Abstract
Chimeric antigen receptor T (CAR-T) cells are a type of tumor immunotherapy that is a breakthrough technology in the clinical treatment of tumors. The basic principle of this method is to extract the patient's T cells and equip them with targeting recognition receptors of tumor cells and return them to the patient's body to recognize and kill tumor cells specifically. Most CAR-T cell therapies treat hematological diseases such as leukemia or lymphoma and achieved encouraging results. The safety and effectiveness of CAR-T cell technology in solid tumor treatment require to be improved, although it has demonstrated promising efficacy in treating hematological malignancies. It is worth noting that certain patients may experience fatal adverse reactions after receiving CAR-T cell therapy. At present, the difficulty of this therapy mainly lies in how to reduce adverse reactions and target escape effects during the course of treatment. The improvement of CAR-T cell therapy mainly focuses on improving CAR-T structure, finding suitable tumor targets and combining them with immune checkpoint inhibitors to the enhance efficacy and safety of treatment. The problems in the rapid development of CAR-T cell therapy provide both obstacles and opportunities. The present review elaborates on the clinical application of CAR-T cell technology to provide a reference for clinical practice and research on tumor treatment.
Keywords: chimeric antigen receptor T cells, tumor treatment, adverse reactions, overcoming strategies
1. Introduction
According to the Global Health Assessment released by the World Health Organization in 2020, it is estimated that in the next 20 years, the number of global cancer cases may increase by 60% and the tumor burden is also increasing (1). The traditional methods for treating tumors, such as chemotherapy or radiotherapy, cause significant harm to patients and the curative effect is not satisfactory. A novel treatment method different from traditional therapy has been invented, namely chimeric antigen receptor T-cell (CAR-T) therapy. CAR-T cell therapy is a method of adoptive cell therapy that modifies a patient's peripheral T cells in vitro by genetic engineering, which are then infused into the patient to recognize and kill tumor cells for treatment, as illustrated in Fig. 1. CAR-T cells have achieved satisfactory results in treating hematopoietic malignancies (2). CAR-T cell therapy (Kymriah), approved by the Food and Drug Administration (FDA) to treat B-cell acute lymphoblastic leukemia (ALL) in 2017, was the world's first approved CAR-T product (3). CAR-T products, including Kymirah, Yescarta, Tecartus, Breyanzi, Abecma, Relma-cel and Carvykti, have been approved for treating hematological malignancies, as summarized in Table I; the CAR-T treatment method and commercialization process of CAR-T have been formally recognized by the FDA.
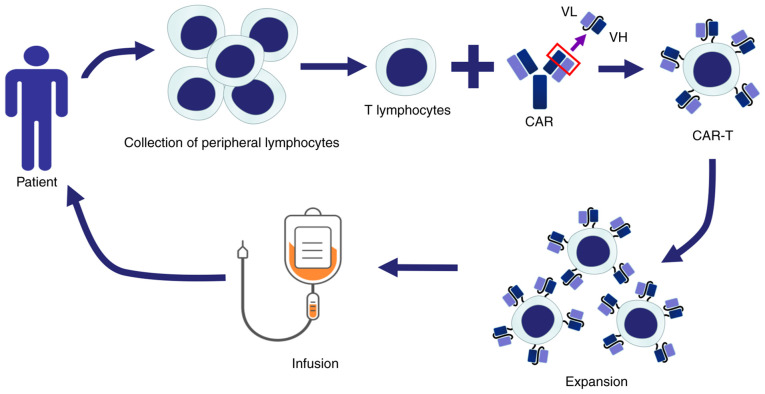
Figure 1.
Schematic illustrating the principle of CAR-T cell therapy: Lymphocytes are obtained from patients and T cells are isolated. These T cells are transferred into specific CAR genes through viral or non-viral vectors and thereby transformed into CAR-T cells. These CAR-T cells are expanded in vitro and returned to the patient's body to identify and kill tumor cells specifically. CAR-T, chimeric antigen receptor T-cell; VL, variable region of light chain; VH, variable region of heavy chain.
Table I.
CAR-T products approved to treat hematological malignancies.
| Drug | Target | Targeted diseases | Time of approval | Listing location | Guide price |
|---|---|---|---|---|---|
| Kymriah | CD19 | B-ALL; DLBCL | August 2017 | USA | 475,000 USD |
| Yescarta | CD19 | DLBCL; RRFL | October 2017 | USA | 373,000 USD |
| Tecartus | CD19 | R/R MCL | July 2020 | USA | 373,000 USD |
| Breyanzi | CD19 | DLBCL | February 2021 | USA | 410,000 USD |
| Abecma | BCMA | R/R MM | March 2021 | USA | 437,000 USD |
| Relma-cel | CD19 | DLBCL | September 2021 | China | 12,00,000 RMB |
| Carvykti | BCMA | R/R MM | February 2022 | USA | 460,000 USD |
The guide price is for the full treatment for one patient. B-ALL, B-cell acute lymphoblastic leukemia; DLBCL, Diffuse large B-cell lymphoma; R/R FL, relapsed or refractory follicular lymphoma; R/R MCL, relapsed or refractory mantle cell lymphoma; R/R MM, relapsed or refractory multiple myeloma.
However, CAR-T cell therapy has limitations in the treatment of solid tumors due to solid tumor cells frequently expressing different tumor antigens, which may reduce its targeting effect, and patients receiving CAR-T cell therapy frequently experience side effects, such as cytopenia, and have an increased risk of infection in the presence of neutropenia, previous immunosuppression, lymphatic clearance, tocilizumab or steroid application (4,5). The antigens selected by CAR-T cell therapy may be expressed on both tumor and normal tissue cells, causing CAR-T cells to attack normal cells mistakenly and produce off-target effects. In addition, CAR-T cells are prone to release cytokines excessively after entering the patient's body, resulting in cytokine release syndrome (CRS). Patients affected by CRS may experience severe inflammation, hypotension, hypoxia and even death, thus significantly reducing the efficacy and safety of CAR-T therapy (6). Therefore, summarizing the current research progress and adverse reactions of CAR-T cell therapy is expected to provide references for improving the efficacy and safety of this method.
2. Development of CAR-T cell products
T cells have an essential role in the process of anti-tumor immunity. The activation of T cells requires multiple signals, including T cell receptor (TCR) recognition of antigenic peptide-major histocompatibility complex (MHC) on the surface of antigen-presenting cells (APC), which may process antigen and present it in conjunction with MHC molecules on the cell surface and interact with appropriate T cell receptors, followed by co-stimulation factor and cytokine signaling. Since the MHC mainly activates TCRs, tumor cells frequently downregulate MHC expression to avoid being recognized by the immune system. The traditional TCRs must recognize MHC-antigen peptide complexes to activate T cells, while CARs may recognize antigens directly and lead to T cell activation. CARs are able to mimic the TCR's function and overcome their limitation. Gross et al (7) constructed immunoglobulin-TCR chimeric molecules as functional receptors with antibody-type specificity in 1989; this receptor is able to recognize pathogenic antigens and transmit T cell activation signals. Since the single-chain antibody fragment (scFv) formed by the variable regions of the heavy chain and light chain of the antibody has the same function of recognizing antigen as the Fab region of the antibody, Eshhar et al (8) later replaced TCR with the scFv fragment, which fused with CD3ζ chain; this fusion protein effectively promoted the activation of T cells and killed lymphoma cells expressing specific antigens in vitro, which is referred to as the first generation of CAR-T cells. In the clinic, the patient's T cells were extracted and integrated with genetically engineered CARs. The cultured CAR-T cells were injected back into the patient to attack tumor cells specifically and achieve the purpose of treatment. The T cells were activated antigen-specifically with no MHC restriction during the treatment. CAR-T cells are able to bind and kill tumors specifically by releasing cytotoxic substances. Unlike cytotoxic T lymphocyte (CTL) cells, CAR-T cells are able to recognize the antigen of tumor cells through scFv. This recognition is not restricted by MHC molecules, which has significant advantages over the traditional CTL cells, as illustrated in Fig. 2.
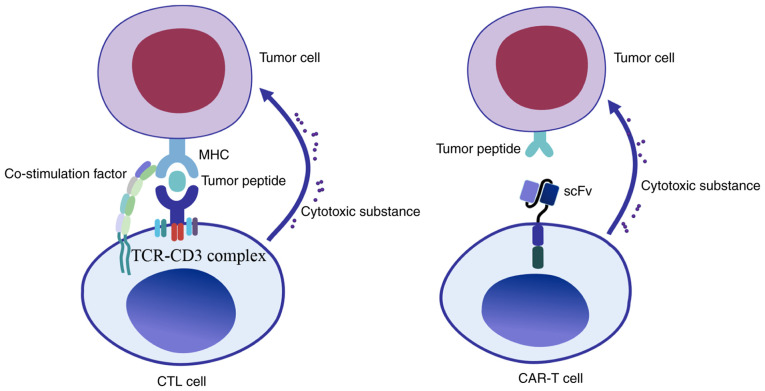
Figure 2.
