Abstract
Objectives:
Treatment of Clostridioides difficile infection (CDI) has undergone significant change in recent years with the introduction of fidaxomicin and bezlotoxumab. This study evaluated the cost-effectiveness of fidaxomicin and bezlotoxumab for initial CDI compared with standard therapy with oral vancomycin.
Methods:
A Markov model with eight health states was built based on transition probabilities, costs and health utilities derived from literature to evaluate the cost-effectiveness of standard fidaxomicin, bezlotoxumab plus vancomycin, and extended-pulsed fidaxomicin versus standard oral vancomycin over a lifetime horizon from the US societal perspective.
Results:
For overall CDI treatment, oral vancomycin had a cost of $39 178 and was associated with a gain of 11.64 quality-adjusted life-years (QALYs). Extended-pulsed fidaxomicin had a higher QALY gain of 11.65 at a lower cost of $37 613, and therefore was dominant over vancomycin. Standard fidaxomicin had a QALY gain of 11.94 versus vancomycin at an incremental cost of $495 per QALY. Bezlotoxumab plus vancomycin led to a QALY gain of 11.77 at an incremental cost of $17 746 per QALY. At the willingness-to-pay (WTP) threshold of $150 000 per QALY, extended-pulsed fidaxomicin, bezlotoxumab plus vancomycin and standard fidaxomicin were more cost-effective compared with vancomycin alone, yielding incremental net monetary benefits of $3248, $17 011 and $44 308, respectively. One-way sensitivity analysis suggested that the probabilities of sustained cure from the initial episode were the most sensitive inputs, and results were overall not particularly sensitive to any drug costs.
Conclusions:
Based on a WTP threshold of $150 000, standard fidaxomicin was estimated to be the most cost-effective treatment. Standard-of-care vancomycin was dominated by extended-pulsed fidaxomicin for treating an episode of CDI and preventing further recurrence, and the addition of bezlotoxumab to vancomycin was dominated by standard fidaxomicin.
Keywords: Bacterial resistance, Bezlotoxumab, Clostridioides difficileinfection, Cost-effectiveness analysis, Extended-pulsed fidaxomicin, Fidaxomicin, Incremental cost-effectiveness ratio
Introduction
The treatment of Clostridioides difficile infection (CDI) has undergone significant change in recent years, most notably with the introduction of fidaxomicin and bezlotoxumab. Fidaxomicin is a macrocyclic antibiotic that has a more targeted anti-C. difficile effect compared with other antibiotics, and can be used as primary therapy [1]. Bezlotoxumab is a monoclonal antibody against C. difficile toxin B that can be used as an adjunct to standard antibiotic therapy by boosting humoral immunity, which has been correlated with a reduction in the risk of recurrence [2]. Although each works by a different mechanism, both were shown in clinical trials to reduce the absolute risk of CDI recurrence by ~10% when compared with standard of care (SOC) [3–5]. Unfortunately, both therapies are costly compared with standard CDI therapy with vancomycin [6]. Because of this, and the uncertainty about whether the reduced recurrence rate compared with vancomycin held for CDI, previous economic analyses have been ambiguous, showing fidaxomicin to be cost-effective compared with vancomycin in 14 of 24 economic evaluations [7]. Studies also supported cost-effectiveness of fidaxomicin in patient subgroups with higher rates of recurrence, such as the elderly, those with severe CDI and those taking concomitant antibiotics [7]. In addition, extended-pulsed fidaxomicin, which extends 20 fidaxomicin doses over a longer time period after initial daily dosing, was shown to be superior to standard vancomycin as the first-line CDI treatment in a clinical trial [8]. The cost-effectiveness of extended-pulsed fidaxomicin was supported by an analysis in England [9], but no study to date has compared the cost-effectiveness of extended-pulsed fidaxomicin with standard fidaxomicin or bezlotoxumab. Similarly, two of three pharmaco-economic analyses found that bezlotoxumab added to standard therapy was cost-effective compared with standard therapy alone, mainly as a result of the reduction in recurrent episodes of CDI seen in the phase 3 trials [10–12]. The only cost-effectiveness analysis that has compared fidaxomicin with standard therapy plus bezlotoxumab is by Lam et al., and it focused solely on recurrent episodes of CDI [12]. Therefore, given the comparable reduction in recurrence rates in clinical trials, it remains uncertain how to best incorporate these therapies into both initial and recurrent CDI management. The objective of this study was to evaluate the cost-effectiveness of standard fidaxomicin, bezlotoxumab in addition to vancomycin (bezlotoxumab-vancomycin), and extended-pulsed fidaxomicin on initial and recurrent CDI compared with standard therapy with oral vancomycin.
