 glyc was modified as follows. For M9 gluc, glucose was substituted, at a final concentration of 0.2%, for glycerol in the standard medium to test a change in carbon source. To determine the effect of high osmolarity, the sodium chloride concentration was increased by 300 mM, compared to 8.5 mM in M9 glyc (making M9 NaCl). High pH was tested by using M9, pH 8.0, consisting of M9 glyc buffered by the addition of TAPS [tris(hydroxymethyl)methylaminopropanesulfonic acid] to a final concentration of 100 mM and adjusted to a pH of 8.0 using 4 M NaOH as described previously (51). Similarly, to make M9 pH 5.5, MES (2-(N-morpholino)ethanesulfonic acid) was added to M9 glyc at a final concentration of 100 mM and the pH was adjusted to 5.5 using 1 N HCl as described previously (51). M9 CAA was M9 glyc supplemented with Casamino Acids at a final concentration of 0.2% to assess the effect of amino acids on transcription. To test the effect of high iron concentrations (in M9 Fe), the concentration of ferric citrate was increased to 98 μM, compared to 2.45 μM in M9 glyc. All cultures except those used to measure the effects of low temperature and low oxygen levels were incubated at 37°C in a shaking water bath.
Measurement of β-galactosidase activity.
For assays determining the effects of environmental stimuli on fimbrial transcription, each bacterial strain was inoculated from a frozen −70°C stock onto M9 glyc agar, incubated at 37°C, and passaged once. After growth for approximately 36 h, a single Lac+ colony was isolated and resuspended in 1 ml of M9 salts. Since expression of β-galactosidase served as a reporter of fimbrial gene transcription in each strain used, a Lac+ colony was chosen to ensure that the cultures were started with bacteria that were actively transcribing the fimbrial genes. Flasks containing 10 ml of the appropriate prewarmed medium were inoculated with 140 μl of the colony suspension. Experiments testing different environmental conditions were frequently conducted in parallel, using the same colony suspension to inoculate different medium.
The bacterial cultures were grown to log phase (optical density at 600 nm of 0.25 to 0.9), and β-galactosidase activities were measured as described previously (37). This inoculation method ensured that all bacterial strains had grown for approximately 9 to 11 generations prior to the measurement of β-galactosidase activity. For cultures grown at pH 5.5 and 7.0, bacteria were centrifuged and then resuspended in M9 salts before proceeding with the assay. Each β-galactosidase activity value represents an average from two or more separate cultures grown under identical conditions.
For experiments assessing the effect of the Δcrp-45 mutation on fan and daa transcription, each bacterial strain was inoculated onto LB agar (DL812 and CWZ369) or TB agar (DL1530 and CWZ370) and incubated at 37°C. A single colony from each strain was isolated and resuspended in 1 ml of LB. Five milliliters of broth (LB or TB) was inoculated from the colony suspension and grown to exponential phase. β-Galactosidase activity was measured as described above, each value representing an average from two separate cultures grown under identical conditions.
Calculation of switch frequencies.
Phase transition rates were calculated as described previously (9). Each switch frequency is based on data from two or more separate colonies. To determine the switch frequency, each strain was streaked on the medium to be tested. An initial colony, phase ON (Lac+) or phase OFF (Lac−), was excised, resuspended and diluted in M9 salts, and plated on the same medium for the determination of switch frequencies.
RESULTS
Experimental design.
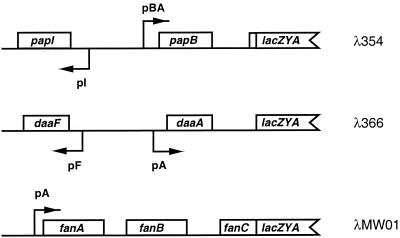
To study transcriptional regulation for the pap, daa, and fan fimbrial genes, we used transcriptional fusions that place lacZYA expression under the control of the promoter that drives transcription of the major fimbrial subunit gene (Fig. 1). In previous studies, three separate strains, each containing one of the fimbrial operon fusions as a lambda lysogen on the chromosome of MC4100, were created (11, 12, 55). DL1504 contains the papBA-lacZYA fusion (λ354), DL1530 contains the daa-lacZYA fusion (λ366), and DL812 contains the fanABC′-lacZYA fusion (λMW01) (Table 1). These fusions were constructed to measure transcription initiated from the pBA (pap), pA (daa), and pA (fan) promoters, respectively.
FIG. 1.
Transcriptional fusions used in this study. λ354 contains the pap regulatory region, λ366 contains the daa regulatory region, and λMW01 contains the fan regulatory region (Table 1). Each transcriptional fusion is carried on the E. coli chromosome as a lambda lysogen. Open boxes indicate fimbrial genes and lacZYA sequences contained within each fusion. Transcription of lacZYA is driven by the pBA promoter of the pap operon, the pA promoter of the daa operon, and the pA promoter of the fan operon.
To assess changes in fimbrial gene expression under different conditions, transcription was quantitated by determining levels of β-galactosidase expression and comparing them to transcription levels of cultures grown in M9 glyc in a 37°C shaking water bath. These conditions were chosen as a reference point, as they yield high levels of fimbrial transcription for each of the operons tested.
To ensure that a given culture was initiated with cells that were transcriptionally active for fimbrial gene expression, the cultures were inoculated with a Lac+ colony grown on M9 glyc agar at 37°C. Because the pap and daa operons are subject to a phase variation mechanism, both phase ON (Lac+) and phase OFF (Lac−) colonies are observed under these conditions. Within a Lac+ colony, the percentage of cells in a phase ON state may vary between approximately 20 and 50 when grown on M9 glyc at 37°C (data not shown). To limit the variability due to phase variation, experiments in which the same colony suspension was used to start several cultures grown under different environmental conditions were conducted in parallel as often as possible. However, because of this inherent variability, changes in transcriptional levels for the pap and daa operons that were less than twofold were not considered significant in this study. fan gene expression is not known to be subject to phase variation, and all colonies displayed a Lac+ phenotype when grown on M9 glyc agar at 37°C.
H-NS controls pap and daa transcription.
Before investigating the effect of environmental stimuli on fimbrial transcription and the potential role of H-NS in these processes, it was important to determine whether H-NS controlled transcription of the daa and fan operons and, if so, if it altered fimbrial transcription in the absence of a change in environmental conditions. It was shown previously that introduction of the hns651 mutation significantly decreases transcription of the pap operon when environmental conditions remain unchanged (54, 57). Our results confirm this, showing an approximately 7.6-fold decrease in pap transcription in the hns651 mutant strain DL1947 compared to the wild-type strain DL1504 when grown in M9 glyc (Fig. 2). Studies have indicated that the repressive effect of the hns651 mutation on pap transcription is due, at least in part, to alterations in the rates at which cell transit between phase ON and phase OFF states, with the overall effect of decreasing the number of cells in a phase ON state at 37°C in M9 glyc (Table 2; references 54 and 57).
