Abstract
Purpose:
Activation of the PI3K/mTOR signaling pathway is common in head and neck squamous cell carcinoma (HNSCC). BYL719 is an α-specific PI3K inhibitor that is synergistic and efficacious when combined with cetuximab, an FDA-approved radiosensitizing agent in the treatment of HNSCC. The agent independently has been shown to enhance radiosensitivity. This study evaluates the addition of BYL719 to cetuximab and radiation in the treatment of locally advanced HNSCC.
Methods and Materials:
This is a single-institution, phase I study. Patients with the AJCC 7th Edition stage III-IVB HNSCC received standard cetuximab (400 mg/m2 intravenous loading dose) prior to intensity-modulated radiation therapy (IMRT) followed by 250 mg/m2 weekly infusions during IMRT. BYL719 was given orally during IMRT in 3 dose levels: 1) 200 mg/day, 2) 250 mg/day, or 3) 300 mg/day in a standard 3 + 3 dose-escalation design.
Results:
Eleven patients were evaluable. Dose level 2 was the maximum tolerated dose for BYL719. Two patients on dose level 3 had dose-limiting toxicities (DLTs) of oral mucositis that required a dose reduction of BYL719. One patient on dose level 2 had a DLT of nausea that led to withdrawal of on-study treatment. Related grade 3 or higher adverse events consisted of decreased lymphocyte count, oral mucositis, dysphagia, hyperglycemia, maculopapular rash, and palmar-plantar erythrodysesthesia syndrome. All 11 patients had a complete response on post-treatment imaging and 10 remain disease free. Of the 8 patients with mutational analysis, 1 had an activating PIK3CA mutation associated with a rapid response on serial intra-treatment magnetic resonance imaging scans.
Conclusion:
The recommended phase II dose of BYL719 is 250 mg/day in combination with cetuximab and IMRT in patients with locally advanced HNSCC. Further evaluation of the addition of BYL719 to the platinum-sparing regimen of cetuximab and IMRT in the treatment of locally advanced HNSCC is warranted.
Summary
This is a single-institution phase I study that evaluated the addition of BYL719, an α-specific PI3K inhibitor, to cetuximab and intensity-modulated radiation therapy (IMRT) in patients with stage III-IVB head and neck squamous cell carcinoma. The recommended phase II dose of BYL719 was found to be 250 mg/day in combination with cetuximab and IMRT. Related grade ≥ 3 adverse events included decreased lymphocyte count, oral mucositis, dysphagia, hyperglycemia, maculopapular rash, and palmar-plantar erythrodysesthesia syndrome.
INTRODUCTION
Cetuximab concurrent with definitive radiation therapy is a Food and Drug Administration (FDA)-approved platinum-sparing treatment regimen for patients with locally advanced head and neck squamous cell carcinoma (HNSCC).1 Although the toxicity profile of cetuximab may be viewed as favorable compared to that of cisplatin, the efficacy of cetuximab with radiation is inferior to that of cisplatin with radiation, especially for human papillomavirus (HPV)-positive disease.2–6 Therefore, there is a need to develop a radiosensitizing regimen that is less toxic than cisplatin yet more efficacious than single-agent cetuximab.
Aberrant activation of the PI3K/mTOR pathway is common in HNSCC. Activating mutations in PIK3CA, the gene encoding the p110α isoform of PI3K, have been reported in approximately 6-9% of HPV-negative tumors and 25-30% of HPV-positive tumors.7–10 Additional activating molecular alterations are harbored throughout the PI3K/mTOR pathway. Specifically, ≤5% of HNSCCs will have mutations in PTEN, AKT1/2/3, TSC2, mTOR, or Raptor.11
BYL719 (Alpelisib) is an oral class I α-specific PI3K inhibitor that strongly inhibits both the wild-type and mutant PI3Kα isoform, with much weaker inhibition of the PI3K β, γ, and δ isoforms. The combined use of BYL719 and an epidermal growth factor receptor (EGFR) inhibitor has demonstrated synergy in HNSCC cell lines.12 Specifically, co-inhibition of EGFR with BYL719 was sufficient to decrease mTOR activity as shown by the suppression of S6 phosphorylation in PIK3CA-mutant cell lines with acquired resistance to BYL719 monotherapy. In in vivo models of BYL719-resistant cell line-derived xenografts, cetuximab was shown to re-sensitize tumors to PI3K inhibition, with BYL719 resulting in decreased EGFR phosphorylation and mTOR activation, along with an associated decrease in proliferation and induction of cell death. Furthermore, in vitro dual inhibition with BYL719 and cetuximab was evaluated in a panel of 34 human HNSCC cell lines.13 In the 34 cell lines, two lines responded to single-agent cetuximab and six lines responded to single-agent BYL719, while the combination of cetuximab and BYL719 resulted in greater than 75% inhibition in 20 cells lines. This response was independent of PIK3CA mutational status, suggesting that this combination is both broadly synergistic and efficacious.
