Abstract
A patient with an ultimate diagnosis of human herpesvirus-6 (HHV-6) encephalitis developed central nervous system (CNS) symptoms 13 days after undergoing myeloablative haploidentical allogeneic hematopoietic stem cell transplant (HSCT). Due to the patient’s body habitus, magnetic resonance (MR) imaging was not obtained until the onset of retrograde amnesia on day +24. MR imaging and other clinical findings eliminated all skepticism of HHV-6 encephalitis and HHV-6 antivirals were initiated on day +28, leading to gradual recovery. This case demonstrates some of the factors that may complicate the diagnosis of post-alloHSCT HHV-6 encephalitis. Because HHV-6 encephalitis and viremia can occur without warning, a single negative study should not exclude future development, especially if CNS symptoms are present. Acute graft-versus-host disease and cord blood transplantation are both significant risk factors for HHV-6 encephalitis. Human leukocyte antigen (HLA) mismatch, engraftment complications, or certain HLA alleles have also been associated with HHV-6 encephalitis. Chromosomally integrated HHV-6 must also be ruled out to prevent inappropriate and potentially harmful administration of antivirals. Due to the severe short- and long-term sequelae of HHV-6 encephalitis, appropriate treatment should be administered as soon as possible.
Keywords: human herpesvirus-6, encephalitis, hematopoietic stem cell transplant, amnesia, HHV-6, reactivation
Introduction
Human herpesvirus-6 (HHV-6), a member of the Herpesviridae family, is generally acquired between the ages of 6 and 15 months, accounting for 20% of all acute fevers in children between 6 and 12 months old, and is the causative agent for exanthem subitem 1 . Like other HHVs, HHV-6 is capable of establishing latency within different anatomic sites such as the salivary glands and brain 1 . Chronic low levels of viral replication result in very low levels of HHV-6 DNA with an estimated seroprevalence of >95% in the adult population 1 . HHV-6 is divided into two similar subtypes, HHV-6A and HHV-6B, with the latter being more associated with reactivation events 2 .
Immunocompromised patients who have undergone allogeneic hematopoietic stem cell transplant (alloHSCT) have demonstrated HHV-6 reactivation at a rate of 40%–50% 3 . However, the true rate of HHV-6 reactivation is unknown as it is infrequently tested. HHV-6 reactivation has the potential to cause encephalitis demonstrated by the presence of HHV-6 in the cerebrospinal fluid (CSF) 4 or the astrocytes of white matter in the cerebral cortex and hippocampus without other pathogens5,6.
HHV-6 encephalitis is an uncommon but serious consequence following alloHSCT, with an overall incidence ranging from 0% to 11.6%, occurring from 2 to 6 weeks following HSCT 7 . Outcomes of HHV-6 encephalitis in this population are poor with a mortality rate of 20%–40%7,8. A high proportion of patients can suffer from short- and long-term central nervous system (CNS) sequelae, ranging from overt seizures to dysthesia 7 . Syndrome of inappropriate antidiuretic hormone with hyponatremia are other possible sequelae 9 . Mortality can also result from complications directly or indirectly associated with HHV-6 reactivation, such as acute graft-versus-host disease (aGVHD) or development of secondary infectious diseases 10 .
We report a case following myeloablative haploidentical HSCT where several factors, including the patient’s body habitus, increased the difficulty of arriving at the ultimate diagnosis of HHV-6 encephalitis. In addition, we briefly review the most recent literature to highlight important clinical nuances of post-alloHSCT HHV-6 encephalitis to consider in this setting.
Case Report
A 36-year-old morbidly obese female with past medical history of acute myeloid leukemia (AML) and status post 7+3 induction chemotherapy achieved a first complete remission without minimal residual disease (MRD) by multicolor flow cytometry. She underwent high-dose cytarabine (HiDAC) consolidation, but unfortunately developed MRD. Additional chemotherapy of decitabine and venetoclax started on day −66 led to eradication of MRD on day −31 and the patient was admitted to the hospital for myeloablative HSCT. The patient received a haploidentical peripheral blood stem cell transplant from her sister, a human leukocyte antigen (HLA)-matched grade to donor at high resolution of 5/10. The CD34+ cell quantity was 5–7 × 106/kg. The patient received fludarabine and melphalan-140 mg/m2 conditioning regimen before infusion of stem cells on days -6 to -1. Graft-versus-host disease (GVHD) prophylaxis consisted of post-transplant cyclophosphamide (PTCy), tacrolimus, and mycophenolate mofetil (MMF) on days +3 and +4. Letermovir was given for cytomegalovirus (CMV) prophylaxis.