Mechanisms of tumor cell recognition by T cells. In traditional CTL cell therapy, the TCR recognizes tumor antigen peptides presented by MHC molecules on the tumor cells' surface through the TCR-CD3 complex, activates with the assistance of co-stimulation molecules and releases cytotoxic substances to kill tumor cells. By contrast, CAR-T is able to recognize the antigen of tumor cells through the scFv; this recognition is not restricted by MHC molecules, which has significant advantages over the traditional CTL cell therapy. CTL, cytotoxic T lymphocyte; TCR, T cell receptor; scFv, single chain fragment variable; MHC, major histocompatibility complex; CAR-T, chimeric antigen receptor T-cell.
The basic structure of CARs includes a tumor-associated antigen (TAA) binding region, an extracellular hinge region, a transmembrane region and an intracellular immunoreceptor tyrosine-based activation motif (ITAM). TAA is abundantly located on the tumor cell surface and seldom expressed in normal tissues, and various TAAs may be used as target antigens for CAR-T cells. The tumor target antigen is crucial for the specificity, efficacy and safety of therapy. CAR-T cells have developed rapidly in recent years; the extracellular regions of these CAR-T cells are V regions of antibodies and the transmembrane part is almost the same. The intracellular structure distinguishes these CAR-T cells. The first-generation CAR-T cell intracellular segment is relatively simple; it is mainly composed of the ITAM region of the ζ chain of the CD3 molecule and there are only three pairs of ITAMs, which lack costimulatory molecules. These first-generation CAR-T cells may only cause transient T cell proliferation and less cytokine secretion, cannot provide a sustained anti-tumor effect and clinical treatment is unsatisfactory. Due to the inefficiency of signal transmission and short survival time of CAR-T cells in patients, various studies have rated them as having ‘no significant effect but great potential’ (9,10). Costimulatory molecules, such as 4-1BB (CD137), CD28, tumor necrosis factor receptor superfamily member 4 (OX40) or inducible costimulatory molecule were added to the intracellular region of the second-generation CAR, these costimulatory molecules activate JNK, ERK and NF-κB signaling pathways in T cells, having a more substantial killing effect on tumor cells. However, most vectors used in second-generation CAR-T cells are retroviruses that may only carry limited gene fragments, which may restrict the function of these CAR-T cells. The third-generation CAR-T cells use a lentivirus as a transfection vector, which may carry larger gene segments into T cells. The intracellular part frequently contains two or more costimulatory signal regions to stimulate T cells to produce numerous cytokines. Third-generation CAR-T cells have a more potent anti-tumor effect compared with second-generation (11,12). However, certain studies have indicated that the killing activity of third-generation CAR-T cells has not been improved significantly; this may be because the activation signal generated by ITAM has reached its threshold in terms of lymphocyte activation and only adding costimulatory molecules may not improve the efficacy for CAR-T cells (13). In the fourth generation of CAR-T technology, the IL-12 gene was introduced into CAR-T cells to enhance the killing ability. When CAR-T cells recognize tumor cells and activate, they secrete a large amount of IL-12 at the tumor site and recruit various innate immune cells to kill the tumor cell. Considering the side effects of CAR-T cells, a suicide gene added to CAR may control the survival time of CAR-T cells in vivo. CAR-T cells are mainly used to treat hematological tumors and have not achieved satisfactory results in treating solid tumor tissue. the main reason is that CAR-T cells cannot enter the solid tumor. Therefore, the receptor of certain cytokines or chemokines is added into CAR to increase the infiltration ability of T lymphocytes in tumor tissue, thereby enhancing the killing effects of solid tumors. This is also one of the design ideas for the fourth generation of CAR-T cells (14–16). The third-generation and fourth-generation CAR-T cells contain more costimulatory signals to secrete more cytokines, which may cause a robust immune response in the body, so the second-generation technology is rather used to treat hematological tumors based on safety considerations. These autologous CAR-T cell therapies have numerous limitations, including high cost, long manufacturing cycle and limited cell sources. Furthermore, certain patients are unsuitable for autologous CAR-T cell therapy due to physical reasons such as an advanced tumour and/or physical weakness, accompanied by severe infection or poor immune activity after repeated chemotherapy and radiotherapy. The fifth-generation CAR-T technology called Universal CAR-T (UCAR-T) cell therapy is an attractive breakthrough (17).
CAR-T cell therapy is expensive, e.g., Kymriah costs $475,000 per patient in the US, which is unaffordable for an ordinary family. The high price is not only due to the increased investment cost of CAR-T, but CAR-T therapy also requires to be prepared according to the individual differences of patients and it is similar to a personalized medical service. To overcome the high cost of CAR-T therapy, scientists proposed the concept of UCAR-T. There are two strategies for UCAR-T: Universal T cells and UCAR. The autologous CAR-T cells for immunotherapy frequently have numerous limitations and it is difficult to generate sufficient numbers of effective CAR-T cells for various unpredictable reasons. Allogeneic CAR-T cells derived from healthy donors may have clinical efficacy; however, these allogeneic cells may induce graft-vs. -host disease (GVHD) after infusion, while UCAR-T cells may overcome this obstacle. The steps include isolation of T lymphocytes from healthy donors, transfer of CAR genes and other genes (such as costimulatory or suicide genes) into T cells through viral vectors and knockout of GVHD-related genes [TCR or human leukocyte antigen (HLA)] through gene-editing technology. T cells edited by transcription activator-like effector nuclease technology performed well and CRISPR/Cas9 technology is also undergoing clinical trials (18,19).
A novel UCAR-T contains a two-receptor system; the universal receptor part is T cells with leucine adapters (Zipcar) and the ligand part is single chain fragment variable fused to a cognate leucine zipper with leucine adapters that is able to target tumor antigens. This UCAR-T may perform receptor switching against different tumor antigens or recognize multiple tumor antigens by combining the universal receptor part without further modification of T cells (20). UCAR T-19 cells developed using lentivirus and CRISPR/Cas9 gene-editing systems express mutated B2M-HLA-E and mutated B2M-HLA-G fusion proteins that may prevent the destruction of host cells by allogeneic NK cells (21). UCAR-T technology currently faces numerous challenges such as GVDH reaction caused by trace amounts of TCR-positive CAR-T cells, off-target effects and difficult amplification in vivo due to immune system rejection. Thus, most UCAR-T projects are in the preclinical stage and perfect technology is expected to ensure the safety and efficacy of UCAR-T cell therapy (22). The development of CAR-T cell products is presented in Fig. 3.
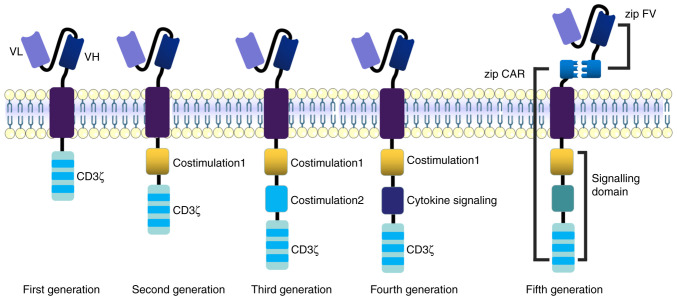
Figure 3.
Evolution of the development of CAR-T cell products: First-generation CAR-T cells only contain CD3ζ-derived signaling modules. Second-generation CAR-T cells contain a CD3ζ-derived signaling module and a co-stimulatory domain. Third-generation CAR-T cells contain a CD3ζ-derived signaling module and two co-stimulatory domains, including 4-1BB, CD28, OX40 or ICOS. Fourth-generation CAR-T cells contain a CD3ζ-derived signaling module, co-stimulatory domain and a cytokine (such as IL-12) producing module. Fifth-generation CAR-T cells consist of Zip CAR and zip FV with leucine adapters. The horizontal membrane in the middle is the cell membrane, which is a lipid bilayer. CAR-T, chimeric antigen receptor T-cell; VL, variable region of light chain; VH, variable region of heavy chain; FV, fragment variable; OX40, tumor necrosis factor receptor superfamily member 4; ICOS, inducible costimulatory molecule.