These pre-clinical results led to the evaluation of the combination of BYL719 and cetuximab in patients with advanced HNSCC. The first in-human, dose-escalation study of BYL719 monotherapy found the maximum tolerated dose (MTD) to be 400 mg once daily; the most common adverse events (AEs) were hyperglycemia, nausea, vomiting, diarrhea, and decreased appetite.14 Based on assessments of tolerability and efficacy, however, the MTD for BYL719 monotherapy was deemed to be 350 mg once daily. The phase Ib portion of the study concluded that the MTD of BYL719 was 300 mg once daily when given with standard weekly cetuximab.
BYL719, encapsulated into P-selectin–targeted nanoparticles to achieve specific accumulation of BYL719 in the tumor milieu, has been found to be a potent radiosensitizing agent.15 Given its radiosensitizing properties and synergy when combined with cetuximab, we sought to enhance the Bonner regimen1 through the addition of BYL719 to cetuximab and intensity-modulated radiation therapy (IMRT) in the treatment of locally advanced HNSCC.
We designed a phase I study to define the phase II recommended dose of BYL719 when added to standard-dose cetuximab and IMRT for the treatment of patients with locally advanced, stage III-IVB HNSCC.
METHODS AND MATERIALS
Patient Eligibility Criteria
This was a single-institution, phase Ib study (ClinicalTrials.gov Identifier: NCT02282371) approved by our hospital’s institutional review board. All patients provided written consent. Eligible patients had previously untreated American Joint Committee on Cancer (AJCC) 7th edition stage III, IVA, or IVB HNSCC (nasopharynx cancer was excluded) regardless of PIK3CA mutational status. Enrolled patients were ≥18 years of age, had an Eastern Cooperative Oncology Group (ECOG) performance status ≤1, and had the ability to swallow capsules. The baseline laboratory requirements were: absolute neutrophil count ≥1.0 x 109/L, platelet count ≥100 x 109/L, hemoglobin ≥90 g/L, serum creatinine ≤1.5 times the upper limit of normal (ULN), and serum bilirubin ≤1.5 times the ULN. Aspartate and alanine aminotransferase levels were required to be ≤2.5 times the ULN.
Patients were excluded if they had diabetes mellitus, including steroid-induced diabetes mellitus. Additional exclusion criteria included an active infection or a serious underlying medical condition that would interfere with the ability to receive treatment on-study. Patients were also ineligible if they had a history of pre-existing interstitial lung disease, New York Heart Association (NYHA) class II or greater congestive heart failure, and poorly controlled gastrointestinal disorders that could affect the absorption of BYL719. Patients who received therapeutic anticoagulation with warfarin were also excluded, as were patients unable to discontinue drugs known to be strong inhibitors or inducers of isoenzyme CYP3A.
Treatment Plan
All patients received definitive IMRT with either a total of 69.96 Gy (2.12 Gy per fraction) delivered over 33 fractions or a total of 70 Gy (2 Gy per fraction) delivered over 35 fractions. Patients received an intravenous (IV) loading dose of 400 mg/m2 cetuximab 4–10 days prior to the start of IMRT with subsequent weekly maintenance dosing of IV 250 mg/m2 for 7 concurrent treatments with radiation therapy. BYL719 was administered orally each day from the start of IMRT to completion of radiotherapy. The three dose levels of BYL719 that were evaluated included: 200 mg/day, 250 mg/day, and 300 mg/day per oral.