Materials and methods
Model structure
A Microsoft Excel-based Markov health state transition model (Fig. 1) was built based on the model by Prabhu et al. to simulate the costs and health effects of treating CDI patients with each of the four CDI therapies from a US societal perspective [10]. The model followed patients over a lifetime horizon, which was further divided into two parts: a 15-day cycle length for the initial 6 months (biweekly cycles), followed by annual cycles for the remaining lifetime, and an annual discount rate of 3% was applied to the future costs and health effects throughout [13]. The model assumed eight health states: initial CDI episode, treatment failure, treatment success (clinical cure), CDI recurrence, colectomy, sustained clinical cure (absorbing state), post-colectomy (absorbing state) and death (absorbing state, not shown in Fig. 1). All patients were assumed to enter the model with their initial CDI episodes and transition thorough the model according to corresponding treatment-specific transition probabilities.
Fig. 1.
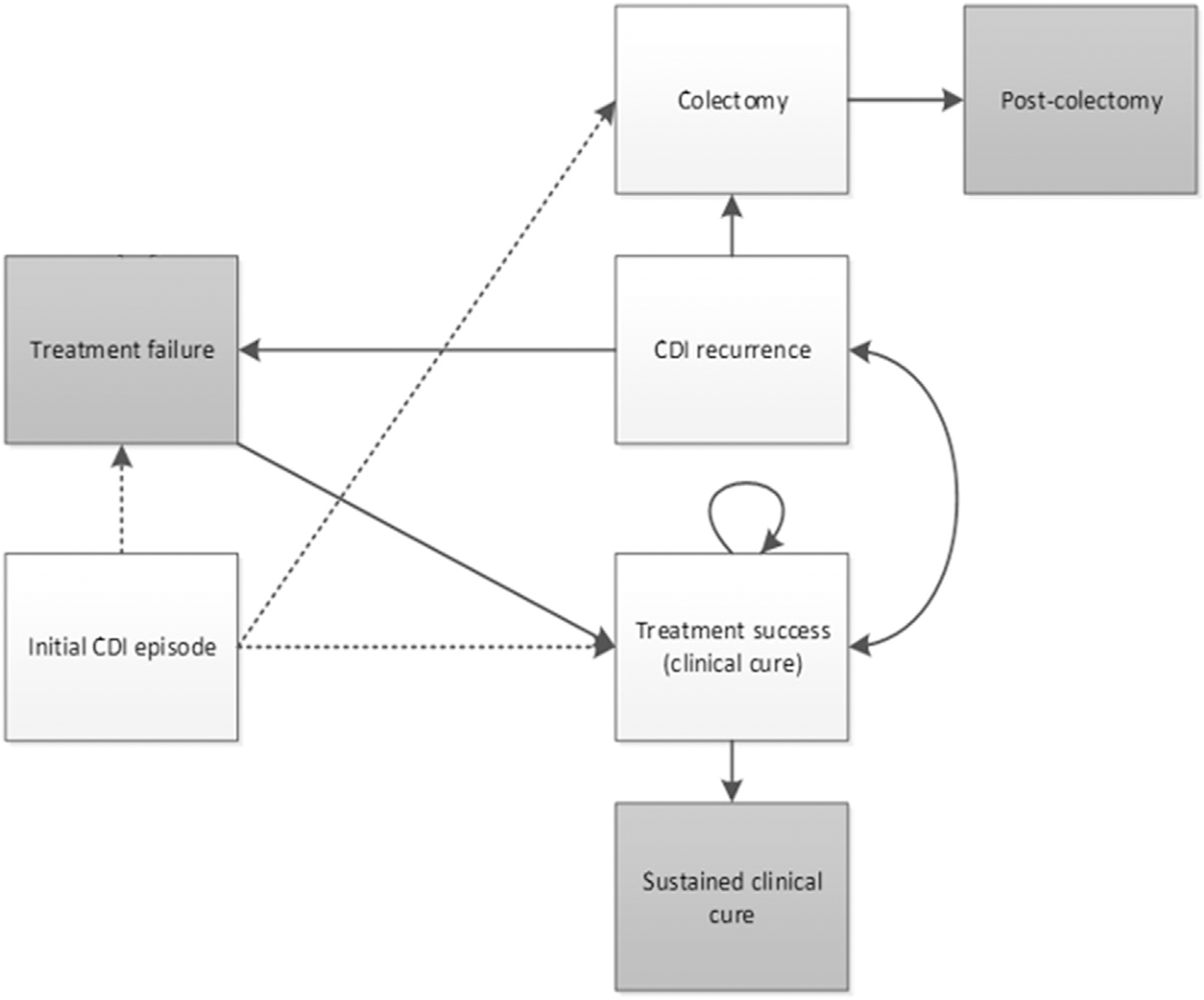
Markov model diagram; CDI, Clostridioides difficile infection.