3. Application of CAR-T cells in hematological tumor treatment
B-cell ALL (B-ALL)
B-ALL is a common hematological disease with a poor prognosis for most patients. Currently, bone marrow transplantation is the best treatment. Early CD19-targeted CAR-T cell therapy was unsatisfactory in treating relapsed or refractory (R/R) B-ALL, as more than half of the patients relapsed after CD19 CAR-T therapy; however, CAR-T cell therapy combined with allogeneic stem cell transplantation (SCT) may increase patient survival (23). In a study including 30 adult patients with B-ALL receiving CD19+ CAR-T therapy, the median time to progression was 5.5 months, median survival was 7.5 months and 13 patients (43%) achieved a complete response (CR). Of note, 7 of 12 patients (58.3%) receiving blinatumomab/inotuzumab after CAR-T treatment failure achieved a CR, and thus, new treatment strategies are required in order to reduce the risk associated with treatment and improve patient prognosis (24). Patients with B-ALL are prone to relapse after receiving CD19-targeted immunotherapy and a new alternative strategy is thus required. CD72 is a cell marker of poor prognosis in patients with B-ALL and synthetic CD72-specific nanobodies incorporated into CARs demonstrated robust activity against B-cell malignancy models, which may offer new ideas for treatment (25). CD19-targeting CAR-T cell therapy is limited in certain conditions, while bispecific CAR-T cells targeting both CD19 and CD22 may overcome this limitation and all 6 patients with R/R B-ALL achieved minimal residual disease (MRD)-negative CR after receiving this bispecific CAR-T cell therapy (26). After patients with B-ALL received allogenic hematopoietic SCT (HSCT), only those who achieved a second remission without MRD were able to obtain satisfactory long-term survival; all of those 5 patients with B-ALL achieved rapid tumor eradication and MRD(−) complete remission (CR) after CD19+ CAR-T cell therapy. Elevated cytokines are directly related to tumor burden during CAR-T cell infusion and certain patients require steroids to alleviate cytokine-mediated toxicity (27). Another study indicated that among 30 patients with relapsed ALL who received CD19+CAR-T therapy, 27 patients (90%) achieved CR, the 6-month event-free survival rate was 67% and the overall survival rate was 78%. However, 27% of patients exhibited severe cytokine release syndrome, which was alleviated after treatment with an anti-IL-6 receptor antibody; this may be associated with a high disease burden prior to infusion (28). Patients with B-ALL are prone to CRS after CAR-T therapy and corticosteroids or IL-6 receptor inhibitors may alleviate it effectively. In addition, serum C-reactive protein is a reliable indicator for judging the severity of CRS (29). The relapses of patients with ALL after single-targeted CAR-T therapy may be related to tumor antigen escape. CD19 and CD22 dual-targeted CAR-T cells (CTA101) based on CRISPR/Cas9 technology have advantages as a novel therapy; the CR was 83.3% after 6 patients with R/R ALL accepted CTA101 infusion and 5 patients achieved CR or CR with incomplete hematologic recovery, remaining MRD negative (30).
T-cell ALL (T-ALL)
Despite encouraging results for CAR-T in treating B-ALL, significant challenges remain in T-ALL treatment. T-ALL is a hematological malignancy caused by the malignant transformation and clonal expansion of T-lineage precursor cells in the bone marrow and thymus. It mainly occurs in children and adolescents and has a higher recurrence rate, lower remission rate and lower long-term survival rate than B-ALL (31). Studies have indicated that anti-CD7 CAR-T has an important role in treating T-ALL. In a phase I clinical trial of T-ALL, 20 participants received anti-CD7 CAR-T treatment and 90% of patients (n=18) achieved CR. Most patients experienced adverse reactions such as cytokine release syndrome; most adverse reactions were reversible and only one patient died of fungal pneumonia-related pulmonary hemorrhage (32). Another study confirmed that 20 patients with R/R T-ALL (n=14) and lymphoblastic lymphoma (n=6) accepted anti-CD7 CAR-T therapy, of which 19 patients achieved MRD negative CR in the bone marrow by day 28 (33). CD7 is a promising therapeutic target due to its wide expression in almost all T-cell malignancies. A study reported that a patient with T-ALL received anti-CD7 CAR-T therapy, was injected with 5×106/kg anti-CD7 CAR-T cells, and the patient's blood and bone marrow achieved remission (34). Another patient with T-ALL received autologous anti-CD7 CAR-T cells and achieved remission on day 17; although symptoms of cytopenias occurred from days 14 to 21, this patient accepted HSCT after achieving CR, suggesting that anti-CD7 CAR-T may be a safe and effective strategy for the treatment of patients with T-ALL (35). In a study using an anti-CD7 nanobody fragment coupled with an endoplasmic reticulum/Golgi retention domain demonstrated that cells transduced with CD7-CAR were able to prevent fratricide and achieve expansion; these cells exhibited antitumor potential in CD7-positive malignant cell lines and patient-derived xenograft models (36). Research has indicated that anti-CD7 CAR-T may cause T cells to fratricide and Png et al (37) conjugated protein expression blocker (PEBL) to CD7 fragment; this anti-CD7 PEBL eliminates CD7 expression in T cells, effectively alleviating CAR-T-mediated fratricide without damaging T cell proliferation or the interferon-γ and tumor necrosis factor secretion ability. PEBL-CAR T cells are cytotoxic to CD7+ leukemia cells in vitro, both in leukemia cell lines and patient-derived T-ALL xenografts.
Non-Hodgkin lymphoma (NHL)
NHL is a heterogeneous disease and most NHLs are B cell types that account for 70–85%. Despite decades of advances in chemotherapy regimens and new treatments, the disease remains incurable. The CD19+ CAR-T cell therapy axicabtagene ciloleucel (Axi-Cel) has a significant effect in patients with refractory large B-cell lymphoma. After failure of conventional therapy, 111 patients received Axi-Cel therapy, the objective response rate was 82%, the CR rate was 54%, 42% of patients had sustained remission and the overall survival rate of all patients was 52% at 18 months of follow-up in this trial. The most common adverse events during treatment were neutropenia (78%), anemia (43%) and thrombocytopenia (38%). Patients who received CAR T-cell therapy with Axi-Cel had high levels of durable response (38). A long-term study indicated that 60% of patients with B-cell lymphoma treated with CD19+ CAR-T cells (CTL019) were still in remission after 5 years and the clinical effect of CTL019 was not affected, although different patients accepted different lymphodepletion regimens (39). CD28 and 4-1BB may activate different signaling pathways and combining them may overcome the limitations of costimulation alone. A total of 16 patients with relapsed/refractory lymphoma accepted infusion of 2G CAR-T cells (with CD28 only) and 3G CAR-T cells (CD28 and 4-1BB); of these, 3 patients achieved CR and 3 patients partial remission. CRS occurred in 6 patients, but the symptoms were mild and did not require anti-IL-6 therapy, indicating CD28 combined with 4-1BB may produce satisfactory therapeutic effects in patients with lymphoma (40). A CAR-T that is able to recognize C-X-C motif chemokine receptor 5 (CXCR5) with high affinity may target B-NHL cells effectively based on the characteristic that CXCR5 is highly expressed on the surface of lymphoma cells; application of CXCR5+ CAR-T cells in a mouse lymphoma model was able to kill malignant B lymphoma cells specifically, providing a promising strategy for the treatment of B-NHL (41).
CAR-T cell therapy in B-NHL may cause various adverse reactions and the prognosis of patients is not as satisfactory as that of ALL; the lower response rate may be related to the physical barrier of the tumor inhibitory microenvironment. In addition, certain patients relapsed after treatment, which may be related to limited CAR-T cell persistence and CD19 antigen escape (42). Studies suggested that about half of the patients with R/R diffuse large B-cell lymphoma (DLBCL) experience severe side effects after CAR-T cell therapy. Axi-Cel and tisagenlecleucel are new CAR-T cell therapies that combine CAR T-cells with a fusion protein between interferon and anti-CD20 monoclonal antibody with checkpoint inhibitors or sensitizers that have apoptotic-regulatory effects; the remission rate of patients may reach 83%, the complete response rate is between 40 and 58% and the side effects of CAR-T cell therapy are significantly reduced (43). Although the therapeutic effect of CAR-T cells in NHL is impressive, the single-targeted CAR-T therapy still has its limitations. The most common reason is tumor cell immune escape due to target antigen loss and multi-targeted CAR-T may achieve a better therapeutic effect (44).