Dose Escalation and Definition of Dose-Defining Toxicity
A standard 3 + 3 dose-escalation plan for three dose levels of BYL719 was followed. Assessment for dose-limiting toxicity (DLT) of BYL719 occurred through 2 weeks following the completion of radiation therapy for each patient; there was no intra-patient dose escalation. The MTD was defined as the highest dose level at which ≤1 of the 6 patients experienced a DLT. Patients who experienced a DLT were removed from the study and managed with standard of care for the treatment of HNSCC. If the MTD was not exceeded among 6 patients treated at dose level 3, then the phase II recommended dose of BYL719 would be 300 mg/day.
A DLT was defined as any toxicity requiring radiation treatment delay of up to 7 days or any AE of grade 3 or higher per version 4.0 of the Common Terminology Criteria for Adverse Events [CTCAE v4.0], felt to be possibly, probably, or definitely related to BYL719. Any patient unable to receive at least 4 weekly infusions of maintenance cetuximab, 30 days of BYL719, and a minimum of 30 fractions of radiation due to toxicity was considered to have experienced a DLT.
Accounting for the common toxicities associated with definitive radiation and cetuximab, the following grade 3 AEs were not considered DLTs: oral mucositis, dysphagia, odynophagia, xerostomia, fatigue, skin rash or radiation dermatitis, nausea and/or vomiting lasting up to 7 days, diarrhea lasting less than 48 hours, hypomagnesemia, hyponatremia if sodium was >125 mmol/L, hypocalcemia, hypophosphatemia, hypokalemia, neutropenia lasting up to 7 days in the absence of neutropenic fever, and non-neutropenic infection requiring IV antibiotics. A grade 3 or higher anaphylactic reaction to cetuximab and grade 3 or 4 lymphopenia were also not considered DLTs. Given the known toxicities of BYL719, grade 3 or 4 hyperglycemia lasting less than 48 hours and hyperlipidemia were not considered DLTs. Hospitalizations did not necessarily reflect a DLT, as patients receiving chemoradiation often require hospitalization for supportive care.
Efficacy Assessments
Radiologic response assessment was performed with cross-sectional imaging of the primary tumor approximately 3–4 months after the completion of treatment with IMRT and concurrent BYL719 with cetuximab. For patients who maintained active follow-up, clinic visits were recommended every 3 months for 2 years, then every 6 months for the next 3 years, and annually thereafter.
Mutational Analysis
Targeted next-generation DNA sequencing was performed to identify mutations along the PI3K/mTOR pathway. For patients with adequate pre-treatment formalin-fixed paraffin-embedded tumor tissue, targeted mutational analysis was performed using MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets). The assay has been previously described and involves massively parallel sequencing, coupled with solution-phase exon capture. Exon capture was performed on barcoded pools of sequence libraries by hybridization (Nimblegen SeqCap Target Enrichment, Roche NimbleGen, Madison, WI, USA) using custom oligonucleotides to capture all exons and select introns of 341 cancer genes.16
RESULTS
Patient Characteristics
Sixteen patients were enrolled on the study from February 2015 to July 2017. Only 11 patients were evaluable for assessment. One patient on dose level 1 withdrew consent prior to starting BLY719. On dose level 2, one patient experienced anaphylaxis to the cetuximab loading dose infusion prior to starting IMRT and BYL719 and 3 patients withdrew consent for grade 1 nausea within the first 2 days of BLY719 administration despite anti-nausea medications.
Baseline characteristics for the 11 evaluable patients are summarized in Table 1. All evaluable patients were men, and the median age was 60 years (range, 36-73 years). The median ECOG performance status was 0 (range, 0-1). AJCC 7th edition staging status was as follows: stage III, 2 patients; stage IVA, 8 patients; and stage IVB, 1 patient. The primary site of disease was the oropharynx in 9 patients and unknown in 2. Ten of the 11 evaluable patients had HPV-associated HNSCC. Six patients were never-smokers, 2 had less than a 10-pack–year history of smoking, and 3 had greater than a 30-pack-year history of smoking.
Table 1.
