Significance
Insular woodiness, the evolution of woodiness in plant species that inhabit islands, is the most conspicuous aspect of island floras, comparable to flightlessness in birds and dwarfism/gigantism in mammals. Yet scientific evidence on insular woodiness has been too fragmented to understand its distribution and evolutionary origins. Here, we identify more than 1,000 insular woody species resulting from at least 175 independent evolutionary transitions on 31 different archipelagos around the world. In combination with global data on island species composition, climate, and environment conditions, we test multiple longstanding hypotheses regarding the origins of insular woodiness and find that the absence of herbivores and traits related to drought stress are best correlated with the occurrence of insular woody species across all islands.
Keywords: drought, island syndrome, phylogenetically derived woodiness, secondary woodiness, wood formation
Abstract
Insular woodiness (IW)—the evolutionary transition from herbaceousness toward woodiness on islands—is one of the most iconic features of island floras. Since pioneering work by Darwin and Wallace, a number of drivers of IW have been proposed, such as 1) competition for sunlight requiring plants with taller and stronger woody stems and 2) drought favoring woodiness to safeguard root-to-shoot water transport. Alternatively, IW may be the indirect result of increased lifespan related to 3) a favorable aseasonal climate and/or 4) a lack of large native herbivores. However, information on the occurrence of IW is fragmented, hampering tests of these potential drivers. Here, we identify 1,097 insular woody species on 375 islands and infer at least 175 evolutionary transitions on 31 archipelagos, concentrated in six angiosperm families. Structural equation models reveal that the insular woody species richness on oceanic islands correlates with a favorable aseasonal climate, followed by increased drought and island isolation (approximating competition). When continental islands are also included, reduced herbivory pressure by large native mammals, increased drought, and island isolation are most relevant. Our results illustrate different trajectories leading to rampant convergent evolution toward IW and further emphasize archipelagos as natural laboratories of evolution, where similar abiotic or biotic conditions replicated evolution of similar traits.
The repeated evolution of peculiar morphological, physiological, and behavioral traits in insular lineages is described in island syndromes (1–4). Iconic examples in the animal kingdom include flightlessness in birds and insects (5, 6), naivety toward predators (7), and changes in body size (8, 9). In angiosperms, the evolution of herbaceousness toward insular woodiness (IW) is one of the most prominent aspects of island floras (4, 10–12). Famous examples include the Hawaiian silverswords (Dubautia and Argyroxiphium) and woody violets (Viola), as well as the Macaronesian tree lettuces (Sonchus) and viper buglosses (Echium). Notably, angiosperms evolved from a woody ancestor, making them ancestrally woody and invoking that nonwoody (herbaceous) angiosperms lost their woodiness during evolutionary history (13). Therefore, IW represents a phylogenetically derived state that is an evolutionary reversion (14).
In contrast to well-documented island syndromes in animals, IW and its evolutionary drivers remain poorly understood (10, 11). Most existing hypotheses postulate that 1) biological competition among colonizing herbs in open island vegetations favors taller stems that need to be mechanically stronger—and hence woodier—to capture more sunlight (15, 16). Alternatively, 2) increased drought stress demands better protection of root-to-shoot water transport against hydraulic dysfunction (17–19). IW may also be an indirect result of selection for longer lifespan induced via 3) a more favorable aseasonal climate buffered by the surrounding oceans, leading to continuous growth that is not interrupted by frost (11), and/or via 4) reduced herbivory due to a lack of large native herbivores on islands, and hence continuous growth without damage to aboveground plant organs before successful reproduction (11). Testing these four hypotheses at the global scale has so far been hampered by fragmented knowledge about IW, missing information on evolutionary relationships, and a lack of standardized descriptions of island environments. For instance, existing IW studies have focused on a few iconic clades on oceanic islands of volcanic origin (20, 21) or a single oceanic archipelago (12, 22), leaving most insular woody clades across the world unidentified. Therefore, documenting and understanding IW remains at the forefront of island biology (23), despite pioneering work by Darwin (15), Hooker (24), Wallace (25), and others.
To reveal the global drivers of IW, we compiled a dataset of insular woody species (IWS) in nonmonocot angiosperms from oceanic islands and islands on the continental shelf. We identified IWS and inferred the timing and number of evolutionary transitions to IW per archipelago and plant family based on information from hundreds of molecular phylogenies, floras, and taxonomic revisions. We then combined this dataset with past and present environmental data on islands worldwide to test four IW hypotheses. We specifically targeted three central themes with respect to IW evolution:
-
1)
Species identity and evolution of IW. How many IWS are there? How many times did IW evolve and in which lineages? How clustered is IW on the angiosperm tree of life?
-
2)
Geographic distribution of IW. Are IWS and evolutionary transitions to IW equally distributed across archipelagos? Is IW prevailing on oceanic compared to continental islands?
-
3)
Drivers of IW distribution. Do the IW drivers differ between island groups (e.g., oceanic islands vs. all islands)? Which IW hypotheses (competition, drought, favorable aseasonal climate, and reduced herbivory) are supported by the global distribution of IW?
We test these hypotheses in two ways by quantifying the correlation of 1) the extant distribution of IWS with environmental conditions and 2) the number of independent evolutionary transitions with long-term environmental conditions on islands worldwide.
Results
Species Identity and Evolution of IW.
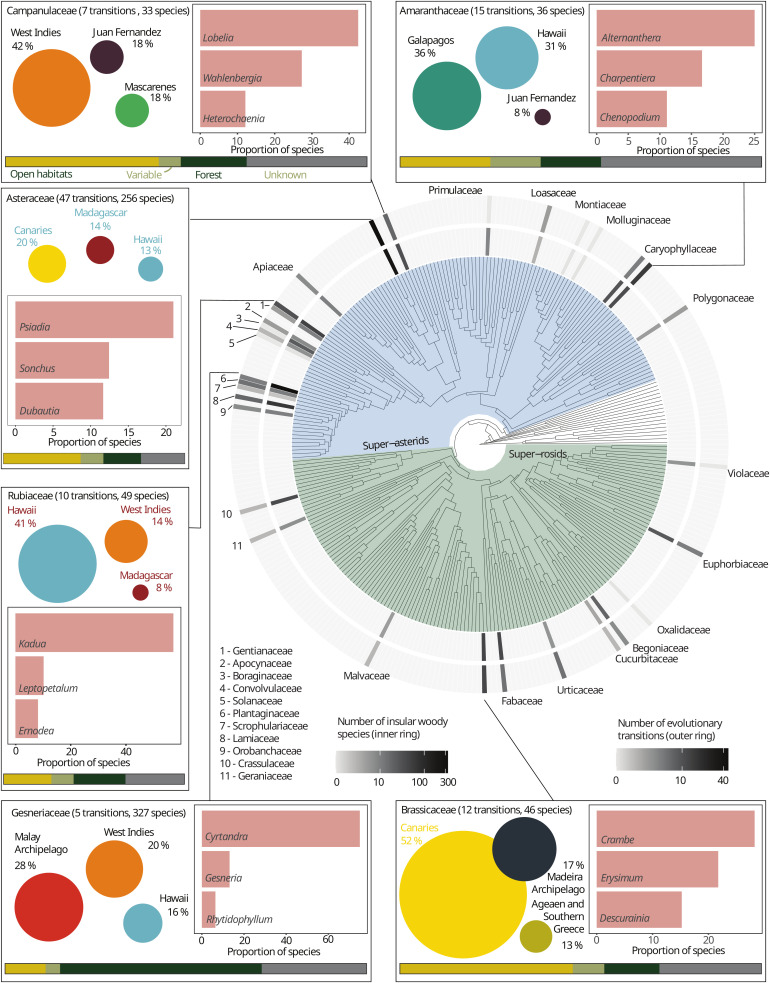
We identified 1,097 IWS belonging to 149 genera and 32 families, representing at least 175 evolutionary transitions. IW was widespread across the tree of life of nonmonocot angiosperms but concentrated in only few plant families (Fig. 1; SI Appendix, Fig. S1; and Dataset S1 for a list of IWS). Most IWS (898 species, or 82% of all IWS) and evolutionary transitions toward IW (131 transitions, or 75% of all recorded transitions) occurred in the superasterids clade, with most species in only two families: Gesneriaceae (327 species, or 30%) and Asteraceae (256 species, or 23%). The top five families with evolutionary transitions to IW (comprising 91 transitions or 52% of all observed transitions) were: Asteraceae (member of the superasterids, 47 transitions or 27% of all observed transitions), Amaranthaceae (superasterids, 15 transitions or 9%), Brassicaceae (superrosids, 12 transitions or 7%), Rubiaceae (superasterids, 10 transitions or 6%), and Campanulaceae (superasterids, 7 transitions or 4%) (Dataset S2 for the number of IWS and minimum number of IW shifts per genus). In Gesneriaceae, a low number of transitions led to at least 245 IWS due to the spectacular radiation of the genus Cyrtandra (Fig. 1). Stem ages for 61 insular woody clades with time-calibrated phylogenies available varied from 19.7 to 0.1 Ma before present, with 51 of the lineages dating back less than 10 Ma; many of these recent IW clades were endemic to the Canary Islands (SI Appendix, Fig. S2).
