Abstract
RNA interference (RNAi), a cellular process by which small RNAs target and manipulate complementary RNA transcripts, has well-characterized roles in post-transcriptional gene regulation and transposon repression. Recent studies have considered RNAi activity beyond these classical roles and revealed critical roles for RNAi in chromosome function. By guiding chromatin modification, RNAi promotes chromosome segregation during both mitosis and meiosis and regulates chromosomal and genomic dosage response. Small RNAs and the RNAi machinery also participate in the resolution of DNA damage. Interestingly, many of these lesser-studied functions seem to be more strongly conserved across eukaryotes than well-characterized functions such as the processing of microRNAs. These findings have implications for the evolution of RNAi since the last eukaryotic common ancestor and provide a more complete view of the function of RNAi.
Introduction
RNA interference (RNAi) is a post-transcriptional gene silencing (PTGS) mechanism mediated by small RNAs that has been found to play essential roles across eukaryotic organisms in genome defence against viruses and transposable elements1–4, development5–7, cellular differentiation8 and disease progression9,10. Effector proteins of the Argonaute family such as AGO and PIWI use the sequence of small RNAs — microRNAs (miRNAs), short interfering RNAs (siRNAs) and PIWI-associated RNAs (piRNAs) — to identify target RNAs with complementary base pairing. The small RNA–effector complex either cleaves the target or recruits other proteins to inhibit the translation or degrade the target RNA.
The strong evolutionary conservation of proteins involved in miRNA biogenesis and RNAi, such as DROSHA–DGCR8, DICER1 and AGO, and the apparent absence of classical miRNAs in the last eukaryotic common ancestor (LECA)11 suggest that these proteins possess specific qualities that enable non-microRNA precursor RNA interactions to perform a broad range of important, conserved functions. Some of these microRNA-independent, often nuclear, RNAi functions have subsequently been elucidated in great mechanistic detail (as reviewed elsewhere3,12,13). Other highly conserved functions of RNAi proteins have consequences specifically for chromosome and genome integrity and emphasize the importance of RNAi outside the production of microRNAs. The molecular mechanisms related to these themes are easier to elucidate in organisms without microRNAs, such as the fission yeast Schizosaccharomyces pombe, as in other organisms the consequences of losing miRNA-mediated gene repression by mutating or manipulating one of the RNAi components can cloud experimental interpretation. Despite this caveat, we demonstrate that a number of functions and themes of the RNAi machinery are conserved from unicellular eukaryotes, such as S. pombe, to higher eukaryotes. Moreover, we explore the possibility that chromatin modification was an early consequence of ancestral function and affected genome integrity at multiple levels.
In this Review, we begin by tracing the evolutionary history of the RNAi components from their origins in prokaryotes to the unification of these proteins into a functional pathway in the LECA. We then provide a brief overview of the known molecular preferences of these proteins to demonstrate the inherent flexibility of RNAi. Next, we detail conserved functions of RNAi in the segregation of chromosomes during cell division. Then we examine the regulation of dosage by RNAi at the RNA, chromatin and DNA levels. We demonstrate how the regulation of dosage at these various stages contributes to chromosome function in general. Finally, we describe the expanding role of RNAi proteins in the detection and resolution of DNA damage. Throughout the review we will draw comparisons to the classic small RNA-driven heterochromatin formation mechanism in S. pombe and delineate between mechanisms that seem to be an adaptation of this pathway and those that are independent of small RNAs.
The evolution and molecular targets of RNAi proteins
The production of the various classes of eukaryotic small RNAs — siRNAs, microRNAs and piRNAs — occurs through defined molecular activity on precursor RNA molecules (Fig. 1a–c). The enzymatic activities that produce small RNAs and generate an effect on RNA targets are highly conserved and maintain a general flexibility in substrates. These qualities and their conservation, which will be discussed further in the following sections, are important to recognize to define the broad range of functions of RNAi and of the molecular targets that generate these functions.
Figure 1 |. Small RNA biogenesis pathways and the evolutionary conservation of their components.
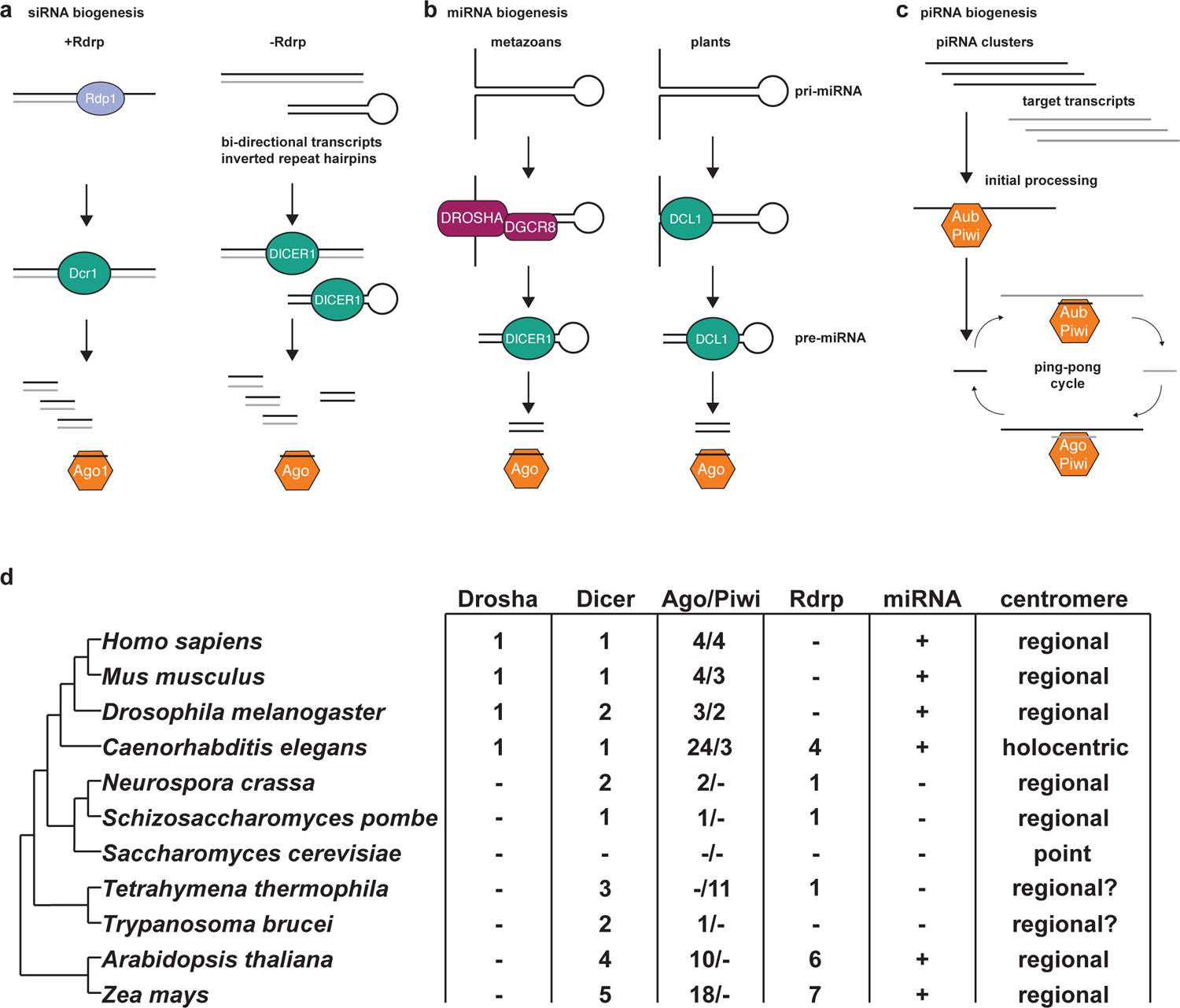
a | Small interfering RNAs (siRNAs) can be produced from either double stranded RNA (dsRNA) generated by RNA-dependent RNA polymerase (Rdrp) or other dsRNA substrates. In S. pombe and other eukaryotes possessing a Rdrp (Rdp1), a transcript is targeted for the synthesis of dsRNA, which is subsequently recognized by Dicer (Dcr1) and cleaved into small RNA duplexes. Additionally, dsRNA can occur without the activity of Rdrp in instances such as bi-directional transcription or inverted repeat hairpin formation. The non-Rdrp precursors are also recognized by Dicer and cleaved into small RNA duplexes. In each case the siRNA duplexes are loaded into Argonaute (Ago1) effectors, the passenger strand is discarded, and the guide strand is then used to recognize RNA targets. b | microRNAs (miRNAs) are produced from hairpin structures formed in the pri-miRNA transcript. In metazoans, such as humans, the hairpin structure is processed initially by the DROSHA/DGCR8 complex into the pre-miRNA, which is further processed by DICER1 into the miRNA duplex. In plants, the processing of pri-miRNA to pre-miRNA to miRNA duplex occurs through two successive cleavages by Dicer-like 1 (Dcl1). In both metazoans and plants, miRNA duplex is bound by an Argonaute effector protein (AGO), the passenger strand is discarded, and the guide strand can then direct the effector to complementary targets. c | PIWI-interacting RNAs (piRNAs) are produced in D. melanogaster and M. musculus from single-stranded RNA (ssRNA) transcripts from piRNA precursor loci and their complementary target loci. The piRNA precursor transcripts are initially processed by PIWI proteins and then enter the ping-pong amplification cycle in which the recognition of the ssRNA target transcript by the PIWI-piRNA effector complex generates secondary piRNAs complementary to the piRNA transcripts. These secondary piRNAs are bound by an Argonaute or PIWI effector protein and can again target the piRNA ssRNA precursor transcripts to produce additional piRNAs derived from the piRNA locus. d | A phylogenetic diagram of humans and selected model organisms displays the conservation of RNAi proteins, microRNAs, and centromere structure.
The evolutionary history of RNAi
RNAi is an ancient pathway that was present in the LECA. This ancestral RNAi pathway most likely had an Ago-like protein, an Ago-related but distinct Piwi-like protein, a Dicer-like protein and an RNA-dependent RNA polymerase (RdRP)11,14. These components and their active domains are highly conserved across eukaryotes (Fig. 1d) and even prokaryotes. For example, the two miRNA-processing enzymes DROSHA and DICER1 (Fig. 1b) possess RNase III domains that are found in bacteria and play important roles in rRNA processing in Escherichia coli15,16. Eukaryotic RdRP likely derived from a bacteriophage protein and may have been a catalytically inactive cofactor for a ribozyme polymerase17. Argonaute proteins are present in all domains of life, and the PIWI domain is always present, indicating the requirement of this domain for Argonaute function18. In prokaryotes, it has been proposed that Argonaute is part of a defence system against novel mobile elements19. Indeed, given the likely presence of both Ago-like and Piwi-like effector proteins, transcriptional and post-transcriptional repression of transposons and viruses are the proposed functions of the ancestral RNAi pathway11,14,18. Other RNAi pathway components such as the DROSHA–DGCR8 complex, also known as Microprocessor, have been considered to be exclusive to metazoans, although homologues have been identified in unicellular Ichthyosporea (also known as Mesomycetozoea), which is indicative of an earlier than previously predicted evolutionary origin20.
The lack of conservation of microRNAs, as well as some of the enzymes that are necessary to produce them in metazoans, is also strong evidence that the ancestral function of RNAi may have been genome defence rather than gene regulation. microRNAs have arisen independently in animals21, plants22, slime molds23, multiple lineages of algae24,25, fungi26 and Ichthyosporea20 (Fig. 1d), which suggests that the LECA did not have microRNAs and indicates strong convergent evolution on the microRNA-generating hairpin. The evolutionary origin of microRNA-generating sequences is unknown and is likely distinct in each acquisition, as it is between animals and plants27. Many microRNA-like structures evolved from transposons28 in a number of organisms, including mammals29,30 and plants31,32. In this evolutionary model of gene regulation by RNAi, the ancestral function of genome defence was adapted through molecular rearrangement of the transposon target sequences, resulting in a hairpin-like structure and the production microRNAs. Thus, though RNAi has evolved important gene regulatory functions in higher eukaryotes, the proposed ancestral function of genome defence has been conserved, and we explore other conserved functions that may predate the multiple innovations of microRNAs.
The targeting activity of RNAi proteins
The molecular mechanism of RNAi is dependent on the capacity of its protein components to recognize an RNA target. In animals, the microRNA production pathway begins when the two RNase III domain-containing proteins DROSHA and DICER1 cleave RNA in a structure-dependent manner (Fig. 1b). DROSHA recognizes the junction of the stem loop structure (that is, pri-miRNA) with the single-stranded RNA (ssRNA) base and cleaves approximately 11 base pairs further into the stem to produce the pre-miRNA33,34. In coordination with DROSHA, DGCR8 interacts with the double-stranded RNA (dsRNA) stem and apical junction to further stabilize the complex and allow for efficient processing34. DICER1 recognizes dsRNA structures in general, including the pre-miRNA hairpin, and preferentially cleaves approximately 21–22 base pairs from the 3’ terminus35–39.
Despite the clear reliance on secondary structure as the primary means of target recognition, these enzymes can have some primary sequence bias, which varies from one species to another35,40,41. The DROSHA–DGCR8 complex shows preferences for a UG motif at the base of the stem loop structure, a UGU motif in the apical loop, and a CNNC motif 16–18 base pairs from the cut site40. Although human DICER1 has less preference for sequence motifs than the Microprocessor complex, not all sequences are cleaved equally efficiently and factors such as the presence of a 2 nucleotide (nt) 3’ overhang, which is bound by the PAZ domain, can have a strong influence on human DICER1 processivity35,36,39. Additionally, the sequence content of the overhang can influence human DICER1 efficiency36. Recent work has identified specific sequence motifs targeted by individual Dicer homologues in the ciliate Paramecium tetraurelia41. These inherent preferences of these ciliate Dicers allow the homologues to precisely target sequences for removal in a process called DNA elimination41, as discussed below.
Many mature microRNA sequences have a terminal 5’ U or A bias. This bias has been attributed to the binding preferences of Argonaute proteins rather than a sequence motif preferentially cleaved by DICER142,43. In general, Argonaute proteins will load dsRNA and then discard one strand (passenger strand), resulting in the single-stranded (guide strand), mature small RNA–AGO protein complex44. However, there are indications in mammals and Drosophila melanogaster that ssRNA can also be loaded into Argonaute proteins45,46, and different Ago homologues seem to possess biases for short sequence motifs45,46. Additionally, recent evidence points to the wide variety of chemical modifications on RNA molecules (>100)47. One modification that has been shown to be functional in small RNAs is 2’O methylation, which stabilizes small RNAs by protecting the molecules from 3’ uridylation48. This modification occurs on microRNAs and siRNAs in plants49, piRNAs in Tetrahymena thermophila50, piRNAs in mammals51, and on piRNAs52,53, siRNAs53–57 and some microRNAs57,58 in D. melanogaster. In fact, in flies, the 2’O methylated siRNAs or microRNAs are associated with Ago2 rather than Ago153,57. Intriguingly, the majority of small RNAs that are 2’O methylated enter the nucleus and participate in small RNA-directed chromatin modification pathways. It will be interesting to determine whether any of the other identified RNA modifications play a role in processing, loading or stability of small RNAs.
In the context of generating microRNAs, DROSHA–DGCR8, DICER1 and AGO proteins principally recognize RNA secondary structure and, to a lesser extent, some primary sequence motifs in selecting their substrates. These qualities of RNAi proteins enable non-microRNA interactions that have a broad range of critical, conserved functions, as we discuss in the following sections.
The role of RNAi in centromere function
Since the discovery of RNAi and of the pathway’s conservation amongst higher eukaryotes, disruption or deletion of RNAi factors has been performed in a wide variety of eukaryotes. Deletion of Drosha–Dgcr8, Dicer or Argonaute homologues is embryonically lethal in many model organisms including mouse59–61, D. melanogaster62,63, Caenorhabditis elegans64,65, T. thermophila66, Arabidopsis thaliana67, maize68 and zebrafish69. The loss of microRNAs certainly plays a prominent part in this phenotype, as some knockout embryos progress through the early stages of development but fail to properly differentiate cell types, which is a well-defined microRNA function, particularly in mammals6,8. However, a closer examination of cellular phenotypes in response to the reduction or deletion of different RNAi proteins demonstrates that these factors have important roles in chromosome function that are unrelated to microRNA-driven gene repression in many eukaryotes.