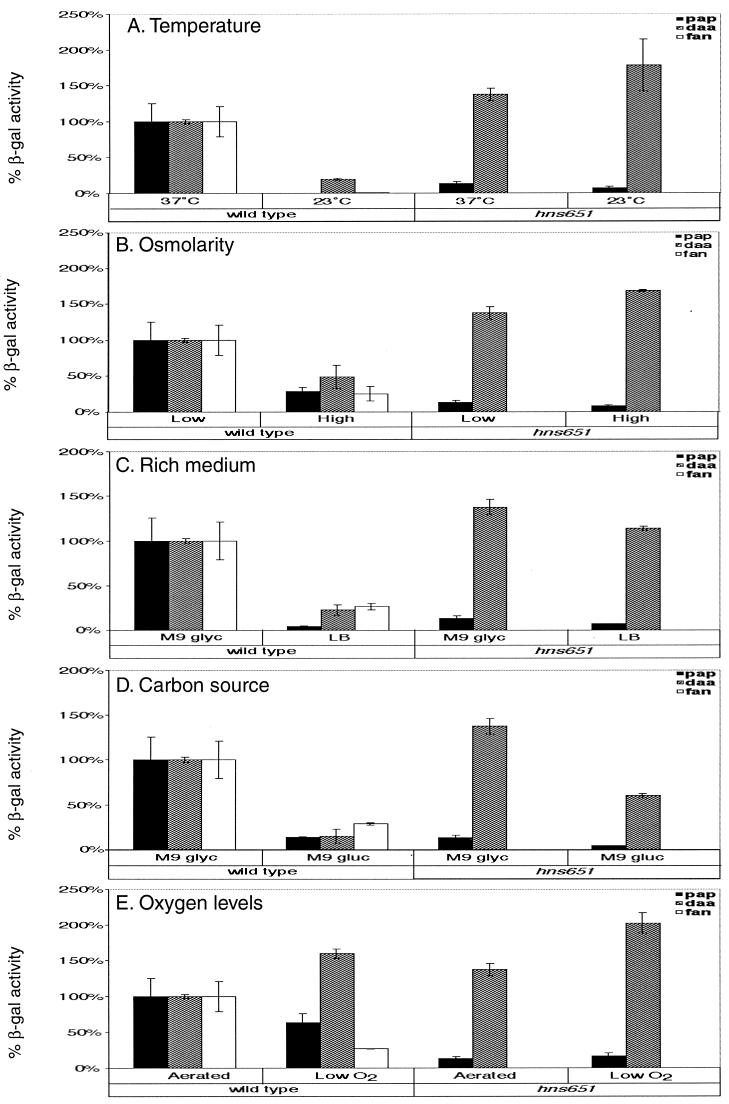
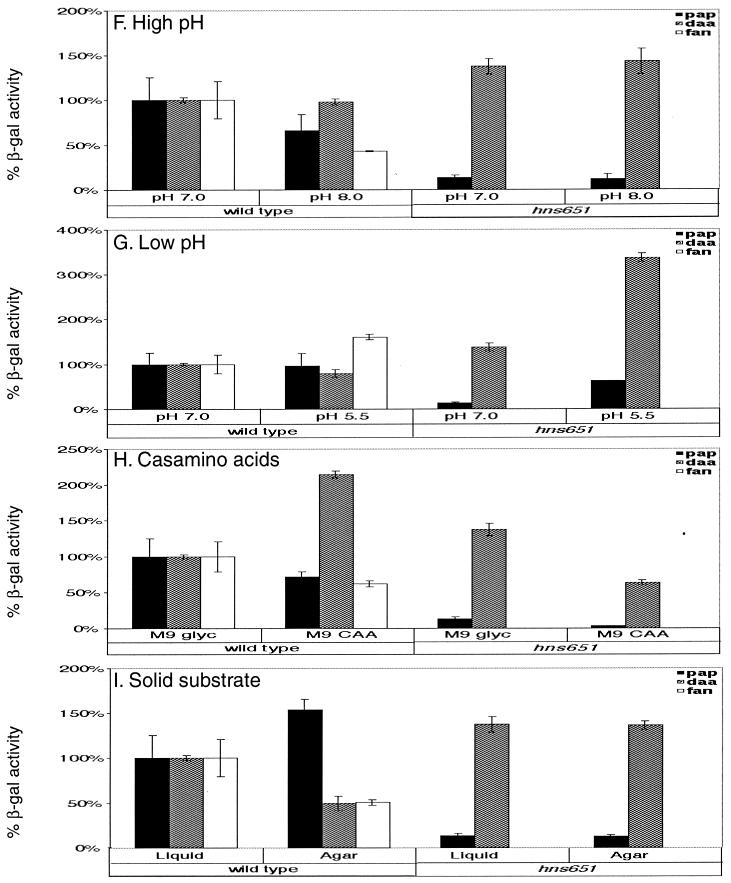
FIG. 2.
Quantitation of the effects of environmental stimuli on pap, daa, and fan fimbrial transcription in wild-type and hns651 mutant strains. β-Galactosidase (β-gal) activities are presented as percentage of the activity after growth of the wild-type strain on M9 glyc at 37°C with aeration. Bars indicate β-galactosidase activities measured in strains containing the pap-lacZYA transcriptional fusion (wild-type DL1504 and hns651 mutant DL1947; pap), in strains containing the daa-lacZYA transcriptional fusion (wild-type DL1530 and hns651 mutant CWZ263; daa), and in the wild-type strain containing the fan-lacZYA transcriptional fusion (DL812; fan). Error bars represent 1 standard deviation from the mean.
TABLE 2.