Fig. 1.
IW across the nonmonocot angiosperm tree of life. The phylogenetic tree shows the number of IWS (inner ring) and the minimum number of evolutionary transitions in each family (outer ring). The inlets show additional information for the families with the highest number of species and transitions: the bubbles show the three archipelagos comprising the highest proportion of IWS in the family (proportion scales with radius, not area; bubbles are to scale across families). The bar chart shows the contribution of the three genera comprising most IWS in each family to the total number of IWS in this family (in percent). Bottom: The stacked bar chart shows the proportion of IWS occurring in open habitats, forest, and forest and open habitats (variable); gray shows the proportion of species for which no habitat information was available.
We found mixed results concerning the phylogenetic clustering of IW. At the family level, the mean pairwise distance (MPD) suggested phylogenetic clustering of the occurrence of IWS against a null model (MPDobserved = 222.2, MPDnull = 238.1, standardized effect size = −3.96, P = 0.01). In contrast, the mean nearest-taxon distance (MNTD) rejected phylogenetic clustering of the occurrence of IWS compared to a null model (MNTDobserved = 136.9, MNTDnull = 154.8, standardized effect size = −1.848, P = 0.07). Since MPD and MNTD quantify phylogenetic structure at different tree depths (MPD describes the basal signal and MNTD describes the more terminal signal) (26), these results likely reflect the concentration of IW in specific clades of the angiosperm tree of life, particularly the superasterids, as well as the more random distribution of IW within these clades (Fig. 1). At the genus level, Pagel’s lambda () indicated weak but significant phylogenetic clustering in the proportion of IWS per genus (mean across replicates = 0.013, with P < 0.05 for difference to = 0 for all replicates). However, Blomberg’s K indicated no phylogenetic signal in the proportion of IWS per genus (mean K across replicates = 0.03, P > 0.05 for all replicates). These results indicate that the proportion of IWS per genus was significantly different from the expectation of a Brownian motion model and the proportion of IWS varies also among closely related genera (i.e., the variances in IWS per genus was within, rather than among, clades). Based on the distribution of IWS across families, we expect stronger phylogenetic clustering at the species level. However, we could not assess phylogenetic clustering at the species level because the currently available angiosperm-wide phylogenies only contain a small fraction of the IWS.
Geographic Distribution of IW.
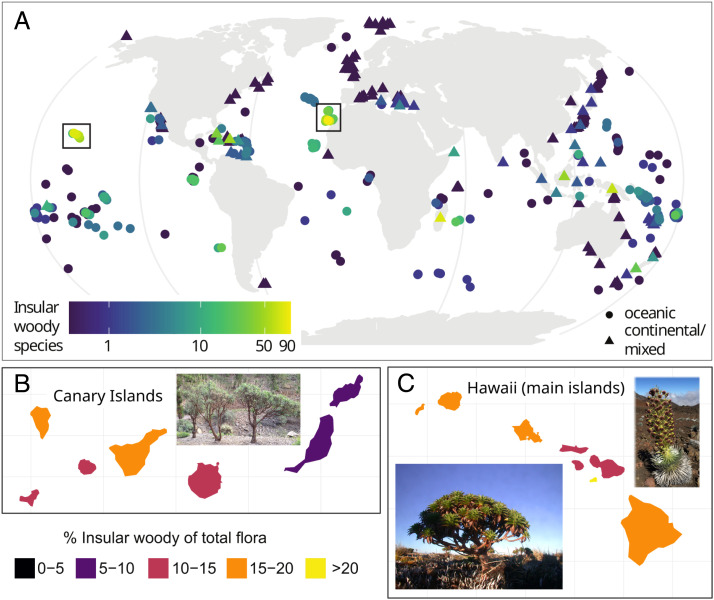
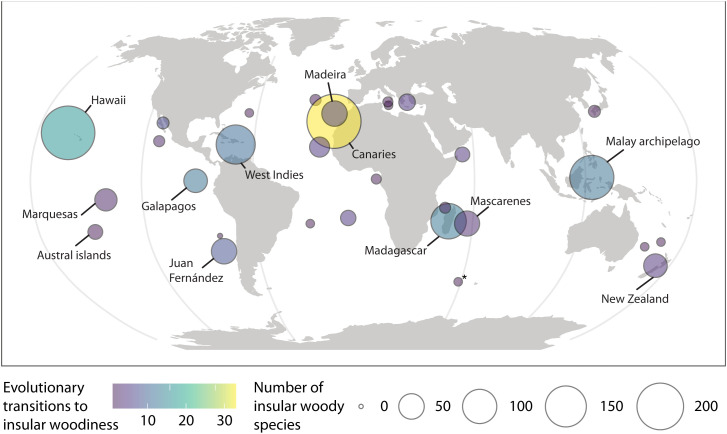
IWS occurred on islands worldwide, but generally, the number and relative representation of IWS were low on islands outside the tropics or subtropics (Fig. 2). Of the 175 evolutionary shifts toward IW, we could confidently assign 162 shifts to 31 individual archipelagos (Fig. 3). Globally, the Canary Islands (204 IWS representing 18% of all IWS and at least 33 evolutionary transitions to IW, accounting for 19% of all transitions) and the Hawaiian archipelago (199 species, or 18%, and 17 transitions, or 10%) emerged as centers of IW, followed by the Malay Archipelago (129 species, or 11%, and 12 transitions, or 7%), and the West Indies (98 species, or 9%, and transitions 11, or 6%). Additional oceanic archipelagos with a considerable number of IWS and evolutionary transitions were Madeira (36 species, or 3%, and six transitions, or 3%), Juan Fernandez Islands (35 species, or 3%, and eight transitions, or 5%), and Mascarenes (34 species, or 3%, and three transitions, or 2%) (Dataset S3). The top three individual islands with the highest number of IWS were Tenerife (97 species, Canary Islands), Kaua’i (79 species, Hawaiian archipelago), and Madagascar (78 species). Of the 10 single islands with the most IWS, 8 belonged to the Canary Islands or the Hawaiian archipelago. The islands with the highest proportion of IWS on the total known angiosperm flora were Santa Fé (25%, Galápagos), Robinson Crusoe Island (25%, Juan Fernández), and Kaho’olawe Island (21%, Hawaiian archipelago). The continental island with the highest proportion of IWS was the South Island of New Zealand (3.2%, rank 61 of all islands). The proportion of IWS varied significantly within archipelagos, in particular for the two archipelagos with the most IWS: from 21% (Kaho’olawe) to 9% (Lana’i) for the Hawaiian archipelago and from 16% (Tenerife) to 7% (Lanzarote) for the Canary Islands (Fig. 2 and SI Appendix, Fig. S3).
Fig. 2.
Global geographic distribution of IW at the level of islands. (A) Number of IWS across all islands. (B and C) Proportion of IWS of the total flora on islands of the two archipelagos with the most IWS: the Canary Islands and the Hawaiian archipelago. The inlet pictures show three iconic examples of IWS: Echium virescens on the Canaries (picture F. Lens, Naturalis Biodiversity Center, Leiden, The Netherlands) and Argyroxiphium sandwicense and Dubautia waialealae on Hawaii (silverswords, pictures by Seana Walsh and Ken Wood, National Tropical Botanical Garden, Kaua’i, HI, USA).
Fig. 3.