On day +13, the patient developed photophobia, hallucinations, and a rash on her back and chest that was erythematous and blanching. Liver enzymes were also elevated. Since the transplant, the patient had also suffered diarrhea. Concerns for liver, gastrointestinal, and skin aGVHD led to initiation of 2 mg/kg methylprednisolone. In addition, tacrolimus was held with concerns for posterior reversible encephalopathy syndrome (PRES). However, a magnetic resonance imaging (MRI) was not done due to the patient’s body habitus. It should also be noted that the patient’s absolute neutrophil count, white blood cells, and platelets were still critical at this point. Frequent platelet infusions were also required, showing minimal response. Engraftment would not occur until day +32. Symptoms and labs appeared to improve over the following 2 days following methylprednisolone and withdrawal of tacrolimus.
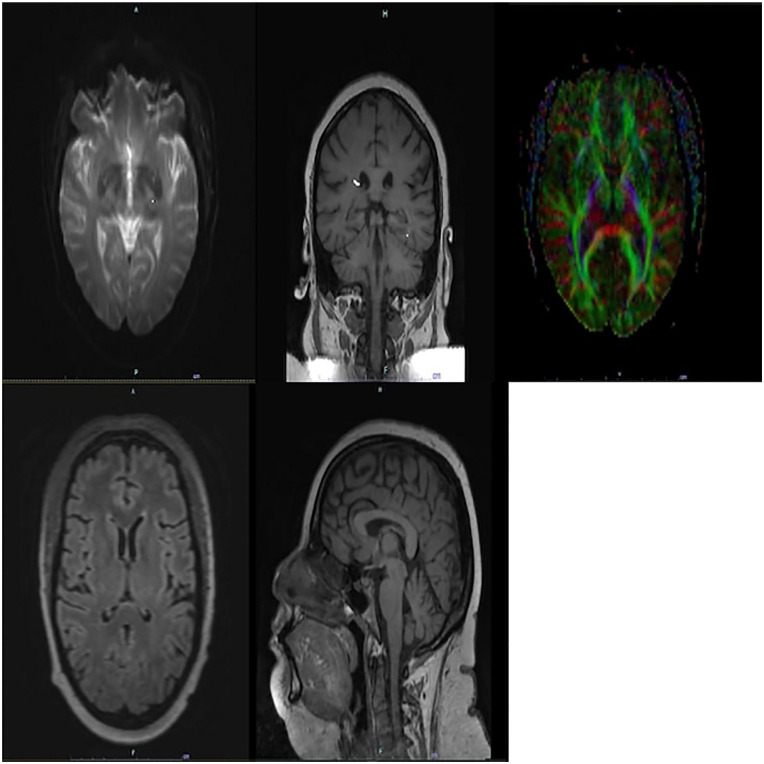
On day +24, the patient experienced new-onset retrograde amnesia. She was not able to recall her cancer diagnosis, what she was in the hospital for or other events within the past 2 years but was able to recall remote memories including her parents’ and significant other’s names. The differential diagnosis included drug-induced altered mental status (e.g., calcineurin inhibition, fludarabine from the conditioning regimen), and infection, now including HHV-6 among other pathogens. On day +24, IgG (1:10) and IgM (<1:20), antibodies for HHV-6 were not suggestive of active infection. A computed tomography scan of the head revealed no acute changes and Electroencephalogram (EEG) was with diffuse slowing, but no specific findings. Tacrolimus was again held, and a lumbar puncture was performed, demonstrating protein 31 mg/dl, glucose 103 mg/dl, red blood cells 180/µl. On day +25, the patient’s CSF was shown to be positive for HHV-6 by qualitative multiplex polymerase chain reaction (PCR) assay and the quantitative assay was pending. Initially, the interdisciplinary team thought viral encephalitis was less likely given the concerns for the specificity of the qualitative assay and the normal CSF. Offsite MR imaging machines that could accommodate the patient’s body habitus were eventually identified. The MRI (Fig. 1) revealed abnormal symmetric diffusion T2 hyperintensity involving the bilateral hippocampus and amygdala, suggesting viral encephalitis. This MRI also ruled out potential fludarabine and tacrolimus neurotoxicity. The imaging and clinical findings were consistent with HHV-6 encephalitis, eliminating previous skepticism.
Figure 1.
Axial, coronal, and sagittal MRIs demonstrating abnormal symmetric T2/Fluid attenuated inversion recovery (FLAIR) hyperintense signal involving the hippocampus and amygdala bilaterally with associated faint restricted diffusion. MRI: magnetic resonance imaging.