Effects of glucose, osmolarity, and the hns651 mutation on phase transition frequencies for pap and daa operons
| Strain | Relevant genotype | Growth mediuma | Weighted avg of phase transition frequenciesb
|
|
|---|---|---|---|---|
| Phase ON→OFF | Phase OFF→ON | |||
| DL1504 | Wild type | M9 glyc | 3.37 × 10−2 | 3.50 × 10−4 |
| pap-lacZYA fusion | M9 gluc | 4.40 × 10−2 | NAc | |
| M9 NaCl | 4.23 × 10−2 | 1.59 × 10−4 | ||
| DL1947 | hns651 | M9 glycd | 3.65 × 10−2 | 1.66 × 10−4 |
| pap-lacZYA fusion | M9 gluc | 3.32 × 10−2 | 4.14 × 10−4 | |
| M9 NaCl | 4.46 × 10−2 | 4.21 × 10−4 | ||
| DL1530 | Wild type | M9 glyc | 3.05 × 10−2 | 1.44 × 10−4 |
| daa-lacZYA fusion | M9 gluc | 2.97 × 10−2 | 1.80 × 10−4 | |
| M9 NaCl | 2.95 × 10−2 | 1.82 × 10−4 | ||
| CWZ263 | hns651 | M9 glyc | 3.98 × 10−2 | 1.66 × 10−3 |
| daa-lacZYA fusion | M9 gluc | 4.28 × 10−2 | 5.66 × 10−4 | |
| M9 NaCl | 3.87 × 10−2 | 1.46 × 10−3 | ||
The same growth medium was used for isolation of the initial colony (Lac+ or Lac−) and for subsequent quantitation of switch frequencies from the initial colony.
Calculated from at least two independent analyses as described by Blyn et al. (9) and given per cell per generation.
NA, not applicable. A weighted average could not be calculated, as no Lac+ colonies were observed in a screening of approximately 37,000 colonies from four independent analyses.
The phase transition rates for DL1947 on M9 glyc were previously published (57).
To determine whether H-NS is involved in controlling transcription of the daa and fan operons, the hns651 mutation was introduced into strains containing each of these transcriptional operons. The hns651 mutation is an insertion element in hns, and no H-NS protein is detected by Western blot analysis in strains containing this mutation (57). The hns651 mutation was introduced into DL1530 to create CWZ263 (Table 1). In contrast to the pap operon, transfer of the hns651 mutation into strain DL1530 containing the daa transcriptional fusion caused an increase in transcription of this operon in M9 glyc, indicating that H-NS plays only a negative role in controlling daa transcription (Fig. 2). Because of the effect of the hns651 mutation on pap phase variation (Table 2; references 54 and 57), phase transition rates were calculated in strain CWZ263 on M9 glyc plates at 37°C to determine if the stimulatory effect of the hns651 mutation on daa transcription was through influencing the phase variation mechanism (Table 2). While the phase ON→OFF transition rate was unchanged, the phase OFF→ON transition rate was 11.5-fold higher in the hns651 mutant strain CWZ263 than in the wild-type strain. Thus, cells transit from a phase OFF to a phase ON state more frequently than in the wild-type strain, accounting for the increased β-galactosidase activities measured in mutant strain CWZ263 compared to wild-type strain DL1530 grown on M9 glyc at 37°C.
In a similar manner, the hns651 mutation was introduced into strain DL812 containing the fan transcriptional fusion. Transductants were initially streaked on MacConkey-salicin to determine if they could utilize salicin, a trait indicative of an hns mutant strain due to derepression of the cryptic bgl operon (19). The hns651 transductants demonstrated an unstable phenotype in which both pink and white colonies were seen; in contrast pap and daa hns651 transductants retained a uniform pink colony phenotype. Because of this result, fan transcription was measured in five separate hns651 transductants after growth in various media to assess whether transcription was consistent between them. The transductants demonstrated a high level of variability for fan transcription (data not shown); thus, no quantitative data are shown. The results suggest that the transductants may have harbored secondary mutations and thus were genetically unstable (see Discussion).
H-NS controls fimbrial transcription in response to multiple environmental cues.
In the wild-type and hns651 mutant strains, various environmental stimuli were tested for their effects on pap, daa, and fan transcription and to determine the role of H-NS in responding to these environmental cues.
Temperature.
Temperature has been shown to be an important regulator of virulence gene expression in several genera of bacteria (34, 36). To determine the effect of temperature on fimbrial transcription, cultures were grown to exponential phase at 18 to 20°C in M9 glyc, and β-galactosidase activities were measured to determine pap, daa, and fan gene expression.
pap and fan transcription had previously been shown to be thermoregulated (9, 26, 53)); results of this study confirm those findings, demonstrating 380- and 121-fold reductions in pap and fan transcription, respectively, in response to low temperature (Fig. 2). In this study, daa transcription was also shown to be thermoregulated, demonstrating that low temperature is an important environmental cue for all three fimbrial operons. Transcription of daa was reduced 5.2-fold at low temperature compared to 37°C (Fig. 2).
Introduction of the hns651 mutation relieves the repression of low temperature on pap and daa fimbrial transcription, demonstrating that H-NS acts as a thermoregulator in each of these operons. For the daa operon, the hns651 mutation caused an increase in daa expression at 23°C to levels equivalent to those seen in the wild-type strain grown at 37°C (Fig. 2). H-NS was shown previously to control thermoregulation of the pap operon, and our results confirm this observation (Fig. 2) (25, 57).
Osmolarity.
Changes in osmolarity have been found to influence the transcription of virulence genes in several genera of bacteria, including Escherichia, Salmonella, Shigella, Pseudomonas, and Vibrio (34). To test the effect of high osmolarity on fimbrial transcription, β-galactosidase activities were measured after growth in M9 NaCl. Transcription of all three fimbrial operons was repressed by growth at high osmolarity. pap, daa, and fan transcription levels were repressed 3.5-, 2.1-, and 3.9-fold, respectively, compared to growth at low osmolarity (in M9 glyc) (Fig. 2).
For the daa operon, introduction of the hns651 mutation fully abrogates the transcriptional repression by high osmolarity. Transcription in the hns651 mutant strain grown under high-osmolarity conditions exceeds that seen for the hns651 mutant strain grown under conditions of low osmolarity (Fig. 2). For the pap operon, the level of transcription in the hns651 mutant strain at high osmolarity is decreased 1.6-fold compared to the hns651 mutant strain under low osmolarity, indicating that in the hns651 mutant strain high osmolarity may still have a repressive effect (Fig. 2). However, the magnitude of the effect of high osmolarity in the hns651 mutant strain is not as great as in the wild-type strain, where pap transcription is decreased 3.5-fold in response to increased osmolarity.
Rich medium.
Growth in LB, a rich medium, was also shown to repress transcription of all three operons tested. LB had the largest effect on pap transcription, which was repressed 22.4-fold compared to growth in M9 glyc (Fig. 2). Growth in LB reduced daa and fan transcription 4.5- and 3.7-fold, respectively, compared to growth in M9 glyc (Fig. 2).
Growth in LB has a repressive effect that is relieved for the pap and daa operons by the hns651 mutation. Transcription levels in the hns651 mutant strains grown in LB were equivalent to those seen when cells were grown in M9 glyc (Fig. 2).
Carbon source.