Minimum number of evolutionary transitions to IW and number of IWS on archipelagos worldwide. Only archipelagos with at least one evolutionary transitions are shown for clarity. An additional 13 transitions could not be linked unambiguously to any of the archipelagos. The asterisk summarizes multiple Southern Indian Ocean islands (Kerguelen, Crozet, Prince Edward, and Heard and MacDonald Islands).
Potential Drivers of IW.
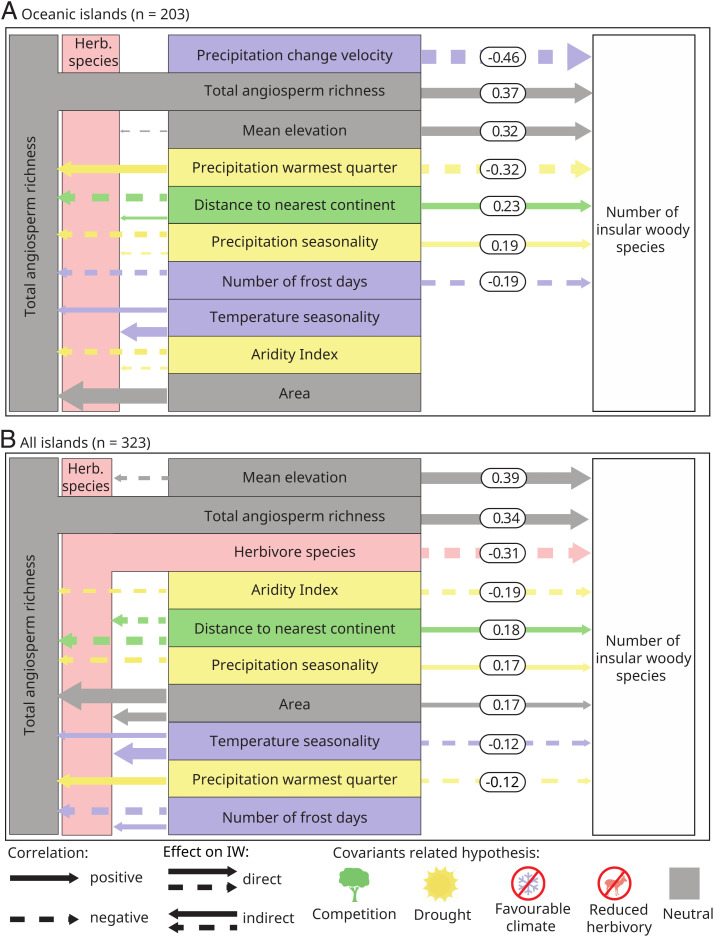
On oceanic islands, we found direct correlations between the number of IWS and explanatory variables related to the competition, drought, and favorable aseasonal climate hypotheses after statistically accounting for differences in island area, mean elevation, and total number of angiosperm species (Fig. 4 and SI Appendix, Fig. S4 and Table S1). As expected, we found direct, negative effects of precipitation change velocity since the last glacial maximum (used as a proxy for long-term favorable aseasonal climate; Table 1 and Methods for details) and the number of frost days (favorable aseasonal climate) on the number of IWS. This shows that more stable or predictive climates—both historical and present day—favored IWS. Furthermore, we detected a positive effect of precipitation seasonality and a negative effect of precipitation of the warmest quarter, supporting the hypothesis that drought may increase the number of IWS. Last, we found that distance to the nearest continent positively affected IWS, suggesting that more isolated islands with fewer woody competitors in the resident population may have more IWS (competition; Fig. 4A).
Fig. 4.
Environmental correlates of IWS richness. (A) Oceanic islands. (B) Oceanic and continental islands. The boxes represent explanatory variables from a structural equation model and are ordered according to the direct effect size (Top to Bottom). The numbers show standardized direct effect sizes, the thickness of arrows is proportional to the effect size, and colors link explanatory variables with hypotheses for the evolution of IW. Herbivore species = time-integrated richness of large-mammal herbivores. See Table 1 for details on the hypotheses, SI Appendix, Table S4 for details on the individual explanatory variables, and SI Appendix, Table S1 for all effect sizes. Only predictors with at least one significant direct or indirect effect are shown. The deer silhouette by Birgit Lang in the public domain from www.phylopic.org.
Table 1.
Existing hypotheses for the evolution of IW, along with resulting predictions and the outcome of the analyses in this study
| Hypothesis | Argumentation | Predictions tested in this study |
|---|---|---|
| Competition | When isolated oceanic islands emerge, they are first colonized by herbaceous lineages that generally have better long-distance dispersal abilities compared to tree lineages. In this initial vegetation without trees, competition for light among dense herbaceous populations selects for taller plants, which need to mechanically reinforce their stems, leading to wood formation (15). This idea is extended by Givnish (16), who argued that natural selection will favor woodiness if pioneer immigrants of open habitats invade denser habitats. | The number of IWS is higher on more isolated oceanic islands (supported). Evolutionary transitions toward IW are more common on more isolated oceanic archipelagos (rejected). |
| Drought | Species with woody stems are more drought tolerant than their herbaceous counterparts and often grow in (seasonally) dry habitats (12, 18). The more lignified wood cell walls and its fine-scale adaptations in IW stems are able to avoid the formation and spread of spontaneous drought-induced air bubbles that can block the long-distance water transport from roots to leaves (18). This air blockage is one of the prime mechanisms leading to drought-induced plant mortality (17) and points to drought as a potential driver of IW (19). | The number of IWS is positively correlated with drought expressed by precipitation seasonality (supported), less precipitation in the warmest quarter of the year (supported), and a lower aridity index (rejected*). IWS occur predominantly in open rather than forest habitats on individual archipelagos (rejected, since habitat importance varies with plant family; Fig. 1). |
| Favorable aseasonal climate | A favorable aseasonal climate (without frost), caused by the buffering effect of the surrounding ocean, leads to a continuous growth period throughout the year. This continuous growth period leads to increased plant age, which in turn favors woodiness in stems (11). | The number of IWS is negatively correlated with number of frost days (supported*), higher past climate change velocity in temperature (rejected) and precipitation (supported*), and higher temperature seasonality (rejected*). Evolutionary transitions toward IW are more common on islands in lower latitudes, characterized by a more stable favorable paleoclimate (rejected). |
| Reduced herbivory | The absence of native large-mammal herbivores on islands allows short-lived herbaceous species to live longer, inducing evolution of woody shrubs (11). | The number of IWS is positively correlated with the time-integrated species richness of large-mammal herbivores richness (rejected*). |
A fifth hypothesis on the role of pollinators and longevity was not tested in this study due to lack of a global island pollinator dataset. The results of the analyses concerning each prediction are shown in the last column and relate to a model including only oceanic islands. Asterisks indicate instances in which the results from the model including only oceanic islands differed from results of a model including oceanic and continental islands (Fig. 4).
When also including continental islands, the importance of the explanatory variables changed considerably (Fig. 4B). Most notably, the direct effects of the explanatory variables related to reduced herbivory were most important, followed by variables related to drought, competition, and favorable aseasonal climate. Specifically, we found a negative effect of mammal herbivore species richness on the number of IWS, suggesting that a lack of mammalian herbivory opened up ecological opportunity for IWS. Furthermore, we found a negative effect of the aridity index (lower aridity index indicating drier climate) and precipitation of the warmest quarter and a positive effect of precipitation seasonality on IWS, thus supporting a positive effect of drought on the number of IWS. Instead of climate velocity or number of frost days, in the all-island model, we detected a negative effect of temperature seasonality on IWS, suggesting that less seasonal climates (for temperature) favored the occurrence of IWS. Finally, and consistent with the results for oceanic islands, we also found a positive effect of island isolation on the number of IWS species per island (Fig. 4B).
Additionally, for both models, we found indirect effects of explanatory variables related to all hypotheses via the total angiosperm richness and the species richness of large-mammal herbivores (Fig. 4). The results were qualitatively similar when excluding the Hawaiian archipelago and the Canary Islands as outliers (SI Appendix, Table S1), suggesting that these enigmatic and classical examples of IW do not drive the global patterns we found. Overall, the model fit was high for all structural equation models and best for oceanic islands alone (including the Canary Islands and the Hawaiian archipelago; R2oceanic = 0.61 vs. R2all = 0.55; SI Appendix, Table S1).