RNAi and mitotic chromosome segregation in unicellular eukaryotes
S. pombe has single-copy homologues of RdRP, Dicer and Argonaute and their deletions — rdp1Δ, dcr1Δ and ago1Δ, respectively — yield strong chromosomal phenotypes70–73. In cycling cells, mutant strains display lagging chromosomes during anaphase and are sensitive to the microtubule poison thiabendazole70–73, indicating the presence of compromised mitotic structures. Dicer and Argonaute mutants are particularly poor at chromosome segregation during mitosis70–73 (Fig. 2a). Additionally, a missegregation phenotype is observed in the cell division that takes place during the transition from cycling cells to quiescent cells in G0 entry caused by nitrogen starvation74.
Figure 2 |. Chromosome segregation phenotypes of RNAi are highly conserved.
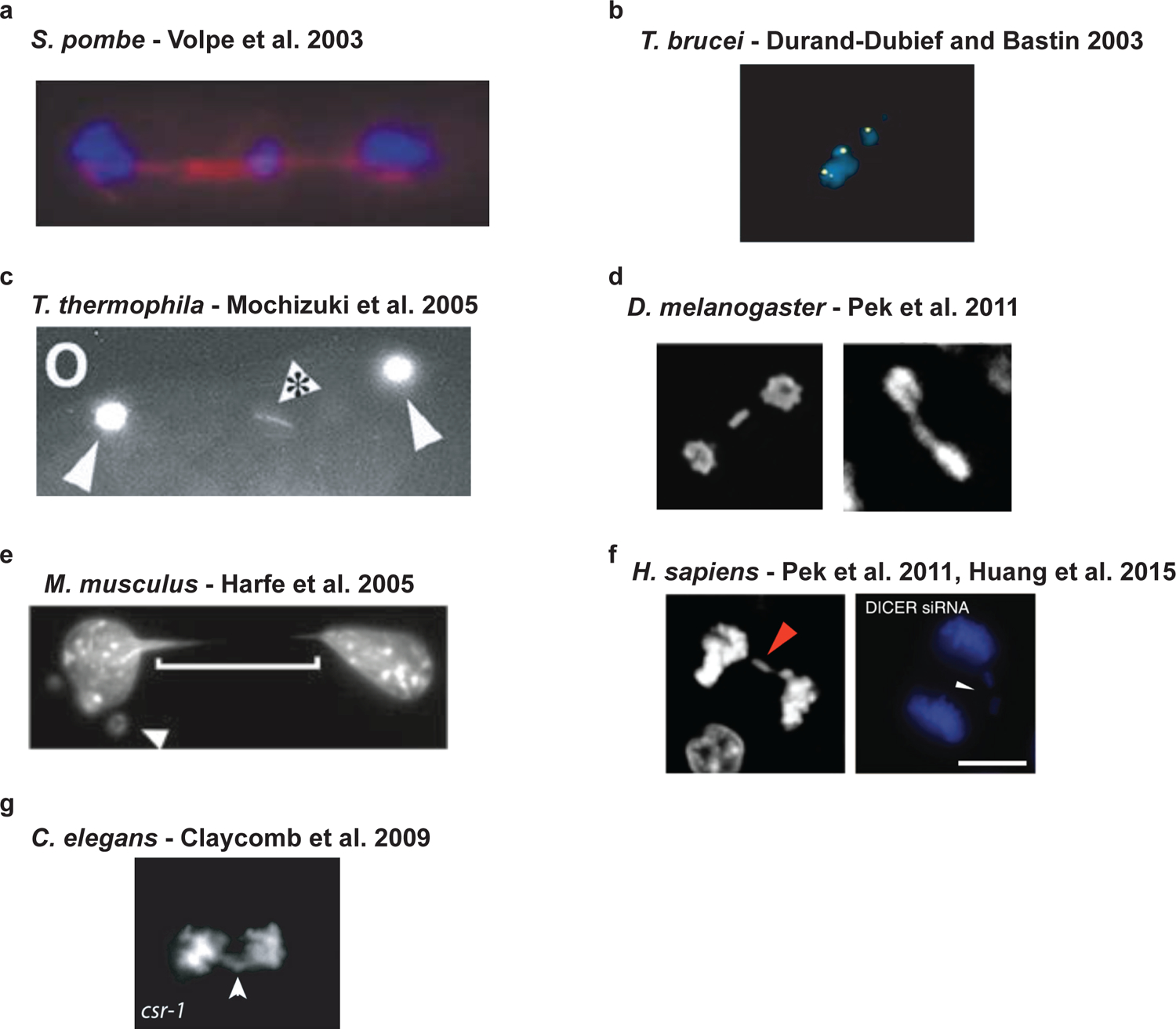
a| Deletion mutants of either the RdRP, Dicer (shown), or Argonaute homologues in S. pombe, result in lagging chromosomes in mitosis and meiosis. b| The deletion of an Argonaute homologue in T. brucei results in lagging chromosomes and segregation defects in mitosis. c| The deletion of a Dicer homologue in T. thermophila results in lagging chromosomes and bridging of genetic material in mitosis and meiosis. d| While the displayed images are of disruptions of belle, the knockout or knockdown of Dicer and Argonaute homologues in D. melanogaster leads to lagging chromosomes in the same cell types - wing disc cells and S2 cell culture. e| The deletion of Dicer1 in mouse fibroblasts results in bridging during mitosis and the formation of micronuclei most likely derived from missegregated chromosomes. f| In human RPE-1 cells, the knockdown of Dicer1 or Ago2 by siRNAs generated lagging chromosomes. In human HeLa cells, the knockdown of Dicer1 also generated lagging chromosomes. g| The knockdown of an Ago homologue (shown) in C. elegans or other factors in the CSR-1 22G-RNA pathway results in lagging chromosomes in the early worm embryo.
Fission yeast has regional centromeres with an inner centromeric region and outer pericentromeric regions flanking either side. This structure enables accurate specification of the centromeric region used for kinetochore attachment. The pericentromeric region provides a boundary between the inner centromere and the rest of the chromosome. In S. pombe, Dicer and Argonaute participate in nuclear co-transcriptional gene silencing at the pericentromeric regions70,71,75 (Fig. 3a). Bi-directional transcription by RNA polymerase II (Pol II) from the repeated elements of this region forms dsRNA that Dcr1 recognizes and cleaves into double-stranded siRNA precursors75,76. To further the reinforcement of this region as a target of siRNAs, Dcr1 and Ago1 interact with the RdRP Rdp1 to generate additional dsRNA and siRNAs from the transcripts of this region70,77,78 (Fig. 1a). Additionally, another species of siRNAs is produced from hairpin-like structured RNA in pericentromeric transcripts. This secondary structure can be recognized and cleaved by Dcr1 independently of the RdRP79. Finally, Dcr1-independent exosome degradation products can be directly loaded into Ago1 and trimmed by the Triman exonuclease to form primal small RNAs (priRNAs)80. Thus, there seem to be multiple distinct mechanisms for producing siRNAs from the pericentromere in fission yeast.
Figure 3 |. Faithful chromosome segregation is ensured by the recruitment and protection of centromeric cohesin.
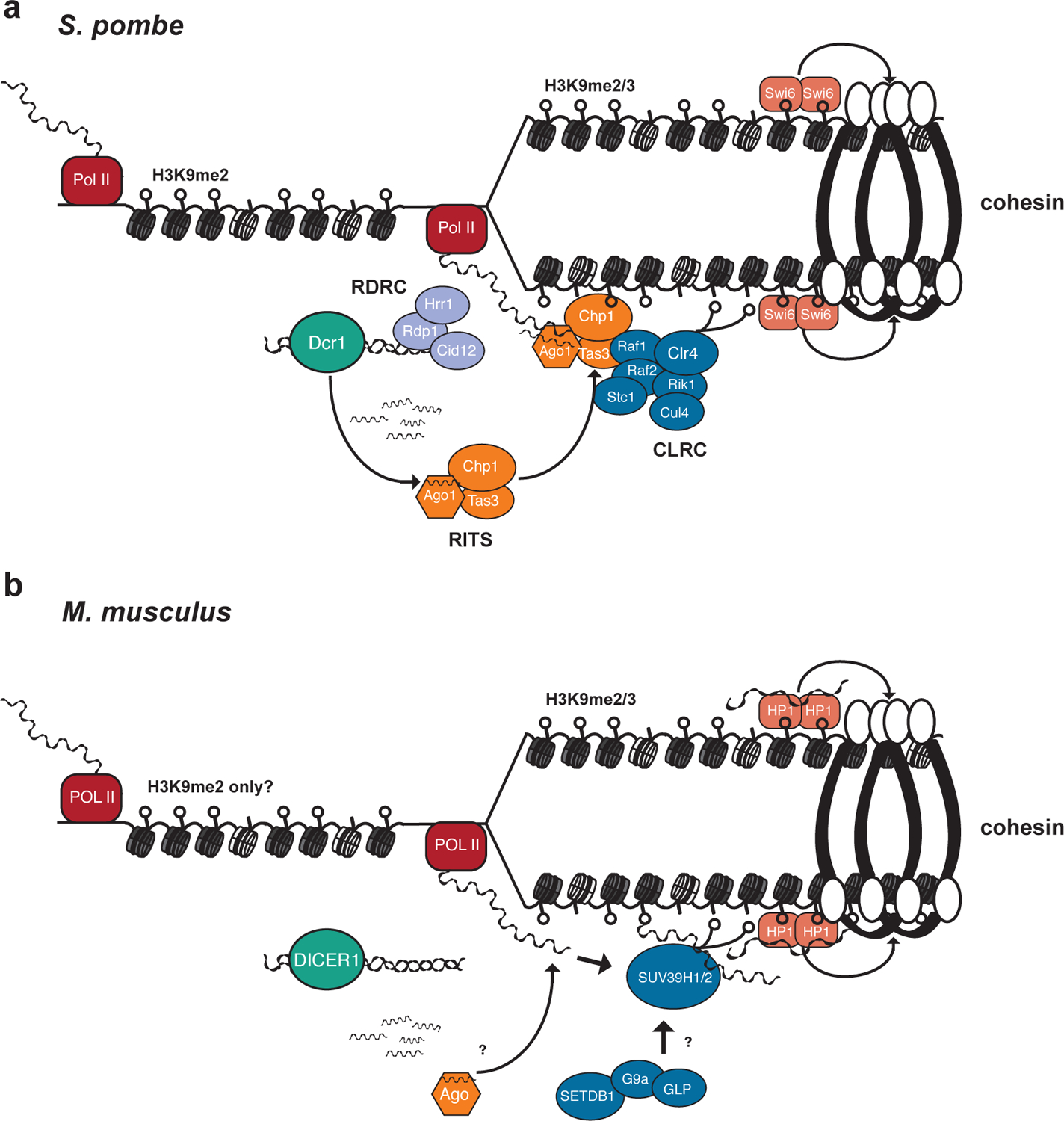
a| In S. pombe the RNAi pathway directs the heterochromatin machinery via small RNAs and the establishment of pericentromeric heterochromatin results in the recruitment and retention of centromeric cohesin during mitosis and meiosis and the proper segregation of chromosomes. The formation of pericentromeric heterochromatin begins with Pol II transcription of long non-coding RNAs (lncRNAs) from the dg/dh pericentromeric repeats. The RDRC complex (Rdp1, Hrr1, and Cid12) targets the lncRNAs for the synthesis of dsRNA. These dsRNAs are recognized and cleaved by Dcr1 into siRNAs. These small RNAs are loaded into Ago1 which then directs the RNA-induced transcriptional silencing complex (RITS – Ago1, Chp1, and Tas3) to the pericentromere through the targeting of nascent lncRNAs. Chp1 then recognizes H3K9me and stabilizes the complex at the site. The RITS complex recruits the Clr4-Rik1-Cul4 complex (CLRC – Clr4, Rik1, Cul4, Stc1, Raf2, Raf2), which furthers the spread of H3K9me heterochromatin through the activity of the methyltransferase Clr4. The homologue of heterochromatin protein 1 (HP1) Swi6 binds to H3K9 methylation and reinforces the heterochromatin state. Additionally, it directly recruits the cohesin complex that holds sister chromatids together until anaphase during mitosis. Thus, the combined activity of RNAi and the CLRC complex generate a chromatin state at the pericentromere that results in the proper segregation of chromosomes during cell division. b| In M. musculus and other mammals, such as humans, the RNAi pathway ensures the proper segregation of chromosomes presumably through the formation of heterochromatin at the centromeric region. RNA Pol II transcribes the major satellite pericentromeric repeats into lncRNAs in a bi-directional fashion. The resulting dsRNAs are recognized by DICER1 and possibly cleaved into a small RNA population. There is some evidence to suggest that AGO2 may play a role potentially by loading satellite small RNAs, but the effect this has on the chromatin in the region is unknown. Rather the regulation of the major satellite lncRNAs by DICER1 may ensure the appropriate recruitment of recruiting chromatin machinery to the region. The H3K9 methyltransferases SUV39H1 and SUVH39H2 can be recruited directly by major satellite lncRNAs. The H3K9 methylation and major satellite lncRNAs are recognized by HP1, which reinforces the heterochromatic state of the pericentromere. HP1 may play a role in recruiting and/or maintaining cohesin at the centromere to ensure proper separation of sister chromatids, though this function is primarily performed by SGO1 in mammals.
The resulting siRNAs are loaded into Ago1 and direct the effector complex, the RNAi-induced transcriptional silencing complex (RITS), to the region. This complex indirectly recruits the histone 3 lysine 9 (H3K9) methyltransferase Clr4 to the region to deposit and spread the methylation mark H3K9me81. The methylation can be recognized by several proteins, including Clr4 itself82, that subsequently induce a state of constitutively repressed heterochromatin. One of these proteins is Swi6, a homologue of the conserved heterochromatin protein 1 (HP1)83. This factor recruits the cohesin complex to centromeric heterochromatin for proper cohesion of sister chromatids and faithful segregation of chromosomes during mitosis84,85. Furthermore, Clr4, Dcr1 and Swi6 facilitate the deposition of the centromere-specific histone variant Cnp1 (CENP-A in mammals) in the inner core of the centromere that defines the location of kinetochore attachment86. Loss of the cohesin subunits Rad21 or Psc3, Swi6, Dcr1, Rdp1 or Ago1 leads to lagging chromosomes and segregation defects during anaphase in fission yeast70–73,75,84,85. This cascade of events beginning with Dcr1 recognizing dsRNA of pericentromeric transcripts has an important role in the process of mitosis and chromosome segregation. Interestingly, experimental evolution experiments in other fungi have recently demonstrated a strong correlation between RNAi and the maintenance of a large repetitive regional centromere87. This correlation is also seen in the presence of point centromeres and the loss of RNAi in Saccharomyces cerevisiae and it may be that the presence of RNAi itself maintains the physical structure of the centromere at the genetic and epigenetic level.
The unicellular eukaryote Trypanosoma brucei possesses an RNAi pathway composed of Dicer and Argonaute homologues. The deletion of TbAgo1 in trypanosome cells slows proliferation and leads to strong mitotic defects that include chromosome missegregation and whole-genome duplication following failed mitosis88 (Fig. 2b). The trypanosome centromere seems to be regional and of similar size and AT-rich composition as that of higher eukaryotes, but the pattern of loading of a CENP-A homologue has yet to be determined. Furthermore, the centromeric regions are flanked by regions of repetitive DNA derived from transposons89. This structure resembles the content of the heterochromatic pericentromeric regions seen in many other eukaryotes. The ciliated protozoa Tetrahymena also possesses a fully functional RNAi system and centromeres that have a regional structure90. These RNAi homologues have a number of different functions. Tetrahymena has a macronucleus that is polyploid (~45n) and transcribed in addition to a micronucleus that is diploid and devoid of transcription in cycling cells91. During cell division, the micronucleus, which possesses centromeres, divides mitotically, whereas the macronucleus, which does not have centromeres, divides amitotically, with random distribution of genetic material. Although the deletion of the Dicer homologue Dicer-like protein 1 (Dcl1) is viable, it leads to smaller micronuclei and chromosome lagging and bridging (a continuous piece of DNA between the two segregating chromosome masses in anaphase) during mitosis66 (Fig. 2c). These mutants are also more susceptible to microtubule poisons, as dcr1Δ strains are in S. pombe, indicating defects in chromosome segregation. Thus, in unicellular eukaryotes, such as fission yeast, trypanosomes and Tetrahymena, RNAi plays important roles in chromosome function and segregation.