Glucose as a carbon source serves as another stimulus that controls transcription of all three fimbrial operons tested. The effect of glucose was tested by growing cultures in M9 gluc. Glucose was shown to repress fan transcription 3.5-fold (Fig. 2). Similarly, pap transcription was reduced 7.3-fold and daa transcription was reduced 6.6-fold compared to growth at 37°C in M9 glyc medium (Fig. 2), confirming results of previous studies (3, 5).
pap transcription has been shown to be dependent on the cAMP-catabolite gene activator protein (CAP) complex (3, 21, 22). To determine if daa and fan transcription was dependent on CAP, the Δcrp-45 deletion mutation (48) was transduced into strains DL812 and DL1530 to create CWZ369 and CWZ370, respectively. fan transcription in CWZ369 (295 ± 14 Miller units [MU]) was reduced 5.1-fold compared to transcription in the wild-type strain DL812 (1,502 ± 96 MU). Similarly, daa transcription was reduced in the Δcrp-45 strain CWZ370 (1 ± 0 MU) compared to the wild-type strain DL1530 (19 ± 2 MU). These results indicate that fan and daa transcription is dependent on CAP and are supported by other studies indicating cAMP-CAP is an important regulator of daa and fan gene expression (6, 32).
For the pap and daa operons, glucose is still somewhat repressive in the absence of H-NS. pap and daa transcriptional levels are 2.9- and 2.3-fold lower, respectively, in the hns651 mutant strains grown in M9 gluc compared to the mutant strains grown in M9 glyc (Fig. 2). However, while transcription is still decreased in the hns651 mutant strains grown in glucose, the repression is not as extensive as that in the wild-type strains, where transcriptional levels are reduced 7.3- and 6.6-fold, respectively, in response to glucose (Fig. 2).
Low oxygen.
Low oxygen levels were tested to determine their effects on fimbrial transcription by growing standing cultures in M9 glyc. The cultures were harvested in exponential phase, with the growth times being two to three times as long as in the cultures grown in M9 glyc with aeration. Low oxygen levels were shown to decrease fan transcription 3.7-fold (Fig. 2) compared to growth with aeration, whereas for the pap and daa operons, transcription levels varied less than 2-fold between conditions of low and high aeration (Fig. 2). Introduction of the hns651 mutation did not significantly alter pap or daa transcription in response to low-oxygen conditions.
pH.
Because pH has been shown to regulate the expression of several virulence genes (34), we tested the effects of low and high pH on fimbrial transcription. High pH (8.0) decreased fan transcription 2.3-fold, whereas pap and daa transcription was similar to that measured after growth at pH 7.0 (in M9 glyc) (Fig. 2). While high pH repressed fan transcription, low pH increased fan transcriptional levels 1.6-fold compared to growth at pH 7.0 (Fig. 2). pap and daa transcription at pH 5.5 was equivalent to that seen at pH 7.0 (Fig. 2).
While low pH did not greatly influence transcription of the fimbrial operons in the wild-type strains, it had a greater effect on fimbrial transcription in the hns651 mutant strains. Growth at a pH of 5.5 was found to stimulate pap and daa transcription 4.7- and 2.4-fold, respectively, above the level measured in the hns651 mutant strains grown at pH 7.0, indicating that low pH stimulates pap and daa transcription in the absence of H-NS (Fig. 2).
Casamino Acids.
The addition of Casamino Acids to M9 glyc was found to cause a 2.1-fold increase in daa transcription compared to growth in M9 glyc (Fig. 2). pap and fan transcription levels varied less than twofold after growth in M9 CAA compared to growth in M9 glyc (Fig. 2).
In contrast, growth of the hns651 mutant strains in Casamino Acids repressed fimbrial transcription for the pap and daa operons. Transcription in the respective hns651 mutant strains was decreased 3.9-fold for pap and 2.2-fold for daa in response to the addition of Casamino Acids compared to transcription of the hns651 mutant strains in M9 glyc (Fig. 2).
Solid medium.
For S pili, growth on solid medium was shown to stimulate sfa transcription fourfold above growth in liquid medium (49). For the pap operon, transcriptional levels were similar to those measured after growth in M9 glyc liquid medium (Fig. 2). In contrast, growth on solid agar decreased transcription twofold for the fan and daa operons (Fig. 2).
For the daa operon, introduction of the hns651 mutation caused a loss of repression such that transcriptional levels in the hns651 mutant strain grown on solid medium were equivalent to those when cells were grown in liquid M9 glyc (Fig. 2).
Iron.
In the case of CFA/I fimbriae, high iron levels have been shown to repress production at the bacterial surface (28). In contrast, high iron levels did not dramatically alter transcription of the operons tested in this study (data not shown). In the hns651 mutant strains, pap transcription levels were increased 2.2-fold in response to increased iron concentration, whereas daa transcription levels in the hns651 mutant strain remained consistent with those measured for cells grown in M9 glyc (data not shown).
Effect of environment and H-NS on pap and daa phase transition rates.
Because transcription of both pap and daa is subject to a methylation-dependent phase variation mechanism (33, 55), we analyzed pap and daa phase transition rates to determine if the repressive effects of glucose and high osmolarity could be attributed to alterations in switch frequencies. In addition, the phase transition rates were determined for strains DL1947 and CWZ263 on M9 glyc, M9 gluc, and M9 NaCl to assess the influence of H-NS on switch frequencies under these conditions. While growth on LB is another condition that is repressive for pap and daa transcription, all of the colonies had a uniform colony phenotype on LB such that phase transition rates could not be calculated on this medium.
For both pap and daa, the phase ON→OFF transition rates are not greatly influenced by the hns651 mutation, glucose as a carbon source, or high osmolarity. The phase ON→OFF rates are similar for the hns651 mutant strains DL1947 and CWZ263 compared to their respective wild-type strains DL1504 and DL1530 grown under all conditions tested (Table 2).
In contrast, the phase OFF→ON transition rate for the pap operon is altered by glucose as a carbon source, high osmolarity, and the hns651 mutation (Table 2). On glucose, a phase transition rate could not be calculated for the wild-type strain DL1504, as no phase ON (Lac+) colonies were observed in a screening of over 37,000 colonies plated from four individual phase OFF (Lac−) colonies grown on glucose. This result is in agreement with earlier results for a similar, but not identical, papBA-lacZYA transcriptional fusion in which only three Lac+ colonies were seen in the screening of 119,000 colonies, yielding a phase transition frequency of 4.51 × 10−6/cell/generation (9). Growth at high osmolarity also decreases the phase OFF→ON transition rate 2.2-fold compared to growth on M9 glyc in DL1504, indicating that high osmolarity, like glucose, inhibits transcription by decreasing the rate at which cells transition to a phase ON state (Table 2). As described previously (54, 57) and shown in Table 2, the phase OFF→ON transition rate is lower in the hns651 mutant strain DL1947 than in the wild-type strain grown on M9 glyc.