Baseline Characteristics of All Evaluable Patients
| Characteristic | Number of patients |
|---|---|
|
| |
| Median Age, years | 60 (range, 36-73) |
|
| |
| Median ECOG Performance Status | 0 (range, 0-1) |
|
| |
| Sex | |
| Men | 11 |
| Women | 0 |
|
| |
| Stage | |
| III | 2 |
| IVA | 8 |
| IVB | 1 |
|
| |
| Subsite of primary tumor | |
| Oropharynx | 9 |
| Unknown primary | 2 |
|
| |
| HPV status (per p16 IHC) | |
| Positive | 10 |
| Negative | 1 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HPV, human papillomavirus; IHC, immunohistochemistry.
Dose Escalation and Dose-Limiting Toxicities
None of the 3 evaluable patients enrolled at dose level 1 (BYL719 200 mg/day) experienced a DLT. There were no DLTs among the first 3 patients enrolled at dose level 2 (BYL719 250 mg/day). Two patients enrolled at dose level 3 (BYL719 300 mg/day) experienced grade 3 oral mucositis requiring a dose reduction of BYL719; therefore, these AEs were considered DLTs. One patient with grade 3 oral mucositis was subsequently hospitalized for aspiration pneumonia.
Three additional evaluable patients were enrolled at dose level 2. One of the 3 patients was noted as having a DLT, as the patient withdrew from the study just prior to receiving 30 days of BYL719 due to grade 2 nausea. In total, 1 of the 6 evaluable patients on dose level 2 experienced a DLT; therefore, the recommended phase II dose of BYL719 is 250 mg/day in combination with cetuximab and IMRT.
Adverse Events
All AEs per CTCAE v4.0 for the 11 evaluable patients while on-study treatment through 2 weeks following the completion of radiation were reported. Table 2 lists AEs possibly, probably, or definitely related to BYL719. Related grade 3 or higher AEs consisted of decreased lymphocyte count (10 patients), oral mucositis (4), dysphagia (2), hyperglycemia (1), maculopapular rash (1), and palmar-plantar erythrodysesthesia syndrome (1). Decreased lymphocyte count, hyperglycemia, and palmar-plantar erythrodysesthesia syndrome were the only AEs uniquely attributed to BYL719, while the other grade 3 AEs thought to be enhanced by BYL719 were also related to IMRT and/or cetuximab. Although there were 4 grade 3 oral mucositis events reported, only 2 were considered DLTs due to the required dose reductions of BYL719. All AEs attributed to or enhanced by BYL719 resolved within a timeframe that would be expected following completion of IMRT with concurrent radiosensitizing therapy.
Table 2.
Adverse Events Possibly, Probably, or Definitely Related to BYL719 present in 2 or more patients
| Toxicity | All Grades | Grade 3 | Grade 4 |
|---|---|---|---|
| Lymphocyte Count Decreased | 10 | 9 | 1 |
| Hyperglycemia | 9 | 1 | 0 |
| Nausea | 9 | 1 | 0 |
| Rash maculo-papular | 9 | 1 | 0 |
| Weight loss | 9 | 0 | 0 |
| Dysphagia | 8 | 2 | 0 |
| Fatigue | 8 | 0 | 0 |
| Mucositis Oral | 8 | 4 | 0 |
| Vomiting | 8 | 0 | 0 |
| Oral pain | 7 | 0 | 0 |
| Alanine aminotransferase increased | 6 | 0 | 0 |
| Dysgeusia | 6 | 0 | 0 |
| Anemia | 5 | 0 | 0 |
| Dyspepsia | 5 | 0 | 0 |
| Headache | 5 | 0 | 0 |
| Radiation dermatitis (worsening) | 5 | 0 | 0 |
| Alkaline phosphatase increased | 4 | 0 | 0 |
| Diarrhea | 4 | 0 | 0 |
| Dizziness | 4 | 0 | 0 |
| Aspartate aminotransferase increased | 3 | 0 | 0 |
| Anorexia | 2 | 0 | 0 |
| Dry mouth | 2 | 0 | 0 |
| Dry skin | 2 | 0 | 0 |
| Fever | 2 | 0 | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 2 | 1 | 0 |
| Pre-syncope | 2 | 0 | 0 |
| Throat pain | 2 | 0 | 0 |
Efficacy
All 11 evaluable patients showed no evidence of persistent disease on the required radiologic assessment 3–4 months following the completion of radiation per a computed tomography (CT) or magnetic resonance imaging (MRI) scan of the primary tumor or neck and a standard-of-care whole-body fluorine-18-fluorodeoxyglucose positron emission tomography (FDG-PET) scan. Additional physical examinations with endoscopy showed no evidence of mucosal disease in all of the evaluable patients 3-4 months post-treatment.