Concerns for the high mortality and morbidity for HHV-6 encephalitis led to initiation of a course of foscarnet from day +28 to +38 initially at 60 mg/kg q8h and then 90 mg/kg q12h to facilitate discharge planning. The medication was switched to ganciclovir 5 mg/kg q12h (adjusted for renal dosing) on day +38 to complete the 3-week course primarily due to acute kidney injury and electrolyte abnormalities. After initiation of foscarnet, mentation improved, and the patient was able to form new memories, but remained unable to recall the past 2 years of her life. She was able to gain perspective on what she had gone through by reviewing social media posts.
On day +31, 3 days after the initiation of foscarnet, repeat HHV-6 serum was again negative. The first quantitative assay returned a week later revealing a positive HHV-6 viral load of 51,200 copies/ml. As an aside, CMV was also detected in the serum at 49.9 copies/ml, but not the CSF.
Of note, during this process, the patient also exhibited another herpesvirus, CMV, reactivation, with fluctuating, but overall up-trending titers throughout the course of antiviral treatment. However, this was low-level positivity and, therefore, unconcerning. CMV tests ranged from 52.4 to 776 copies/ml from days +28 to +55.
On day +41, the patient was discharged from the hospital. The patient was tapered off steroid treatment on day +55 for the now resolved episode of GVHD and is continuing tacrolimus and completing the course of ganciclovir for both CMV viremia and HHV-6 encephalitis. Due to poor kidney function, ganciclovir dosing was adjusted again to 2.5 mg/kg q12h. The patient still exhibited some residual cognitive deficits, but with significant clinical improvement. Repeat quantitative and qualitative LPs were negative on day +66.
Diagnosis of HHV-6 Encephalitis
Diagnosing HHV-6 in post-HSCT patients can be challenging due to the possibility of neurologic manifestations being attributed to other causes with similar presentations, such as PRES 11 , other infection, or drug toxicity 12 . In addition, the presence of HHV-6 in the CSF may not always be associated with CNS dysfunction and encephalitis 12 . Thus, diagnosis of post-HSCT HHV-6 encephalitis should meet all the following criteria: (1) presence of CNS symptoms, (2) positive CSF PCR results for HHV-6 DNA, and (3) the absence of other identified causes of CNS dysfunction 13 . It is also worth noting that a qualitative CSF assay may give a positive result because of a low, clinically insignificant viral load. A quantitative CSF assay better characterizes the degree of HHV-6 involvement in the CSF.
MRI can also be a very useful diagnostic tool. The MRI findings of this patient are consistent with previous reports of HHV-6 encephalopathy, including, but not limited to, medial temporal lobe involvement14–17. However, HHV-6 encephalitis may not always be accompanied by radiologic correlates, especially when MRI is performed shortly after symptom onset17–19, so negative MRIs do not exclude HHV-6 encephalitis. Thus, repeating an early negative MRI may be beneficial.
Besides positive HHV-6 DNA by PCR, an otherwise normal CSF examination, such as with our case, is possible as well and does not exclude HHV-6 encephalitis 17 . In addition, increased levels of plasma HHV-6 DNA have been associated with an increased risk of developing HHV-6 encephalitis. Plasma HHV-6 DNA ≥10,000 copies/ml has offered a 100% sensitivity rate for identifying HHV-6 encephalitis 20 . Regular monitoring of HHV-6 DNA in plasma in high-risk patients may assist in the early diagnosis of HHV-6 encephalitis. However, the kinetics of HHV-6 encephalitis are dynamic. It is possible for HHV-6 encephalitis to develop suddenly before the presence of high levels of HHV-6 DNA in the plasma 4 . Clinical signs of HHV-6 encephalitis may develop even before a rise in plasma viremia levels. This is likely why monitoring HHV-6 plasma viremia levels is not always successful at predicting or preventing the development of HHV-6 encephalitis 21 . Viral burden within brain tissue as high as 10,000 copies/million cells without any presence of viral load in the plasma or serum is possible 22 . Thus, low-plasma HHV-6 viremia levels do not exclude development of HHV-6 encephalitis and higher-plasma HHV-6 viremia levels increase the risk of HHV-6 encephalitis. A recent retrospective database study also found that the CSF/blood HHV-6 viral replication ratio to be >1 exclusively in post-HSCT patients diagnosed with HHV-6 encephalitis. Thus, this clinical value was suggested to potentially distinguish some cases of HHV-6 encephalitis in patients having low CSF viral loads, lack of radiologic features, or otherwise ambiguous cases. However, in this study, there were only 7 individuals out of 926 who were diagnosed with HHV-6 encephalitis. More studies may be needed to verify this finding 18 .