In the hns651 mutant strain DL1947, the phase OFF→ON transition rates on M9 glucose and M9 NaCl are increased to levels similar to those for wild-type strain DL1504 grown on M9 glyc, demonstrating that the hns651 mutant transitions more frequently to a phase ON state on these media compared to the wild-type strain. However, while the phase OFF→ON transition rates are increased, the overall level of transcription does not increase to that seen for wild-type strain DL1504 grown in M9 glyc. Instead, it approximates transcription seen for the hns651 strain grown on M9 glyc. These results suggest that while phase ON colonies are seen, the level of transcription within the phase ON cells of DL1947 cannot be at the same level as in the wild-type strain (see Discussion).
For the daa operon, the phase OFF→ON transition rates are increased by the hns651 mutation under each of the conditions tested. In hns651 mutant strain CWZ263 grown on M9 glyc, the phase OFF→ON rate is increased 11.5-fold compared to the wild-type strain DL1504. Similarly, under conditions of high osmolarity and glucose as a carbon source, the phase OFF→ON rates are increased 8.0- and 3.1-fold, respectively, in the hns651 mutant strain compared to wild-type strain DL1530 grown under the same conditions. The phase OFF→ON rate for CWZ263 grown on glucose is not as high as that seen in the hns651 strain grown on M9 glyc, supporting the transcriptional evidence that glucose is still partially repressive in an hns651 mutant strain (Fig. 2). Taken together, these results indicate that the hns651 mutation relieves the repression of glucose and high osmolarity, at least in part, by increasing the rate at which cells transit into the phase ON state. While it is clear that the hns651 mutation influences the phase OFF→ON transition rates, it is not evident that the environmental stimuli affect the phase transition rates in the wild-type strain. The phase OFF→ON rates do not vary significantly between growth on M9 glyc, M9 gluc, or M9 NaCl.
DISCUSSION
While this study investigated the role of several environmental stimuli on fimbrial transcription, four environmental cues—high osmolarity, low temperature, glucose as a carbon source, and rich medium (LB)—were found to repress transcription of all three fimbrial operons studied. These results suggest that there are common environmental cues used by these fimbrial operons in E. coli to regulate transcription.
The importance of these environmental cues is supported by the commonality of their use by these three fimbrial operons as well as other virulence genes in E. coli. In this study, maximal expression of fimbrial transcription occurs at 37°C, corresponding to the internal temperature of most mammalian hosts. In E. coli, several fimbriae in addition to the ones studied here, including type I, 987P, CFA/I, S pili, K88, and Bfp, are not transcribed at low temperature (16, 18, 24, 27, 41, 47, 49).
Fimbrial transcription was also maximal when strains were grown in M9 glyc, where the osmolality is similar to that measured in the small intestine, supporting the hypothesis that osmolality is an important physiological cue used by the bacterium to control fimbrial transcription. Osmolality measured within the small intestine of a variety of mammals varies relatively little throughout the intestine, ranging from 316 to 379 mosmol/kg in the animals tested, indicating the luminal contents are isotonic to modestly hypertonic (20). This is in contrast to the stomach, where osmolalities can fluctuate greatly (20). Using a vapor pressure osmometer, we measured the osmolality of M9 glyc at 239 mosmol/kg, approximating levels seen physiologically in the mammalian intestine. Several other examples in E. coli follow this same pattern of regulation, in which the expression of virulence determinants is repressed by high osmolarity (2, 13, 17, 39, 49).
Because glucose has been shown to repress 987P fimbrial transcription through the action of cAMP-CAP, Edwards and Schifferli (18) have suggested a model in which the concentration of glucose would serve as a physiological cue to the bacterium's location within the intestine. In a study of the intestinal tracts of several types of mammals, glucose concentrations were measured to be below 0.0072% (0.4 mM), on average, within the distal small intestine (20). Edwards and Schifferli propose that this low concentration of glucose in the distal small intestine causes an increased expression of 987P fimbriae, correlating with studies showing that cells expressing 987P bind to this site in vivo (40). The data showing that all three fimbrial operons in this study are repressed by glucose through the action of cAMP-CAP support this model. Bacteria expressing K99 have been shown to bind within the distal intestine (38), while bacteria producing F1845 bind to the cecal and colonic mucosa in an infant pig model (6), locations where glucose concentrations would be lower. Limiting amounts of glucose in urine would support the expression of P pili in the urogenital tract and also the large intestine, a location that has been hypothesized to be a major reservoir for uropathogenic E. coli (56).
Together, these environmental cues may be used by E. coli to correctly time the expression of fimbriae. We propose that the environmental cue of 37°C would serve as a primary signal that the bacterium is within the host, whereas the cues of osmolality and glucose concentration would be used to more specifically signal the environment of the intestine. It is likely that there may be other, as yet unrecognized environmental cues that may be utilized to finely regulate fimbrial expression within the host.
We do not know why LB represses fimbrial transcription, but it has been demonstrated that LB also decreases transcription of 987P fimbriae and bundle-forming pili and increases the rate at which cells expressing type I fimbriae transit to a phase OFF state (18, 24, 35). We measured the osmolality of LB at 254 mosmol/kg, which is very similar to the 239 mosmol/kg measured in M9 glyc, indicating that the osmolality of LB would not be repressive. Similarly, supplementation of Casamino Acids to M9 glyc had little effect on fimbrial transcription, suggesting that the high amino acid content of LB would not be repressive. The carbohydrate content of LB is 0.16%, and thus glucose could contribute to the repression seen. However, at least in the case of pap, the level of transcription is significantly lower in LB than in M9 gluc.
An alternative hypothesis is that the critical factor may be the influence of LB on Lrp levels, as they have been shown to decrease approximately three- to fourfold in response to growth in rich medium (15, 30). Because transcription in all three of these operons is dependent on Lrp (10, 12, 55), decreased levels of Lrp would be expected to lower transcription. In addition, the interaction of the amino acid leucine with Lrp has been shown to decrease transcription of the fan operon and would contribute to repression in this system (12). However, transcription of 987P is also repressed by LB (18), but 987P expression is not Lrp dependent, suggesting that there must be another mechanism by which LB controls fimbrial transcription. Further studies are needed to determine the role of rich medium in controlling fimbrial transcription.