Multiple myeloma (MM)
MM is a hematological malignancy of B cells caused by the abnormal proliferation of plasma cells. Tumor cells express a large amount of B cell maturation antigen (BCMA) instead of CD19, which is different from B cell leukemia and lymphoma. Therefore, BCMA is an important therapeutic target for MM. A variety of BCMA-targeted therapies have achieved encouraging results in the treatment of MM (45). The first human trial of BCMA-CAR-T cells in MM was performed by the National Cancer Institute in 2016, in which 12 patients received BCMA-CAR-T cell treatment. In this trial, two patients accepted a dose level of 9×106 CAR-T cells/kg body weight treatment, bone marrow plasma cells became undetectable by flow cytometry after treatment and they entered a stringent complete remission. However, two patients experienced toxicities related to cytokine release, including fever, hypotension and dyspnea. These results were the first to indicate the anti-tumor activity of CAR-BCMA-T cells in patients with MM (46). The FDA approved idecabtagene vicleucel for the treatment of patients with myeloma in 2021. It is the first FDA-approved cell therapy for MM, which is expected to prolong the remission period and even cure patients with MM (47). In a clinical trial conducted by the Shanghai Institute of Hematology targeting BCMA (LCAR-B38M) in 17 patients with R/R MM, the overall response rate was 88.2% and 13 patients reached CR, 2 patients had a very good partial response (VGPR) and 1 was a nonresponder. Of note, 8 patients maintained CR or VGPR after 417 days of follow-up. It was also observed that anti-CAR antibody positivity is a high-risk factor for relapse. Bi-epitopic CAR-T cells targeting BCMA represent a promising therapy for R/R MM and most adverse effects were manageable (48).
Since the changes in BCMA expression levels reduce the activity of CAR-T cells, methods to minimize tumor cell escape are under consideration. Antigens other than BCMA on the surface of plasma cells may be new targets for CAR-T cell recognition. CS1 (also known as CD319, CRACC or SLAMF7) is a newly discovered target and BCMA-CS1 bispecific CAR-T cells demonstrated a superior tumor cell clearance ability as compared with BCMA and CS1 single-targeted CAR-T therapy. Combining this treatment with anti-programmed death 1 (PD-1) antibodies may accelerate tumor cell clearance in vivo (49). Targeting CD38 antigen in patients with MM using CAR-T cells in which the intracellular region is composed of CD28 and 4-1BB may stimulate T cell proliferation and improve the anti-tumor function significantly, which may provide a new target for MM therapy (50).
4. Application of CAR-T cells in solid tumor treatment
The latest global cancer data in 2021 pointed out that most cancers are solid tumors, which are therefore the major focus in the fight against cancer (51). However, CAR-T cell therapy has not received a substantial breakthrough in treating solid tumors and most clinical trials for solid tumors are at an early stage. One of the important reasons is that solid tumor cells frequently express multiple tumor antigens, which are less targeted than hematological tumors. Tumor cells and surrounding non-tumor cells form a tumor microenvironment (TME) with hypoxia and insufficient nutrients. Furthermore, inhibitory factors of tumor cells, high glucose consumption and competition of amino acids in the TME deprive T cells of adequate energy support and lead to cell unresponsiveness or exhaustion (52–55). The TME is thus unfavorable for the survival of CAR-T cells, as outlined in Fig. 4. Currently, the improvement of CAR-T cells in the treatment of solid tumors mainly focuses on finding suitable targets to enhance safety and efficacy.
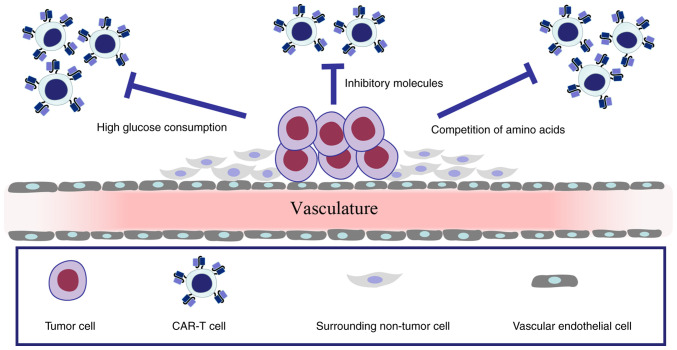
Figure 4.
Tumor cells and surrounding non-tumor cells form a TME; the functions of CAR-T cells are impaired by inhibitory molecules, high glucose consumption or competition of amino acids by tumor cells in the TME. TME, tumor microenvironment; CAR-T cell, chimeric antigen receptor T-cell.
Solid tumors that are responsible for >85% of cancer-associated deaths require angiogenesis for tumor cell growth and metastasis. Targeting vascular endothelial growth factor receptor 2, which is overexpressed in the tumor vasculature, is a promising strategy for anti-angiogenic tumor treatment; the solid tumor may be inhibited by targeting blood vessels in clinical treatment (56,57). The heterogeneity of specific tumor antigens is an obstacle to CAR-T cell therapy. Tumor antigen escape may be overcome by multispecific targeting and CAR-T cells with three specific antigen recognition domains may recognize the tumor antigens specifically. Epidermal growth factor receptor (EGFR), epithelial cell adhesion molecule and human epidermal growth factor receptor 2 (HER2) have been linked together in a single CAR via ankyrin repeat proteins and these CAR-T cells had a strong killing ability of tumor cells such as K562, MDA-MB-231 and NCI-H1975 (58). The immunosuppressive TME is an environment for tumor cells to survive and tumor-associated macrophages (TAM) are an important part of the TME. TAMs expressing folate receptor beta (FRβ) may promote the growth of tumor cells. mFRβ CAR-T cells may selectively reduce the enrichment of pro-inflammatory monocytes, delay tumor progression and prolong survival in an ovarian cancer mouse model (59). OX40 is a potent CAR-T enhancer that may reduce CAR-T cell apoptosis by upregulating Bcl-2 family members and enhancing CAR-T cell cytotoxicity through NF-κB, MAPK and PI3K-AKT signaling. CAR-T cells with OX40 exhibited enhanced amplification and antitumor activity in mouse tumor models (60). CAR-T cells targeting prostate-specific membrane antigen (PSMA) are able to mediate tumor vascular destruction, inhibit tumor cell growth and accelerate the regression of prostate cancer. These CAR-T cells may also ablate PSMA+ blood vessels, leading to the depletion of tumor cells and reducing the tumor burden in the ovarian cancer mouse model (61). Reports have confirmed that CAR-T cells targeting tumor endothelial marker TEM-8 have a satisfactory effect in triple-negative breast cancer treatment, as the patients' tumor volume was significantly reduced after CAR-T cell therapy and the blood vessels in the cancer had decreased significantly after 2 months (62).
Glypican-3 (GPC3) is a carcinoembryonic glycoprotein highly expressed in hepatocellular carcinoma (HCC). GPC3 CAR-T cells exhibited potent cytotoxicity against HCC cell lines, reduced the tumor burden in the Huh-7 ×enograft mouse model and secreted high levels of IFN-γ to inhibit tumor cell growth (63,64). Natural killer group 2 member D (NKG2D) ligand (NKG2DL) is low expressed on the normal cell surface but overexpressed on malignant cells, providing an ideal target for CAR-T therapy. A CAR-T cell based on NKG2D, 4-1BB and CD3ζ was able to kill the HCC cell lines SMMC-7721 and MHCC97H with high expression levels of NKG2DL effectively, which may provide new treatment ideas for NKG2DL+ HCC (65). Tumor-associated-mucin 1 (tMUC1) is a mucosal mucin molecule abnormally expressed in pancreatic ductal adenocarcinoma (PDA). tMUC1-CAR T cells are able to bind to tMUC1 on PDA cells specifically and control the growth of pancreatic tumors in vivo effectively (66). Carbonic anhydrase IX (CAIX) is a membrane protein that is highly expressed in glioblastoma. CAR-T cells targeting CAIX inhibited the growth of glioblastoma cell lines LN229, T98G, A172 and U251 in vitro, and intratumoral injection of CAIX CAR-T cells significantly inhibited tumor growth in a glioblastoma mouse model, indicating the feasibility of the use of CAIX CAR-T cells in the treatment of glioblastoma (67). MAGE family member A1 (MAGE-A1) is a member of the cancer-testis antigen family, which is highly expressed in lung adenocarcinoma (LUAD). MAGE-A1 CAR-T cells can inhibit the growth of LUAD cell lines PC9, H1299, GLC82 and A549, which may be a strategy for the treatment of MAGE-A1+ LUAD (68). Carcinoembryonic antigen (CEA) is a tissue non-specific tumor antigen expressed in numerous tumor types, such as colon cancer, esophageal cancer and non-small cell lung cancer. In a study, 10 patients with CEA+ colorectal cancer received CEA CAR-T cell therapy, of which 7 patients reached stable disease and 2 patients remained stable for >30 weeks; the serum CEA level decreased significantly (69).