The median follow-up period beginning from the completion of radiation treatment was 23.5 months (range, 8.4-37.7 months). All but one of the 11 patients remain disease free per physical examination and imaging. One patient, who was a never-smoker and treated on study for stage III HPV-positive oropharynx cancer, developed recurrent, distant metastatic disease involving pulmonary nodules without evidence of locoregional recurrence.
Mutational analysis was performed on a pre-treatment specimen from 8 of the 11 patients. A patient who was a never smoker with HPV-positive, AJCC 7th Ed. Stage IVA (cT2N2a) oropharynx squamous cell carcinoma that arose from the left base of tongue and enrolled at dose level 3 was found to have an activating PIK3CA E542K mutation. This patient was found to have a rapid response to treatment on intra-treatment MRI scans, with a near complete response within 8 days of initiating IMRT (Figure 1). None of the other 7 patients were found to have a mutation along the PI3K/mTOR pathway per mutational analysis of a primary tumor or locoregional lymph node biopsy. Interestingly, the patient who was diagnosed with distant, metastatic disease developed an acquired PIK3CA N345K mutation that was seen on molecular analysis of a lung biopsy, which was not present on analysis of the oropharynx biopsy.
Figure 1:
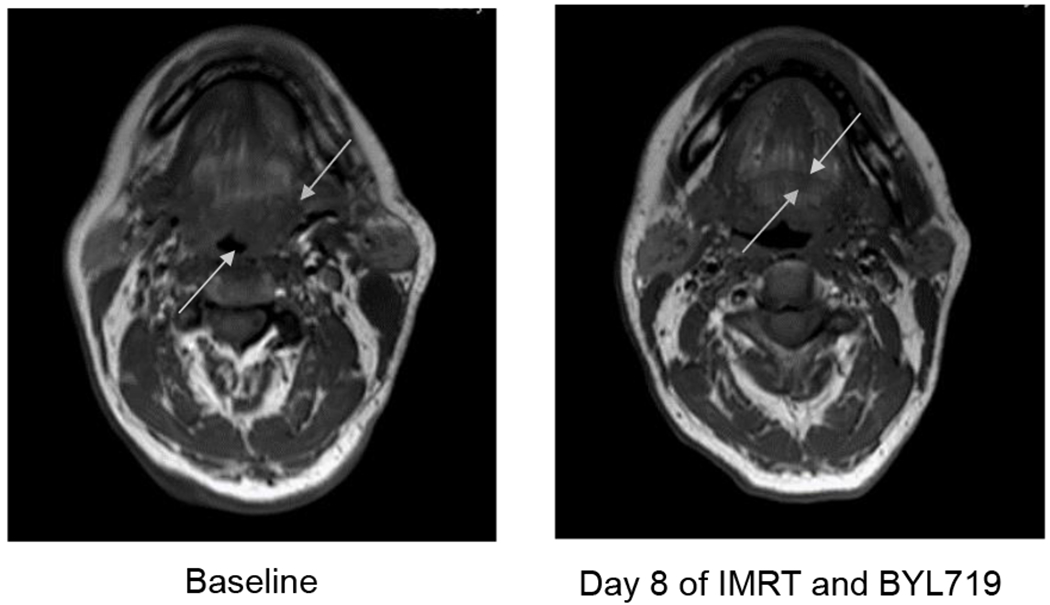
The images depict a rapid response in a patient with HPV-positive, AJCC 7th Ed. Stage IVA (cT2N2a) oropharynx squamous cell carcinoma that arouse from the left base of tongue harboring a PIK3CA E542K mutation. This patient received a loading dose of cetuximab 400mg/m2 IV prior to IMRT to a total of 70 Gy to the gross disease delivered in 35 fractions with concurrent cetuximab 250mg/m2 x 7 weekly treatments and BYL719 300mg/day. A near complete radiologic response was noted after receiving 16 Gy over 8 fractions with BYL719 300mg/day x 8 days.