In this study, H-NS has been shown to be central regulator in response to the environmental cues of temperature, osmolarity, glucose as a carbon source, and rich medium for the pap and daa operons. The genetic evidence presented here expands the number of fimbrial operons in which H-NS represses transcription in response to low temperature. The thermoregulated expression of type I, 987P, and CFA/I fimbriae is also dependent on H-NS (16, 18, 27, 46), pointing to H-NS as an important player in this process. Mutations within hns also relieved the repression on fimbrial transcription due to high osmolarity, demonstrating the importance of H-NS in osmoregulation of these operons and corroborating other studies in which H-NS has been identified as an osmosensor (1). It is interesting that several H-NS-controlled genes demonstrate a pattern of regulation opposite that of the fimbrial operons studied here, where high osmolarity has been found to activate transcription (4, 31, 34). Mutations within hns partially relieve the repression due to glucose for the pap and daa fimbrial operons tested in this study and, to our knowledge, represent the only examples of fimbrial genes in which mutations in hns have been shown to relieve catabolite repression.
While we did not obtain reproducible results between separate hns651 transductants of strain DL812 containing the fan transcriptional fusion, it is possible that H-NS controls transcription in this operon as well. Transcriptional levels measured in the five transductants in M9 glucose and at low temperature, while variable, were all greater than those for the wild-type strain, suggesting that at least for these two stimuli, H-NS may control fan transcription (data not shown). The variability in transcriptional levels between separate transductants suggests that this strain may harbor secondary mutations that influence transcription. We hypothesize that the selection for secondary mutations may be due to the strength of the fan promoter in combination with the hns651 mutation. In the wild-type strain, the average β-galactosidase level produced in DL812 in M9 glyc is 14,733 MU, approximately 10- and 100-fold higher than the transcriptional levels seen for the pap and daa operons, respectively, under the same conditions. If introduction of the hns651 mutation further increases fan transcription, the high level of β-galactosidase expression may be toxic to the cell. Thus, secondary mutations may have occurred that counterbalance the loss of transcriptional repression due to the hns651 mutation and allow survival of the strain.
Our analyses of pap and daa phase transition rates suggest two different models for how environmental stimuli influence transcription in each of the operons studied. For the pap operon, both glucose as a carbon source and high osmolarity decrease the rate at which cells transition from a phase OFF to a phase ON state, accounting for the decrease in transcription seen under these conditions. In contrast, the phase transition rates for the daa operon are not greatly influenced by these stimuli. These results suggest that the repression due to these stimuli in the daa operon may be due to decreased efficiency of transcriptional initiation or elongation within the cell, rather than to an effect on phase transition rates.
Our data indicate that H-NS functions primarily as a negative regulator of daa transcription. Introduction of an hns651 mutation causes transcriptional levels to increase above that seen in the wild-type strain grows in M9 glyc, by stimulating the rate at which cells transit from a phase OFF to a phase ON transcriptional state. The increased phase OFF→ON transition rate in the hns651 mutant strain occurs not just in response to the repressive environmental stimuli but also when the strain is grown on M9 glyc, demonstrating that H-NS has an inhibitory effect on transcription even in the absence of a repressive stimulus such as glucose or high osmolarity.
It has been proposed that the role of cAMP-CAP, along with the activator PapB, is to antagonize the repressive effects of H-NS on pap transcription (22). Our data and previous studies support a greater role for H-NS in transcription of the pap operon (57). If the only function of H-NS in the pap operon was to antagonize activation, transcriptional levels in the hns651 mutant strain would be expected to be equivalent to or greater than those at 37°C in the wild-type strain. However, the levels of transcription are decreased in an hns651 mutant strain at 37°C in M9 glyc compared to the wild-type strain, demonstrating that H-NS plays a positive role in transcription. Additionally, while the hns651 mutation relieves the repression due to several environmental stimuli, transcription only approximates the levels seen for the hns651 mutant in M9 glyc, suggesting that even in the absence of the repressive stimulus, H-NS is needed for maximal pap transcription.
Because the phase OFF→ON rates in DL1947 grown both on glucose and at high osmolarity are similar to rates for wild-type DL1504 grown in M9 glyc without an increase in transcriptional levels, it seems unlikely that phase variation occurs by the same mechanism as in the wild-type strain. This is most clearly demonstrated by growth of the hns651 mutant strain DL1947 on glucose. In the wild-type strain, cAMP-CAP is required for pap transcription (3, 21, 22). Thus, a phase ON colony in DL1947 grown on glucose initiates transcription from the papBA promoter by a cAMP-CAP-independent mechanism since little cAMP-CAP complex is present in cells grown on glucose. It is not clear how switching occurs under this condition, but this result suggests that even in the absence of H-NS, cAMP-CAP is required for maximal transcription. Further study is needed to understand the mechanism by which H-NS controls pap transcription and phase variation, particularly in response to environmental cues.
A picture is emerging from the findings of this study and others that the production of many fimbriae in E. coli is controlled by the environmental cues of low temperature, high osmolarity, carbon source, and rich medium and that H-NS is a central regulator in response to these common environmental cues. Such studies may be important for designing therapeutic strategies that target E. coli infections in which the expression of fimbriae plays an important role in colonization.
ACKNOWLEDGMENTS
We thank David Low, Marjan van der Woude, and N. Patrick Higgins for generous gifts of bacterial strains. In addition, we thank Marjan van der Woude for critical reading of the manuscript. We also thank the Smith College students who provided technical assistance on this project, including Angela Rasmussen, Stacie Eliades, Jennifer Hoot, Deborah Cwalina, and Alanna Morris.
This work was supported by the Albert F. Blakeslee Trust and by Smith College.