RNAi and chromosome segregation in higher eukaryotes
Participation of RNAi proteins in chromosome functions such as segregation is conserved in higher eukaryotes, though the evidence for small RNAs or heterochromatin formation in this mechanism remains controversial (Fig. 2). Invertebrate genomes frequently have multiple copies of RNAi genes that have diverged in sequence and function, with one set dedicated to producing microRNAs and the other dedicated to producing endogenous siRNAs (endo-siRNAs) for the purposes of transposon or viral targeting and heterochromatin formation62,92. In D. melanogaster, which also has regional centromeres, the deletion mutants of the endo-siRNA-producing proteins Dcr-2 and Ago2 have a lagging chromosome phenotype in wing disc cells93. S2 cultured cells treated with siRNAs that target Dcr-2 and Ago2 also display the lagging chromosome phenotype93 (Fig. 2d). Furthermore, deletion of Ago2 in fly embryos results in heterochromatin defects as well as mitotic and chromosomal disruptions94. In one accession of the model plant Arabidopsis thaliana, mutants in AGO4 have been reported to have lagging chromosomes and bridging in mitosis95, although this phenotype has not been reported in other studies.
In vertebrate and particularly mammalian cells, the role of RNAi in chromosome segregation is more controversial. The chicken DT40 cell line harbours a human chromosome 21, and conditional deletion of the chicken Dicer homologue results in missegregation of the human chromosome, along with disruption of the centromeric heterochromatin and reduced enrichment of HP1 to heterochromatic loci96. These hybrid Dicer knockout cells accumulate long RNAs from the human pericentromeric α-satellite repeats, but they do not produce an abundant small RNA population in the presence of Dicer. This finding may be due to the fact that the small RNA machinery in this cell line is chicken-derived, and there is no RdRP present. Mouse fibroblasts prepared from Dicer1 knockout embryos display high incidences of both bridging chromosomes in anaphase and micronuclei, most likely formed from lagging chromosomes failing to enter the new nucleus97 (Fig. 2e). Mutation of Dicer1 in mouse embryonic stem cells (mESCs) yields a strong increase in the RNA transcribed from the major and minor satellite sequences, which occupy the pericentromeric and centromeric regions, respectively, accompanied by a reduction in small RNA from these regions98. It is important to note that this population was in the 25–30 nucleotide size range and unlikely to be DICER1-dependent. However, these deletion cell lines exhibit either no change99 or a decrease98 in H3K9me3 at the pericentromere, without evidence of chromosomal segregation defects. In NIH-3T3 mouse fibroblasts, DICER1 binds directly to major satellite RNA in concert with WDHD1 (WD repeat and HMG-box DNA binding protein 1) and localizes to the pericentromere, as detected by chromatin immunoprecipitation (ChIP)100. The association of nuclear DICER1 with satellite loci in human HEK293 cells is also detectable by ChIP101. In human RPE-1 cells, the knockdown of DICER1 or AGO2 induces lagging chromosomes, an increase in centromeric α-satellite transcripts and mislocalization of a kinetochore-recruiting protein, CENPC1102 (Fig. 2f). Furthermore, the knockdown of Dicer1 in HeLa cells also induces lagging chromosomes and a disruption of the localization of a component of the condensin complex93 (Fig. 2f). These phenotypes are not seen in Dgcr8 knockout cell lines, which indicates that this phenotype is not due to the loss of canonical microRNAs102.
The consistent increase in centromeric satellite transcripts upon loss of DICER1 and the localization of DICER1 to centromeric satellite loci in both mouse and human cells suggests that DICER1 binds to either secondary structures in these nascent RNAs or to dsRNA structures arising at the locus from bi-directional transcription. Indeed, both the major and minor satellite transcripts in mouse exist preferentially as dsRNA103. In mammals, the long non-coding centromeric and pericentromeric RNAs have a pronounced role in centromere formation, as they are integral parts of the pericentromeric and centromeric heterochromatin106–109. The RNAs can interact with a number of heterochromatin-forming and centromere-specific factors such as the H3K9 methyltransferases SUV39H1 and SUV39H2104–106, HP1107,108, CENPA109,110, the CENPA chaperone protein HJURP109, and AURORA B111–113, which is necessary for additional epigenetic marks identifying the centromere. Misregulation of satellite RNA levels in mammals can lead to lagging chromosomes and segregation defects111,113,114. Similarly, Dicer1 knockdowns or knockouts can lead to an accumulation of the same satellite RNAs, such as the minor satellite RNA in mouse, but a causal link has not been established98.
A model built around the regulation of long non-coding satellite RNA transcripts or transcription by DICER1, rather than the loading and targeting by small RNAs, could explain the appearance of lagging chromosomes and segregation defects in mammalian Dicer1 knockouts (Fig. 3b). Whether the accumulation of long non-coding satellite RNAs in Dicer1 mutants leads to an alteration of centromeric chromatin by recruiting the previously mentioned factors remains to be seen. Furthermore, in mammals there is conflicting evidence concerning the role of the SUV39-deposited H3K9me3-HP1 complex in recruiting or maintaining centromeric cohesin, which ensures proper chromosome segregation. While some groups report experimental evidence to suggest a role for H3K9me or H4K20me marks at pericentromeric heterochromatin in centromeric cohesin function in mouse embryonic fibroblast115, human HeLa cells116,117, and mESCs118, others have reported there to be no change in centromeric cohesin following disruption of heterochromatin factors in MEFs119 or HeLa cells120. It may be that these cell types differ in other heterochromatin features such as DNA methylation. There is also conflicting evidence of HP1 proteins interacting with the primary centromeric cohesin maintenance factor shugoshin (SGO1) in mammalian cells with one group reporting a necessary interaction121 and other groups showing that this interaction is dispensable117,122.
Small RNAs, whether satellite-derived or not, may participate in heterochromatin formation and/or chromosome segregation in mammals, but evidence is scarce. The fact that a lagging chromosome phenotype is seen in Ago2 knockdown in one cell line does suggest that small RNAs play some targeting role102. However, as mammals lack an RdRP-like enzyme to amplify small RNAs from their precursor transcripts, they do not possess all of the machinery of the co-transcriptional gene silencing mechanism present in S. pombe and, therefore, formation, spreading and maintenance of heterochromatin at the pericentromere might occur through different mechanisms. The absence of RdRP might be compensated by an increase in bi-directional transcription and/or by additional hairpin-like structures in the major satellite transcripts. It is important to note that, in humans, a complex involving hTERT (telomerase reverse transcriptase), which is an enzyme that maintains telomere length, exhibits RdRP-like function123. Indeed, this complex can produce siRNAs in a Dicer-independent fashion that contribute to heterochromatin formation at centromeres124. However, the role of the long non-coding centromeric transcripts in heterochromatin formation appears to be far more pronounced in mammals as detailed above. Additionally, there is evidence in other eukaryotes, such as maize, for long non-coding centromeric transcripts associating with centromeric chromatin125.
The chromosomes of C. elegans do not have regional centromeres, but instead are holocentric. Holocentric chromosomes have multiple regions throughout the chromosome that are specified as centromeres and serve as points of kinetochore attachment during cell division. Despite this fundamental difference, much of the machinery in centromere specification and kinetochore attachment seems to be conserved126, and RNAi also has an essential role in chromosome segregation. C. elegans DRH-3, a Dicer associated RNA-helicase, has a chromosome segregation phenotype when knocked down in embryos127–129. The worm DCR-1 also participates in proper gamete formation; deletion of DCR-1 results in misshapen oocytes that have high DNA content caused by failure to divide following replication130. This phenotype, however, seems to be due to loss of DCR-1 in the soma rather than in the germline131. The Argonaute family member CSR-1 has important functions in chromosome segregation and cell division, as both knockdown of CSR-1 and deletion mutants show disrupted chromosome segregation in the early embryo65,129 (Fig. 2g). In C. elegans, CSR-1 and DRH-3 are part of a pathway that includes an RdRP (EGO-1) and a Tudor-domain protein called EKL-1. Knockdown of any of these factors induces lagging chromosomes in the early embryo129. Additionally, the CENP-A centromere specific histone homologue HCP-3, components of both Condensin I and Condensin II, as well as two cohesin subunits, SCC-1 and SCC-3, all show a disrupted localization in embryos when these factors are knocked down129. The molecular mechanism for ensuring proper segregation of chromosomes is dependent on the production of 22 nt small RNAs (22G-RNAs) that are ultimately loaded into CSR-1. The 22G-RNAs target a specific subset of genes, but do not lead to the repression of these genes at the RNA or protein level. Instead, these small RNAs guide CSR-1 to the chromatin associated with this subset of protein-coding genes. These genes are widely distributed throughout all chromosomes, except for the X chromosome. Interestingly, the X chromosome is frequently missegregated in meiosis and is the only chromosome whose loss is tolerated by the worm129.
The CSR-1–22G-RNA pathway appears to act at the chromatin level, though how much it resembles the co-transcriptional gene silencing pathway in S. pombe remains to be described. One idea is that the targeting of a subset of protein-coding loci dispersed across chromosomes at the chromatin level may be analogous to targeting the pericentromeric repeats in fission yeast. Furthermore, given the holocentric nature of C. elegans chromosomes and the need to define multiple centromeres along the length of the chromosomes and the missegregation phenotypes observed in mutants, CSR-1 targeting has been proposed to play a role in centromere formation. Supporting this notion, it has been shown that the CSR-1 22G-RNA targets are inversely correlated with C. elegans HCP-3 occupancy, raising the possibility that this targeting could limit the spread of HCP-3 by establishing chromatin borders132,133. The role of CSR-1 targeting seems to be maintenance of Pol II sense transcription of the germline target loci in cis. The loss of CSR-1 activity results in a global decrease in sense target loci transcription, an increase in antisense target loci transcription and a concomitant increase in aberrant transcription from normally transcriptionally-silent regions, such as HCP-3-enriched regions133. This aberrant transcription may interfere with HCP-3 deposition along the chromosomes, but the general distribution appears to be maintained in csr-1 mutants134. Thus, CSR-1 and 22G-RNAs seem to exert general effects on the transcriptome and the directionality of transcription from the genome of the early worm embryo at the chromatin level. Whether the CSR-1–22G-RNA pathway participates in the chromatin organization of mitotic chromosomes remains to be seen, but the requirement for this pathway for the proper distribution of H3K9me2 on meiotic chromosomes135 is intriguing. However, the role of the CSR-1/22G-RNA pathway in chromatin modification that promotes chromosome segregation in the early worm embryo is complicated by an indirect genic regulation of KLP-7, which is a microtubule depolymerase that, when misregulated, generates a portion of the chromosome missegregation observed in mutants in this pathway134.
The role of RNAi in chromosome segregation is widely conserved in unicellular eukaryotes, plants and animals. However, the full small RNA-generating pathway does not always seem to be necessary for this function. The conservation of this role argues that it may have been an important ancestral function of RNAi. The only molecular conservation that is necessary to maintain this function in all of these organisms is Dicer’s recognition of a dsRNA structure that derives from the DNA sequence of the pericentromere or centromere-adjacent regions. This function of Dicer in coordination with small RNAs and/or other proteins can alter chromatin at the region and ultimately assist in defining the true centromeric locus. The establishment of pericentromeric heterochromatin and the proper specification of the centromere then ensures the inheritance of the genetic material on that chromosome.
RNAi and the segregation of meiotic chromosomes
In addition to ensuring the transmission of genetic material during mitosis, this function of RNAi also extends to the transmission of genetic material from the parental generation to progeny. RNAi mutants in S. pombe, such as dcr1Δ, rdp1Δ and ago1Δ, show missegregation of chromosomes in the second division of meiosis73. This observation is again likely due to malformation of heterochromatin at the centromere and disruption of cohesins. An additional meiotic phenotype of RNAi mutants is increased recombination in the centromere region136, which is again mirrored in chromatin mutants, but it is not known if this contributes to missegregation. This mirroring of the mitotic chromosome missegregation phenotype in meiosis is a phenomenon seen in many of the organisms already discussed. In Tetrahymena, Dicer homologue DCL1 knockouts exhibit disrupted chromosome condensation and missegregation of the micronucleus chromosomes during meiotic divisions66. The C. elegans CSR-1 small RNA pathway, which is required for mitotic chromosome segregation in the early embryo, is also needed for meiotic chromosome segregation. The germlines of mutants in this pathway show malformed nuclei with abnormal DNA content127,135,137. A hermaphrodite mating population displays a sex skewing to XO males as a result of the spontaneous loss of the X chromosome, which strongly implicates chromosome missegregation phenotypes in meiosis of CSR-1 mutants129. Furthermore, male DCR-1 mutants have defects in sperm function and X chromosome segregation138. The participation of the same pathway in both mitosis and meiosis in C. elegans suggests a similar small RNA-directed mechanism may underlie the role of RNAi in ensuring chromosome segregation in cell division.
RNAi components have important roles in meiotic progression in model plants such as Arabidopsis, rice and maize. The loss-of-function mutant of the rice Argonaute gene MEIOSIS ARRESTED AT LEPTOTENE 1 (MEL1) disrupts chromosome condensation and loses a class of 21 nucleotide small RNAs called phased siRNAs (phasiRNAs), which are produced from specific loci – PHAS loci – and require a microRNA trigger for biogenesis. The MEL1 mutant is male sterile, but does not show major disruption of the H3K9 methylation of the pericentromeres, as determined by immunocytology139–141. In maize, an Argonaute homologue also participates in chromosome segregation during male meiosis. Mutant alleles of AGO104 show disruption of chromosome condensation, chromosome segregation and spindle formation. Early in meiosis I these mutants have abnormal chromosome condensation and spindle defects, whereas later in meiosis multinucleated progeny cells and micronuclei are present142. Interestingly, AGO104 seems to be closely related to both Arabidopsis AGO9 and AGO4. As previously discussed, ago4 is the Argonaute mutant reported to have both mitotic and meiotic chromosome segregation defects95, whereas ago9 mutants have diploid female gametes, although most likely due to apospory rather than defects in meiosis143. In the maize AGO104 mutant, the centromeric and pericentromeric regions are hypomethylated at the DNA level in a non-CG context, which indicates a disruption of the chromatin in this region142. This leads to an increase in transcription of the repetitive elements present at the centromere that AGO104 normally silences. The fact that chromatin disruption at the centromere in these Argonaute mutants leads to chromosomal defects again resembles the consequences of losing co-transcriptional gene silencing in S. pombe.
The role of RNAi in regulating dosage
Condensation, alignment and segregation are important chromosome-level functions that RNAi factors clearly play major parts in during cell division. However, chromosomes must also perform the crucial task of supplying the cell with the appropriate dosage of the genic products they encode. Dosage can be determined either at the RNA level by regulating transcripts, the chromatin level by regulating modification, or the DNA level by regulating copy number.
Controlling dosage at the transcript level
RNAi plays a significant role in determining the transcriptional output of loci, including those subject to gene dosage, even at the level of an entire chromosome. Polyploidy is common in plants, and it is important to prevent the formation of deleterious polyploids that arise from the union of a tetraploid-derived diploid gamete with a normal haploid gamete. In Arabidopsis, small RNAs are involved in sensing this imbalance in genome dosage. This mechanism begins when a highly conserved microRNA, miR845, targets the tRNAMet primer binding site (PBS) of long-terminal repeat (LTR) retrotransposons in the pollen. This targeting induces 21–22 nt secondary small RNA production by RNA polymerase IV. This class of RNAs is referred to as epigenetically activated small interfering RNA (easiRNA), and their increased abundance in diploid pollen mediates the response to a genomic imbalance32,144. In the presence of easiRNAs, triploid seeds abort due to gene misregulation (triploid block). The misregulation seems to take place at the level of genes with a parent-of-origin imprinting expression pattern in the endosperm145. It is not currently known exactly how easiRNAs generate this misregulation. Interestingly, miR845 is derived from the truncation and inversion of a transposon-derived 5’ LTR containing a PBS site32. Other factors required for the triploid block include the histone H3K9 methyltransferases in plants146, which suggests that transcriptional regulation is involved in this process. Thus, RNAi is important for sensing the copy number of the genome after meiosis.