DISCUSSION
This phase I trial establishes that the recommended phase 2 dose of BYL719 in combination with cetuximab and IMRT is 250 mg oral per day. Overall, this combination was found to be safe. BYL719 likely enhances common toxicities associated with definitive radiation and cetuximab. However, the contribution of BYL719 to these treatment-associated AEs is subjective given that grade 3 oral mucositis and dysphagia can occur with radiation alone and a grade 3 maculopapular rash can occur with cetuximab alone. BYL719 monotherapy has been reported to be associated with the AE of nausea, diarrhea, hyperglycemia, and a maculopapular rash, and less commonly can cause oral mucositis. Given that 3 patients withdrew for grade 1 nausea within the first two days of BYL719 administration, all patients treated with this drug in future studies should be provided with an effective anti-nausea regimen upon initiation of treatment. Cetuximab frequently causes a maculopapular rash and infrequently causes diarrhea, but otherwise has no further overlapping toxicities. Other than GI toxicities, the AE profile for cisplatin, commonly associated with ototoxicity, neuropathy, and renal insufficiency, differs from BYL719. Therefore, a large population of patients who would benefit from a platinum-sparing radiosensitizing regimen are those with diabetes mellitus due to associated neuropathy and nephropathy. However, patients with diabetes mellitus were excluded from this study due to the concern for hyperglycemia with BYL719. Given that most patients had mild hyperglycemia on study—8 patients had grade 1 or 2 hyperglycemia and 1 patient had grade 3 hyperglycemia—and that moderate hyperglycemia can be treated with insulin, suggests that patients with diabetes mellitus can be managed and safely included if further studies are conducted to develop this regimen.
Emerging data from the Radiation Therapy Oncology Group (RTOG) 1016 and De-ESCALaTE HPV studies have shown inferior efficacy with concurrent cetuximab, compared to cisplatin with radiation for HPV-positive, locally advanced oropharynx cancer.5,6 These studies highlight the need to develop platinum-sparing radiosensitizing regimens by either replacing cetuximab or enhancing the activity of cetuximab monotherapy as a radiosensitizing regimen for patients who are not platinum candidates. NRG-HN004 is a Randomized Phase II/III Trial of Radiotherapy with Concurrent MEDI4736 (Durvalumab) vs. Radiotherapy with Concurrent Cetuximab in Patients with Stage III-IVB Head and Neck Cancer with a Contraindication to Cisplatin (NCI-2017-01522). The outcome of NRG-HN004 will likely inform the direction of development of platinum-sparing radiosensitizing regimens in locally advanced HNSCC, as immunotherapy may be an appealing replacement for EGFR-directed therapy.
If further development of radiosensitizing regimens that enhance the activity of cetuximab is pursued, there is rationale for combining dual EGFR and PI3K pathway inhibition. The activation of EGFR through dimerization stimulates tyrosine kinase activity in the C terminal domain and results in the initiation of multiple downstream signal transduction cascades, including the PI3K/Akt/mTOR pathway. Resistance to EGFR inhibition is often due to the compensatory hyperactivation of downstream PI3K/AKT/mTOR signaling.12 Preclinical data have implicated that dual EGFR and PI3K inhibition in HNSCC cell lines and xenograft models is synergistic in inhibiting cell proliferation. Single targeting of the EGFR suppressed cell growth in pre-clinical models, while dual inhibition with the combination of a PI3K inhibitor additionally induced apoptosis and inhibited cell survival.17–19 Furthermore, HNSCC cell lines with a constitutively active AKT were sensitized to EGFR inhibition through targeting of the PI3K/AKT pathway.20 Given the pre-clinical evidence in HNSCC models, inhibition of the PI3K pathway in addition to cetuximab, regardless of tumor mutational status, should be beneficial. Although BYL719 potently inhibits both the wild-type and mutant PI3Kα isoforms, with weaker inhibition of the PI3K β, γ, and δ isoforms, it is plausible that a pan-PI3K inhibitor or an mTOR inhibitor would have improved synergy with cetuximab, depending on the site of PI3K/mTOR pathway activation resulting from acquired resistance to EGFR inhibition.