REFERENCES
- 1.Atlung T, Ingmer H. H-NS: a modulator of environmentally regulated gene expression. Mol Microbiol. 1997;24:7–17. doi: 10.1046/j.1365-2958.1997.3151679.x. [DOI] [PubMed] [Google Scholar]
- 2.Badger J L, Kim K S. Environmental growth conditions influence the ability of Escherichia coli K1 to invade brain microvascular endothelial cells and confer serum resistance. Infect Immun. 1998;66:5692–5697. doi: 10.1128/iai.66.12.5692-5697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Båaga M, Göransson M, Normark S, Uhlin B E. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 1985;4:3887–3893. doi: 10.1002/j.1460-2075.1985.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltrametti F, Kresse A U, Guzman C A. Transcriptional regulation of the esp genes of enterohemorrhagic Escherichia coli. J Bacteriol. 1999;181:3409–3418. doi: 10.1128/jb.181.11.3409-3418.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilge S S, Apostol J M, Jr, Aldape M A, Moseley S L. mRNA processing independent of RNase III and RNase E in the expression of the F1845 fimbrial adhesin of Escherichia coli. Proc Natl Acad Sci USA. 1993;90:1455–1459. doi: 10.1073/pnas.90.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilge S S, Apostol J M, Jr, Fullner K J, Moseley S L. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1993;7:993–1006. doi: 10.1111/j.1365-2958.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 7.Bilge S S, Clausen C R, Lau W, Moseley S L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989;171:4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blyn L B, Braaten B A, Low D A. Regulation of pap pilin phase variation by a mechanism involving differential Dam methylation states. EMBO J. 1990;9:4045–4054. doi: 10.1002/j.1460-2075.1990.tb07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blyn L B, Braaten B A, White-Ziegler C A, Rolfson D H, Low D A. Phase-variation of pyelonephritis-associated pili in Escherichia coli: evidence for transcriptional regulation. EMBO J. 1989;8:613–620. doi: 10.1002/j.1460-2075.1989.tb03416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braaten B A, Blyn L B, Skinner B S, Low D A. Evidence for a methylation-blocking factor (mbf) locus involved in pap pilus expression and phase variation in Escherichia coli. J Bacteriol. 1991;173:1789–1800. doi: 10.1128/jb.173.5.1789-1800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braaten B A, Nou X, Kaltenbach L S, Low D A. Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E. coli. Cell. 1994;76:577–588. doi: 10.1016/0092-8674(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 12.Braaten B A, Platko J V, vanderWoude M W, Simons B H, DeGraaf F K, Calvo J M, Low D A. Leucine-responsive regulatory protein controls the expression of both the pap and fan pili operons in Escherichia coli. Proc Natl Acad Sci USA. 1992;89:4250–4254. doi: 10.1073/pnas.89.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmona M, Balsalobre C, Munoa F, Mourino M, Jubete Y, de la Cruz F, Juarez A. Escherichia coli hha mutants, DNA supercoiling and expression of the haemolysin genes from the recombinant plasmid pANN202–312. Mol Microbiol. 1993;9:1011–1018. doi: 10.1111/j.1365-2958.1993.tb01230.x. [DOI] [PubMed] [Google Scholar]
- 14.Casadaban M. Transposition and fusion of the lac genes to selected promoters in E. coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 15.Chen C F, Lan J, Korovine M, Shao Z Q, Tao L, Zhang J, Newman E B. Metabolic regulation of lrp gene expression in Escherichia coli K-12. Microbiology. 1997;143:2079–2084. doi: 10.1099/00221287-143-6-2079. [DOI] [PubMed] [Google Scholar]
- 16.Dorman C J, Bhriain N N. Thermal regulation of fimA, the Escherichia coli gene coding for the type 1 fimbrial subunit protein. FEMS Microbiol Lett. 1992;78:125–130. doi: 10.1016/0378-1097(92)90013-e. [DOI] [PubMed] [Google Scholar]
- 17.Edwards R A, Keller L H, Schifferli D M. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene. 1998;207:149–157. doi: 10.1016/s0378-1119(97)00619-7. [DOI] [PubMed] [Google Scholar]
- 18.Edwards R A, Schifferli D M. Differential regulation of fasA and fasH expression of Escherichia coli 987P fimbriae by environmental cues. Mol Microbiol. 1997;25:797–809. doi: 10.1046/j.1365-2958.1997.5161875.x. [DOI] [PubMed] [Google Scholar]
- 19.Falconi M, McGovern V, Gualerzi C, Hillyard D, Higgins N P. Mutations altering chromosomal protein H-NS induce mini-Mu transposition. New Biol. 1991;3:615–625. [PubMed] [Google Scholar]
- 20.Ferraris R P, Yasharpour S, Lloyd K C, Mirzayan R, Diamond J M. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol. 1990;259:G822–G837. doi: 10.1152/ajpgi.1990.259.5.G822. [DOI] [PubMed] [Google Scholar]
- 21.Forsman K, Görannson M, Uhlin B E. Autoregulation and multiple DNA interactions by a transcriptional regulatory protein in E. coli pili biogenesis. EMBO J. 1989;8:1271–1277. doi: 10.1002/j.1460-2075.1989.tb03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forsman K, Sonden B, Goransson M, Uhlin B E. Antirepression function in Escherichia coli for the cAMP-cAMP receptor protein transcriptional activator. Proc Natl Acad Sci USA. 1992;89:9880–9884. doi: 10.1073/pnas.89.20.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaastra W, de Graaf F K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982;46:129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gally D L, Bogan J A, Eisenstein B I, Blomfield I C. Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J Bacteriol. 1993;175:6186–6193. doi: 10.1128/jb.175.19.6186-6193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Göransson M, Sonden B, Nilsson P, Dagberg B, Forsman K, Emanuelsson K, Uhlin B E. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990;344:682–685. doi: 10.1038/344682a0. [DOI] [PubMed] [Google Scholar]
- 26.Göransson M, Uhlin B E. Environmental temperature regulates transcription of a virulence pili operon in E. coli. EMBO J. 1984;3:2885–2888. doi: 10.1002/j.1460-2075.1984.tb02225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordi B J, Dagberg B, de Haan L A, Hamers A M, van der Zeijst B A, Gaastra W, Uhlin B E. The positive regulator CfaD overcomes the repression mediated by histone-like protein H-NS (H1) in the CFA/I fimbrial operon of Escherichia coli. EMBO J. 1992;11:2627–2632. doi: 10.1002/j.1460-2075.1992.tb05328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karjalainen T K, Evans D G, Evans D J, Jr, Graham D Y, Lee C H. Iron represses the expression of CFA/I fimbriae of enterotoxigenic E. coli. Microb Pathog. 1991;11:317–323. doi: 10.1016/0882-4010(91)90017-5. [DOI] [PubMed] [Google Scholar]