Malignant glioma is a common brain tumor type and the traditional treatment, such as surgical resection, radiotherapy or chemotherapy, is not satisfactory. A novel CAR-T cell (GCT02) that may recognize epidermal growth factor receptor variant III (EGFRvIII) on the tumor cells' surface may mediate tumor cell elimination, indicating a promising treatment for glioblastoma (70). In a study, 10 patients with recurrent glioblastoma accepted EGFRvIII-CAR-T cell infusion; the EGFRv expression level decreased in 5 patients, and 1 patient had no disease progression at the 18-month follow-up (71). However, certain glioblastoma tumor cells do not express any mutated forms of EGFR but express normal EGFR abundantly due to the heterogeneity of tumor cells. Only relying on CAR-T cells that recognize EGFRvIII alone may not eliminate tumors. A novel type of CAR-T cells carry a bispecific T-cell engager (BiTE) that may target both mutant EGFR and wild-type EGFR, enhancing the binding ability between effector T cells and tumor cells and significantly attenuating the effect of EGFRvIII antigen loss on CAR-T cells effectively. Model mice with glioblastoma received BiTE CAR-T cell infusion and tumor cells were no longer visible in 80% of the mice. Furthermore, these BiTE CAR-T cells had no toxicity to human skin grafts in vivo (72). A new type of synNotch CAR-T cells is able to selectively kill neuroblastoma cells; the synNotch protein on the surface of T cells is modified to recognize tumor antigen GD2 and then instructs T cells to recognize the tumor antigen B7H3, leading to the tumor cells' regression. These CAR-T cells had high specificity in mouse models and did not exhaust quickly after killing tumor cells, demonstrating a long-lasting anti-tumor effect, indicating that this new therapy may help patients achieve long-term survival (73). The popular targets of CAR-T cells in solid tumors are summarized in Table II.
Table II.
Popular targets in solid tumor treatment.
| Tumor site/origin | Potential therapeutic target for treatment |
|---|---|
| Skin | GD2, VEGFR |
| Head and neck | EGFR |
| Brain | EGFRvIII, HER2, IL-13RA |
| Nerve cells | GD2, PHOX2B |
| Lungs | CEA, EGFR, HER2, MSLN, CLDN18, ROR, GD2 |
| Breast | GD2, EGFR, ROR, TEM8, HER2, MSLN |
| Stomach | CEA, HER2, EpCAM, CLDN18, MSLN |
| Liver | CEA, GPC3, HER2 |
| Pancreas | CEA, MSLN, MUC1, HER2, CLDN18, EGFR |
| Ovary | CEA, MSLN, L1CAM, MUC16, CLDN18, PSCA |
| Kidney | CAIX, VEGFR |
| Prostate | PSMA, PSCA |
| Colon | CEA, GUCY2C, CLDN18 |
| Soft tissue | GD2, HER2 |
GD2, diasialoganglioside 2; VEGFR, vascular endothelial growth factor receptor; EGFR, epidermal growth factor receptor; EGFRvIII, EGFR variant III; HER2, human epidermal growth factor receptor 2; IL-13RA, interleukin-13RA; PHOX2B, paired-like homebox 2B; MSLN, mesothelin; CLDN18, claudin 18; ROR, tyrosine protein kinase transmembrane receptor; TEM8, tumor endothelial marker 8; CEA, carcinoembryonic antigen; EpCAM, epithelial cell adhesion molecule; GPC3, glycipan 3; MUC1, mucin; L1CAM, L1 cell adhesion molecule; PSCA, prostate stem cell antigen; CAIX, carbonic anhydrase IX; PSMA, prostate-specific membrane antigen; GUCY2C, guanylate cyclase 2C.
5. Side effects of CAR-T cell therapy and strategies to overcome them
Although CAR-T cell therapy has made significant progress in clinical trials, adverse reactions during the treatment also affected the efficacy of this treatment. CRS, on-target off-tumor effects, allergic reactions and neurological toxicity syndrome are common adverse reactions during the therapy. This may be due to the insufficient structure of CAR-T cells, differences in patient constitution and the dose of CAR-T cells used. The side effects during CAR-T treatment may lead to different responses in different systems and most of the toxic and side effects are reversible if patients receive a timely and correct intervention.
CRS
CRS is the most common side effect, including neutropenia, anemia and thrombocytopenia. CRS may be divided into four grades according to its severity. The symptoms of first-grade CRS are mild and do not threaten the patient's life; the patient will get relief after receiving antipyretics, antiemetics and other treatments. Symptoms of grade 2 CRS include fever, neutropenia and elevated creatinine and patients require hospitalization. Symptoms of grade 3 CRS include hypotension, coagulation disorders and hypoxia, and affected patients require supportive treatments such as vasopressors, supplemental oxygen and fibrinogen infusion. Grade 4 CRS may cause hypotension or severe organ toxicity and is frequently life-threatening; patients require mechanical ventilation and high-dose vasopressor therapy (74). Certain patients experience cytopenias after receiving CAR-T cell therapy; neutropenia or thrombocytopenia is the most common cytopenia and is called prolonged hematologic toxicity (PHT) if persisting for >30 days. In a clinical trial of CD19 CAR-T cell treatment of patients with R/R DLBCL, PHT was reported in 18 of 31 (58%) patients and the 1-year overall survival was significantly reduced in patients with PHT compared to patients without PHT (75). Red blood cell and platelet transfusions are recommended for patients with prolonged severe anemia and thrombocytopenia (76). There are 2 strategies for CRS, a preventive strategy to reduce the risk and a remedial strategy in the event of lethal toxicity. Cytoreductive chemotherapy to reduce the disease burden of patients prior to CAR-T cell infusion is recommended for the prevention strategies. The dosage of CAR-T cells should be strictly controlled in the treatment of patients with a high disease burden, predictive biomarkers such as IFN-γ, IL-2, IL-2Rα, IL-6, TNF-α, C-reactive protein should be closely monitored and in high-risk patients, intervention should be performed early (77). Tocilizumab, an IL-6 receptor antagonist, is recommended for patients with severe CRS as a remedial strategy. In a study, the symptoms were rapidly alleviated in all patients after receiving tocilizumab infusion; however, this may increase the possibility of infection in certain patients and corticosteroids may be considered if patients have no response to tocilizumab (78). In addition, Koristka et al (79) designed a technology to incorporate an elimination tag (E-tag) into CAR-T cells; if there are extra CAR-T cells in the patient, a single-chain antibody corresponding to E-tag may be infused to kill E-tagged CAR-T cells while sparing cells lacking the E-tag, reducing the number of CAR-T cells in patients in a short time. In most cases, CRS is alleviated after administration of steroids or tocilizumab treatment. CAR-T cells may also be eliminated selectively by introducing suicide genes, which induce apoptosis of CAR-T cells to reduce their toxicity if adverse reactions occur. The suicide gene encodes herpes simplex virus thymidine kinase, which may act on DNA polymerase, prevent DNA synthesis and lead to cell apoptosis, preventing the overactivation of CAR-T cells (80). The common cell suicide gene Caspase 9 may also be introduced into CAR-T cells and external chemicals such as FK506-binding protein may induce Caspase 9 dimerization and initiate the mitochondrial apoptosis pathway, promoting CAR-T cell apoptosis and improving the safety of CAR-T cell therapy (81). The occurrence of CRS is closely related to the infusion dose and the patient's physical condition. Due to the difference in CAR-T cell culture methods and patient inclusion criteria in each clinical trial, it is difficult to set a uniform dose and clinicians should make corresponding adjustments based on clinical test results, combined with the tumor type and the patient's tumor burden.
On-target off-tumor effects
Antigens on tumor cell surfaces may be divided into tumor-specific antigens and TAAs. TAAs are recognized by CAR-T cells and these antigens are not unique to tumor cells; when CAR-T cells get in contact with non-tumor tissue, target antigens cause ‘on-target off-tumor effects’. This adverse reaction affects the gastrointestinal tract, blood, respiratory and other systems. The main method to manage the impact of adverse reactions on patients is to control the amount of CAR-T cells and attenuate the function of CAR-T cells. However, this also weakens the anti-tumor effect of CAR-T cells simultaneously. One of the promising approaches is to find intracellular targets to overcome the off-target effects, which may overcome the limitations of tumor cell surface targets. Recognizing intracellular target peptides presented by MHC molecules on the tumor cell surface by CAR-T cells may be a promising therapeutic direction (82). Only targeting a single tumor antigen may increase the risk of targeting normal tissues; a case report indicated that a patient with colon cancer died after receiving HER2 CAR-T cell therapy, possibly due to CAR-T cells immediately localizing to the lung and triggering the release of cytokines by recognizing ERBB2 on lung epithelial cells (83). Fedorov et al (84) designed antigen-specific inhibitory CARs derived from PD-1 or CTLA-4 molecules, which may competitively inhibit the function of CAR-T cells. This molecule is able to effectively prevent CAR-T cells' toxicity while maintaining their anti-tumor activity. Designing a CAR-T cell that targets multiple tumor antigens simultaneously appears to be a feasible way to avoid the off-target effects. Combining antibodies that may recognize different tumor antigens in the same CAR-T cell may significantly enhance the specificity. A co-transduced CAR-T T cell targeting two prostate tumor antigens, PSMA and prostate stem cell antigen, is able to destroy tumors that express both antigens but does not affect tumors expressing either antigen alone; this ‘tumor-sensing’ strategy may help avoid certain side effects of single-targeted therapy (85). The novel BCMA-OR-CD38 Tan CAR-T cell that exhibits cytotoxicity against BCMA or CD38-positive tumor cells exhibited superior cytotoxicity and proliferation ability in vitro. Importantly, these CAR-T cells achieved complete tumor clearance in myeloma-bearing mice and no recurrence was observed (86).