Concerning the number of evolutionary transitions to IW at the archipelago level, we tested the competition and favorable aseasonal climate hypotheses. Due to a lack of relevant measurements or proxies of environmental variables across evolutionary time, we could not test the other hypotheses here (Methods for details). We found a strong and significant positive effect of the archipelago area on the number of transitions (but not on the presence or absence of any shifts, i.e., in the count but not the zero hurdle components of the model). In contrast, we did not find a significant effect of distance to nearest continent, vicinity to the equator, or minimum archipelago age (SI Appendix, Table S2 and Fig. S5), neither for the number nor for the occurrence of evolutionary transitions. Consequently, our results did not support the predictions from the competition hypothesis (more transitions on more isolated islands) and the favorable climate hypothesis (more transitions on islands closer to the equator with more favorable climate on evolutionary timescales).
Discussion
Here, we provide a comprehensive global overview of the taxonomy, geography, and evolution of IW and use global data in a correlative approach to test IW hypotheses originally postulated based on incomplete data from only iconic taxa, individual islands, and archipelagos. We identified at least 175 evolutionary transitions from herbaceousness toward IW across angiosperms, giving rise to over 1,000 IWS. This more than triples the known number of IWS and IW transitions [e.g., (20, 22, 27)]. A large majority of these IWS are nested in the superasterids clade (particularly in the families Gesneriaceae and Asteraceae; Fig. 1) and mainly belong to relatively young lineages that originated less than 10 Ma ago (SI Appendix, Fig. S2). Our results show IW as a widespread phenomenon and reveal oceanic (i.e., typically volcanic) islands in the meridional and tropical climatic belts as centers of IWS richness (Fig. 2) and as main locations for evolutionary transitions toward IW (Fig. 3). Among oceanic islands, the Canary and Hawaiian Islands prevail in terms of number of IWS (204 vs. 199) as well as transitions (17 vs. 33) (12, 20, 28) (Dataset S2), even when excluding the spectacular Hawaiian lobelioid radiation with an unclear origin of woodiness. Among continental islands, the flora of Madagascar stands out (78 IWS and 13 transitions). New Caledonia, in contrast, harbors surprisingly few IWS and evolutionary transitions (3 IWS and 0 transitions), despite its tropical climate and the total submergence of the island during the Late Cretaceous and Early Paleogene (29) (resulting in the chance of herbaceous populations to colonize the island after reemergence and evolve IW as a response to competition). The occurrence of 395 IWS on continental islands, as well as 808 derived woody island species that evolved their woodiness on nearby continents prior to island colonization, invokes the question of how common evolutionary transitions toward woodiness occurred on continents. For instance, we consider the Hawaiian lobelioid clade (Campanulaceae)—the most conspicuous radiation of (woody) species on the archipelago, with 126 species—provisionally as derived woody until molecular phylogenies provide more insight into the habit of the ancestral lineage that colonized the archipelago (30). A prime example of a woody island radiation that developed its woodiness on adjacent continents is Veronica (Plantaginaceae), with 118 derived woody island species, mainly native to New Zealand (former genera Hebe and Parahebe).
We used IWS richness and its correlation with island environmental characteristics to test four hypotheses that explore why plants evolved their woodiness on islands. The direct (and most indirect) effects observed in our structural equation models indicate that variables that prolong plant longevity—due to favorable aseasonal climatic conditions and perhaps also reduced herbivory (11)—as well as variables linked with drought (12, 22) and perhaps also competition (15, 16) (approximated by island isolation), likely acted as global drivers of IW (Fig. 4 and Table 1). A positive correlation between increased lifespan and woodiness is expected based on differences in longevity between nonwoody species (annual or short-lived perennials) vs. woody species (longer-lived perennials) in nature. Moreover, there is evidence from the AT-HOOK MOTIF NUCLEAR LOCALIZED 15 gene that promotes longevity in flowering plants and at the same time controls vascular cambium initiation and activity (31, 32). Similarly, the link between increased woodiness or lignification and increased drought tolerance has been experimentally validated in a number of studies that combine detailed anatomical observations with xylem physiological measurements in stems of multiple lineages (18, 33, 34). In contrast, the results from the competition hypothesis are more complex, because 1) the effect of competition (approximated as island isolation) does not change on more isolated oceanic islands with initially open vegetation compared to all islands, including continental islands with remains of initial dense forests in which herbaceous species are outcompeted for light (Fig. 4), and 2) Darwin’s central assumption for the competition hypothesis was that trees rarely reach islands due to dispersal limitation (35), which is questionable (36–38). In addition to the hypotheses tested in this study, further biotic interactions may also affect IW evolution. This has been formalized in the additional promotion-of-outcrossing hypothesis, suggesting that increased plant lifespan and related IW are a consequence of longer flowering times that are essential in an insect-poor island environment to promote cross-pollination (25, 39). However, this hypothesis may be only applicable to isolated islands with low occurrence of pollinators, while high pollinator diversity does not necessarily impact the number of IWS, as exemplified by the Canary Islands (40).
When comparing the importance of the explanatory variables in the structural equation models, including oceanic islands vs. all islands (Fig. 4), some additional differences with respect to potential IW drivers apply specifically to oceanic islands. For instance, the reduced importance of island area and time-integrated richness of large-mammal herbivores on oceanic islands is likely caused by their small area and the rare occurrence of native mammal herbivores. In contrast, the precipitation change velocity since the last glacial maximum and the number of frost days increase in importance, suggesting that long-term climate stability in the more isolated oceanic islands is a more relevant driver of IW compared to continental islands that are often closer to continents and often larger, with more potential refugia under climate change (Fig. 4 and SI Appendix, Fig. S6). Our results indicate that the mechanisms behind the evolution of IW are complex, including involvement of multiple variables related to plant longevity (i.e., stable climate and reduced herbivore pressure), drought, and arguably island isolation. Although the global IW hypotheses are similarly supported when comparing oceanic vs. all islands, the type of island does seem to affect the extent to which individual variables, as proxies for these hypotheses, act on wood formation (Fig. 4). This may be linked to ecological and evolutionary processes that are simultaneously affected, such as higher speciation rates on oceanic islands (41).
At a finer scale, the mechanisms driving IW likely become even more complex, as different environmental conditions within and across archipelagos may drive IW. For instance, in addition to the different age (42, 43) and isolation of the Canary Islands vs. the Hawaiian archipelago (100 vs. 3,200 km to the nearest continent, respectively), the majority of IWS on the Canary Islands are native to dry, open habitats that receive less than 500 mm of precipitation per year (12). In contrast, many IWS on the Hawaiian archipelago are thriving in wet rainforests (up to 10,000 mm of mean annual precipitation; Fig. 1) (44). The number of IWS in these contrasting vegetation types likely reflects general patterns of angiosperm diversification on these archipelagos, since dry habitats on the Canary Islands are common and harbor most angiosperm species (45), whereas on the Hawaii archipelago, wet forests have the highest angiosperm species richness (44). Moreover, drivers can be taxon specific, since the habitat preferences of IWS may be unique for each family (Fig. 1). Additional complexity arises from the intersection of different environmental drivers and IW hypotheses, since isolation, for instance, may affect competition, as well as herbivore pressure and pollinator abundance/diversity. One may assume that more detailed information about contemporary distribution patterns of IWS would lead to precise estimation of these finer-scale drivers, but this may not necessarily be true. The geologically dynamic nature of the islands, the presence of contrasting habitats on a single island, and the uncertainties involved in any dating study (SI Appendix, Fig. S3) are important bottlenecks for accurately assessing in which habitat and paleoclimate insular woody lineages have evolved (22, 46).