- 29.Kawula T H, Orndorff P E. Rapid site-specific DNA inversion in Escherichia coli mutants lacking the histone-like protein H-NS. J Bacteriol. 1991;173:4116–4123. doi: 10.1128/jb.173.13.4116-4123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landgraf J R, Wu J, Calvo J M. Effects of nutrition and growth rate on Lrp levels in Escherichia coli. J Bacteriol. 1996;178:6930–6936. doi: 10.1128/jb.178.23.6930-6936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leclerc G J, Tartera C, Metcalf E S. Environmental regulation of Salmonella typhi invasion-defective mutants. Infect Immun. 1998;66:682–691. doi: 10.1128/iai.66.2.682-691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo-Tseng T, Lee J, Isaacson R E. Regulators of Escherichia coli K99 region 1 genes. Adv Exp Med Biol. 1997;412:303–310. doi: 10.1007/978-1-4899-1828-4_50. [DOI] [PubMed] [Google Scholar]
- 33.Low D A, Robinson E N, Jr, McGee Z A, Falkow S. The frequency of expression of pyelonephritis-associated pili is under regulatory control. Mol Microbiol. 1987;1:335–346. doi: 10.1111/j.1365-2958.1987.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 34.Mahan M J, Slauch J M, Mekalanos J J. Environmental regulation of virulence gene expression in Escherichia, Salmonella, and Shigella spp. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 2803–2816. [Google Scholar]
- 35.Martinez-Laguna Y, Calva E, Puente J L. Autoactivation and environmental regulation of bfpT expression, the gene coding for the transcriptional activator of bfpA in enteropathogenic Escherichia coli. Mol Microbiol. 1999;33:153–166. doi: 10.1046/j.1365-2958.1999.01460.x. [DOI] [PubMed] [Google Scholar]
- 36.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 38.Moon H W, Isaacson R E, Pohlenz J. Mechanisms of association of enteropathogenic Escherichia coli with intestinal epithelium. Am J Clin Nutr. 1979;32:119–127. doi: 10.1093/ajcn/32.1.119. [DOI] [PubMed] [Google Scholar]
- 39.Mourino M, Munoa F, Balsalobre C, Diaz P, Madrid C, Juarez A. Environmental regulation of alpha-haemolysin expression in Escherichia coli. Microb Pathog. 1994;16:249–259. doi: 10.1006/mpat.1994.1026. [DOI] [PubMed] [Google Scholar]
- 40.Nagy B, Moon H W, Isaacson R E. Colonization of porcine small intestine by Escherichia coli: ileal colonization and adhesion by pig enteropathogens that lack K88 antigen and by some acapsular mutants. Infect Immun. 1976;13:1214–1220. doi: 10.1128/iai.13.4.1214-1220.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagy L K, Mackenzie T, Pickard D J, Dougan G. Effects of immune colostrum on the expression of a K88 plasmid encoded determinant: role of plasmid stability and influence of phenotypic expression of K88 fimbriae. J Gen Microbiol. 1986;132:2497–2503. doi: 10.1099/00221287-132-9-2497. [DOI] [PubMed] [Google Scholar]
- 42.Normark S, Lark D, Hull R, Norgren M, Båga M, O'Hanley P, Schoolnik G, Falkow S. Genetics of digalactoside-binding adhesion from a uropathogenic Escherichia coli. Infect Immun. 1983;41:942–949. doi: 10.1128/iai.41.3.942-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nou X, Braaten B, Kaltenbach L, Low D A. Differential binding of Lrp to two sets of pap DNA binding sites mediated by Pap I regulates Pap phase variation in Escherichia coli. EMBO J. 1995;14:5785–5797. doi: 10.1002/j.1460-2075.1995.tb00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Hanley P, Low D A, Romero I, Lark D, Vosti K, Falkow S, Schoolnik G. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med. 1985;313:414–420. doi: 10.1056/NEJM198508153130704. [DOI] [PubMed] [Google Scholar]
- 45.Olsen P B, Klemm P. Localization of promoters in the fim gene cluster and the effect of H-NS on the transcription of fimB and fimE. FEMS Microbiol Lett. 1994;116:95–100. doi: 10.1111/j.1574-6968.1994.tb06681.x. [DOI] [PubMed] [Google Scholar]
- 46.Olsen P B, Schembri M A, Gally D L, Klemm P. Differential temperature modulation by H-NS of the fimB and fimE recombinase genes which control the orientation of the type 1 fimbrial phase switch. FEMS Microbiol Lett. 1998;162:17–23. doi: 10.1111/j.1574-6968.1998.tb12973.x. [DOI] [PubMed] [Google Scholar]
- 47.Puente J L, Bieber D, Ramer S W, Murray W, Schoolnik G K. The bundle-forming pili of enteropathogenic Escherichia coli: transcriptional regulation by environmental signals. Mol Microbiol. 1996;20:87–100. doi: 10.1111/j.1365-2958.1996.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 48.Sabourn D, Beckwith J. Deletion of the Escherichia coli crp gene. J Bacteriol. 1975;122:338–340. doi: 10.1128/jb.122.1.338-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmoll T, Ott M, Oudega B, Hacker J. Use of a wild-type gene fusion to determine the influence of environmental conditions on expression of the S fimbrial adhesin in an Escherichia coli pathogen. J Bacteriol. 1990;172:5103–5111. doi: 10.1128/jb.172.9.5103-5111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 51.Slonczewski J L, Gonzalez T N, Bartholomew F M, Holt N J. Mu d-directed fusion regulated by low pH in Escherichia coli. J Bacteriol. 1987;169:3001–3006. doi: 10.1128/jb.169.7.3001-3006.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ussery D W, Hinton J C D, Jordi B J A M, Granum P E, Seirafi A, Stephen R J, Tupper A E, Berridge G, Sidebotham J M, Higgins C F. The chromatin-associated protein H-NS. Biochimie. 1994;76:968–980. doi: 10.1016/0300-9084(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 53.van der Woude M W. Ph.D. thesis. Amsterdam, The Netherlands: Free University of Amsterdam; 1990. [Google Scholar]
- 54.van der Woude M W, Kaltenbach L S, Low D A. Leucine-responsive regulatory protein plays dual roles as both an activator and a repressor of the Escherichia coli pap fimbrial operon. Mol Microbiol. 1995;17:303–312. doi: 10.1111/j.1365-2958.1995.mmi_17020303.x. [DOI] [PubMed] [Google Scholar]
- 55.van der Woude M W, Low D A. Leucine-responsive regulatory protein and deoxyadenosine methylase control phase variation and expression of the sfa and the daa pili operons in Escherichia coli. Mol Microbiol. 1994;11:605–618. doi: 10.1111/j.1365-2958.1994.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 56.Vosti K L, Goldberg L M, Momto A S, Rantz L A. Host-parasite interaction in patients with infections due to Escherichia coli. I. The serogrouping of E. coli from intestinal and extraintestinal sources. J Clin Investig. 1964;43:2377–2385. doi: 10.1172/JCI105112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.White-Ziegler C A, Angus Hill M L, Braaten B A, van der Woude M W, Low D A. Thermoregulation of Escherichia coli pap transcription: H-NS is a temperature-dependent DNA methylation blocking factor. Mol Microbiol. 1998;28:1121–1137. doi: 10.1046/j.1365-2958.1998.00872.x. [DOI] [PubMed] [Google Scholar]
- 58.Williams R M, Rimsky S. Molecular aspects of the E. coli nucleoid protein, H-NS: a central controller of gene regulatory networks. FEMS Microbiol Lett. 1997;156:175–185. doi: 10.1111/j.1574-6968.1997.tb12724.x. [DOI] [PubMed] [Google Scholar]