Other toxic and side effects
CAR-T cells may cause tumor lysis syndrome (TLS) once entering the patient's body. Intracellular substances and metabolites are released into the blood if tumor cells are killed by CAR-T cells. Kidneys are not able to filter these substances, ultimately resulting in severe metabolic disorders, the main clinical manifestation of which include hyperkalemia, hyperphosphatemia, hypocalcemia, acute renal failure and severe cardiac arrhythmias that may occur in extreme cases. The incidence of TLS in hematological malignancies is significantly higher than that in patients with solid tumors, and metabolically active cancers, such as B-cell lymphoma, have the highest risk of TLS. Early identification of patients with a risk of TLS and targeted prevention may reduce patient mortality (87). Rasburicase is a recombinant uric acid oxidase that converts uric acid to soluble allantoin; it is the first-choice therapeutic drug for patients with TLS. Allopurinol may prevent uric acid formation by inhibiting xanthine and hypoxanthine oxidase and may also be used in TLS (88). Neurotoxic syndrome is frequently associated with CAR-T cell therapy, including somnolence, aphasia, ataxia, disorientation and seizures; the occurrence of seizures may be related to corticosteroids or IL-1/IL-6 signaling antagonists applied during treatment (89). The lentiviral vectors are mainly used to mediate the expression of chimeric antigen receptors. However, lentiviruses are not safe in certain cases and lentivirus may cause T lymphoma in mice, considering the characteristics of lentivirus integration into the host's chromosomes (90). There is a specific error probability in the production of CAR-T cells using the lentiviral packaging system; it has been reported that the TET2 gene is integrated randomly into CAR and this integration endows CAR-T cells with enhanced proliferation and anti-tumor ability, indicating that the TET2 gene may help to improve CAR-T cell immunotherapy (91). A report described a patient with B-ALL who accepted CAR-T cell therapy, and during T cell manufacturing, the CAR gene was unintentionally introduced into a single leukemic B cell and its product was bound in cis mode to the CD19 epitope on the surface of leukemic cells, masking it from recognition by CAR-T cells. The patient ultimately died of complications related to progressive leukemia. Therefore, finding a safer delivery system for producing CAR-T cells is necessary for the future (92). DNA-carrying biodegradable nanoparticles may introduce leukemia-targeting CAR genes into the nucleus of T cells effectively compared with the traditional process; these nanoparticles are effective for transfection of CAR genes, and they are safer and less expensive (93). Electro-transfection is a theoretically safer method for CAR-T cell production that does not promote the transgene integration into the host genome; CAR-T cells may be generated within 6 days and exhibit potent cytotoxicity against target cells (94). With the development of modern medical technology, CAR-T cell therapy has undergone rigorous clinical trials prior to application and its safety has been guaranteed. A small number of patients may experience severe side effects; although most of the side effects are relatively mild, patients require to receive timely and effective interventions to alleviate the toxic effects once adverse effects occur.
6. Summary and outlook
Clinical trials have proved that CAR-T cell therapy is encouraging in the treatment of hematological malignancies. However, it is still difficult in solid tumors. The problems in the rapid development of CAR-T cell therapy are both obstacles and opportunities. Target antigen escape, a tumor-suppressive microenvironment and adverse reactions during the treatment may influence the efficacy and safety. Improving the anti-tumor effect and reducing the incidence of adverse reactions is necessary for future applications, and a more detailed understanding of CAR-T and tumor cells is essential. The next generation of CAR-T cells should solve the problems of expensive treatment, side effects during the treatment and long preparation time. The application prospect of CAR-T therapy is broad and it will bring a breakthrough in treating human tumors and other diseases.
Acknowledgements
Not applicable.
Funding Statement
This research was supported by the Shandong Province Health Department (grant nos. 2019WS589 and 2017WS407) and the Shandong Province Traditional Chinese Medicine Science and Technology Development Plan (grant no. 2017-216).
Availability of data and materials
Not applicable.
Authors' contributions
DLD conceived and designed the review. LC wrote the first draft. LC, TX and BW participated in writing the manuscript. All authors contributed to the article and read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rafei H, Kantarjian HM, Jabbour EJ. Recent advances in the treatment of acute lymphoblastic leukemia. Leuk Lymphoma. 2019;60:2606–2621. doi: 10.1080/10428194.2019.1605071. [DOI] [PubMed] [Google Scholar]
- 3.Kansagra AJ, Frey NV, Bar M, Laetsch TW, Carpenter PA, Savani BN, Heslop HE, Bollard CM, Komanduri KV, Gastineau DA, et al. Clinical utilization of chimeric antigen receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)-an expert opinion from the European society for blood and marrow transplantation (EBMT) and the American society for blood and marrow transplantation (ASBMT) Bone Marrow Transplant. 2019;54:1868–1880. doi: 10.1038/s41409-019-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson RC, Maus MV. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat Rev Cancer. 2021;21:145–161. doi: 10.1038/s41568-020-00323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bupha-Intr O, Haeusler G, Chee L, Thursky K, Slavin M, Teh B. CAR-T cell therapy and infection: A review. Expert Rev Anti Infect Ther. 2021;19:749–758. doi: 10.1080/14787210.2021.1855143. [DOI] [PubMed] [Google Scholar]
- 6.Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegler EL, Wang P. Preclinical models in chimeric antigen receptor-engineered T-cell therapy. Hum Gene Ther. 2018;29:534–546. doi: 10.1089/hum.2017.243. [DOI] [PubMed] [Google Scholar]
- 10.Grigor EJM, Fergusson D, Kekre N, Montroy J, Atkins H, Seftel MD, Daugaard M, Presseau J, Thavorn K, Hutton B, et al. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: A systematic review and meta-analysis. Transfus Med Rev. 2019;33:98–110. doi: 10.1016/j.tmrv.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Roselli E, Frieling JS, Thorner K, Ramello MC, Lynch CC, Abate-Daga D. CAR-T engineering: Optimizing signal transduction and effector mechanisms. BioDrugs. 2019;33:647–659. doi: 10.1007/s40259-019-00384-z. [DOI] [PubMed] [Google Scholar]
- 12.Hu W, Huang X, Huang X, Chen W, Hao L, Chen Z. Chimeric antigen receptor modified T cell (CAR-T) co-expressed with ICOSL-41BB promote CAR-T proliferation and tumor rejection. Biomed Pharmacother. 2019;118:109333. doi: 10.1016/j.biopha.2019.109333. [DOI] [PubMed] [Google Scholar]
- 13.Ramello MC, Benzaïd I, Kuenzi BM, Lienlaf-Moreno M, Kandell WM, Santiago DN, Pabón-Saldaña M, Darville L, Fang B, Rix U, et al. An immunoproteomic approach to characterize the CAR interactome and signalosome. Sci Signal. 2019;12:eaap9777. doi: 10.1126/scisignal.aap9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abramson JS. Anti-CD19 CAR T-cell therapy for B-cell non-hodgkin lymphoma. Transfus Med Rev. 2020;34:29–33. doi: 10.1016/j.tmrv.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Chmielewski M, Abken H. TRUCKs: The fourth generation of CARs. Expert Opin Biol Ther. 2015;15:1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 16.Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang CH, Saso K, Butler MO, Minden MD, Hirano N. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018;24:352–359. doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin H, Cheng J, Mu W, Zhou J, Zhu L. Advances in universal CAR-T cell therapy. Front Immunol. 2021;12:744823. doi: 10.3389/fimmu.2021.744823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Lin Q, Song Y, Liu D. Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol. 2018;11:132. doi: 10.1186/s13045-018-0677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qasim W, Zhan H, Samarasinghe S, Adams S, Amrolia P, Stafford S, Butler K, Rivat C, Wright G, Somana K, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9:eaaj2013. doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 20.Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173:1426–1438.e11. doi: 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Y, Xu B, Wu Z, Bo J, Tong C, Chen D, Wang J, Wang H, Wang Y, Han W. Mutant B2M-HLA-E and B2M-HLA-G fusion proteins protects universal chimeric antigen receptor-modified T cells from allogeneic NK cell-mediated lysis. Eur J Immunol. 2021;51:2513–2521. doi: 10.1002/eji.202049107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: Development and challenges. Nat Rev Drug Discov. 2020;19:185–199. doi: 10.1038/s41573-019-0051-2. [DOI] [PubMed] [Google Scholar]