In addition to the environmental conditions potentially favoring the evolution to IW on a specific archipelago, intrinsic aspects of the herbaceous colonizing population are important to determine whether this lineage has the potential to develop IW (35). For instance, monocots never produce wood (47, 48), implying that IW is per definition not possible [although a unique type of secondary growth without wood development occurs in a number of monocot genera (49)]. Furthermore, herbaceous colonizers belonging to nonmonocot angiosperm lineages that are preadapted to radiate into the available island niches and that have already undergone multiple IW shifts elsewhere in the world (e.g., Euphorbia, Begonia, Bidens, Lobelia, and Sonchus; Dataset S2) will be more likely to evolve into multiple IWS compared to herbaceous colonizers belonging to lineages with a more trait-conservative evolutionary trajectory. Interestingly, a high number of IW transitions per genus does not necessarily induce increased diversification rates on different archipelagos, as exemplified by Plantago (7 IWS and 5 transitions), Urtica (5 IWS and 5 transitions), and Salvia (3 IWS and 3 transitions). Likewise, 77 out of the 149 genera (51%) identified in our IW study only include a single IWS (38%) or two IWS (13%) descending from the same colonization event (Dataset S2). This contrasts with the idea of IW as a key innovation that links higher diversity of plant growth forms and the opportunity to occupy a greater phenotypic trait space with accelerated rates of lineage diversification in comparison to herbaceous continental relatives (20). Our observation that some insular woody lineages do not diversify, whereas others undergo spectacular radiations [e.g., Cyrtandra with 245 species (27)], shows that other traits than IW must play an important role in their diversification. Identifying traits that promote diversification remains a challenge in evolutionary biology (23, 50), but there is growing evidence that hybridization—which can be regarded as the most extreme form of outcrossing and may overcome loss of genetic variation via founder effects in the colonizing population—is a promising candidate (51, 52).
Our global synthesis of the phylogenetic and geographical distribution of IW fills the gap in understanding global patterns of rampant reversions from herbaceousness toward woodiness on islands, particularly in the tropic and meridional climatic belts. Our results show that multiple drivers, associated with variables increasing drought and plant longevity as a result of more favorable aseasonal climatic conditions and/or reduced herbivory, affect the distribution and evolution of IW, while island isolations (as proxies for competition) seems less important. The unexpected high number of evolutionary transitions toward IW in distantly related lineages confirms earlier studies in model plants, suggesting that the gene regulatory mechanism or mechanisms controlling the wood pathway or pathways must be simple (53, 54) and potentially conserved through evolutionary time. Our results open a route forward to investigate these mechanisms (47, 55) that have shaped the more than 1,000 iconic woody island species.
Methods
Species Identity and Evolution of IW.
Wood is defined as the secondary xylem produced by a vascular cambium (56). In angiosperms that are able to produce wood, i.e., nonmonocot angiosperms, the exact boundary between woody and herbaceous species is sometimes hard to define because of the continuous variation in wood development in the aboveground stem among species (57, 58). We considered only woody island species that 1) produced a distinct wood cylinder extending toward the upper stem parts (i.e., shrubs, trees, and lianas), excluding species with only a woody stem base (woody herbs or suffrutescent species, which are not woody enough according to our definition) (59), and that 2) evolved from a herbaceous ancestor on an island, as inferred from the available phylogenetic literature (see below for details). Our method allowed us to distinguish woody island species that evolved woodiness on the islands (IWS), other woody island species that evolved their woodiness on nearby continents and then expanded their range to islands (derived woody species), and members of lineages with only woody ancestors (ancestrally woody species).
To identify IWS worldwide, we first screened molecular (and when available time-calibrated) phylogenies from over 100 angiosperm families with a dense sampling of woody and herbaceous species and retrieved information on habit (woody vs. herbaceous) and geographic distribution (island vs. continental) of these species based on 416 publications from the floristic and taxonomic literature (literature list in SI Appendix). Subsequently, we visually traced character evolution on these published phylogenies following a maximum parsimony approach to assess whether the woody island species are insular woody, derived woody, or ancestrally woody. We relied on a manual approach based on individual published phylogenies rather than modeled ancestral state reconstruction based on a supertree or supermatrix phylogeny of angiosperms because available large-scale phylogenies of angiosperms are based on few genetic markers and therefore represent a simplistic version of evolutionary history, often leading to insufficient resolution or topologies conflicting with phylogenetic reconstructions from individual groups (60–62). Furthermore, because most IWS are rare, most of them are not included in available large-scale phylogenies, biasing model reconstructions and making a global assessment impossible. Finally, the identification of island species on a global phylogeny (to differentiate insular woody from derived woody species) is challenging due to the presence of widespread and introduced species and the incompleteness of the Global Inventory of Floras and Traits (GIFT) database. To illustrate these issues and verify our approach, we have reconstructed ancestral states of woody/herbaceous and island colonization based on stochastic character mapping (63) using 19 selected clades extracted from a large-scale species-level phylogeny (60) (SI Appendix, Table S3 and Dataset S4). We selected these clades based on genera to comprise samples of woody and herbaceous species on islands worldwide. These examples illustrate the above-mentioned issues of problematic topologies [e.g., in the Hawaiian silverswords (clade 1 in Dataset S4) and Echium (clade 8)] (20), insufficiently resolved topologies [e.g., in Lotus (clade 19)], insufficient representation of IWS [e.g., in Bidens (clade 6), Wahlbergia (clade 7), and Didymocarpus (clade 13), which do not include a single woody species, respectively], and problems identifying and reconstructing insular lineages [e.g., in Echium (clade 8) and Daucus (clade 9)]. Furthermore, our approach enabled us to ensure a conservative estimate of the global number of IWS and number of transitions to IW, and we emphasize that our estimates represent minimum numbers. Therefore, we also ignored lineages with incomplete information on evolutionary relationships, especially in the family Asteraceae, where at least several dozen IWS could be added once future phylogenies allow distinguishing between IW or derived woodiness in some island clades (e.g., the iconic Scalesia trees on the Galapagos Islands). In addition to the IWS, we found 808 species which were derived woody and occurred on islands but did not classify as insular woody, either because the evolutionary origin of woodiness could not be unambiguously placed on islands or because they clearly evolved woodiness on a continent with subsequent dispersal to islands. When in doubt about habit, we assessed growth form using herbarium specimens at the Naturalis Biodiversity Centre (L, U, WAG), and observed handmade cross-sections of stems at different heights. We then used the taxonomic information of the Leipzig Catalogue of Vascular Plants (64) for taxonomic resolution (SI Appendix, Methods). In addition, we recorded habitat preferences of IWS, which can inform on the drivers of IW, specifically with regard to the drought hypothesis predicting more IWS in open habitats (which are on average drier compared to forests within the same archipelago). We therefore recorded habitat information from free-text habitat descriptions found in the literature and classified species into forest- vs. open-habitat species. We considered all species occurring in both forest and any open habitat as variable. Hence, our dataset comprised 1) the identity of IWS; 2) information on growth form, habitat preferences, and geographic distribution of IWS; and 3) the number and age of evolutionary transitions toward IW per lineage and archipelago.
To test if IW was phylogenetically clustered on the nonmonocot angiosperm tree of life, we calculated the phylogenetic signal in the proportion of IWS per taxon using Blomberg’s K (65) and Pagel’s (66) as implemented in phytools (63). We calculated the phylogenetic signal for the proportion of IWS species per tip on two taxonomic levels: 1) the family level, using a family-level phylogeny (67), and 2) the genus level, using a genus-level phylogeny obtained by randomly pruning all but one species per genus from a large-scale phylogeny of seed plants (60). We repeated the genus-level analysis 100 times to account for paraphyletic genera. Furthermore, we calculated the phylogenetic signal in the presence of IWS (binary: yes/no) across plant families (as a binary trait) using the MPD and MNTD (68).
Geographic Distribution of IW.
To identify global hot spots and cold spots of IW, we obtained information on island location (latitude and longitude of the island centroid), area, mean elevation, geologic origin, and archipelago affiliation via the GIFT database (69). Additionally, we obtained the total number of native angiosperm species per island from GIFT. We classified islands based on their geological origin into oceanic (including volcanic islands, atolls, and raised ocean floor), continental (shelf islands and continental fragments), and any combination of oceanic and continental origin as mixed. For 83 islands where no information on the geological origin and 4 islands for which no information on total angiosperm richness was available from GIFT, we filled in this information from publicly available sources (SI Appendix, Methods). We then summarized the number of unambiguous independent evolutionary transitions toward IW per archipelago, as well as the number of IWS per archipelago and individual island based on the consulted phylogenetic papers and following the archipelago scheme of GIFT (69) (SI Appendix, Methods). Because the Hawaiian archipelago and the Canary Islands are well-studied IW hotspots, we additionally calculated the proportion of IWS on individual islands of these archipelagos to illustrate the variation of IW across islands of individual archipelagos.