A number of sexually reproducing organisms have evolved to use RNAi for a process called meiotic silencing of unpaired DNA (MSUD) or meiotic silencing of unpaired chromatin (MSUC). This phenomenon was first described in the filamentous fungus Neurospora crassa in which the unpaired state of a gene causes it to be repressed during meiosis, typically detected via ascospore colour and shape. The RdRP encoded by SAD-1 is required for MSUD147, as are the Dicer homologue DCL-1 and an Argonaute homologue SMS-2, all of which generate small RNAs corresponding to unpaired regions148–150 (Fig. 4a). Homologues of S. pombe helicases Hrr1 and Rad54 are also required for MSUD151,152 and for RNAi-mediated silencing in fission yeast78,153. Although little is known about the recognition mechanism that identifies unpaired DNA with remarkable accuracy, the downstream effects clearly employ RNAi to target and silence the detected unpaired regions. Once an unpaired region is specified, it is hypothesized that it is transcribed into an aberrant RNA. This RNA specifying the unpaired region is most likely targeted by SAD-1 for the formation of dsRNA, which is then used as the precursor for the generation of the MSUD-associated small interfering RNAs (masiRNAs) by DCL-1 and other associated factors154. SMS-2 will then load the masiRNAs and target any unpaired transcripts. The mechanism of repression seems to be post-transcriptional, as SMS-2 has been shown to be exclusively perinuclear and cytoplasmic155. When an additional copy of a gene is present, but unpaired, all transcripts from all three copies of this gene are silenced, presumably by small RNA silencing. Thus, although MSUD may be a mechanism to control excessive gene dosage resulting from deleterious chromosomal rearrangements, it has also been proposed that this process represses transposons and meiotic drive elements to maintain genomic integrity147,156,157.
Figure 4 |. RNAi regulates dosage at the transcript, chromatin, and DNA levels.
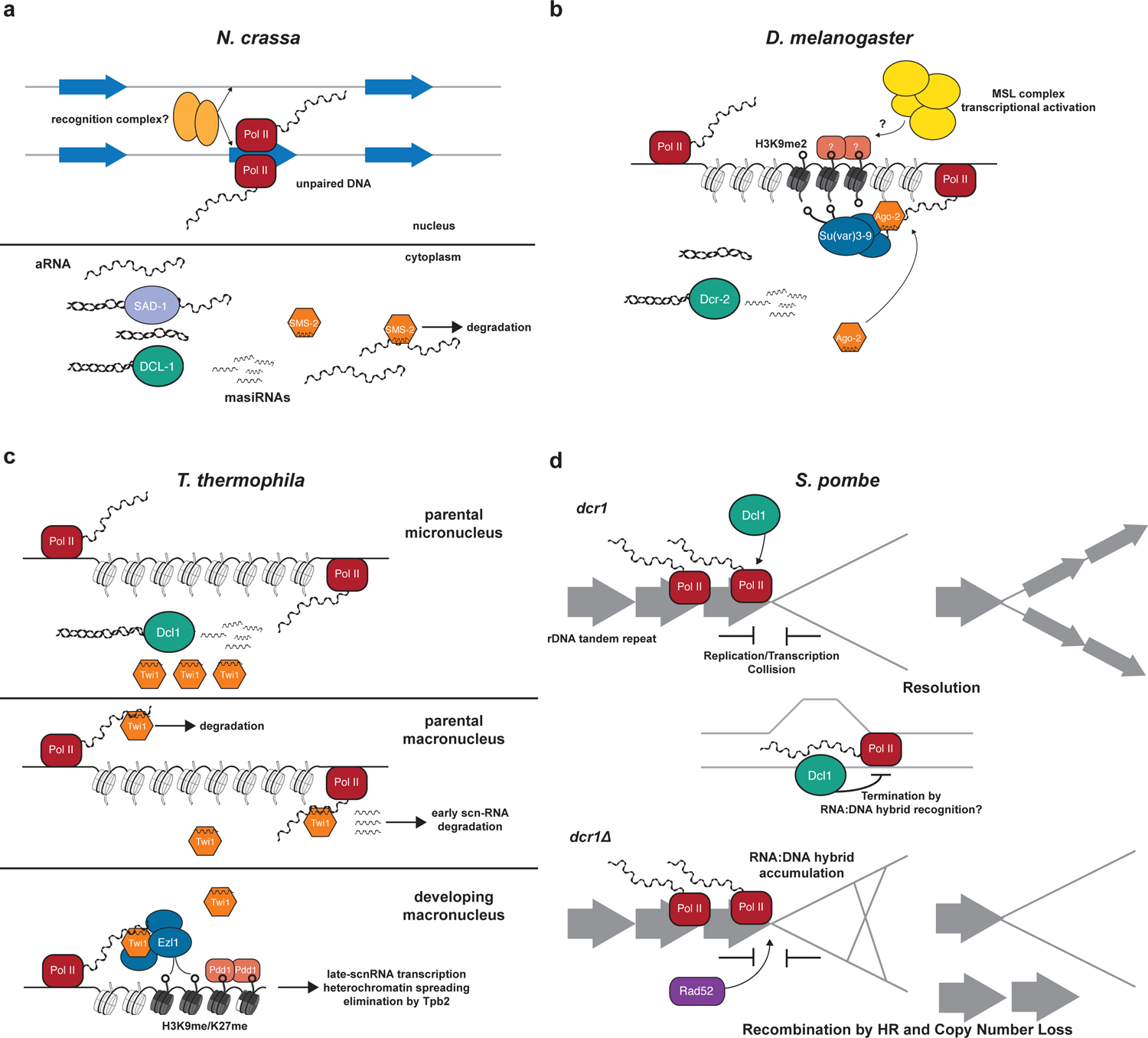
a| Meiotic silencing of unpaired DNA (MSUD) is an RNAi-based mechanism that identifies and silences transcription products of unpaired loci at the transcript level. During the pairing of homologous chromosomes in meiosis an unidentified recognition complex identifies regions that are unpaired. This stimulates bi-directional transcription by RNA Pol II of aberrant RNAs. The aberrant RNAs are exported to the out of the nucleus and then used as a template by RdRP homologue SAD-1 to generate dsRNA. A Dicer homolog, DCL-1, then targets the dsRNA and generates siRNAs called MSUD-associated small interfering RNAs or (masiRNAs). The Argonaute homologue SMS-2 loads the masiRNAs and targets any transcript from the unpaired locus for degradation. b| The X chromosome dosage compensation mechanism in Drosophila uses endo-siRNAs targeting the 1.688X satellite repeat to direct chromatin modifications that contribute to increasing transcription from the male X chromosome to match the dosage of the two X chromosomes in female flies. The mechanism most likely begins with RNA Pol II bi-directionally transcribing the 1.688X loci. The resulting dsRNA is recognized by Dcr-2, which generates 1.688X endo-siRNAs. Ago2 loads the endo-siRNAs and directs the H3K9 methyltransferase Su(var)3–9 to the 1.688X loci on the X chromosome. The presence of this mark may recruit the Male Specific Lethal (MSL) complex to the surrounding region, which in turn increases transcription from the X chromosome. c| The meiotic process of DNA elimination relies on the direction of heterochromatin formation by RNAi to identify sequences to be removed from the new macronucleus. The bi-directional transcription of the parental micronuclear genome, most likely by RNA Pol II, generates dsRNA that are processed by the Dicer homologue Dcl1 into early scan-RNAs (scn-RNAs). The PIWI-like effector Twi1 binds the early scnRNAs and translocates to the parental macronucleus where the effector protein and small RNA search for complementary sequences on nascent transcripts. If a Twi1/scn-RNA complex finds a complementary transcript the scn-RNA is degraded. If a target is not found the Twi1/scn-RNA complex translocates to the developing macronucleus and again searches for complementary nascent transcripts. When a target sequence is identified, Twi1 recruits the H3K9 and H3K27 methyltransferase Ezl1 to the region. The recognition of H3K9me/H3K27me by the HP1 homologue Pdd1 strengthens the chromatin state of the region by inducing the transcription of late-scnRNAs which then feedback on the region to spread heterochromatin. This leads to the excision of this DNA sequence by Tpb2. d| The copy number of the ribosomal DNA tandem array loci is maintained by Dcr1 in S. pombe through the resolution of replication/transcription collisions. In the presence of Dcr1 (top panel) the collision of a replication fork with RNA Pol II can be resolved through the action of Dcr1 in Pol II transcription termination and importantly neither the catalytic activity Dcr1 nor small RNAs are required for this function. Rather it may be an RNA:DNA hybrid that stimulates this function of Dcr1. In the absence of Dcr1 (bottom panel), Rad52 and RNA:DNA hybrids accumulate at sites of unresolved replication stress. This leads to repair by homologous recombination and as a consequence a loss of rDNA copy number.
MSUC occurs in other organisms that need to silence unpaired sex chromosomes in male meiosis. In C. elegans, the aforementioned CSR-1–22G-RNA small RNA pathway clearly acts to silence the unpaired male X chromosome. Loss of CSR-1 or other associated factors leads to a global reduction of H3K9me2 on the male X chromosome, concomitant with an increase in H3K9me2 on the autosomes135. Interestingly, further analysis of CSR-1–22G-RNA targets indicated that the X chromosome is relatively devoid of small RNA targets129. There may be other X chromosome-specific factors that regulate the deposition of H3K9me2 in concert with CSR-1–22G-RNA targeting. The loss of this targeting would then generate additional free H3K9 methylation machinery that acts on the autosomes. In any case, RNAi seems to direct H3K9 methylation in this context as in S. pombe. Additionally, in mouse, AGO4 might direct small RNAs for the purpose of MSUC in early prophase of meiosis158.
In somatic cells of animals with an XY sex chromosome pair, one of the two sexes needs to alter the gene expression from its X chromosome to generate the correct dosage of X-linked genes. The solution is to either increase dosage from the X chromosome in males to match the levels of XX females, or silence one of the X chromosomes in females to reduce dosage to that of a single X. Sex chromosome dosage compensation in Drosophila employs the former strategy by upregulating genes on the X chromosome in males. A protein complex called Male Specific Lethal (MSL) works in concert with two long non-coding RNAs, roX1 and roX2, to achieve this compensation159. The long non-coding RNAs are required for proper localization of the MSL complex as deletion of both roX1 and roX2 will lead to mislocalization of the complex to autosomal loci160. In addition, RNAi has an integral role in localizing the complex to the X chromosome (Fig. 4b). Mutations in Dcr-2 and Ago2 display synthetic lethality with roX1– roX2 double mutants, indicating that siRNAs contribute to X chromosome recognition161. These siRNAs are derived from the 1.688X satellite repeat, which is highly enriched on the X chromosome162. Expression of a hairpin from this satellite repeat is sufficient to generate siRNAs and substantially suppresses the male lethality phenotype in roX1–roX2 mutants. Ago2 is enriched at the 1.688X satellite repeat loci and recruits Clr4 H3K9 methyltransferase homologue Su(var)3–9 to deposit H3K9me2163. This chromatin mark, although generally considered a repressive mark, may direct the MSL complex to the X chromosome to increase expression. The dosage compensation mechanism in Drosophila seems to be an adaptation of the RITS complex for the purpose of transcriptional activation rather than silencing. The reversal of the output occurs downstream of the histone methyltransferase and may be due to a direct or indirect interaction with the MSL complex. The ability of RITS-like complexes to effectively identify specific DNA loci makes it highly useful in processes related to chromosome function.
The proclivity of RNAi to recognize transposon sequences, which is the proposed ancestral function of the pathway, can of course serve to protect the integrity of the genome from these potential invaders, but this function has clearly been adapted for other purposes. The preceding instances exemplify the ways in which eukaryotic genomes, through the capabilities of the RNAi pathway, have co-opted transposon and other repetitive sequences for broad chromosomal functions such as the recognition of interspecies or interploidy hybridization events and regulation of chromosome dosage.
DNA elimination – controlling chromosome content and function through RNAi
Tetrahymena and other ciliates possess both a polyploid macronucleus that is transcriptionally active and a diploid micronucleus that remains transcriptionally silent until meiosis. During conjugation, which is the sexual reproduction phase of Tetrahymena, meiosis takes place but only the micronucleus divides to form two haploid pronuclei. After fertilization, the diploid zygote unites the two pronuclei, divides twice to produce a new macronucleus and a new micronucleus, and then eliminates the remaining parental macronucleus. During the formation of the new macronucleus, a process called DNA elimination takes place. This process involves a small RNA-mediated mechanism of the removal of internal DNA segments or internal eliminated sequences (IESs)164 (Fig. 4c).
DNA elimination begins in the meiotic prophase when the micronucleus is transcribed bi-directionally. DCL-1 recognizes the dsRNA products of this transcription and cleaves them to produce ‘scan RNAs’ (scnRNAs)66. The Tetrahymena Argonaute homologue TWI1 then loads the scnRNAs for target recognition165. The primed TWI1 enters the parental macronucleus searching for complementary target transcripts. This initial scnRNA targeting is a method of negative selection. Any scnRNAs that find target transcripts in the macronucleus are degraded. This degradation prevents subsequent targeting of potentially essential transcripts and facilitates the inclusion of these transcript loci in the new macronucleus. The remaining scnRNAs that have no target transcripts in the parental macronucleus translocate to the new macronucleus166. TWI1, loaded with these pre-selected scnRNAs, targets nascent transcripts and recruits the histone methyltransferase EZL1. scnRNAs can also target IESs containing similar sequences in trans167. This in trans targeting confers redundancy and robustness to the scnRNA network. The recruitment of EZL1 nucleates the formation of heterochromatin through the addition of H3K9me and H3K27me168. An HP1 homologue, PDD1, recognizes these marks and binds to these regions169–171. This step marks the nucleation of heterochromatin in these regions. This state is further reinforced by additional transcription and small RNA production specifically from the regions flanking these newly marked heterochromatic regions. TWI1 loads these new small RNAs, termed late-scnRNAs, and reinitiates the process so that the heterochromatic state spreads over the intended IES172. Exactly how the boundaries of this region are maintained is yet to be described. A domesticated piggyBac transposase, TPB2, recognizes the heterochromatic regions in the new macronucleus and removes them from the new genome173,174. Similar RNAi and heterochromatin driven mechanisms of DNA elimination take place in other ciliates. In Paramecium tetraurelia, the DNA elimination process is entirely dependent on a very similar scnRNA-based mechanism of targeting chromatin modifications to indicate sequences to be removed as in Tetrahymena thermophila175,176, whereas in Oxytricha trifallax177,178 and Stylonichia lemnae177,178 piRNAs are used to label sequences for retention in the new macronucleus rather than for removal.
These DNA elimination mechanisms closely resemble the small-RNA directed heterochromatin formation strategies that take place at the pericentromeric regions of S. pombe and other organisms. Instead of marking the genetic material for proper segregation as in fission yeast, this heterochromatin formation mechanism has been adapted in ciliates to alter the content of their chromosomes via DNA elimination. Interestingly, many of the IESs that are removed correspond to transposon sequences and so it seems this mechanism is also an adaptation of the proposed ancestral function of RNAi and a strategy for maintaining genomic integrity179. Despite the obvious difference in outcome, the ultimate function of RNAi in both chromosome segregation and DNA elimination is to maintain chromosome stability and ensure the faithful segregation and propagation of the necessary genetic material.
Maintaining copy number
In S. pombe, the Dicer homologue Dcr1 interacts with RNA polymerases and associated factors to facilitate transcription termination and polymerase release74,180. These interactions have important roles in maintaining the genetic and epigenetic stability of the genome. Loss of Dcr1 results in the failure of Pol II to release from the 3’ end of transcripts and this leads to DNA damage at the ribosomal DNA (rDNA) locus, pericentromeric regions, tRNA-encoding DNA loci (tDNA) and highly-expressed genes180,181. These phenotypes are due to the collisions of the replisome and the transcription machinery during S-phase at these highly-transcribed loci. In the absence of Dcr1 the stalled replication forks are restarted through the activity of homologous recombination (HR), which leads to loss of heterochromatin180. Outside of heterochromatic regions, small RNAs are produced in wild-type strains from the regions where Pol II stalls in dcr1 mutants, but there is little change to the H3K9me2 level at these loci, indicating that the small RNAs are not being loaded into Ago1 and directing heterochromatin formation. In the absence of Dcr1, R-loops accumulate at these sites caused by polymerase collision181. R-loops are three-stranded structures formed when a nascent transcript invades the DNA duplex182. One consequence of the failure to properly resolve the collisions of the replication and transcription machinery is an accumulation of DNA damage at these sites and the loss of copy number at tandem arrays such as the rDNA181 (Fig. 4d). The dramatic copy number loss of the rDNA in Dcr1 deletion mutants occurs as a result of the recruitment of HR machinery to the site, demonstrated by ChIP with Rad52. However, the catalytic activity of neither Dcr1 nor Ago1 was required to maintain copy number181, suggesting interaction with other structures, such as R-loops. Thus, in S. pombe Dcr1 is required to maintain the copy number of highly transcribed tandem repeat regions in the genome, such as the rDNA.