In our patient, there was no sign of active HHV-6 infection at the onset of amnesia by serology. However, serology is not as useful in determining active infection as detection of viral DNA or transcripts by reverse transcriptase-polymerase chain reaction (RT-PCR) 23 , so it is unknown whether our patient reactivated HHV-6 and developed encephalitis very suddenly, or whether active infection in the CSF/plasma could have been detected at the first signs of CNS symptomology at day +13. The patient’s body habitus also deterred imaging studies early on, which may have suggested HHV-6 encephalitis sooner. Given the dynamic nature of the development of HHV-6 encephalitis, vigilance is required for all patients.
HHV-6 Reactivation Post-HSCT and Risk Factors for HHV-6 Encephalitis
HHV-6 reactivation following HSCT has been linked with adverse outcomes such as aGVHD [hazard ratio (HR) = 2.65 95% confidence interval (CI): 1.89–3.72; P < 0.001] 24 or engraftment complications7,25. A large-scale retrospective study found acute GVHD grades II to IV to be a significant risk factor for HHV-6 post-transplantation acute limbic encephalitis (aHR = 7.5, P < 0.001) 26 . Engraftment syndrome has also been associated with the development of HHV-6 encephalitis. 7 HHV-6 reactivation is also becoming increasingly associated with delayed T-cell reconstitution, CMV reactivation following HHV-6 reactivation, and idiopathic pneumonia syndrome 25 . Similar to engraftment syndrome and aGVHD, other HHV-6-related sequelae may also have a relationship with HHV-6 encephalitis, which warrants further investigation. While end-organ disease has been reported in relation to HHV-6, the relationship is not well supported25,27.
Presence of a HLA-mismatched donor was found to be an independent significant risk factor for HHV-6 reactivation [odds ratio (OR) = 4.5, P = 0.008], but not encephalitis 28 . However, a subsequent larger study found that HLA-mismatch was an independent risk factor of HHV-6 encephalitis (aHR 4.3, P = 0.04) 26 . Small-scale studies also show a higher incidence of HHV-6 encephalitis in haploidentical as compared to HLA-identical HSCT patients29,30. In this context, an HLA-mismatch is defined as the mismatch of at least one of six antigen classes among HLA-A, B, and DR 31 . Yamamoto et al. 31 also found that patients with the HLA*B40:06 allele were at high risk for developing HHV-6 encephalitis (OR = 31.1, P = 0.027).
Umbilical cord blood transplant (CBT) has been identified as a significant independent risk factor for HHV-6 encephalitis in a large retrospective study of 1,344 patients [aHR = 20.0; 95% CI: 7.3–55.0; P < 0.001] 26 and a subsequent meta-analysis (8.3% in CBT recipients vs 0.5% in patients receiving another stem cell source, P < 0.001) 32 . Furthermore, there was no association between CMV recipient seropositivity and HHV-6 encephalitis, indicating a unique predisposition of HHV-6 to reactivate after CBT 26 . The reason for preferential HHV-6 reactivation in CBT is unclear. A partially mismatched HLA status may also have compounded to further increase these odds. It should also be noted our patient’s requirement for frequent platelet transfusions and delayed engraftment are factors that are also associated with HHV-6 infection 25 .
Chromosomally Integrated HHV-6
Chromosomally integrated HHV-6 (ciHHV-6) occurs in approximately 1% of the population. Individuals with ciHHV-6 have an HHV-6 genome integrated into the telomere of every chromosome, which can be passed down in a Mendelian fashion in a condition known as inherited chromosomally integrated-HHV-6 (iciHHV-6). As a result, these individuals consistently test positive for HHV-6 (one copy per nucleated cell), which can be mistaken for an active infection 33 . For patients with high viral loads, iciHHV-6/ciHHV-6 must be ruled out to prevent the unnecessary administration of drugs that may have adverse effects or even inadvertently activate the virus33,34. In our patient, ciHHV-6 was incidentally ruled out because of a negative serum HHV-6 test by PCR after initiation of antivirals. In ciHHV-6 patients, HHV-6 serum and plasma DNA by PCR will always be positive, and DNA levels in serum will typically be higher than plasma 28 . However, both plasma and serum viral load will be at lower numbers than in whole blood28,33. CiHHV-6 status can be confirmed by whole-blood quantitative polymerase chain reaction (qPCR). A patient with a whole blood load greater than 5.5 log10 can be assumed to have ciHHV-6 33 . ciHHV-6 status cannot be determined from qPCR testing of cell-free samples such as plasma or CSF 33 . PCR testing in somatic cells, such as hair or nail follicles, will also confirm ciHHV-6 status since this form of HHV-6 is found in all nucleated cells 28 . Quantitative (RT-PCR) monitoring of the expression of HHV-6 DNA has been suggested to monitor active infection in ciHHV-6 patients, but it is not generally available, and its clinical utility is not well defined 33 . For suspected ciHHV-6 cases, guidelines set forth by the 2017 European Conference on Infections in Leukemia (ECIL) recommend ciHHV-6 testing in both donor and recipient pre-HSCT samples. To test for ciHHV-6 reactivation, viral culture plus viral genome sequencing to test for reactivated ciHHV-6 infection is suggested 27 .