For all analyses, we only included islands with a size larger than 10 km2 and with 20 or more angiosperm species or at least one known IWS to avoid biased results based on outliers with a low overall angiosperm species richness. In addition, for the structural equation models (but not for visualization in the figures), we only included individual islands for which an estimate of the total angiosperm species richness was available from GIFT. As a result, we obtained a dataset with 425 islands for visualization and, of those, 323 islands from 94 archipelagos as data for the structural equation models (SI Appendix, Fig. S6 and Dataset S5).
Potential Drivers of IW.
To test the four hypotheses on the drivers of IW (Table 1), we used structural equation models to relate the number of IWS per island to different island environmental characteristics. We selected as explanatory variables environmental conditions for which we postulate a direct influence on the number of IWS per island under each of the hypotheses (Table 1 and SI Appendix, Table S4) and used the structural equation models to identify statistical significance and to rank explanatory variables by effect size. We interpret a significant correlation of an explanatory variable with the number IWS, in the expected direction (positive or negative), as corroboration for the linked hypothesis. In addition to the direct effect of the environmental conditions on the number of IWS, we included their indirect effect via the total angiosperm richness and the time-integrated number of large-mammal herbivores in the model when appropriate (SI Appendix, Fig. S7). We obtained per-island values for all environmental variables via GIFT, which were originally derived from gridded products of various climate and elevation models or calculated based on the islands’ shapes (69).
To test the competition hypothesis, we included island isolation (the distance to the nearest continent obtained from GIFT), as the explanatory variable in the structural equation model. Based on Darwin’s assumption that trees are dispersal limited compared to herbs and thus rarely reach isolated islands (35), we expected more open niches for woodiness to evolve on isolated islands due to the absence of woody competitors and resulting competition of herbaceous colonizers to fill these niches. We thus expected a positive effect of island isolation on IWS.
To test the drought hypothesis, we included the aridity index (mean annual precipitation/mean annual evapotranspiration; lower values indicate drier conditions) (70), the precipitation of the warmest quarter (71), and the precipitation seasonality in the structural equation model. Based on the assumption that IW increased the resistance of plants against drought-induced gas bubble formation inside the water-conducting cells in the wood cylinder (18, 19) (Table 1), we expected more IWS under drier conditions. Hence, we expected more IWS on islands with a lower aridity index, higher precipitation seasonality, and less precipitation in the warmest quarter (as approximation for the season with the most plant growth).
To test the favorable aseasonal climate hypothesis, we included the average number of frost days (72), temperature seasonality (71), and temperature and precipitation change velocity since the last glacial maximum (73, 74) (the latter two calculated as the ratio between the temporal change from 21,000 y before present to today and the contemporary spatial change per island) in the structural equation model. Based on the assumption that a more stable favorable climate (particularly the absence of frost) favors an increased plant lifespan and therefore wood formation (11), we expected more IWS under more stable climate conditions. Hence, we expected more IWS on islands with fewer frost days, lower temperature seasonality, and less temperature and precipitation change velocity since the last glacial maximum. We included precipitation and temperature change velocity since the last glacial maximum as proxies for long-term climate stability, which we assume is important for the extinction rate of IW lineages. In this context, we included precipitation change velocity since the last glacial maximum as linked to the favorable aseasonal climate hypothesis, rather than the drought hypothesis, since the index captures climate stability over thousands of years, rather than specific drought conditions.
To test the reduced herbivory hypothesis, we included the time-integrated species richness of large-mammal herbivores in the structural equation model. We combined this index as a measure of herbivore pressure from two sources. First, we obtained the present natural distributions of all terrestrial mammal species with a body mass above 1 kg, at least 20% of plant material in their diet, and potential natural distribution on islands via the Phylogenetic Atlas of Mammal Macroecology version 1.2 (Phylacine) (75). From Phylacine, we include mammal species that lived from the Last Interglacial (∼130,000 y ago) until the present. We selected the diet threshold to also include species with a small percentage of vegetative plant material in their diet, since on islands, where herbivore pressure is generally low, even species with a small fraction of plant material in their diet may have a strong impact on plant life history. Furthermore, we excluded species that exclusively eat seeds and fruits, such as many rodents, jackals, badgers, and foxes, which have little impact on a plant’s vegetative growth. Second, to also account for the impact of herbivory at deeper timescales, we complemented the species list from Phylacine with a list of extinct terrestrial mammalian herbivores and omnivores from fossils recorded in scientific literature (literature list in SI Appendix). We included selected fossil taxa with a high amount of plant material in their diet and more than 1 kg of body mass that occurred over the past 20 Ma. We manually removed duplicate entries that arose in some cases from species present in the fossil and Phylacine data and used this list as time-integrated species richness of native large-mammal herbivores per island (Dataset S6). We focused on mammal herbivore pressure, since bird herbivory has distinctly different effects on plant adaptations (76). Based on the assumption that reduced mammal herbivore pressure increases plant lifespan and therefore wood formation (11), we expected more IWS on islands with a lower time-integrated species richness of large-mammal herbivores.
To control for the effects expected under a neutral model of IW evolution, we included the direct effect of island area, mean elevation, and total angiosperm richness in the structural equation model. We expect more IWS on larger islands (due to lower extinction rates), on islands with a higher mean elevation (due to increased habitat heterogeneity caused by geological heterogeneity), and on islands with a higher overall angiosperm richness (due to a higher pool of evolutionary lineages and potential higher background diversification rates).
We designed our structural equation models following Onstein et al. (77). First, we normalized all variables between 0 and 1. We started with an a priori structural equation model that included all hypothesized pathways among all predictor variables (SI Appendix, Fig. S3) and evaluated the model’s modification indices, model fits, and residual correlations among those (78). To ensure adequate fit of structural equation models, we made sure that P values of χ2 tests were greater than 0.05, with comparative fit index > 0.90 and confidence intervals of the root-mean-square error of approximation < 0.05. We progressively deleted paths with the least statistical significance from the structural equation model until our final model only consisted of significant pathways (at P < 0.05), for which we extracted the standardized coefficients. We tested the model residuals for normality and equal variances, and we checked for extreme outliers that could affect the results. Since IW has until recently mostly been discussed using volcanic islands, we fitted one structural equation model focusing on oceanic islands only (203 islands) and a second model including all islands (i.e., also islands of continental origin; 323 islands). Furthermore, as a sensitivity analysis to investigate drivers of IW beyond the most iconic archipelagos, which potentially are outliers with regard to the number of IW species, we repeated both analyses excluding the Hawaiian archipelago and the Canary Islands (resulting in datasets with 188 and 307 islands for oceanic and all islands, respectively). We fitted the structural equation models using the R package lavaan version 0.6.8 (79). Since multiple islands were missing climatic information, we used a random forest multiple imputation method to fill these gaps (80). Specifically, we estimated information on temperature seasonality, precipitation seasonality and precipitation of the warmest quarter for 4 islands (1% of the islands included in the analysis), mean aridity index for 9 islands (2.1%), mean elevation for 12 islands (2.9%), climate change velocity of temperature for 13 islands (3.1%), and climate change velocity of precipitation for 27 islands (6.5%).
Spatial autocorrelation can affect results from nonspatial analyses (81). To assess the extent of spatial autocorrelation in our data, we used correlograms and visualized changes in Moran’s I values in the raw data response variables and in the residuals when fitting an ordinary least squares (OLS) regression model with the same set of predictor variables on IWS richness as in the structural equation model. Moran’s I values indicated that there was little (nonsignificant) spatial autocorrelation in the residuals of the OLS. The OLS model residuals were computed using the R package ncf version 1.2.9 (82), and Moran’s I values were calculated using the R package spdep version 1.1.5 (83).