Other than androgen deprivation therapy in prostate cancer, targeted therapies have not been widely developed as radiosensitizing agents. In patients with PIK3CA-mutant, locally advanced HNSCC, single-agent BYL719, as a radiosensitizing agent, may be more effective than when combined with cetuximab, given that further activation along the PI3K/AKT/mTOR axis may occur as resistance to EGFR inhibition develops. Although prior to this phase I study, the only data of the radiosensitizing properties of BYL719 came from the P-selectin–targeted nanoparticle form to achieve high concentrations within the tumor milieu,15 the uncharacteristic rapid radiologic response in the patient with a known PIK3CA mutation suggests that the oral formulation of BYL719 can enhance radiosensitivity as a single agent.
At the time the study was conducted, selecting patients by mutational status to receive targeted therapy as a radiosensitizing agent with curative-intent radiation had limitations due to feasibility. Large-scale whole-exome sequencing can have a turnaround time of weeks, while more focused molecular diagnostic assays that evaluate the presence of fewer alterations have been difficult to run on a large scale and are not currently Clinical Laboratory Improvement Amendments (CLIA) approved, and thus, therapeutic management has not been based on results from these techniques. Radiosensitizing agent selection by mutation status on a large-scale basis may become feasible, such that patients can be selected for targeted therapies without an unacceptable delay in curative-intent treatment. As molecular diagnostic assays evolve, selecting radiosensitizing regimens may become more biologically based in the future.
Further development of the combination of cetuximab and BYL719 or a radiosensitizing regimen containing dual EGFR and PI3K inhibition would be rational in two patient populations. One would be in patients in whom a platinum agent or cytotoxic chemotherapy is contraindicated or are unwilling to accept the standard toxicity profile of cisplatin or chemotherapy in standard radiosensitizing regimens. The second group would be comprised of patients with an activating alternation in the PI3K/AKT/mTOR pathway. Given the rapid, marked response demonstrated in the patient with an activating PIK3CA E542K mutation, a biologically-based radiosensitization approach in selected patients should be pursued regardless of platinum-eligibility and HPV-status once technically feasible to identify patients by mutation status on a large-scale basis.
In summary, this study demonstrated that the recommended phase 2 dose of BYL719 in combination with cetuximab and IMRT is 250 mg oral per day. BYL719 and cetuximab appears to be a safe platinum-sparing radiosensitizing regimen.
Acknowledgments
Funding: Supported in part by the MSK Cancer Center Support Grant P30 CA008748 and Novartis.
Conflicts of interest: L.A.D. received research support from Regeneron Pharmaceuticals and Esai Pharmaceuticals and advisory board fees from Regeneron Pharmaceuticals and CUE Biopharma. S.S.B. is a full-time employee at Flatiron Health. M.G.F. and family member are full-time employees and stock shareholders of Regeneron Pharmaceuticals. A.L.H. receives research support from Novartis, Kura Oncology, AstraZeneca, Merck, Bristol-Myers Squibb, Genentech/Roche, Celldex Therapeutics, Bayer, Eli Lilly, Ayala Pharmaceuticals, Astellas Pharma, Daiichi Sankyo, Eisai, Allos Therapeutics, and Pfizer; advisory board fees from Sanofi Genzyme, Novartis, AstraZeneca, Merck, Bristol-Myers Squibb, Sun Pharmaceuticals, Ayala Pharmaceuticals, Regeneron Pharmaceuticals, TRM Oncology, and Eisai; consulting fees from Genentech/Roche and CureVac, ; travel reimbursement from Kura Oncology, AstraZeneca, Merck, Ignyta, Ayala Pharmaceuticals, and Janssen Pharmaceutica; speaker fees from Novartis and Medscape; and food reimbursement from Klus Pharma. N.Y.L. is on the steering committee for Pfizer (unpaid position); reports spouse is a stock shareholder of AstraZeneca; receives research support from Merck, Pfizer, and AstraZeneca; and advisory board fees from Merck, Merck Serono, and Sanofi Aventis. S.M.M. receives advisory board fees from AstraZeneca. D.G.P. receives research support from Novartis and Pfizer and advisory board fees from Boehringer-Ingelheim. N.R. receives research support from Bristol-Myers Squibb, Pfizer, AstraZeneca; and advisory board fees from Mirati Therapeutics. E.J.S. receives research support from Roche, Plexxikon, and Eisai; and consulting fees from Novartis, Loxo Oncology, Bristol-Myers Squibb, and Eisai. All remaining authors have declared no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2006;354(6):567–578. [DOI] [PubMed] [Google Scholar]