- 23.Martino M, Alati C, Canale FA, Musuraca G, Martinelli G, Cerchione C. A review of clinical outcomes of CAR T-cell therapies for B-acute lymphoblastic leukemia. Int J Mol Sci. 2021;22:2150. doi: 10.3390/ijms22042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wudhikarn K, Flynn JR, Rivière I, Gönen M, Wang X, Senechal B, Curran KJ, Roshal M, Maslak PG, Geyer MB, et al. Interventions and outcomes of adult patients with B-ALL progressing after CD19 chimeric antigen receptor T-cell therapy. Blood. 2021;138:531–543. doi: 10.1182/blood.2020009515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nix MA, Mandal K, Geng H, Paranjape N, Lin YT, Rivera JM, Marcoulis M, White KL, Whitman JD, Bapat SP, et al. Surface proteomics reveals CD72 as a target for in vitro-evolved nanobody-based CAR-T cells in KMT2A/MLL1-rearranged B-ALL. Cancer Discov. 2021;11:2032–2049. doi: 10.1158/2159-8290.CD-20-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, Han X, Liu Y, Zhang W, Wang C, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13:30. doi: 10.1186/s13045-020-00856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Zhou Y, Zhang M, Ge W, Li Y, Yang L, Wei G, Han L, Wang H, Yu S, et al. CRISPR/Cas9-engineered universal CD19/CD22 dual-targeted CAR-T cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia. Clin Cancer Res. 2021;27:2764–2772. doi: 10.1158/1078-0432.CCR-20-3863. [DOI] [PubMed] [Google Scholar]
- 31.Raetz EA, Teachey DT. T-cell acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016:580–588. doi: 10.1182/asheducation-2016.1.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pan J, Tan Y, Wang G, Deng B, Ling Z, Song W, Seery S, Zhang Y, Peng S, Xu J, et al. Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: First-in-human, phase I trial. J Clin Oncol. 2021;39:3340–3351. doi: 10.1200/JCO.21.00389. [DOI] [PubMed] [Google Scholar]
- 33.Lu P, Liu Y, Yang J, Zhang X, Yang X, Wang H, Wang L, Wang Q, Jin D, Li J, Huang X. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: First-in-human phase 1 clinical trial. Blood. 2022;140:321–334. doi: 10.1182/blood.2021014498. [DOI] [PubMed] [Google Scholar]
- 34.Dai HP, Cui W, Cui QY, Zhu WJ, Meng HM, Zhu MQ, Zhu XM, Yang L, Wu DP, Tang XW. Haploidentical CD7 CAR T-cells induced remission in a patient with TP53 mutated relapsed and refractory early T-cell precursor lymphoblastic leukemia/lymphoma. Biomark Res. 2022;10:6. doi: 10.1186/s40364-022-00352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie L, Ma L, Liu S, Chang L, Wen F. Chimeric antigen receptor T cells targeting CD7 in a child with high-risk T-cell acute lymphoblastic leukemia. Int Immunopharmacol. 2021;96:107731. doi: 10.1016/j.intimp.2021.107731. [DOI] [PubMed] [Google Scholar]
- 36.Chen D, You F, Xiang S, Wang Y, Li Y, Meng H, An G, Zhang T, Li Z, Jiang L, et al. Chimeric antigen receptor T cells derived from CD7 nanobody exhibit robust antitumor potential against CD7-positive malignancies. Am J Cancer Res. 2021;11:5263–5281. [PMC free article] [PubMed] [Google Scholar]
- 37.Png YT, Vinanica N, Kamiya T, Shimasaki N, Coustan-Smith E, Campana D. Blockade of CD7 expression in T cells for effective chimeric antigen receptor targeting of T-cell malignancies. Blood Adv. 2017;1:2348–2360. doi: 10.1182/bloodadvances.2017009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chong EA, Ruella M, Schuster SJ, Lymphoma Program Investigators at the University of Pennsylvania Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med. 2021;384:673–674. doi: 10.1056/NEJMc2030164. [DOI] [PubMed] [Google Scholar]
- 40.Ramos CA, Rouce R, Robertson CS, Reyna A, Narala N, Vyas G, Mehta B, Zhang H, Dakhova O, Carrum G, et al. In vivo fate and activity of second-versus third-generation CD19-specific CAR-T cells in B cell non-Hodgkin's lymphomas. Mol Ther. 2018;26:2727–2737. doi: 10.1016/j.ymthe.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bunse M, Pfeilschifter J, Bluhm J, Zschummel M, Joedicke JJ, Wirges A, Stark H, Kretschmer V, Chmielewski M, Uckert W, et al. CXCR5 CAR-T cells simultaneously target B cell non-Hodgkin's lymphoma and tumor-supportive follicular T helper cells. Nat Commun. 2021;12:240. doi: 10.1038/s41467-020-20488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yin Z, Zhang Y, Wang X. Advances in chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Biomark Res. 2021;9:58. doi: 10.1186/s40364-021-00309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mihăilă RG. Chimeric antigen receptor-engineered T-cells-a new way and era for lymphoma treatment. Recent Pat Anticancer Drug Discov. 2019;14:312–323. doi: 10.2174/1574892814666191022164641. [DOI] [PubMed] [Google Scholar]
- 44.Shah NN, Maatman T, Hari P, Johnson B. Multi targeted CAR-T cell therapies for B-cell malignancies. Front Oncol. 2019;9:146. doi: 10.3389/fonc.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu B, Jiang T, Liu D. BCMA-targeted immunotherapy for multiple myeloma. J Hematol Oncol. 2020;13:125. doi: 10.1186/s13045-020-00962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ali SA, Shi V, Maric I, Wang M, Stroncek DF, Rose JJ, Brudno JN, Stetler-Stevenson M, Feldman SA, Hansen BG, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferment B, Arnulf B. CAR-T cells immunotherapy in multiple myeloma: Present and future. Bull Cancer. 2021;108((10 Suppl)):S65–S72. doi: 10.1016/j.bulcan.2021.09.005. (In French) [DOI] [PubMed] [Google Scholar]
- 48.Xu J, Chen LJ, Yang SS, Sun Y, Wu W, Liu YF, Xu J, Zhuang Y, Zhang W, Weng XQ, et al. Exploratory trial of a biepitopic CAR T-targeting B cell maturation antigen in relapsed/refractory multiple myeloma. Proc Natl Acad Sci USA. 2019;116:9543–9551. doi: 10.1073/pnas.1819745116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zah E, Nam E, Bhuvan V, Tran U, Ji BY, Gosliner SB, Wang X, Brown CE, Chen YY. Systematically optimized BCMA/CS1 bispecific CAR-T cells robustly control heterogeneous multiple myeloma. Nat Commun. 2020;11:2283. doi: 10.1038/s41467-020-16160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drent E, Poels R, Ruiter R, van de Donk NWCJ, Zweegman S, Yuan H, de Bruijn J, Sadelain M, Lokhorst HM, Groen RWJ, et al. Combined CD28 and 4-1BB costimulation potentiates affinity-tuned chimeric antigen receptor-engineered T cells. Clin Cancer Res. 2019;25:4014–4025. doi: 10.1158/1078-0432.CCR-18-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 52.Springuel L, Lonez C, Alexandre B, Van Cutsem E, Machiels JH, Van Den Eynde M, Prenen H, Hendlisz A, Shaza L, Carrasco J, et al. Chimeric antigen receptor-T cells for targeting solid tumors: Current challenges and existing strategies. Biodrugs. 2019;33:515–537. doi: 10.1007/s40259-019-00368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang X, Xu J, Liu M, Xing H, Wang Z, Huang L, Mellor AL, Wang W, Wu S. Adoptive CD8+ T cell therapy against cancer: Challenges and opportunities. Cancer Lett. 2019;462:23–32. doi: 10.1016/j.canlet.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 54.Irving M, Vuillefroy de Silly R, Scholten K, Dilek N, Coukos G. Engineering chimeric antigen receptor T-cells for racing in solid tumors: Don't forget the fuel. Front Immunol. 2017;8:267. doi: 10.3389/fimmu.2017.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 56.Hajari Taheri F, Hassani M, Sharifzadeh Z, Behdani M, Arashkia A, Abolhassani M. T cell engineered with a novel nanobody-based chimeric antigen receptor against VEGFR2 as a candidate for tumor immunotherapy. IUBMB Life. 2019;71:1259–1267. doi: 10.1002/iub.2019. [DOI] [PubMed] [Google Scholar]