To determine under which conditions evolutionary transitions to woodiness on islands may have occurred (as compared to under which conditions recent IWS occur, as tested with the structural equation model), in a second step, we related the number of independent evolutionary transitions per archipelago with archipelago characteristics (SI Appendix, Table S5) relevant under our research hypotheses using generalized linear regression models. We did this analysis on the archipelago rather than the island level, since we could rarely trace back transitions to individual islands and because volcanic archipelagos persist longer than their individual islands and hence are more relevant on evolutionary timescales. To cope with zero inflation due to a large number of archipelagos without any shifts, we used a hurdle model (i.e., a regression framework fitting separate models to the binary outcome—Was there any transition present: yes or no?—and to the count reflecting the number of transitions) as implemented in the R package pscl version 1.5.5 (84). We used a truncated Poisson generalized linear model with log-link modeling counts above zero (i.e., the number of IW transitions, if any occurred) and a hurdle component modeling zero vs. larger counts (i.e., On which archipelagos did IW transitions occur at all?) using a binomial model and a logit link. SI Appendix, Methods give details on model structure (84). Similar to the explanatory variables described above, we included isolation (the minimum distance to the nearest continent) to test the competition hypothesis and mean absolute latitude of the archipelago centroid to test the favorable aseasonal climate hypothesis. This assumes that climate was more stable closer to the equator on evolutionary timescales, and we thus expected more evolutionary transitions to IW on islands at lower latitudes. To control for the effects expected under a neutral model of evolution, we also included total archipelago land area and minimum archipelago age as explanatory variables in the model, expecting more evolutionary transitions toward IW on larger and older archipelagos. We did not test the herbivory and drought hypotheses in the context of evolutionary transitions because of a lack of suitable proxies on the archipelago level: Due to island submergence caused by erosion and island emergence caused by volcanic activity, there is continuous turnover of islands on many oceanic archipelagos. Hence, plant lineages that can disperse among islands may be present on archipelagos much longer than the age of contemporary islands. As a result, transitions to IW may have occurred on previously existing but now-submerged islands for which aridity and mammal diversity cannot be reliably reconstructed.
Supplementary Material
Acknowledgments
We dedicate this manuscript to the late Sherwin Carlquist for his seminal contributions to island biology and comparative wood anatomy. A.Z., H.B., R.E.O., and R.R. acknowledge the support of the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, funded by the German Research Foundation (DFG-FZT 118, grant 202548816). A.Z. and R.R. acknowledge funding by sDiv, the synthesis centre of iDiv. H.K. acknowledges funding from the German Research Foundation (research unit FOR 2716 DynaCom).
Footnotes
Preprint Servers: A previous version of the manuscript is available as pre-print here: https://doi.org/10.1101/2022.01.22.477210.
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2208629119/-/DCSupplemental.
Data, Materials, and Software Availability
The data and analytical scripts necessary to reproduce the analyses have been deposited in Zenodo (10.5281/zenodo.6325640) (85). Furthermore, the list of IWS, the list of mammal island herbivore fossils, and data on island characteristics used for the structural equation models are available in supporting information. Previously published data were used for this work (https://gift.uni-goettingen.de/home) (69). All other study data are included in the article and/or supporting information.
References
- 1.Baeckens S., Van Damme R., The island syndrome. Curr. Biol. 30, R338–R339 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Lomolino M. V., The unifying, fundamental principles of biogeography: Understanding Island Life. Front. Biogeogr. 8, e29920 (2016). [Google Scholar]
- 3.Losos J. B., Ricklefs R. E., Adaptation and diversification on islands. Nature 457, 830–836 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Whittaker R. J., Fernández-Palacios J. M., Matthews T. J., Borregaard M. K., Triantis K. A., Island biogeography: Taking the long view of nature’s laboratories. Science 357, eaam8326 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Sayol F., Steinbauer M. J., Blackburn T. M., Antonelli A., Faurby S., Anthropogenic extinctions conceal widespread evolution of flightlessness in birds. Sci. Adv. 6, eabb6095 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright N. A., Steadman D. W., Witt C. C., Predictable evolution toward flightlessness in volant island birds. Proc. Natl. Acad. Sci. U.S.A. 113, 4765–4770 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carthey A. J. R., Banks P. B., Naïveté in novel ecological interactions: Lessons from theory and experimental evidence. Biol. Rev. Camb. Philos. Soc. 89, 932–949 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Benítez-López A., et al. , The island rule explains consistent patterns of body size evolution in terrestrial vertebrates. Nat. Ecol. Evol. 5, 768–786 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Lomolino M. V., et al. , Of mice and mammoths: Generality and antiquity of the island rule. J. Biogeogr. 40, 1427–1439 (2013). [Google Scholar]
- 10.Burns K. C., Evolution in Isolation: The Search for an Island Syndrome in Plants (Cambridge University Press, 2019). [Google Scholar]
- 11.Carlquist S. J., Island Biology (Columbia University Press, 1974). [Google Scholar]
- 12.Lens F., Davin N., Smets E., del Arco M., Insular woodiness on the Canary Islands: A remarkable case of convergent evolution. Int. J. Plant Sci. 174, 992–1013 (2013). [Google Scholar]
- 13.Doyle J. A., Molecular and fossil evidence on the origin of angiosperms. Annu. Rev. Earth Planet. Sci. 40, 301–326 (2012). [Google Scholar]
- 14.Porter M. L., Crandall K. A., Lost along the way: The significance of evolution in reverse. Trends Ecol. Evol. 18, 541–547 (2003). [Google Scholar]
- 15.Darwin C., On the Origin of Species by Means of Natural Selection (John Murray, 1859). [Google Scholar]
- 16.Givnish T. J., “Adaptive plant evolution on islands: Classical patterns, molecular data, new insights” in Evolution on Islands (Oxford University Press, 1998), pp. 281–304. [Google Scholar]
- 17.Choat B., et al. , Triggers of tree mortality under drought. Nature 558, 531–539 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Dória L. C., et al. , Insular woody daisies (Argyranthemum, Asteraceae) are more resistant to drought-induced hydraulic failure than their herbaceous relatives. Funct. Ecol. 32, 1467–1478 (2018). [Google Scholar]
- 19.Lens F., et al. , Embolism resistance as a key mechanism to understand adaptive plant strategies. Curr. Opin. Plant Biol. 16, 287–292 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Nürk N. M., Atchison G. W., Hughes C. E., Island woodiness underpins accelerated disparification in plant radiations. New Phytol. 224, 518–531 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S. C., et al. , Timing and tempo of early and successive adaptive radiations in Macaronesia. PLoS One 3, e2139 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooft van Huysduynen A., et al. , Temporal and palaeoclimatic context of the evolution of insular woodiness in the Canary Islands. Ecol. Evol. 11, 12220–12231 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patiño J., et al. , A roadmap for island biology: 50 fundamental questions after 50 years of The Theory of Island Biogeography. J. Biogeogr. 44, 963–983 (2017). [Google Scholar]
- 24.Hooker J. D., On insular floras: A lecture. J. Bot. 5, 23–31 (1867). [Google Scholar]
- 25.Wallace A. R., Island Life: Or, the Phenomena and Causes of Insular Faunas and Floras, Including a Revision and Attempted Solution of the Problem of Geological Climates (Macmillan, 1880). [Google Scholar]
- 26.Mazel F., et al. , Influence of tree shape and evolutionary time-scale on phylogenetic diversity metrics. Ecography 39, 913–920 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson M. A., Clark J. R., Wagner W. L., McDade L. A., A molecular phylogeny of the Pacific clade of Cyrtandra (Gesneriaceae) reveals a Fijian origin, recent diversification, and the importance of founder events. Mol. Phylogenet. Evol. 116, 30–48 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Price J. P., Wagner W. L., Speciation in Hawaiian angiosperm lineages: Cause, consequence, and mode. Evolution 58, 2185–2200 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Garrouste R., et al. , New fossil discoveries illustrate the diversity of past terrestrial ecosystems in New Caledonia. Sci. Rep. 11, 18388 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knox E. B., Li C., The East Asian origin of the giant lobelias. Am. J. Bot. 104, 924–938 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Karami O., et al. , A suppressor of axillary meristem maturation promotes longevity in flowering plants. Nat. Plants 6, 368–376 (2020). [DOI] [PubMed] [Google Scholar]