- 2.Koutcher L, Sherman E, Fury M, et al. Concurrent cisplatin and radiation versus cetuximab and radiation for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):915–922. [DOI] [PubMed] [Google Scholar]
- 3.Riaz N, Sherman EJ, Fury M, Lee N. Should cetuximab replace Cisplatin for definitive chemoradiotherapy in locally advanced head and neck cancer? J Clin Oncol. 2013;31(2):287–288. [DOI] [PubMed] [Google Scholar]
- 4.Zandberg DPCKJ, Varki V, Suzuki I, Bentzen S, Goloubeva OG Definitive cetuximab-based (CRT-CX) vs. non-cetuximab based chemoradiation (CRT) in older patients with squamous cell carcinoma of the head and neck (HNSCC): Analysis of the SEER-Medicare linked database. J Clin Oncol. 2018;36(suppl; abstr 6001). [Google Scholar]
- 5.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols AC, Palma DA, Chow W, et al. High frequency of activating PIK3CA mutations in human papillomavirus-positive oropharyngeal cancer. JAMA otolaryngology-- head & neck surgery. 2013;139(6):617–622. [DOI] [PubMed] [Google Scholar]
- 8.Lui VW, Hedberg ML, Li H, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer discovery. 2013;3(7):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333(6046):1157–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris LG, Chandramohan R, West L, et al. The Molecular Landscape of Recurrent and Metastatic Head and Neck Cancers: Insights From a Precision Oncology Sequencing Platform. JAMA Oncol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung CH, Guthrie VB, Masica DL, et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann Oncol. 2015;26(6):1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkabets M, Pazarentzos E, Juric D, et al. AXL mediates resistance to PI3Kalpha inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell. 2015;27(4):533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bootle D BYL719 Investigator’s Brochure, Edition 5, 29 July 2013.
- 14.Gonzalez-Angulo AM, Juric D, Argilés G, et al. Safety, pharmacokinetics, and preliminary activity of the α-specific PI3K inhibitor BYL719: Results from the first-in-human study. Journal of Clinical Oncology. 2013;31(15_suppl):2531–2531. [Google Scholar]
- 15.Mizrachi A, Shamay Y, Shah J, et al. Tumour-specific PI3K inhibition via nanoparticle-targeted delivery in head and neck squamous cell carcinoma. Nat Commun. 2017;8:14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won HH, Scott SN, Brannon AR, Shah RH, Berger MF. Detecting somatic genetic alterations in tumor specimens by exon capture and massively parallel sequencing. J Vis Exp. 2013;e50710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anisuzzaman AS, Haque A, Wang D, et al. In Vitro and In Vivo Synergistic Antitumor Activity of the Combination of BKM120 and Erlotinib in Head and Neck Cancer: Mechanism of Apoptosis and Resistance. Mol Cancer Ther. 2017;16(4):729–738. [DOI] [PubMed] [Google Scholar]
- 18.D’Amato V, Rosa R, D’Amato C, et al. The dual PI3K/mTOR inhibitor PKI-587 enhances sensitivity to cetuximab in EGFR-resistant human head and neck cancer models. Br J Cancer. 2014;110(12):2887–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lattanzio L, Tonissi F, Monteverde M, et al. Treatment effect of buparlisib, cetuximab and irradiation in wild-type or PI3KCA-mutated head and neck cancer cell lines. Invest New Drugs. 2015;33(2):310–320. [DOI] [PubMed] [Google Scholar]
- 20.Young NR, Liu J, Pierce C, et al. Molecular phenotype predicts sensitivity of squamous cell carcinoma of the head and neck to epidermal growth factor receptor inhibition. Mol Oncol. 2013;7(3):359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]