Management for Positive or Suspected Cases of Post-alloHSCT HHV-6 Encephalitis
Previous recommendations for handling viral infection in this setting sparsely discuss HHV-6 without any strong recommendations 35 . However, in the past decade, substantial advances have been made in the understanding of HHV-6 prevalence, severity, and treatment, leading to updated guidelines. Current recommendations from both ECIL and the Guidelines Committee of the Japan Society for Hematopoietic Cell Transplantation (JSHCT) have recommended the use of IV foscarnet or ganciclovir10,27 as the first-line therapies for HHV-6 encephalitis with a weak preference toward foscarnet 10 . However, ECIL strongly recommends that drug selection should be dictated by adverse effects and patient comorbidities 27 . Due to the severe sequelae and potential for high morbidity and mortality of HHV-6 encephalitis, beginning foscarnet before confirmation of HHV-6 DNA in CSF for cases of suspected HHV-6 encephalitis has been strongly recommended by JSHCT 10 . In our case, it took a full week for quantitative CSF assays to return. Combination therapy with both foscarnet and ganciclovir may reduce HHV-6 encephalitis sequelae, but not mortality compared with either foscarnet or ganciclovir monotherapy 36 . A weak recommendation is made for combination therapy10,27, especially in select patients with rapidly progressing cases of HHV-6 encephalitis (e.g., with convulsions) 10 . Weak recommendations have also been made for the antiviral course to last for at least 3 weeks10,27. ECIL extends this to continuing antivirals until clearance of HHV-6 DNA from blood and, if possible, CSF 27 . In addition, maximum doses of 180 and 10 mg/kg/day of foscarnet and ganciclovir, respectively, resulted in significantly better responses than non-maximum doses 37 and are thus recommended strongly by both groups10,27. ECIL also moderately supports reduction of immunosuppressive medications if possible 27 . The use of cidofovir has been demonstrated only anecdotally10,27 and has been recommended for use only after standard treatment has failed 10 .
Besides the poor short-term prognosis of HHV-6 encephalitis, there are also long-term sequelae. A prospective study that examined long-term effects of HHV-6 encephalitis survivors found that four out of five survivors were unable to return to school/work or social life because of persistent neuropsychological disorders 38 . Thus, prevention and early treatment of HHV-6 encephalitis is critical. In HSCT recipients, HHV-6 CSF testing by PCR should be done if any CNS symptoms, such as memory deficits, altered mental status, vigilance impairment, or seizures, are observed. A single negative study also does not exclude development of HHV-6 encephalitis, so vigilance must be taken especially if the patient has an identifiable risk factor. Due to the dynamic nature of HHV-6 reactivation previously discussed, there is currently no recommendation for prophylactic monitoring of HHV-6 viremia10,27. However, while further studies are required to validate this claim, it is the expert opinion of the JSHCT that prophylactic monitoring of high-risk patients (e.g., CBT recipients) may assist in early diagnosis 10 . While prophylactic administration of ganciclovir has been demonstrated to prevent HHV-6 reactivation, the severe myelosuppression it causes in some patients limits usefulness of prophylactic use 38 . Foscarnet prophylaxis has been shown to reduce the severity of encephalitis and incidence of high-level reactivation, but it does not prevent encephalitis 39 and also causes nephrotoxicity39,40. Thus, both ECIL and JSHCT do not recommend HHV-6 prophylaxis10,27, but prophylactic therapy is worth future study. With these factors in mind, it is apparent that there is an urgent need for improved antiviral treatments for HHV-6 encephalitis.