- 57.Akbari P, Huijbers EJM, Themeli M, Griffioen AW, van Beijnum JR. The tumor vasculature an attractive CAR T cell target in solid tumors. Angiogenesis. 2019;22:473–475. doi: 10.1007/s10456-019-09687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balakrishnan A, Rajan A, Salter AI, Kosasih PL, Wu Q, Voutsinas J, Jensen MC, Plückthun A, Riddell SR. Multispecific targeting with synthetic ankyrin repeat motif chimeric antigen receptors. Clin Cancer Res. 2019;25:7506–7516. doi: 10.1158/1078-0432.CCR-19-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez-Garcia A, Lynn RC, Poussin M, Eiva MA, Shaw LC, O'Connor RS, Minutolo NG, Casado-Medrano V, Lopez G, Matsuyama T, Powell DJ. J: CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. 2021;12:877. doi: 10.1038/s41467-021-20893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang H, Li F, Cao J, Wang X, Cheng H, Qi K, Wang G, Xu K, Zheng J, Fu YX, Yang X. A chimeric antigen receptor with antigen-independent OX40 signaling mediates potent antitumor activity. Sci Transl Med. 2021;13:eaba7308. doi: 10.1126/scitranslmed.aba7308. [DOI] [PubMed] [Google Scholar]
- 61.Santoro SP, Kim S, Motz GT, Alatzoglou D, Li C, Irving M, Powell DJ, Jr, Coukos G. T cells bearing a chimeric antigen receptor against prostate-specific membrane antigen mediate vascular disruption and result in tumor regression. Cancer Immunol Res. 2015;3:68–84. doi: 10.1158/2326-6066.CIR-14-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Byrd TT, Fousek K, Pignata A, Szot C, Samaha H, Seaman S, Dobrolecki L, Salsman VS, Oo HZ, Bielamowicz K, et al. TEM8/ANTXR1-specific CAR T cells as a targeted therapy for triple-negative breast cancer. Cancer Res. 2018;78:489–500. doi: 10.1158/0008-5472.CAN-16-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishida T, Kataoka H. Glypican 3-targeted therapy in hepatocellular carcinoma. Cancers (Basel) 2019;11:1339. doi: 10.3390/cancers11091339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang P, Qin W, Liu T, Jiang D, Cui L, Liu X, Fang Y, Tang X, Jin H, Qian Q. PiggyBac-engineered T cells expressing a glypican-3-specific chimeric antigen receptor show potent activities against hepatocellular carcinoma. Immunobiology. 2020;225:151850. doi: 10.1016/j.imbio.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 65.Sun B, Yang D, Dai H, Liu X, Jia R, Cui X, Li W, Cai C, Xu J, Zhao X. Eradication of hepatocellular carcinoma by NKG2D-based CAR-T cells. Cancer Immunol Res. 2019;7:1813–1823. doi: 10.1158/2326-6066.CIR-19-0026. [DOI] [PubMed] [Google Scholar]
- 66.Yazdanifar M, Zhou R, Grover P, Williams C, Bose M, Moore LJ, Wu ST, Maher J, Dreau D, Mukherjee AP. Overcoming immunological resistance enhances the efficacy of a novel anti-tMUC1-CAR T cell treatment against pancreatic ductal adenocarcinoma. Cells. 2019;8:1070. doi: 10.3390/cells8091070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui J, Zhang Q, Song Q, Wang H, Dmitriev P, Sun MY, Cao X, Wang Y, Guo L, Indig IH, et al. Targeting hypoxia downstream signaling protein, CAIX, for CAR T-cell therapy against glioblastoma. Neuro Oncol. 2019;21:1436–1446. doi: 10.1093/neuonc/noz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mao Y, Fan W, Hu H, Zhang L, Michel J, Wu Y, Wang J, Jia L, Tang X, Xu L, et al. MAGE-A1 in lung adenocarcinoma as a promising target of chimeric antigen receptor T cells. J Hematol Oncol. 2019;12:106. doi: 10.1186/s13045-019-0793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang C, Wang Z, Yang Z, Wang M, Li S, Li Y, Zhang R, Xiong Z, Wei Z, Shen J, et al. Phase I escalating-dose trial of CAR-T therapy targeting CEA+ metastatic colorectal cancers. Mol Ther. 2017;25:1248–1258. doi: 10.1016/j.ymthe.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abbott RC, Verdon DJ, Gracey FM, Hughes-Parry HE, Iliopoulos M, Watson KA, Mulazzani M, Luong K, D'Arcy C, Sullivan LC, et al. Novel high-affinity EGFRvIII-specific chimeric antigen receptor T cells effectively eliminate human glioblastoma. Clin Transl Immunology. 2021;10:e1283. doi: 10.1002/cti2.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9:eaaa0984. doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi BD, Yu X, Castano AP, Bouffard AA, Schmidts A, Larson RC, Bailey SR, Boroughs AC, Frigault MJ, Leick MB, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol. 2019;37:1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 73.Moghimi B, Muthugounder S, Jambon S, Tibbetts R, Hung L, Bassiri H, Hogarty MD, Barrett DM, Shimada H, Asgharzadeh S. Preclinical assessment of the efficacy and specificity of GD2-B7H3 SynNotch CAR-T in metastatic neuroblastoma. Nat Commun. 2021;12:511. doi: 10.1038/s41467-020-20785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Porter D, Frey N, Wood PA, Weng Y, Grupp SA. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol. 2018;11:35. doi: 10.1186/s13045-018-0571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagle SJ, Murphree C, Raess PW, Schachter L, Chen A, Hayes-Lattin B, Nemecek E, Maziarz RT. Prolonged hematologic toxicity following treatment with chimeric antigen receptor T cells in patients with hematologic malignancies. Am J Hematol. 2021;96:455–461. doi: 10.1002/ajh.26113. [DOI] [PubMed] [Google Scholar]
- 76.Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, Dreger P. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32:34–48. doi: 10.1016/j.annonc.2020.10.478. [DOI] [PubMed] [Google Scholar]
- 77.Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6:4. doi: 10.1186/s40364-018-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szenes V, Curran KJ. Utilization of CAR T cell therapy in pediatric patients. Semin Oncol Nurs. 2019;35:150929. doi: 10.1016/j.soncn.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 79.Koristka S, Ziller-Walter P, Bergmann R, Arndt C, Feldmann A, Kegler A, Cartellieri M, Ehninger A, Ehninger G, Bornhäuser M, Bachmann MP. Anti-CAR-engineered T cells for epitope-based elimination of autologous CAR T cells. Cancer Immunol Immunother. 2019;68:1401–1415. doi: 10.1007/s00262-019-02376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonini C, Ferrari G, Verzeletti S, Servida P, Zappone E, Ruggieri L, Ponzoni M, Rossini S, Mavilio F, Traversari C, Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 81.Straathof KC, Pulè MA, Yotnda P, Dotti G, Vanin EF, Brenner MK, Heslop HE, Spencer DM, Rooney CM. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gerber HP, Sibener LV, Lee LJ, Gee M. Intracellular targets as source for cleaner targets for the treatment of solid tumors. Biochem Pharmacol. 2019;168:275–284. doi: 10.1016/j.bcp.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 83.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng Y, Liu X, Li X, Zhou Y, Song Z, Zhang J, Shi B, Wang J. Novel BCMA-OR-CD38 tandem-dual chimeric antigen receptor T cells robustly control multiple myeloma. Oncoimmunology. 2021;10:1959102. doi: 10.1080/2162402X.2021.1959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilson FP, Berns JS. Tumor lysis syndrome: New challenges and recent advances. Adv Chronic Kidney Dis. 2014;21:18–26. doi: 10.1053/j.ackd.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miao L, Zhang Z, Ren Z, Li Y. Reactions related to CAR-T cell therapy. Front Immunol. 2021;12:663201. doi: 10.3389/fimmu.2021.663201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gust J, Ponce R, Liles WC, Garden GA, Turtle CJ. Cytokines in CAR T cell-associated neurotoxicity. Front Immunol. 2020;11:577027. doi: 10.3389/fimmu.2020.577027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Newrzela S, Cornils K, Li Z, Baum C, Brugman MH, Hartmann M, Meyer J, Hartmann S, Hansmann ML, Fehse B, von Laer D. Resistance of mature T cells to oncogene transformation. Blood. 2008;112:2278–2286. doi: 10.1182/blood-2007-12-128751. [DOI] [PubMed] [Google Scholar]
- 91.Fraietta JA, Nobles CL, Sammons MA, Lundh S, Carty SA, Reich TJ, Cogdill AP, Morrissette JJD, Denizio JE, Reddy S, et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558:307–312. doi: 10.1038/s41586-018-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, Klichinsky M, Shestova O, Patel PR, Kulikovskaya I, et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat Med. 2018;24:1499–1503. doi: 10.1038/s41591-018-0201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, Bonagofski E, Wohlfahrt ME, Pillai SPS, Stephan MT. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Z, Qiu S, Zhang X, Chen W. Optimized DNA electroporation for primary human T cell engineering. Bmc Biotechnol. 2018;18:4. doi: 10.1186/s12896-018-0419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.