S. pombe can be induced to enter a non-proliferative, quiescent G0 state through nitrogen starvation, and RNAi is required to maintain the G0 state over time. Deletion of Dcr1 has the most dramatic effect on G0 maintenance74, and a number of genetic suppressors of this phenotype have been identified. The suppressors of the G0 maintenance phenotype of Dicer deletion point to the suppression of toxic H3K9me heterochromatin build-up at the rDNA that leads to cell death. Ultimately, Dcr1 is responsible for the effective release of RNA polymerase I (Pol I) at the rDNA74. The failure to release Pol I leads to stalling, the build-up of γH2A.X, the toxic H3K9 methylation, and an eventual loss of viability due to decreased dosage of the transcribed rRNA. Thus, in quiescence, the RNAi and heterochromatin pathways genetically interact, albeit in a different mechanistic manner than in proliferating cells.
Other organisms also rely on RNAi for genome integrity and copy number maintenance at sites such as rDNA loci. For example, the ciliate Oxytricha trifallax has a reduction in rDNA copy number in dcl-1 mutants183. This loss of rDNA copies is part of a broader mechanism of small RNA-regulated DNA dosage and genome maintenance that occurs during asexual growth of O. trifallax. Neurospora crassa utilizes RNAi to respond to DNA damage at the rDNA via siRNAs, sometimes also known as quelling-associated qiRNAs184. Mutants in this pathway exhibit a decrease in copy number at the rDNA without altering H3K9me levels185, closely resembling RNAi mutants in S. pombe181, although levels at embedded reporter genes have been reported to go down186. The role of RNAi in the genomic maintenance of rDNA copy number has been proposed to be due to the interaction of siRNAs with the HR repair machinery187. In Drosophila melanogaster, mutants in RNAi lead to an increase in the numbers of nucleoli (dense nuclear structures composed of rDNA repeats); Dcr-2 mutants have reduced H3K9me2 levels at the rDNA and increased extrachromosomal circular DNA (eccDNA) derived from the rDNA188. eccDNA formation is suppressed by mutation of the non-homologous end joining (NHEJ) factor Lig4 and should also result in the loss of copies of rDNA from the array, although this has not been determined experimentally in Drosophila melanogaster. The discrepancy between H3K9me changes at the rDNA in Drosophila melanogaster, Neurospora crassa and S. pombe likely indicates that additional mechanisms maintain H3K9me2 at the rDNA in fungi in the absence of RNAi, whereas DNA repair might be more directly impacted in all three organisms. RNAi maintains genomic integrity and thus the dosage of tandemly repeated regions largely by preventing or responding to DNA damage.
RNAi and DNA damage – maintaining genome integrity
In addition to protecting the genome from invaders, RNAi protects the genome from other insults. There is a clear link between RNAi and DNA damage. For example, knockout or knockdown of Dicer promotes the increase in γH2A.X DNA damage signalling in S. pombe74,180,181, Drosophila melanogaster189, zebrafish190, human HEK293T cells or fibroblasts190–192, mouse cerebellum, or NIH2/4 cells190,193 and mESCs193. This section will focus on the functions of RNAi in the general DNA damage response.
Small RNA and the DNA damage response pathway
Eukaryotic genomes have evolved a number of redundant pathways for repairing DNA after damage. For instance, double strand breaks (DSBs) can be repaired by either NHEJ or HR. NHEJ takes the two free DNA ends and then resects and/or adds bases by polymerase activity before ligating the ends back together. This process does not require a long homologous template and therefore can be more error-prone than HR. However, it is the primary mechanism of repair in mammalian cells because it can occur at any phase of the cell cycle. The HR process requires the homologous sequence from the sister chromatid to faithfully reproduce the original sequence that was damaged and, thus, can only occur in G2 or S phase194. HR can take a number of different paths depending on the type of damage and on the machinery that identifies and repairs the break. Briefly, the molecular events following a DSB begin with the phosphorylation of H2A.X (γH2A.X) by ATM and the recruitment of MDC1194. Depending on the method of repair, more proteins will accumulate such as BRCA1, 53BP1 and RIF1 in HR. BRCA1 resects the ssDNA ends, and then RAD51 binds these ends to mediate the exchange of strands194. From here there are a number of options for finishing the repair. All pathways involve recognition and copying mechanisms that may or may not result in a crossover of genetic information from one sister chromatid to the other. Importantly, the first step of NHEJ and HR is recognition of the site of damage. It may be at this stage that RNAi assists.
Small RNA and DNA damage in lower eukaryotes
In S. pombe, the knockouts dcr1Δ and ago1Δ are sensitive to the genotoxic agent hydroxyurea (HU)195 and small RNAs from centromeric repeats are strongly induced on HU treatment, accumulating in the S phase196. The dcr1Δ mutant accumulates high levels of DNA damage and double mutants of either dcr1Δ or ago1Δ with rad51Δ are synthetic lethal, whereas double mutants with the fork protection complex are lethal under genotoxic stress180. Mutants inRad51/54 DNA damage machinery also lose silencing at the centromere153, whereas rDNA accumulates very high levels of Rad52 in RNAi mutants181. In Neurospora crassa, exogenous DNA damage induces the expression of the Argonaute homologue QDE-2 and a class of small RNAs termed QDE-2-associated (qiRNAs)184 (Fig. 5a). The qiRNAs are 21–22 nucleotides long and are distinct in size from other siRNAs in Neurospora, which are on average 25 nucleotides and silence multi-copy transgenes by a process called quelling197. The production of these small RNAs is dependent on the Dicer homologues DCL-1 and DCL-2, the RdRP homologue QDE-1, and a RecQ DNA helicase homologue QDE-3184. These small RNAs are loaded into the Ago QDE-2185. The vast majority of these small RNAs are derived from the rDNA locus and are also dependent on the homologues of the HR machinery, such as the classic DNA damage resolution proteins Rad51, Rad52, and Rad54, and other chromatin remodellers that participate in HR198. The fact that HR factors are required for the production of qiRNAs at repetitive loci may be because these sequences are the only ones in the genome that can offer many donor templates for the HR machinery. Interestingly, loss of the fork protection complex, which is responsible for the prevention of fork collapse in regions where transcription and replication machinery collide, results in a significant increase in qiRNA production198, consistent with the genetic interactions of rad51Δ and the fork protection complex with RNAi in S. pombe180. The rDNA is potentially the most important region of the genome for fork protection as it is both highly transcribed and composed of repeated tandem arrays. Although HR seems to be required for qiRNA production, qiRNA production may also be required for efficient HR repair, as mutations in the qiRNA–quelling pathway lead to a decrease in rDNA copy number185, as they do in S. pombe181.
Figure 5 |. The roles of RNAi in the recognition and resolution of DNA damage.
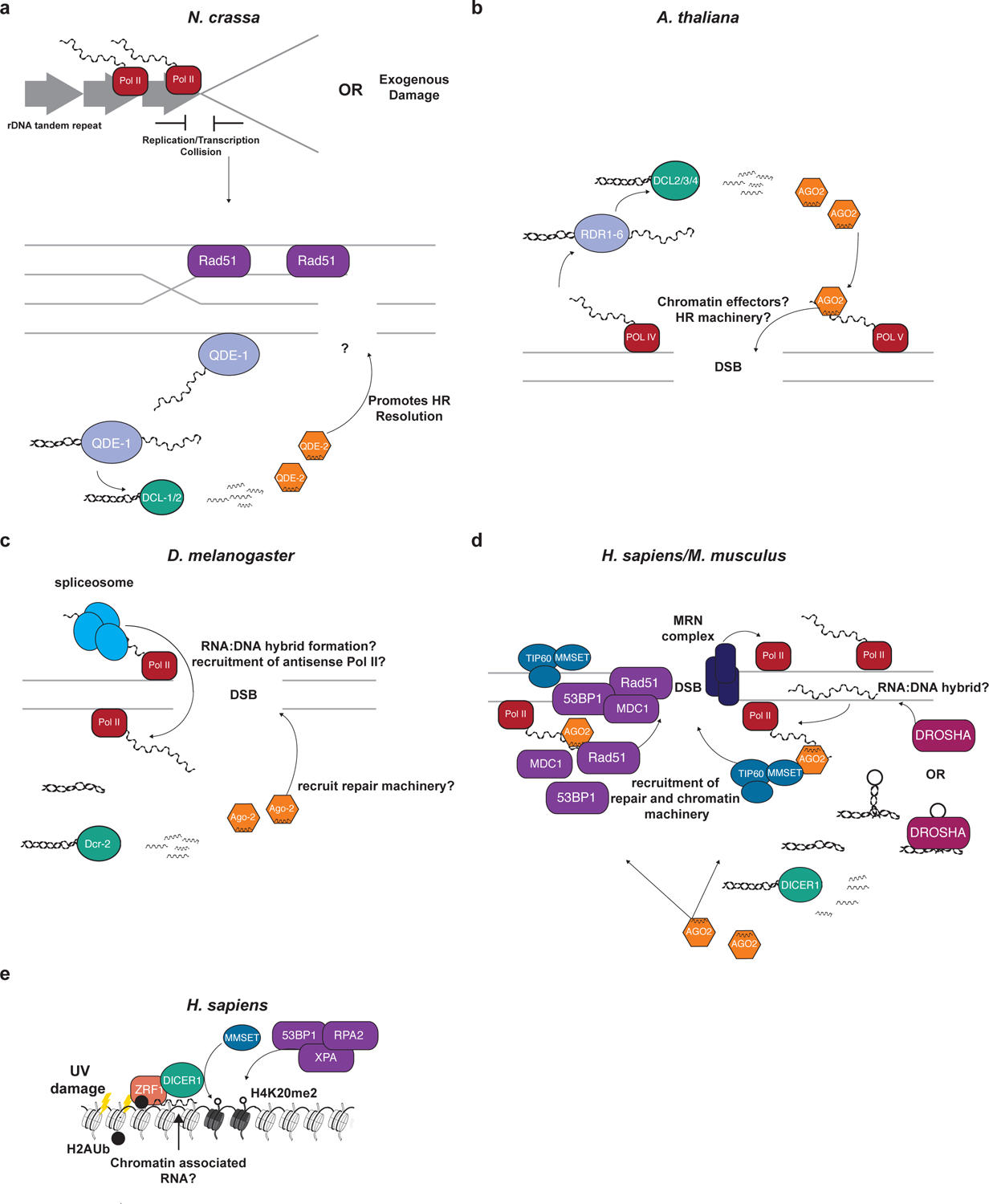
a| In Neurospora QDE-2-associated siRNAs (qiRNAs) participate in the DNA damage response generally through the HR pathway. The RdRP homologue QDE-1, which has DNA-dependent RNA polymerase activity as well, transcribes aberrant RNA from sites of damage and then converts it to dsRNA. The dsRNA is recognized by DCL-1/2 and qiRNAs are produced. These small RNAs are loaded into Argonaute homologue QDE-2 and then promote the resolution of damage through HR. b| The production of DSB-induced siRNAs (diRNAs) in Arabidopsis is dependent on transcription by RNA Pol IV from the site of the damage. These transcripts serve as the template for RdRP homologues RDR2/6 to generate dsRNA. Dicer-like homologues generate the diRNAs, which are then loaded into Ago2. The Ago2/diRNA complex can then recognize nascent transcripts from RNA Pol V and may direct chromatin effectors or repair machinery to the site of damage. c| The endo-siRNA pathway of Drosophila can generate damage-induced small RNAs (and the production of these small RNAs may be enhanced by the splicing machinery. The production of dsRNA recognized by Dcr-2 is enhanced at sites of active transcription, especially sites where the spliceosome is present indicating that the spliceosome may promote antisense transcription. The diRNAs are loaded into Ago2 and it is proposed that the Ago2/diRNA complex may direct downstream repair machinery to the site of the break. d| In mammals, the RNAi pathway can direct both chromatin modifiers and repair machinery to the site of a DNA double-strand break. Pol II transcribes the regions around the break and it may be recruited there by the early-responding MRN complex. The production of anti-sense transcripts may be promoted by the formation of RNA:DNA hybrids in the region and DROSHA may recognize these hybrids. The production of DNA damage-response RNAS (DDRNAs) is dependent on DROSHA and DICER processing dsRNA from the site of the break. These small RNAs can be loaded into AGO2, which can then target the site of DSB and recruit the chromatin effectors MMSET and TIP60 as well as the DNA damage response factors 53BP1 and RAD51 among others to resolve the damage. e| In human cell lines, DICER1 plays a role in the recognition and resolution of DNA damage caused by UV irradiation. In a complex with ZRF1, which can recognize ubiquitinated H2A, DICER1 localizes to the site of damage in an RNA-dependent manner. DICER1 then recruits chromatin effectors, such as MMSET, and repair factors, such as XPA, 53BP1, and RPA2 to the site to promote the resolution of damage.
Small RNA and DNA damage in higher eukaryote models
DNA damage-induced small RNAs have also been found in Arabidopsis thaliana and termed DSB-induced small RNA or (diRNAs) (Fig. 5b). These diRNAs are dependent on Dicer homologues, RNA polymerase IV, and the PI3 kinase ATR199. In general, small RNA of this size in Arabidopsis are generated beginning with Pol IV transcription. The RdRP homologue RDR2 synthesizes dsRNA from the Pol IV transcript and Dicer-like proteins cleave the dsRNA. The diRNAs are then loaded into AGO2. The regulation of diRNAs by ATR indicates they may also be responsive to replication fork failure, as ATR is a primary response mechanism for stalled replication forks199. diRNAs play a notable role in DSB repair, as mutants in diRNA-generating proteins such as Dicer perform significantly worse in a DSB reporter assay199. However, the diRNAs do not seem to be required for the incorporation of γH2A.X, which is an early signal for DNA damage. These diRNAs probably function downstream or alongside of the original signalling of the occurrence of a DSB. The diRNAs that are produced are from both strands and are complementary to the region immediately surrounding the break. In Arabidopsis, these diRNAs do not seem to require HR as in Neurospora200. They certainly play a role in DNA repair, but this role is not specific to one pathway. It should be noted that a follow up study did not find that diRNAs were required for DNA repair as the original report200.
In cultured Drosophila melanogaster cells, damage-induced small RNAs are produced through the canonical endo-siRNA pathway (Fig. 5c). They require Dcr-2 for generation and Ago2 as the effector protein. These small RNAs derive from the region around the break and act in trans through the normal RNAi mechanisms201. The production of these small RNAs may rely on a unique property of Drosophila melanogaster RNA polymerase. At the site of the DSB, the polymerase initiates and transcribes the regions around the break201. Presumably, the double-stranded product of the break-induced RNA and the normal transcript can produce dsRNA for Dcr-2 to act on. However, at this time it is not known if this dsRNA is the relevant substrate of Dcr-2. This mechanism for producing these damage-induced endo-siRNA would require that the break take place in a region that is transcribed to begin with. The generation of these small RNAs does seem to be affected by splicing machinery as well. Substantially more endo-siRNAs were induced in regions in which a break was downstream of an intron compared to non-intronic regions201. The stalling of the splicing machinery seems to be able to serve as mechanism for promoting damage-induced endo-siRNA by providing an unspliced template RNA that can lead to dsRNA by convergent transcription202. However, these endo-siRNAs do not seem to be required for the repair of DSBs by HR, according to cell culture-based artificial assays203.
Small RNA and DNA damage in mammals
The role of RNAi in the DNA damage response seems to be conserved in mammals. Multiple exogenous insults, such as oncogenic stress, ionizing radiation and site-specific endonucleases, activate the DNA damage response in human and mouse cells dependent on enzymatic activity from DICER1 and DROSHA190 (Fig. 5d). DNA damage induces the phosphorylation of DICER1 and subsequent shuttling of the protein to the nucleus in mouse and human cells192,204. As in Arabidopsis thaliana, the involvement of RNAi factors in the DNA damage response in mouse205 and human206,207 cell culture is independent of γH2A.X incorporation at the site of damage. Instead, DICER1 and DROSHA promote the accumulation of other DNA damage response proteins, namely 53BP1206,208 and MCD1206,208. In the NIH2/4 mouse cell line, the DSB response factor 53BP1 is recruited to breaks in a RNA-dependent fashion205. When a DSB forms, the MRN complex (MRE11-RAD50-NBS1) recognizes the break and may promote the association of Pol II on the free ends205. Additionally, the CTD of RNA Pol II is Tyr1-phosphorylated by ABL1, and strand-specific transcription occurs from the site. The transcripts seem to form RNA:DNA hybrids at the site of the break and lead to the appearance of DROSHA and DICER1 RNA substrates208. These precursor transcripts lead to a species of 22–23 nucleotide small RNAs that are dependent on DICER1 and produced from the local region of DSBs190,199. The role of DICER1 and DROSHA is not a primary response, as the loss of DICER1 or DROSHA leads to a decline in the specific DNA damage response mediators MDC1 and 53BP1, which are generally considered to be the secondary level of response to a DSB206,209. These interactions take place on a timescale of hours after the formation of a DSB and are, therefore, not likely to be related to the production of microRNAs by these enzymes because microRNA repression will in general still be active.