- 32.Rahimi A., et al. , Control of cambium initiation and activity in Arabidopsis by the transcriptional regulator AHL15. Curr. Biol. 32, P1764–1775 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Lens F., et al. , Herbaceous Angiosperms are not more vulnerable to drought-induced embolism than Angiosperm trees. Plant Physiol. 172, 661–667 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thonglim A., et al. , Intervessel pit membrane thickness best explains variation in embolism resistance amongst stems of Arabidopsis thaliana accessions. Ann. Bot. 128, 171–182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlquist S., Darwin on island plants. Bot. J. Linn. Soc. 161, 20–25 (2009). [Google Scholar]
- 36.Bullock J. M., et al. , A synthesis of empirical plant dispersal kernels. J. Ecol. 105, 6–19 (2017). [Google Scholar]
- 37.Higgins S. I., Nathan R., Cain M. L., Are long-distance dispersal events in plants usually caused by nonstandard means of dispersal? Ecology 84, 1945–1956 (2003). [Google Scholar]
- 38.Carlquist S., Chance Dispersal: Long-distance dispersal of organisms, widely accepted as a major cause of distribution patterns, poses challenging problems of analysis. Am. Sci. 69, 509–516 (1981). [Google Scholar]
- 39.Böhle U. R., Hilger H. H., Martin W. F., Island colonization and evolution of the insular woody habit in Echium L. (Boraginaceae). Proc. Natl. Acad. Sci. U.S.A. 93, 11740–11745 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whittaker R., Fernández-Palacios J., Island Biogeography: Ecology, Evolution, and Conservation (Oxford University Press, 2007). [Google Scholar]
- 41.Takayama K., Crawford D. J., López-Sepúlveda P., Greimler J., Stuessy T. F., Factors driving adaptive radiation in plants of oceanic islands: A case study from the Juan Fernández Archipelago. J. Plant Res. 131, 469–485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clague D. A., et al. , The maximum age of Hawaiian terrestrial lineages: Geological constraints from Kōko Seamount. J. Biogeogr. 37, 1022–1033 (2010). [Google Scholar]
- 43.Fernández-Palacios J. M., et al. , A reconstruction of Palaeo-Macaronesia, with particular reference to the long-term biogeography of the Atlantic island laurel forests. J. Biogeogr. 38, 226–246 (2011). [Google Scholar]
- 44.Wagner W., Herbst D., Sohmer, Manual of the Flowering Plants of Hawaii (University of Hawai‘i Press/Bishop Museum Press, Revised edition, 1999). [Google Scholar]
- 45.Bramwell D., Bramwell Z. I., Wild Flowers of the Canary Islands (Stanley Thornes Limited, 1974). [Google Scholar]
- 46.García-Verdugo C., Caujapé-Castells J., Sanmartín I., Colonization time on island settings: Lessons from the Hawaiian and Canary Island floras. Bot. J. Linn. Soc. 191, 155–163 (2019). [Google Scholar]
- 47.Spicer R., Groover A., Evolution of development of vascular cambia and secondary growth. New Phytol. 186, 577–592 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Roodt D., Li Z., Van de Peer Y., Mizrachi E., Loss of wood formation genes in monocot genomes. Genome Biol. Evol. 11, 1986–1996 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carlquist S., Monocot xylem revisited: New information, new paradigms. Bot. Rev. 78, 87–153 (2012). [Google Scholar]
- 50.Sauquet H., Magallón S., Key questions and challenges in angiosperm macroevolution. New Phytol. 219, 1170–1187 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Baldwin B. G., A new look at phenotypic disparity and diversification rates in island plant radiations. New Phytol. 224, 8–10 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Marques D. A., Meier J. I., Seehausen O., A combinatorial view on speciation and adaptive radiation. Trends Ecol. Evol. 34, 531–544 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Melzer S., et al. , Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat. Genet. 40, 1489–1492 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Klimes A., Simova I., Zizka A., Antonelli A., Herben T., The ecological drivers of growth form evolution in flowering plants. J. Ecol. 110, 1525–1536 (2022). [Google Scholar]
- 55.Davin N., et al. , Functional network analysis of genes differentially expressed during xylogenesis in soc1ful woody Arabidopsis plants. Plant J. 86, 376–390 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Larson P. R., The Vascular Cambium—Developement and Structure (Springer, 1994). [Google Scholar]
- 57.Lens F., Eeckhout S., Zwartjes R., Smets E., Janssens S. B., The multiple fuzzy origins of woodiness within Balsaminaceae using an integrated approach. Where do we draw the line? Ann. Bot. 109, 783–799 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lens F., Smets E., Melzer S., Stem anatomy supports Arabidopsis thaliana as a model for insular woodiness. New Phytol. 193, 12–17 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Kidner C., et al. , First steps in studying the origins of secondary woodiness in Begonia (Begoniaceae): Combining anatomy, phylogenetics, and stem transcriptomics. Biol. J. Linn. Soc. Lond. 117, 121–138 (2016). [Google Scholar]
- 60.Smith S. A., Brown J. W., Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 105, 302–314 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Bravo G. A., et al. , Embracing heterogeneity: Coalescing the Tree of Life and the future of phylogenomics. PeerJ 7, e6399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eiserhardt W. L., et al. , A roadmap for global synthesis of the plant tree of life. Am. J. Bot. 105, 614–622 (2018). [DOI] [PubMed] [Google Scholar]
- 63.Revell L. J., phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012). [Google Scholar]
- 64.Freiberg M., et al. , LCVP, The Leipzig catalogue of vascular plants, a new taxonomic reference list for all known vascular plants. Sci. Data 7, 416 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blomberg S. P., T. Garland, Jr., Ives A. R., Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 57, 717–745 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Pagel M., Inferring the historical patterns of biological evolution. Nature 401, 877–884 (1999). [DOI] [PubMed] [Google Scholar]
- 67.Harris L. W., Davies T. J., A complete fossil-calibrated phylogeny of seed plant families as a tool for comparative analyses: Testing the ‘time for speciation’ hypothesis. PLoS One 11, e0162907 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kembel S. W., et al. , Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (2010). [DOI] [PubMed] [Google Scholar]
- 69.Weigelt P., König C., Kreft H., GIFT—A global inventory of Floras and Traits for macroecology and biogeography. J. Biogeogr. 47, 16–43 (2020). [Google Scholar]
- 70.Zomer R. J., Trabucco A., Bossio D. A., Verchot L. V., Climate change mitigation: A spatial analysis of global land suitability for clean development mechanism afforestation and reforestation. Agric. Ecosyst. Environ. 126, 67–80 (2008). [Google Scholar]
- 71.Fick S. E., Hijmans R. J., WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017). [Google Scholar]
- 72.Karger D. N., et al. , Climatologies at high resolution for the earth’s land surface areas. Sci. Data 4, 170122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 74.Weigelt P., Steinbauer M. J., Cabral J. S., Kreft H., Late Quaternary climate change shapes island biodiversity. Nature 532, 99–102 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Faurby S., et al. , PHYLACINE 1.2: The phylogenetic atlas of mammal macroecology. Ecology 99, 2626 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Bond W. J., Lee W. G., Craine J. M., Plant structural defences against browsing birds: A legacy of New Zealand’s extinct moas. Oikos 104, 500–508 (2004). [Google Scholar]
- 77.Onstein R. E., et al. , Palm fruit colours are linked to the broad-scale distribution and diversification of primate colour vision systems. Proc. Biol. Sci. 287, 20192731 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grace J. B., et al. , Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere 3, art73 (2012). [Google Scholar]
- 79.Rosseel Y., lavaan: An R package for structural equation modeling. J. Stat. Softw. 48, 1–36 (2012). [Google Scholar]
- 80.Stekhoven D. J., Bühlmann P., MissForest—Non-parametric missing value imputation for mixed-type data. Bioinformatics 28, 112–118 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Kissling W. D., Carl G., Spatial autocorrelation and the selection of simultaneous autoregressive models. Glob. Ecol. Biogeogr. 17, 59–71 (2008). [Google Scholar]
- 82.Bjornstad O. N., ncf: Spatial Covariance Functions. v1.2.9. (2020). https://cran.r-project.org/web/packages/ncf/index.html. Accessed 23 August 2022.
- 83.Bivand R. S., Wong D. W. S., Comparing implementations of global and local indicators of spatial association. Test 27, 716–748 (2018). [Google Scholar]
- 84.Zeileis A., Kleiber C., Jackman S., Regression models for count data in R. J. Stat. Softw. 27, 1–25 (2008). [Google Scholar]
- 85.A. Zizka et al., Data and scripts for 'The evolution of insular woodiness'. Zenodo. https://zenodo.org/record/6325640#.YwT4wxxByUk. Deposited 1 August 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and analytical scripts necessary to reproduce the analyses have been deposited in Zenodo (10.5281/zenodo.6325640) (85). Furthermore, the list of IWS, the list of mammal island herbivore fossils, and data on island characteristics used for the structural equation models are available in supporting information. Previously published data were used for this work (https://gift.uni-goettingen.de/home) (69). All other study data are included in the article and/or supporting information.