Presentations such as this case are often associated with a complex clinical context, possibly resulting in HHV-6 encephalitis being overlooked and delays in diagnosis/treatment. Thus, we have summarized the most recent data and recommendations regarding the criteria, diagnostic nuances, risk factors, and management of HHV-6 encephalitis that should be considered following alloHSCT. There is a need to promptly identify risk factors, eliminate other causes of CNS complication (e.g., calcineurin inhibitor toxicity), obtain early imaging and laboratory tests, begin treatment as soon as possible, and consistently remain vigilant for HHV-6 encephalitis in this setting.
Acknowledgments
The authors are indebted to Kristin Loomis (HHV-6 Foundation) for facilitating the network among the authors of this study and for her continuous support of HHV-6 research.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: Written informed consent was obtained from the patient for their anonymized information to be published in this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Harrison Zhu  https://orcid.org/0000-0002-0458-044X
https://orcid.org/0000-0002-0458-044X
References
- 1. De Bolle L, Naesens L, De Clercq E. Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev. 2005;18(1):217–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zerr DM. Human herpesvirus 6 (HHV-6) disease in the setting of transplantation. Curr Opin Infect Dis. 2012;25(4):438–44. [DOI] [PubMed] [Google Scholar]
- 3. Zerr DM. Human herpesvirus 6 and central nervous system disease in hematopoietic cell transplantation. J Clin Virol. 2006;37(Suppl 1):S52–56. [DOI] [PubMed] [Google Scholar]
- 4. Zerr DM, Gupta D, Huang ML, Carter R, Corey L. Effect of antivirals on human herpesvirus 6 replication in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34(3):309–17. [DOI] [PubMed] [Google Scholar]
- 5. Drobyski WR, Knox KK, Majewski D, Carrigan DR. Brief report: fatal encephalitis due to variant B human herpesvirus-6 infection in a bone marrow-transplant recipient. N Engl J Med. 1994;330(19):1356–60. [DOI] [PubMed] [Google Scholar]
- 6. Wainwright MS, Martin PL, Morse RP, Lacaze M, Provenzale JM, Coleman RE, Morgan MA, Hulette C, Kurtzberg J, Bushnell C, Epstein L, et al. Human herpesvirus 6 limbic encephalitis after stem cell transplantation. Ann Neurol. 2001; 50(5):612–19. [DOI] [PubMed] [Google Scholar]
- 7. Ogata M, Fukuda T, Teshima T. Human herpesvirus-6 encephalitis after allogeneic hematopoietic cell transplantation: what we do and do not know. Bone Marrow Transplant. 2015;50(8): 1030–36. [DOI] [PubMed] [Google Scholar]
- 8. Voigt M, Sinn K, Malouhi A, Gecks T, Zinke J, Hilgendorf I, Scholl S, Hochhaus A, Schnetzke U. HHV-6 encephalitis in a non-transplanted adult acute myeloid leukemia patient. Ann Hematol. 2021;100(7):1895–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toriumi N, Kobayashi R, Yoshida M, Iguchi A, Sarashina T, Okubo H, Suzuki D, Sano H, Ogata M, Azuma H. Risk factors for human herpesvirus 6 reactivation and its relationship with syndrome of inappropriate antidiuretic hormone secretion after stem cell transplantation in pediatric patients. J Pediatr Hematol Oncol. 2014;36(5):379–83. [DOI] [PubMed] [Google Scholar]
- 10. Ogata M, Uchida N, Fukuda T, Ikegame K, Kamimura T, Onizuka M, Kato K, Kobayashi H, Sasahara Y, Sawa M, Sawada A, et al. Clinical practice recommendations for the diagnosis and management of human herpesvirus-6B encephalitis after allogeneic hematopoietic stem cell transplantation: the Japan Society for Hematopoietic Cell Transplantation. Bone Marrow Transplant. 2020;55(6):1004–13. [DOI] [PubMed] [Google Scholar]
- 11. Siegal D, Keller A, Xu W, Bhuta S, Kim DH, Kuruvilla J, Lipton JH, Messner H, Gupta V. Central nervous system complications after allogeneic hematopoietic stem cell transplantation: incidence, manifestations, and clinical significance. Biol Blood Marrow Transplant. 2007;13(11):1369–79. [DOI] [PubMed] [Google Scholar]