HR-based repair is inhibited by the knockdown of DICER1 and AGO2 in human cells as measured by the repair of a GFP marker199. The role of AGO2 at the break occurs through interactions with Rad51. Without AGO2, the accumulation of Rad51 at DSBs is greatly reduced207. Therefore, the guiding of Ago2 by diRNAs assists in the localization of Rad51 and promotes proper resolution of the DSB. A number of histone modifications also have a role in identifying the site of DSB. In human cells DICER1 or DROSHA knockdown reduces the levels of H4K20me2, H4K20me3 and H4K16ac at the site of the DSB2101. The reduction in these modifications is caused by a decrease in the localization of the H4K20 methyltransferase MMSET and the H4K16 acetyltransferase Tip60 to the DSB. The localization of these chromatin modifiers is dependent on their interaction with AGO2210. Furthermore, the binding of AGO2 to diRNAs is responsible for this localization. Ultimately, the modification of chromatin assists in directing RAD51 and BRCA1 to the DSB for resolution of the break. In human U2OS cells, the loss of DROSHA also reduces the accumulation RAD51 and BRCA1 and the extent of resection at DSBs209. Small RNAs from the site of DSBs were not observed after the induction of breaks in endogenous loci. This may indicate that diRNAs are specific for repetitive or highly transcribed loci such as artificial reporters. In these human cells, DROSHA promotes DNA repair, accompanied by RNA invasion by DSBs, although an indirect role via microRNA has not been ruled out209. Interestingly, it has also been demonstrated that antisense transcription generated by the formation of R-loops induces dsRNA formation and the subsequent recruitment of DICER1, AGO1, AGO2, the H3K9 methyltransferase G9a, and HP1γ to form heterochromatin over Pol II pausing and termination sites211. Thus, the relationship between RNAi and R-loops in mammals may be more complicated. An R-loop-based HR mechanism is also present in S. pombe212 where Dcr1 has a role in RNA:DNA hybrid formation and/or stability as well as in copy number maintenance181.
Dicer and nucleotide excision repair: localization of chromatin machinery leads to resolution
The previous sections have described the role of RNAi components and small RNAs in the repair of DSBs. However, Dicer also responds to other forms of DNA damage such as the formation of 6–4 photoproducts and cyclobutene pyrimidine dimers (CPDs) caused by exposure to ultraviolet light as will be discussed below. The primary method for repairing this type of damage is called nucleotide excision repair (NER)213. It is a mechanism of scanning the genome for these bulky helix distortions, removing and repairing them. One method of NER that can operate in any region of the genome is termed global genome NER (GG-NER). The other method is called transcription-coupled NER (TC-NER) and takes place specifically when a polymerase runs into one of these adducts.
In C. elegans, Dcr-1 mutant worms phenocopy strains deficient in NER when exposed to UV treatment214. In human HEK293T cells, DICER1 is recruited to chromatin after UV treatment. This interaction is dependent on a known NER-participating protein ZRF1 as well as RNA. Together with PARP1, DICER1 and these associating factors decondense the chromatin in the area surrounding the damage214 (Fig. 5e). DICER1 brings the methyltransferase MMSET to sites of UV-induced damage, as well as DSBs, and the recruitment of this chromatin modifier is required for efficient NER. MMSET deposits H4K20me2, which in this case provides a signalling mark for the binding of 53BP1 and XPA, which is a factor of the NER repair machinery215. This function seems to be independent of DICER1’s catalytic activity. The mechanism bears some resemblance to the role of DICER1 in the DSB repair pathway as well with MMSET and 53BP1 as the common recruited factors. It will be interesting to see exactly how DICER1 is recruited to these sites of damage and whether the mechanism is the same in NER as it is in DSB repair. Another RNAi factor, DGCR8, participates in the TC-NER pathway in human cells. This occurs after S153 phosphorylation of DGCR8 and the mechanism is independent of DGCR8 binding to DROSHA or RNA216. Although it is clear DGCR8 has an effect on TC-NER, through which factors it operates remains unknown. In Arabidopsis, upon UV damage RNAi participates in GG-NER by producing 21 nt siRNAs at intergenic sites of damage. The production of UV-induced siRNAs (uviRNAs) is dependent on RNA POL IV, RDR2 and DCL-4; upon loading into AGO1 in complex with DDB2, they direct repair machinery to DNA lesions like CPDs217.
DICER1 and DROSHA are clearly involved in the DNA damage response. Some of these roles involve small RNAs bound to an Argonaute effector protein, and are involved in the very initial stages of the DNA damage response, namely the recognition of damage and recruiting other proteins such as the repair proteins themselves or chromatin modifiers, such as MMSET, to further the signal of damage via epigenetic modifications in the region. In the mechanisms that seem to be independent of small RNAs, it is not entirely clear what localizes the RNAi factors to damage. Candidates include RNA or DNA:RNA hybrid structures that form in response to damage, but the catalytic activity or RNA-binding properties of these proteins are not always required for their function in the DNA damage response. The highly conserved nature of these interactions suggests an early role in the DNA damage response independent of the function in silencing.
Conclusions
Although roles for RNAi in chromosomal behaviour and genome integrity were originally described in S. pombe, it is clear that these roles are conserved across eukaryotes (Table 1). It may be that the LECA employed RNAi in some capacity for these functions. Or, more likely, RNAi has been independently recruited to perform these functions through convergent evolution. The role RNAi plays in chromosome segregation can most often be attributed to the formation of heterochromatin at the pericentromere. The alteration of chromatin at this locus can disrupt the dynamics of sister chromatid attachment and release in mitosis. This disruption leads to lagging chromosomes and missegregation phenotypes commonly described in RNAi mutants across eukaryotic species. Many of these mechanisms mirror the classic small-RNA directed heterochromatin formation strategy of S. pombe. The piRNA-mediated heterochromatin formation in the germlines of D. melanogaster and mammals also bears a striking resemblance to S. pombe CTGS218. While mutants in the piRNA pathway do have dramatic genome instability phenotypes the role of chromosome segregation in these phenotypes is far from clear. However, the evidence for DICER1-generated pericentromere-derived small RNAs directing the formation of heterochromatin in somatic mammalian cells is scarce. Rather, the regulation of long pericentromeric transcripts or the regulation of the act of transcription itself may be sufficient to generate the proper chromatin environment required to accurately segregate chromosomes. Future investigations will be able to more clearly delineate between these mechanisms.
Table 1 |.
The functions and phenotypes of RNAi in eukaryotes
| Organism | Chromosomal functions and phenotypes | Genome integrity functions and phenotypes |
|---|---|---|
| S. pombe |
dcr1Δ, ago1Δ, and rdp1Δ - Lagging chromosomes and missegregation in dividing cells, quiescence entry, and meiosis (small RNA)71–75 dcr1Δ - reduction in rDNA copy number181 |
dcr1Δ and ago1Δ - sensitive to HU (indicative of replication stress-induced damage), accumulate DNA damage (Rad22 foci), and are synthetic lethal with rad51A180,195,196 dcr1Δ - damage at repetitive loci and highly expressed genes, loses rDNA copy number180,181 |
| N. crassa |
SAD-1 (Rdrp), DCL-1, SMS-2 (Ago) – required for MSUD post-transcriptional dosage control (small RNA)147–150 DCL-1/2, QDE-2 (Ago), and QDE-1 (Rdrp) – loss of this pathway reduces copy number at repetitive loci such as the rDNA (small RNA)185 |
DCL-1/2, QDE-2 (Ago), and QDE-1 (Rdrp) – generate damage induced siRNAs (qiRNAs) that interact with repair machinery, (small RNA)184 |
| T. thermophila |
Dcl1 knockout - lagging/bridging during mitosis and meiosis66 Dcl1, Twi1P (Ago/Piwi) drive the DNA elimination pathway (small rna)66,165,172 |
|
| T. brucei | TbAgo1 knockout – chromosome missegregation, whole genome aneuploidy (small RNA)88 | |
| H. sapiens | DICER1 or AGO2 knockdown – lagging chromosomes in RPE-1, HeLa cells93,102 |
DICER1/DROSHA – knockdown increases DNA damage in fibroblasts (γH2A.X)190 DICER1/DROSHA/AGO2 – production of DNA damage-induced small RNAs and direction of chromatin/repair machinery to DSB (small RNA)199,206,207,209,210 DICER1/DGCR8 – assist in nucleotide excision repair possibly at the chromatin level214–216 |
| M. musculus | Dicer1 knockout – lagging chromosomes in fibroblasts97 |
DICER1/DROSHA – knockdown increases DNA damage (γH2A.X) in NIH2/4 cells, cerebellum, and mESCs193,190 DICER1/DROSHA/AGO2 – production of DNA damage-induced small RNAs and direction of repair machinery to DSB in NIH2/4 cells (small RNA)205 |
| D. melanogaster |
Dcr-2 and Ago2 deletion/knockdown – lagging chromosomes in wing disc, S2 cells, and embryos (small RNA)93,94 Dcr-2 – mutant has reduced H3K9me2 at rDNA and an increase in rDNA circles188 Dcr-2 and Ago2 loss of function mutations – synthetic lethal with roX1/roX2 doubles, indicating role in X chromosome recognition for dosage compensation through 1.688X satellite repeat siRNAs (small RNA)161,162 |
Dcr2 knockout – increase in DNA damage (γH2A.X)189 Dcr-2 and Ago2 – generate damage-induced small RNAs (small RNA)201 |
| C. elegans |
CSR-1 (Ago) knockdown – chromosome segregation defects in embryo and meiosis (small RNA)65,129 DCR-1 knockout – misshapen oocytes with high DNA content, X chromosome missegregation in male meiosis130,131,138 CSR-1 mutants have reduced H3K9me2 on unpaired X chromosome and increased H3K9me2 on autosomes in early meiosis135 |
DCR-1 – loss of function mutant shows disruption of nucleotide excision repair upon UV treatment214 |
| A. thaliana |
AGO4 knockout – lagging/bridging chromosomes reported during mitosis and male meiosis (small RNA)95 easiRNAs (dependent on microRNA to trigger formation) sense whole genome dosage in interploidy hybrids (small RNA)32,144,145 |
DCLs and AGO2 - generation of damage induced siRNAs (diRNAs) (small RNA)199 RDR2, DCL-4, and AGO1 – production of uviRNAs in GG-NER UV damage response (small aRNA)217 |
| Z. mays | AGO104 loss of function mutation – disruption of chromosome condensation and segregation during male meiosis, also shows the formation of micronuclei and multinucleated cells (small RNA)142 |
Small RNA-directed heterochromatin formation mechanisms have been adapted to regulate dosage at both the RNA transcript and genome levels in a wide variety of eukaryotes. Many eukaryotes use similar mechanisms for regulating dosage at the transcriptional level by targeting the transcripts themselves. Interestingly, the genomic loci regulated by these dosage response mechanisms often overlap with transposon loci, which suggests that the ancestral function of RNAi is conserved but adapted for specialized chromosomal and genomic functions. In ciliate DNA elimination, the generation of small RNAs from specific genomic loci and subsequent targeting of nascent transcripts can direct the chromatin machinery to epigenetically mark specific loci for removal or retention. Furthermore, the maintenance of genomic dosage of tandem repeat regions, such as rDNA, has revealed important functions of Dicer in preventing DNA damage. In addition to maintaining genome integrity at tandem repeats, RNAi also has important roles in recognition and repair of DNA damage. In both small RNA-targeted and small RNA-independent pathways, RNAi recognizes dsDNA breaks and UV-induced CPDs and recruits chromatin modifiers as well as repair factors to promote the resolution of DNA damage.
The more we examine the conserved functions of RNAi beyond gene regulation by microRNAs, the more we will come to recognize its pivotal roles in chromosome and genome integrity. A better understanding of these roles will undoubtedly further our understanding of the consequences that arise from mutating RNAi components in human diseases such as cancer.
References
- 1.Dumesic PA & Madhani HD Recognizing the enemy within: licensing RNA-guided genome defense. Trends in Biochemical Sciences 39, 25–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malone CD & Hannon GJ Small RNAs as Guardians of the Genome. Cell 136, 656–668 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castel SE & Martienssen RA RNA interference in the nucleus: roles for small RNAs in transcription, epigenetics and beyond. Nat. Rev. Genet 14, 100–112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siomi MC, Sato K, Pezic D & Aravin AA PIWI-interacting small RNAs: the vanguard of genome defence. Nat Rev Mol Cell Biol 12, 246–258 (2011). [DOI] [PubMed] [Google Scholar]
- 5.D’Ario M, Griffiths-Jones S & Kim M Small RNAs: Big Impact on Plant Development. Trends Plant Sci. 22, 1056–1068 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Alberti C & Cochella L A framework for understanding the roles of miRNAs in animal development. Development 144, 2548–2559 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Suh N & Blelloch R Small RNAs in early mammalian development: from gametes to gastrulation. Development 138, 1653–1661 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivey KN & Srivastava D MicroRNAs as regulators of differentiation and cell fate decisions. Cell Stem Cell 7, 36–41 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Nicoloso MS, Spizzo R, Shimizu M, Rossi S & Calin GA MicroRNAs--the micro steering wheel of tumour metastases. Nat Rev Cancer 9, 293–302 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Foulkes WD, Priest JR & Duchaine TF DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer 14, 662–672 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Cerutti H & Casas-Mollano JA On the origin and functions of RNA-mediated silencing: from protists to man. Curr Genet 50, 81–99 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pong SK & Gullerova M Noncanonical functions of microRNA pathway enzymes - Drosha, DGCR8, Dicer and Ago proteins. FEBS Letters 592, 2973–2986 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Burger K & Gullerova M Swiss army knives: non-canonical functions of nuclear Drosha and Dicer. Nat Rev Mol Cell Biol 16, 417–430 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Shabalina SA & Koonin EV Origins and evolution of eukaryotic RNA interference. Trends Ecol. Evol. (Amst.) 23, 578–587 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Court DL et al. RNase III: Genetics and function; structure and mechanism. Annu. Rev. Genet 47, 405–431 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacRae IJ & Doudna JA Ribonuclease revisited: structural insights into ribonuclease III family enzymes. Curr. Opin. Struct. Biol 17, 138–145 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Iyer LM, Koonin EV & Aravind L Evolutionary connection between the catalytic subunits of DNA-dependent RNA polymerases and eukaryotic RNA-dependent RNA polymerases and the origin of RNA polymerases. BMC Struct. Biol 3, 1 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swarts DC et al. The evolutionary journey of Argonaute proteins. Nat Struct Mol Biol 21, 743–753 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makarova KS, Wolf YI, van der Oost J & Koonin EV Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biol Direct 4, 29 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bråte J et al. Unicellular Origin of the Animal MicroRNA Machinery. Curr. Biol 28, 3288–3295.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimson A et al. Early origins and evolution of microRNAs and Piwi-interacting RNAs in animals. Nature 455, 1193–1197 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones-Rhoades MW, Bartel DP & Bartel B MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol 57, 19–53 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Avesson L, Reimegard J, Wagner EGH & Soderbom F MicroRNAs in Amoebozoa: Deep sequencing of the small RNA population in the social amoeba Dictyostelium discoideum reveals developmentally regulated microRNAs. RNA 18, 1771–1782 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molnar A, Schwach F, Studholme DJ, Thuenemann EC & Baulcombe DC miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature 447, 1126–1129 (2007). [DOI] [PubMed] [Google Scholar]
- 25.Cock JM et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 465, 617–621 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Lee H-C et al. Diverse Pathways Generate MicroRNA-like RNAs and Dicer-Independent Small Interfering RNAs in Fungi. Mol Cell 38, 803–814 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartel DP Metazoan MicroRNAs. Cell 173, 20–51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts JT, Cardin SE & Borchert GM Burgeoning evidence indicates that microRNAs were initially formed from transposable element sequences. mge 4, e29255 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smalheiser NR & Torvik VI Mammalian microRNAs derived from genomic repeats. Trends in Genetics 21, 322–326 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Qin S, Jin P, Zhou X, Chen L & Ma F The Role of Transposable Elements in the Origin and Evolution of MicroRNAs in Human. PLoS ONE 10, e0131365–10 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piriyapongsa J & Jordan IK Dual coding of siRNAs and miRNAs by plant transposable elements. RNA 14, 814–821 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borges F et al. Transposon-derived small RNAs triggered by miR845 mediate genome dosage response in Arabidopsis. Nat Genet 50, 186–192 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han J et al. Molecular Basis for the Recognition of Primary microRNAs by the Drosha-DGCR8 Complex. Cell 125, 887–901 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Nguyen TA et al. Functional Anatomy of the Human Microprocessor. Cell 161, 1374–1387 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Warf MB, Johnson WE & Bass BL Improved annotation of C. elegans microRNAs by deep sequencing reveals structures associated with processing by Drosha and Dicer. RNA 17, 563–577 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vermeulen A et al. The contributions of dsRNA structure to Dicer specificity and efficiency. RNA 11, 674–682 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J-E et al. Dicer recognizes the 5’ end of RNA for efficient and accurate processing. Nature 475, 201–205 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Kolb FA, Brondani V, Billy E & Filipowicz W Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J 21, 5875–5885 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Kolb FA, Jaskiewicz L, Westhof E & Filipowicz W Single processing center models for human Dicer and bacterial RNase III. Cell 118, 57–68 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Auyeung VC, Ulitsky I, McGeary SE & Bartel DP Beyond Secondary Structure: Primary-Sequence Determinants License Pri-miRNA Hairpins for Processing. Cell 152, 844–858 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoehener C, Hug I & Nowacki M Dicer-like Enzymes with Sequence Cleavage Preferences. Cell 173, 234–247.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu H et al. Sequence features associated with microRNA strand selection in humans and flies. BMC Genomics 10, 413–11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frank F, Sonenberg N & Nagar B Structural basis for 5ʹ-nucleotide base-specific recognition of guide RNA by human AGO2. Nature 465, 818–822 (2010). [DOI] [PubMed] [Google Scholar]
- 44.Kawamata T & Tomari Y Making RISC. Trends in Biochemical Sciences 35, 368–376 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Okamura K, Ladewig E, Zhou L & Lai EC Functional small RNAs are generated from select miRNA hairpin loops in flies and mammals. Genes & Development 27, 778–792 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goh E & Okamura K Hidden sequence specificity in loading of single-stranded RNAs onto Drosophila Argonautes. Nucleic Acids Res. 47, 3101–3116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao BS, Roundtree IA & He C Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol 18, 31–42 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Cozen AE, Liu Y, Chen Q & Lowe TM Small RNA Modifications: Integral to Function and Disease. Trends Mol Med 22, 1025–1034 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji L & Chen X Regulation of small RNA stability: Methylation and beyond. Cell Res. 22, 624–636 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurth HM & Mochizuki K 2’-O-methylation stabilizes Piwi-associated small RNAs and ensures DNA elimination in Tetrahymena. RNA 15, 675–685 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirino Y & Mourelatos Z Mouse Piwi-interacting RNAs are 2’-O-methylated at their 3’ termini. Nat Struct Mol Biol 14, 347–348 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Saito K et al. Pimet, the Drosophila homolog of HEN1, mediates 2’-O-methylation of Piwi- interacting RNAs at their 3’ ends. Genes & Development 21, 1603–1608 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horwich MD et al. The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr. Biol 17, 1265–1272 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Ghildiyal M et al. Endogenous siRNAs derived from transposons and mRNAs in Drosophila somatic cells. Science 320, 1077–1081 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawamura Y et al. Drosophila endogenous small RNAs bind to Argonaute 2 in somatic cells. Nature 453, 793–797 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Okamura K et al. The Drosophila hairpin RNA pathway generates endogenous short interfering RNAs. Nature 453, 803–806 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Czech B et al. Hierarchical Rules for Argonaute Loading in Drosophila. Mol Cell 36, 445–456 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seitz H, Ghildiyal M & Zamore PD Argonaute Loading Improves the 5ʹ Precision of Both MicroRNAs and Their miRNA* Strands in Flies. Curr. Biol 18, 147–151 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bernstein E et al. Dicer is essential for mouse development. Nat Genet 35, 215–217 (2003). [DOI] [PubMed] [Google Scholar]
- 60.Morita S et al. One Argonaute family member, Eif2c2 (Ago2), is essential for development and appears not to be involved in DNA methylation. Genomics 89, 687–696 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Medvid R, Melton C, Jaenisch R & Blelloch R DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet 39, 380–385 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee YS et al. Distinct Roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA Silencing Pathways. Cell 117, 69–81 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Kataoka Y, Takeichi M & Uemura T Developmental roles and molecular characterization of a Drosophila homologue of Arabidopsis Argonaute1, the founder of a novel gene superfamily. Genes Cells 6, 313–325 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Grishok A et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106, 23–34 (2001). [DOI] [PubMed] [Google Scholar]
- 65.Yigit E et al. Analysis of the C. elegans Argonaute Family Reveals that Distinct Argonautes Act Sequentially during RNAi. Cell 127, 747–757 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Mochizuki K & Gorovsky MA A Dicer-like protein in Tetrahymena has distinct functions in genome rearrangement, chromosome segregation, and meiotic prophase. Genes & Development 19, 77–89 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Golden TA et al. SHORT INTEGUMENTS1/SUSPENSOR1/CARPEL FACTORY, a Dicer homolog, is a maternal effect gene required for embryo development in Arabidopsis. PLANT PHYSIOLOGY 130, 808–822 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson BE et al. The dicer-like1 homolog fuzzy tassel is required for the regulation of meristem determinacy in the inflorescence and vegetative growth in maize. Plant Cell 26, 4702–4717 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wienholds E, Koudijs MJ, van Eeden FJM, Cuppen E & Plasterk RHA The microRNA-producing enzyme Dicer1 is essential for zebrafish development. Nat Genet 35, 217–218 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Sugiyama T, Cam H, Verdel A, Moazed D & Grewal SIS RNA-dependent RNA polymerase is an essential component of a self-enforcing loop coupling heterochromatin assembly to siRNA production. Proc. Natl. Acad. Sci. U.S.A 102, 152–157 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Provost P et al. Dicer is required for chromosome segregation and gene silencing in fission yeast cells. Proc. Natl. Acad. Sci. U.S.A 99, 16648–16653 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volpe T et al. RNA interference is required for normal centromere function in fission yeast. Chromosome Res 11, 137–146 (2003). [DOI] [PubMed] [Google Scholar]
- 73.Hall IM, Noma K-I & Grewal SIS RNA interference machinery regulates chromosome dynamics during mitosis and meiosis in fission yeast. Proc. Natl. Acad. Sci. U.S.A 100, 193–198 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roche B, Arcangioli B & Martienssen RA RNA interference is essential for cellular quiescence. Science 354, aah5651–10 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Volpe TA et al. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297, 1833–1837 (2002). [DOI] [PubMed] [Google Scholar]
- 76.Djupedal I et al. RNA Pol II subunit Rpb7 promotes centromeric transcription and RNAi-directed chromatin silencing. Genes & Development 19, 2301–2306 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colmenares SU, Buker SM, Bühler M, Dlakić M & Moazed D Coupling of double-stranded RNA synthesis and siRNA generation in fission yeast RNAi. Mol Cell 27, 449–461 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Motamedi MR et al. Two RNAi Complexes, RITS and RDRC, Physically Interact and Localize to Noncoding Centromeric RNAs. Cell 119, 789–802 (2004). [DOI] [PubMed] [Google Scholar]
- 79.Djupedal I et al. Analysis of small RNA in fission yeast; centromeric siRNAs are potentially generated through a structured RNA. EMBO J 28, 3832–3844 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marasovic M, Zocco M & Halic M Argonaute and triman generate dicer-independent prirnas and mature siRNAs to initiate heterochromatin formation. Mol Cell 52, 173–183 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Verdel A et al. RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303, 672–676 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang K, Mosch K, Fischle W & Grewal SIS Roles of the Clr4 methyltransferase complex in nucleation, spreading and maintenance of heterochromatin. Nat Struct Mol Biol 15, 381–388 (2008). [DOI] [PubMed] [Google Scholar]
- 83.Nakayama J, Rice JC, Strahl BD, Allis CD & Grewal SI Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292, 110–113 (2001). [DOI] [PubMed] [Google Scholar]
- 84.Nonaka N et al. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat Cell Biol 4, 89–93 (2001). [DOI] [PubMed] [Google Scholar]
- 85.Bernard P et al. Requirement of Heterochromatin for Cohesion at Centromeres. Science 294, 2539–2542 (2001). [DOI] [PubMed] [Google Scholar]
- 86.Folco HD, Pidoux AL, Urano T & Allshire RC Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres. Science 319, 94–97 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yadav V et al. RNAi is a critical determinant of centromere evolution in closely related fungi. Proc. Natl. Acad. Sci. U.S.A 115, 3108–3113 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Durand-Dubief M & Bastin P TbAGO1, an argonaute protein required for RNA interference, is involved in mitosis and chromosome segregation in Trypanosoma brucei. BMC Biol. 1, 2 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Echeverry MC, Bot C, Obado SO, Taylor MC & Kelly JM Centromere-associated repeat arrays on Trypanosoma brucei chromosomes are much more extensive than predicted. BMC Genomics 13, 29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elde NC, Roach KC, Yao M-C & Malik HS Absence of Positive Selection on Centromeric Histones in Tetrahymena Suggests Unsuppressed Centromere-Drive in Lineages Lacking Male Meiosis. J Mol Evol 72, 510–520 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruehle MD, Orias E & Pearson CG Tetrahymena as a Unicellular Model Eukaryote: Genetic and Genomic Tools. Genetics 203, 649–665 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mukherjee K, Campos H & Kolaczkowski B Evolution of Animal and Plant Dicers: Early Parallel Duplications and Recurrent Adaptation of Antiviral RNA Binding in Plants. Molecular Biology and Evolution 30, 627–641 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pek JW & Kai T DEAD-box RNA helicase Belle/DDX3 and the RNA interference pathway promote mitotic chromosome segregation. Proc. Natl. Acad. Sci. U.S.A 108, 12007–12012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Deshpande G, Calhoun G & Schedl P Drosophila argonaute-2 is required early in embryogenesis for the assembly of centric/centromeric heterochromatin, nuclear division, nuclear migration, and germ-cell formation. Genes & Development 19, 1680–1685 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oliver C, Santos JL & Pradillo M Accurate Chromosome Segregation at First Meiotic Division Requires AGO4, a Protein Involved in RNA-Dependent DNA Methylation in Arabidopsis thaliana. Genetics 204, 543–553 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fukagawa T et al. Dicer is essential for formation of the heterochromatin structure in vertebrate cells. Nat Cell Biol 6, 784–791 (2004). [DOI] [PubMed] [Google Scholar]
- 97.Harfe BD, McManus MT, Mansfield JH, Hornstein E & Tabin CJ The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc. Natl. Acad. Sci. U.S.A 102, 10898–10903 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanellopoulou C et al. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes & Development 19, 489–501 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murchison EP, Partridge JF, Tam OH, Cheloufi S & Hannon GJ Characterization of Dicer-deficient murine embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A 102, 12135–12140 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hsieh C-L et al. WDHD1 modulates the post-transcriptional step of the centromeric silencing pathway. Nucleic Acids Res. 39, 4048–4062 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.White E, Schlackow M, Kamieniarz-Gdula K, Proudfoot NJ & Gullerova M Human nuclear Dicer restricts the deleterious accumulation of endogenous double-stranded RNA. Nat Struct Mol Biol 21, 552–559 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang C, Wang X, Liu X, Cao S & Shan G RNAi pathway participates in chromosome segregation in mammalian cells. Cell Discov 1, 15029 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martens JHA et al. The profile of repeat-associated histone lysine methylation states in the mouse epigenome. EMBO J 24, 800–812 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnson WL et al. RNA-dependent stabilization of SUV39H1 at constitutive heterochromatin. eLife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shirai A et al. Impact of nucleic acid and methylated H3K9 binding activities of Suv39h1 on its heterochromatin assembly. eLife 6, 80–23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Velazquez Camacho O et al. Major satellite repeat RNA stabilize heterochromatin retention of Suv39h enzymes by RNA-nucleosome association and RNA:DNA hybrid formation. eLife 6, 817 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muchardt C et al. Coordinated methyl and RNA binding is required for heterochromatin localization of mammalian HP1α. EMBO Rep 3, 975–981 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maison C et al. SUMOylation promotes de novo targeting of HP1α to pericentric heterochromatin. Nat Genet 43, 220–227 (2011). [DOI] [PubMed] [Google Scholar]
- 109.Quénet D & Dalal Y A long non-coding RNA is required for targeting centromeric protein A to the human centromere. eLife 3, 267–18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McNulty SM, Sullivan LL & Sullivan BA Human Centromeres Produce Chromosome- Specific and Array-Specific Alpha Satellite Transcripts that Are Complexed with CENP-A and CENP-C. DEVCEL 42, 226–240.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bouzinba-Segard H, Guais A & Francastel C Accumulation of small murine minor satellite transcripts leads to impaired centromeric architecture and function. Proc. Natl. Acad. Sci. U.S.A 103, 8709–8714 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Blower MD Centromeric Transcription Regulates Aurora-B Localization and Activation. Cell Rep 15, 1624–1633 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ideue T, Cho Y, Nishimura K & Tani T Involvement of satellite I noncoding RNA in regulation of chromosome segregation. Genes Cells 19, 528–538 (2014). [DOI] [PubMed] [Google Scholar]