- 12. Hill JA, Boeckh MJ, Sedlak RH, Jerome KR, Zerr DM. Human herpesvirus 6 can be detected in cerebrospinal fluid without associated symptoms after allogeneic hematopoietic cell transplantation. J Clin Virol. 2014;61(2):289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhanushali MJ, Kranick SM, Freeman AF, Cuellar-Rodriguez JM, Battiwalla M, Gea-Banacloche JC, Hickstein DD, Pavletic S, Fahle G, Nath A. Human herpes 6 virus encephalitis complicating allogeneic hematopoietic stem cell transplantation. Neurology. 2013;80(16):1494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacLean HJ, Douen AG. Severe amnesia associated with human herpesvirus 6 encephalitis after bone marrow transplantation. Transplantation. 2002;73(7):1086–89. [DOI] [PubMed] [Google Scholar]
- 15. Gorniak RJ, Young GS, Wiese DE, Marty FM, Schwartz RB. MR imaging of human herpesvirus-6-associated encephalitis in 4 patients with anterograde amnesia after allogeneic hematopoietic stem-cell transplantation. Am J Neuroradiol. 2006;27(4):887–91. [PMC free article] [PubMed] [Google Scholar]
- 16. Noguchi T, Yoshiura T, Hiwatashi A, Togao O, Yamashita K, Nagao E, Uchino A, Hasuo K, Atsumi K, Matsuura T, Kuroiwa T, et al. CT and MRI findings of human herpesvirus 6-associated encephalopathy: comparison with findings of herpes simplex virus encephalitis. Am J Roentgenol. 2010;194(3):754–60. [DOI] [PubMed] [Google Scholar]
- 17. Visser AM, van Doornum GJ, Cornelissen JJ, van den Bent MJ. Severe amnesia due to HHV-6 encephalitis after allogenic stem cell transplantation. Eur Neurol. 2005;54(4):233–34. [DOI] [PubMed] [Google Scholar]
- 18. Berzero G, Campanini G, Vegezzi E, Paoletti M, Pichiecchio A, Simoncelli AM, Colombo AA, Bernasconi P, Borsani O, Di Matteo A, Rossi V, et al. Human herpesvirus 6 encephalitis in immunocompetent and immunocompromised hosts. Neurol Neuroimmunol Neuroinflamm. 2021;8(2):e942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frey JW, Cherabie JN, Assi MA. Human herpesvirus-6 encephalitis following chemotherapy induction for acute myelogenous leukemia. Transpl Infect Dis. 2017;19(6):e12756. [DOI] [PubMed] [Google Scholar]
- 20. Ogata M, Satou T, Kadota J, Saito N, Yoshida T, Okumura H, Ueki T, Nagafuji K, Kako S, Uoshima N, Tsudo M, et al. Human herpesvirus 6 (HHV-6) reactivation and HHV-6 encephalitis after allogeneic hematopoietic cell transplantation: a multicenter, prospective study. Clin Infect Dis. 2013;57(5):671–81. [DOI] [PubMed] [Google Scholar]
- 21. Ogata M, Satou T, Kawano R, Goto K, Ikewaki J, Kohno K, Ando T, Miyazaki Y, Ohtsuka E, Saburi Y, Saikawa T, et al. Plasma HHV-6 viral load-guided preemptive therapy against HHV-6 encephalopathy after allogeneic stem cell transplantation: a prospective evaluation. Bone Marrow Transplant. 2008;41(3):279–85. [DOI] [PubMed] [Google Scholar]
- 22. Yao K, Honarmand S, Espinosa A, Akhyani N, Glaser C, Jacobson S. Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann Neurol. 2009;65(3): 257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Agut H, Bonnafous P, Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev. 2015;28(2):313–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phan TL, Carlin K, Ljungman P, Politikos I, Boussiotis V, Boeckh M, Shaffer ML, Zerr DM. Human herpesvirus-6B reactivation is a risk factor for grades II to IV acute graft-versus-host disease after hematopoietic stem cell transplantation: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2018;24(11):2324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang X, Patel SA, Haddadin M, Cerny J. Post-allogeneic hematopoietic stem cell transplantation viral reactivations and viremias: a focused review on human herpesvirus-6, BK virus and adenovirus. Ther Adv Infect Dis. 2021;8:20499361211018027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hill JA, Koo S, Guzman Suarez BB, Ho VT, Cutler C, Koreth J, Armand P, Alyea EP, 3rd, Baden LR, Antin JH, Soiffer RJ, et al. Cord-blood hematopoietic stem cell transplant confers an increased risk for human herpesvirus-6-associated acute limbic encephalitis: a cohort analysis. Biol Blood Marrow Transplant. 