- 114.Chan FL et al. Active transcription and essential role of RNA polymerase II at the centromere during mitosis. Proc. Natl. Acad. Sci. U.S.A 109, 1979–1984 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guenatri M, Bailly D, Maison C & Almouzni G Mouse centric and pericentric satellite repeats form distinct functional heterochromatin. The Journal of Cell Biology 166, 493–505 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kong X et al. Cohesin associates with spindle poles in a mitosis-specific manner and functions in spindle assembly in vertebrate cells. Mol Biol Cell 20, 1289–1301 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yi Q et al. HP1 links centromeric heterochromatin to centromere cohesion in mammals. EMBO Rep 19, e45484–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hahn M et al. Suv4–20h2 mediates chromatin compaction and is important for cohesin recruitment to heterochromatin. Genes & Development 27, 859–872 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koch B, Kueng S, Ruckenbauer C, Wendt KS & Peters J-M The Suv39h-HP1 histone methylation pathway is dispensable for enrichment and protection of cohesin at centromeres in mammalian cells. Chromosoma 117, 199–210 (2008). [DOI] [PubMed] [Google Scholar]
- 120.Serrano Á, Rodríguez-Corsino M & Losada A Heterochromatin protein 1 (HP1) proteins do not drive pericentromeric cohesin enrichment in human cells. PLoS ONE 4, e5118 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamagishi Y, Sakuno T, Shimura M & Watanabe Y Heterochromatin links to centromeric protection by recruiting shugoshin. Nature 455, 251–255 (2008). [DOI] [PubMed] [Google Scholar]
- 122.Kang J et al. Mitotic centromeric targeting of HP1 and its binding to Sgo1 are dispensable for sister-chromatid cohesion in human cells. Mol Biol Cell 22, 1181–1190 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maida Y et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature 461, 230–235 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maida Y et al. Involvement of Telomerase Reverse Transcriptase in Heterochromatin Maintenance. Molecular and Cellular Biology 34, 1576–1593 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Topp CN, Zhong CX & Dawe RK Centromere-encoded RNAs are integral components of the maize kinetochore. Proc. Natl. Acad. Sci. U.S.A 101, 15986–15991 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maddox PS, Oegema K, Desai A & Cheeseman IM ‘Holo’er than thou: chromosome segregation and kinetochore function in C. elegans. Chromosome Res 12, 641–653 (2004). [DOI] [PubMed] [Google Scholar]
- 127.Duchaine TF et al. Functional Proteomics Reveals the Biochemical Niche of C. elegans DCR-1 in Multiple Small-RNA-Mediated Pathways. Cell 124, 343–354 (2006). [DOI] [PubMed] [Google Scholar]
- 128.Nakamura M et al. Dicer-related drh-3 gene functions in germ-line development by maintenance of chromosomal integrity in Caenorhabditis elegans. Genes Cells 12, 997–1010 (2007). [DOI] [PubMed] [Google Scholar]
- 129.Claycomb JM et al. The Argonaute CSR-1 and Its 22G-RNA Cofactors Are Required for Holocentric Chromosome Segregation. Cell 139, 123–134 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Knight SW & Bass BL A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science 293, 2269–2271 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Drake M et al. A requirement for ERK-dependent Dicer phosphorylation in coordinating oocyte-to-embryo transition in C. elegans. Dev Cell 31, 614–628 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gassmann R et al. An inverse relationship to germline transcription defines centromeric chromatin in C. elegans. Nature 484, 534–537 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cecere G, Hoersch S, O’Keeffe S, Sachidanandam R & Grishok A Global effects of the CSR-1 RNA interference pathway on the transcriptional landscape. Nat Struct Mol Biol 21, 358–365 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gerson-Gurwitz A et al. A Small RNA-Catalytic Argonaute Pathway Tunes Germline Transcript Levels to Ensure Embryonic Divisions. Cell 165, 396–409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.She X, Xu X, Fedotov A, Kelly WG & Maine EM Regulation of Heterochromatin Assembly on Unpaired Chromosomes during Caenorhabditis elegans Meiosis by Components of a Small RNA-Mediated Pathway. PLoS Genet 5, e1000624–15 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ellermeier C et al. RNAi and heterochromatin repress centromeric meiotic recombination. Proc. Natl. Acad. Sci. U.S.A 107, 8701–8705 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maine EM et al. EGO-1, a putative RNA-dependent RNA polymerase, is required for heterochromatin assembly on unpaired DNA during C. elegans meiosis. Curr. Biol 15, 1972–1978 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pavelec DM, Lachowiec J, Duchaine TF, Smith HE & Kennedy S Requirement for the ERI/DICER Complex in Endogenous RNA Interference and Sperm Development in Caenorhabditis elegans. Genetics 183, 1283–1295 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nonomura K-I et al. A germ cell specific gene of the ARGONAUTE family is essential for the progression of premeiotic mitosis and meiosis during sporogenesis in rice. Plant Cell 19, 2583–2594 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fan Y et al. PMS1T, producing phased small-interfering RNAs, regulates photoperiod-sensitive male sterility in rice. Proc. Natl. Acad. Sci. U.S.A 113, 15144–15149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Komiya R et al. Rice germline-specific Argonaute MEL1 protein binds to phasiRNAs generated from more than 700 lincRNAs. Plant J. 78, 385–397 (2014). [DOI] [PubMed] [Google Scholar]
- 142.Singh M et al. Production of Viable Gametes without Meiosis in Maize Deficient for an ARGONAUTE Protein. Plant Cell 23, 443–458 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Olmedo-Monfil V et al. Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature 464, 628–632 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martínez G et al. Paternal easiRNAs regulate parental genome dosage in Arabidopsis. Nat Genet 50, 193–198 (2018). [DOI] [PubMed] [Google Scholar]
- 145.Erdmann RM, Satyaki PRV, Klosinska M & Gehring M A Small RNA Pathway Mediates Allelic Dosage in Endosperm. Cell Rep 21, 3364–3372 (2017). [DOI] [PubMed] [Google Scholar]
- 146.Jiang H et al. Ectopic application of the repressive histone modification H3K9me2 establishes post-zygotic reproductive isolation in Arabidopsis thaliana. Genes & Development 31, 1272–1287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shiu PKT, Raju NB, Zickler D & Metzenberg RL Meiotic silencing by unpaired DNA. Cell 107, 905–916 (2001). [DOI] [PubMed] [Google Scholar]
- 148.Alexander WG et al. DCL-1 colocalizes with other components of the MSUD machinery and is required for silencing. Fungal Genet. Biol 45, 719–727 (2008). [DOI] [PubMed] [Google Scholar]
- 149.Lee DW, Pratt RJ, McLaughlin M & Aramayo R An argonaute-like protein is required for meiotic silencing. Genetics 164, 821–828 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hammond TM et al. Identification of small RNAs associated with meiotic silencing by unpaired DNA. Genetics 194, 279–284 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hammond TM et al. SAD-3, a Putative Helicase Required for Meiotic Silencing by Unpaired DNA, Interacts with Other Components of the Silencing Machinery. G3 (Bethesda) 1, 369–376 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Samarajeewa DA et al. Efficient detection of unpaired DNA requires a member of the rad54-like family of homologous recombination proteins. Genetics 198, 895–904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Onaka AT et al. Rad51 and Rad54 promote noncrossover recombination between centromere repeats on the same chromatid to prevent isochromosome formation. Nucleic Acids Res. 44, 10744–10757 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hammond TM Sixteen Years of Meiotic Silencing by Unpaired DNA. Adv. Genet 97, 1–42 (2017). [DOI] [PubMed] [Google Scholar]
- 155.Hammond TM et al. Fluorescent and bimolecular-fluorescent protein tagging of genes at their native loci in Neurospora crassa using specialized double-joint PCR plasmids. Fungal Genet. Biol 48, 866–873 (2011). [DOI] [PubMed] [Google Scholar]
- 156.Wang Y, Smith KM, Taylor JW, Freitag M & Stajich JE Endogenous small RNA mediates meiotic silencing of a novel DNA transposon. G3 (Bethesda) 5, 1949–1960 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Raju NB, Metzenberg RL & Shiu PKT Neurospora Spore Killers Sk-2and Sk-3Suppress Meiotic Silencing by Unpaired DNA. Genetics 176, 43–52 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Modzelewski AJ, Holmes RJ, Hilz S, Grimson A & Cohen PE AGO4 Regulates Entry into Meiosis and Influences Silencing of Sex Chromosomes in the Male Mouse Germline. Dev Cell 23, 251–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lucchesi JC & Kuroda MI Dosage compensation in drosophila. Cold Spring Harbor Perspectives in Biology 7, 1–21 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deng X & Meller VH roXRNAs Are Required for Increased Expression of X-Linked Genes in Drosophila melanogasterMales. Genetics 174, 1859–1866 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Menon DU & Meller VH A role for siRNA in X-chromosome dosage compensation in Drosophila melanogaster. Genetics 191, 1023–1028 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Menon DU, Coarfa C, Xiao W, Gunaratne PH & Meller VH siRNAs from an X-linked satellite repeat promote X-chromosome recognition in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A 111, 16460–16465 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Deshpande N & Meller VH Chromatin That Guides Dosage Compensation Is Modulated by the siRNA Pathway in Drosophila melanogaster. Genetics 209, 1085–1097 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Noto T & Mochizuki K Whats, hows and whys of programmed DNA elimination in Tetrahymena. Open Biol. 7, 170172–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mochizuki K Conjugation-specific small RNAs in Tetrahymena have predicted properties of scan (scn) RNAs involved in genome rearrangement. Genes & Development 18, 2068–2073 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Noto T et al. The Tetrahymena Argonaute-Binding Protein Giw1p Directs a Mature Argonaute-siRNA Complex to the Nucleus. Cell 140, 692–703 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Noto T & Mochizuki K Small RNA-Mediated trans-Nuclear and trans- Element Communications in Tetrahymena DNA Elimination. Curr. Biol 28, 1938–1949.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Liu Y et al. RNAi-dependent H3K27 methylation is required for heterochromatin formation and DNA elimination in Tetrahymena. Genes & Development 21, 1530–1545 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Madireddi MT et al. Pdd1p, a novel chromodomain-containing protein, links heterochromatin assembly and DNA elimination in Tetrahymena. Cell 87, 75–84 (1996). [DOI] [PubMed] [Google Scholar]
- 170.Coyne RS, Nikiforov MA, Smothers JF, Allis CD & Yao MC Parental expression of the chromodomain protein Pdd1p is required for completion of programmed DNA elimination and nuclear differentiation. Mol Cell 4, 865–872 (1999). [DOI] [PubMed] [Google Scholar]
- 171.Taverna SD, Coyne RS & Allis CD Methylation of Histone H3 at Lysine 9 Targets Programmed DNA Elimination in Tetrahymena. Cell 110, 701–711 (2002). [DOI] [PubMed] [Google Scholar]
- 172.Noto T et al. Small-RNA-Mediated Genome-wide trans-Recognition Network in Tetrahymena DNA Elimination. Mol Cell 59, 229–242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vogt A & Mochizuki K A Domesticated PiggyBac Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in Tetrahymena. PLoS Genet 9, e1004032–14 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cheng C-Y, Vogt A, Mochizuki K & Yao M-C A domesticated piggyBac transposase plays key roles in heterochromatin dynamics and DNA cleavage during programmed DNA deletion in Tetrahymena thermophila. Mol Biol Cell 21, 1753–1762 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lhuillier-Akakpo M et al. Local Effect of Enhancer of Zeste-Like Reveals Cooperation of Epigenetic and cis-Acting Determinants for Zygotic Genome Rearrangements. PLoS Genet 10, e1004665–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sandoval PY, Swart EC, Arambasic M & Nowacki M Functional diversification of Dicer-like proteins and small RNAs required for genome sculpting. Dev Cell 28, 174–188 (2014). [DOI] [PubMed] [Google Scholar]
- 177.Fang W, Wang X, Bracht JR, Nowacki M & Landweber LF Piwi-Interacting RNAs Protect DNA against Loss during Oxytricha Genome Rearrangement. Cell 151, 1243–1255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Postberg J et al. 27nt-RNAs guide histone variant deposition via ‘RNA-induced DNA replication interference’ and thus transmit parental genome partitioning in Stylonychia. Epigenetics & Chromatin 11, 31 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Noto T & Mochizuki K Whats, hows and whys of programmed DNA elimination in Tetrahymena. Open Biol. 7, 170172–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zaratiegui M et al. RNAi promotes heterochromatic silencing through replication-coupled release of RNA Pol II. Nature 479, 135–138 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Castel SE et al. Dicer Promotes Transcription Termination at Sites of Replication Stress to Maintain Genome Stability. Cell 159, 572–583 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aguilera A & García-Muse T R loops: from transcription byproducts to threats to genome stability. Mol Cell 46, 115–124 (2012). [DOI] [PubMed] [Google Scholar]
- 183.Khurana JS, Clay DM, Moreira S, Wang X & Landweber LF Small RNA-mediated regulation of DNA dosage in the ciliate Oxytricha. RNA 24, 18–29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee H-C et al. qiRNA is a new type of small interfering RNA induced by DNA damage. Nature 459, 274–277 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cecere G & Cogoni C Quelling targets the rDNA locus and functions in rDNA copy number control. BMC Microbiol 9, 44–10 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cam HP et al. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat Genet 37, 809–819 (2005). [DOI] [PubMed] [Google Scholar]
- 187.Yang Q, Ye QA & Liu Y Mechanism of siRNA production from repetitive DNA. Genes & Development 29, 526–537 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Peng JC & Karpen GH H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability. Nat Cell Biol 9, 25–35 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Peng JC & Karpen GH Heterochromatic Genome Stability Requires Regulators of Histone H3 K9 Methylation. PLoS Genet 5, e1000435–14 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Francia S et al. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature 488, 231–235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tang K-F et al. Decreased Dicer expression elicits DNA damage and up-regulation of MICA and MICB. The Journal of Cell Biology 182, 233–239 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Burger K et al. Nuclear phosphorylated Dicer processes doublestranded RNA in response to DNA damage. J. Cell Biol 216, 2373–2389 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Swahari V et al. Essential Function of Dicer in Resolving DNA Damage in the Rapidly Dividing Cells of the Developing and Malignant Cerebellum. Cell Rep 14, 216–224 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ciccia A & Elledge SJ The DNA damage response: making it safe to play with knives. Mol Cell 40, 179–204 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Carmichael JB, Provost P, Ekwall K & Hobman TC Ago1 and Dcr1, Two Core Components of the RNA Interference Pathway, Functionally Diverge from Rdp1 in Regulating Cell Cycle Events in Schizosaccharomyces pombe. Mol Biol Cell 15, 1425–1435 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kloc A, Zaratiegui M, Nora E & Martienssen R RNA interference guides histone modification during the S phase of chromosomal replication. Curr. Biol 18, 490–495 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Catalanotto C, Nolan T & Cogoni C Homology effects in Neurospora crassa. FEMS Microbiology Letters 254, 182–189 (2006). [DOI] [PubMed] [Google Scholar]
- 198.Chang SS et al. Homologous recombination as a mechanism to recognize repetitive DNA sequences in an RNAi pathway. Genes & Development 27, 145–150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wei W et al. A Role for Small RNAs in DNA Double-Strand Break Repair. Cell 149, 101–112 (2012). [DOI] [PubMed] [Google Scholar]
- 200.Miki D et al. Efficient Generation of diRNAs Requires Components in the Posttranscriptional Gene Silencing Pathway. Sci. Rep 7, 301 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Michalik KM, Böttcher R & Förstemann K A small RNA response at DNA ends in Drosophila. Nucleic Acids Res. 40, 9596–9603 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Merk K et al. Splicing stimulates siRNA formation at Drosophila DNA double-strand breaks. PLoS Genet 13, e1006861–22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schmidts I, Böttcher R, Mirkovic-Hösle M & Förstemann K Homology directed repair is unaffected by the absence of siRNAs in Drosophila melanogaster. Nucleic Acids Res. 44, 8261–8271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Burger K & Gullerova M Nuclear re-localization of Dicer in primary mouse embryonic fibroblast nuclei following DNA damage. PLoS Genet 14, e1007151 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Michelini F et al. Damage-induced lncRNAs control the DNA damage response through interaction with DDRNAs at individual double-strand breaks. Nat Cell Biol 19, 1400–1411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Francia S, Cabrini M, Matti V, Oldani A & d’Adda di Fagagna F DICER, DROSHA and DNA damage response RNAs are necessary for the secondary recruitment of DNA damage response factors. J Cell Sci 129, 1468–1476 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Gao M et al. Ago2 facilitates Rad51 recruitment and DNA double-strand break repair by homologous recombination. Cell Res. 24, 532–541 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Burger K, Schlackow M & Gullerova M Tyrosine kinase c-Abl couples RNA polymerase II transcription to DNA double-strand breaks. Nucleic Acids Res. 47, 3467–3484 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lu W-T et al. Drosha drives the formation of DNA:RNA hybrids around DNA break sites to facilitate DNA repair. Nat Commun 9, 532 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang Q & Goldstein M Small RNAs Recruit Chromatin-Modifying Enzymes MMSET and Tip60 to Reconfigure Damaged DNA upon Double-Strand Break and Facilitate Repair. Cancer Res 76, 1904–1915 (2016). [DOI] [PubMed] [Google Scholar]
- 211.Skourti-Stathaki K, Kamieniarz-Gdula K & Proudfoot NJ R-loops induce repressive chromatin marks over mammalian gene terminators. Nature 516, 436–439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ohle C et al. Transient RNA-DNA Hybrids Are Required for Efficient Double-Strand Break Repair. Cell 167, 1001–1010.e7 (2016). [DOI] [PubMed] [Google Scholar]
- 213.Marteijn JA, Lans H, Vermeulen W & Hoeijmakers JHJ Understanding nucleotide excision repair and its roles in cancer and ageing. Nat Rev Mol Cell Biol 15, 465–481 (2014). [DOI] [PubMed] [Google Scholar]
- 214.Chitale S & Richly H DICER and ZRF1 contribute to chromatin decondensation during nucleotide excision repair. Nucleic Acids Res. 45, 5901–5912 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chitale S & Richly H DICER- and MMSET-catalyzed H4K20me2 recruits the nucleotide excision repair factor XPA to DNA damage sites. The Journal of Cell Biology 217, 527–540 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Calses PC et al. DGCR8 Mediates Repair of UV-Induced DNA Damage Independently of RNA Processing. Cell Rep 19, 162–174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schalk C et al. Small RNA-mediated repair of UV-induced DNA lesions by the DNA DAMAGE-BINDING PROTEIN 2 and ARGONAUTE 1. Proceedings of the National Academy of Sciences 114, E2965–E2974 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ozata DM, Gainetdinov I, Zoch A, OCarroll D & Zamore PD PIWI-interacting RNAs: small RNAs with big functions. Nat. Rev. Genet 1–20 (2019). doi: 10.1038/s41576-018-0073-3 [DOI] [PubMed] [Google Scholar]