2012;18(11):1638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ward KN, Hill JA, Hubacek P, de la Camara R, Crocchiolo R, Einsele H, Navarro D, Robin C, Cordonnier C, Ljungman P, 2017 European Conference on Infections in Leukaemia (ECIL). Guidelines from the 2017 European Conference on Infections in Leukaemia for management of HHV-6 infection in patients with hematologic malignancies and after hematopoietic stem cell transplantation. Haematologica. 2019;104(11):2155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ward KN, Leong HN, Nacheva EP, Howard J, Atkinson CE, Davies NW, Griffiths PD, Clark DA. Human herpesvirus 6 chromosomal integration in immunocompetent patients results in high levels of viral DNA in blood, sera, and hair follicles. J Clin Microbiol. 2006;44(4):1571–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greco R, Crucitti L, Noviello M, Racca S, Mannina D, Forcina A, Lorentino F, Valtolina V, Rolla S, Dvir R, Morelli M, et al. Human herpesvirus 6 infection following haploidentical transplantation: immune recovery and outcome. Biol Blood Marrow Transplant. 2016;22(12):2250–55. [DOI] [PubMed] [Google Scholar]
- 30. Sisinni L, Gasior M, de Paz R, Querol S, Bueno D, Fernandez L, Marsal J, Sastre A, Gimeno R, Alonso L, Badell I, et al. Unexpected high incidence of human herpesvirus-6 encephalitis after naive T cell-depleted graft of haploidentical stem cell transplantation in pediatric patients. Biol Blood Marrow Transplant. 2018;24(11):2316–23. [DOI] [PubMed] [Google Scholar]
- 31. Yamamoto W, Ogusa E, Matsumoto K, Maruta A, Ishigatsubo Y, Kanamori H. Human herpesvirus-6 encephalopathy after hematopoietic stem cell transplantation and class I human leukocyte antigen. Clin Transplant. 2014;28(5):540–45. [DOI] [PubMed] [Google Scholar]
- 32. Scheurer ME, Pritchett JC, Amirian ES, Zemke NR, Lusso P, Ljungman P. HHV-6 encephalitis in umbilical cord blood transplantation: a systematic review and meta-analysis. Bone Marrow Transplant. 2013;48(4):574–80. [DOI] [PubMed] [Google Scholar]
- 33. Pellett PE, Ablashi DV, Ambros PF, Agut H, Caserta MT, Descamps V, Flamand L, Gautheret-Dejean A, Hall CB, Kamble RT, Kuehl U, et al. Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol. 2012;22(3):144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arbuckle JH, Medveczky MM, Luka J, Hadley SH, Luegmayr A, Ablashi D, Lund TC, Tolar J, De Meirleir K, Montoya JG, Komaroff AL, et al. The latent human herpesvirus-6A genome specifically integrates in telomeres of human chromosomes in vivo and in vitro. Proc Natl Acad Sci USA. 2010;107(12):5563–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ, Center for International Blood and Marrow Research, National Marrow Donor Program, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Toomey D, Phan TL, Nguyen V, Phan TT, Ogata M. Retrospective case analysis of antiviral therapies for HHV-6 encephalitis after hematopoietic stem cell transplantation. Transpl Infect Dis. 2021;23(1):e13443. [DOI] [PubMed] [Google Scholar]
- 37. Ogata M, Oshima K, Ikebe T, Takano K, Kanamori H, Kondo T, Ueda Y, Mori T, Hashimoto H, Ogawa H, Eto T, et al. Clinical characteristics and outcome of human herpesvirus-6 encephalitis after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(11):1563–70. [DOI] [PubMed] [Google Scholar]
- 38. Sakai R, Kanamori H, Motohashi K, Yamamoto W, Matsuura S, Fujita A, Ohshima R, Kuwabara H, Tanaka M, Fujita H, Maruta A, et al. Long-term outcome of human herpesvirus-6 encephalitis after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(9):1389–94. [DOI] [PubMed] [Google Scholar]
- 39. Ogata M, Takano K, Moriuchi Y, Kondo T, Ueki T, Nakano N, Mori T, Uoshima N, Nagafuji K, Yamasaki S, Shibasaki Y, et al. Effects of prophylactic foscarnet on human herpesvirus-6 reactivation and encephalitis in cord blood transplant recipients: a prospective multicenter trial with an historical control group. Biol Blood Marrow Transplant. 2018;24(6):1264–73. [DOI] [PubMed] [Google Scholar]
- 40. Deray G, Martinez F, Katlama C, Levaltier B, Beaufils H, Danis M, Rozenheim M, Baumelou A, Dohin E, Gentilini M. Foscarnet nephrotoxicity: mechanism, incidence and prevention. Am J Nephrol. 1989;9(4):316–21. [DOI] [PubMed] [Google Scholar]