The base-case population characteristics in this model were adapted from clinical trials of fidaxomicin and are summarized in Table 1. Patients were assumed to have no CDI infection for at least 6 months before the initial episode in the model. Patients were treated with one of the following CDI therapies for the initial episode: (a) vancomycin 125 mg making an oral solution from intravenous powder, four times daily for 10 days; (b) fidaxomicin 200 mg by mouth, twice daily for 10 days; (c) bezlotoxumab 10 mg/kg intravenously administered for one dose plus vancomycin 125 mg making an oral solution from intravenous powder, four times daily for 10 days; (d) fidaxomicin 200 mg by mouth, twice daily on days 1–5, then once daily on alternate days from day 7 to day 25 (extended-pulsed) [3–5,8,14,15]. After therapy, patients could either experience clinical success or clinical failure, which were defined according to the corresponding clinical trials [3–5,8,10]. It is assumed that patients will proceed to clinical success or failure only after finishing the second 15-day cycle for extended-pulsed fidaxomicin. Cured patients remained in the short-term clinical cure state before they eventually maintained sustained clinical cure or developed new recurrent CDI episodes. A small proportion of patients whose CDI symptoms were too severe to be treated with any antibiotic therapy proceeded to colectomy, and patients could also die from any health state with a given probability [16,17]. See Technical Appendix for more details of model structure.
Table 1.
Baseline population characteristics
| Variable | Inputs | Range | Reference |
|---|---|---|---|
|
| |||
| Size | 1000 | N/A a | N/A a |
| Average age (year) | 62.4 | 49.9–74.9 | [3,4] |
| % age ≥65 years | 49.5% | 39.6%–59.4% | [3,4] |
| % female | 58.2% | 46.6%–69.8% | [3,4] |
| % inpatient | 63.4% | 50.8%–76.1% | [3,4] |
| % severe CDI | 32.5% | 26.0%–39.0% | [3,4] |
Abbreviations: CDI, Clostridioides difficile infection.
Assumed value.
Input parameters
All parameters and ranges are summarized in Table 2. Most treatment-specific probabilities, including initial cure rates, first and second recurrence rates and sustained clinical cure rates, were derived from fidaxomicin and bezlotoxumab clinical trials [3–5,8]. Other input clinical probabilities, such as probabilities of colectomy and mortality, were assumed to be constant across different therapies and were derived from non-trial clinical studies, Social Security Actuarial life-table data or estimated according to clinicians’ advice [17–20]. The declining exponential approximation of life expectancy (the DEALE) method was used to transfer discount rate and background mortality from annual set to biweekly set [21,22].
Table 2.
Clinical probability, cost and utility inputs
| Description | Base value | Range | Distribution | Ref. |
|---|---|---|---|---|
|
| ||||
| Treatment success from initial, VAN | 0.857 | 0.771–0.943 | Beta | [3,4] |
| Treatment success from initial, FDX | 0.876 | 0.788–0.963 | Beta | [3,4] |
| Treatment success from initial, BEZ | 0.859 | 0.773–0.945 | Beta | [5] |
| Treatment success from initial, EPFX | 0.819 | 0.737–0.900 | Beta | [8] |
|
| ||||
| Treatment success from recurrence, VAN | 0.889 | 0.800–0.978 | Beta | [3,4] |
| Treatment success from recurrence, FDX | 0.898 | 0.808–0.988 | Beta | [3,4] |
| Treatment success from recurrence, BEZ | 0.859 | 0.773–0.945 | Beta | [5] |
| Treatment success from recurrence, EPFX | 0.819 | 0.737–0.900 | Beta | [8] |
|
| ||||
| First recurrence, VAN | 0.248 | 0.198–0.297 | Beta | [3,4] |
| First recurrence, FDX | 0.129 | 0.10–0.155 | Beta | [3,4] |
| First recurrence, BEZ | 0.160 | 0.128–0.192 | Beta | [5] |
| First recurrence, EPFX | 0.078 | 0.062–0.093 | Beta | [8] |
|
| ||||
| Second recurrence, VAN | 0.325 | 0.260–0.390 | Beta | [3,4] |
| Second recurrence, FDX | 0.203 | 0.162–0.243 | Beta | [3,4] |
| Second recurrence, BEZ | 0.198 | 0.158–0.237 | Beta | [5] |
| Second recurrence, EPFX | 0.122 | 0.098–0.146 | Beta | [8] |
|
| ||||
| Third recurrence | 0.45 | 0.25–0.65 | Beta | [18] |
|
| ||||
| Colectomy | 0.015 | 0.012–0.018 | Beta | N/A a |
| Post–colectomy | 0.584 | 0.467–0.701 | Beta | [17] |
|
| ||||
| Sustained clinical cure from initial, VAN | 0.646 | 0.517–0.775 | Beta | [3,4] e |
| Sustained clinical cure from initial, FDX | 0.758 | 0.606–0.910 | Beta | [3,4] e |
| Sustained clinical cure from initial, BEZ | 0.646 | 0.517–0.775 | Beta | N/A b,e |
| Sustained clinical cure from initial, EPFX | 0.758 | 0.606–0.910 | Beta | N/A c,e |
|
| ||||
| Attributable CDI mortality | 0.026 | 0.021–0.031 | Beta | [19] |
|
| ||||
| Background mortality, year 0 | 0.010 | 0.008–0.012 | Beta | [20] e |
| Background mortality, year 1 | 0.011 | 0.009–0.013 | Beta | |
| Background mortality, year 2 | 0.012 | 0.009–0.014 | Beta | |
|
| ||||
| Vancomycin cost | $21 | $17–$26 | N/A d | [24] |
| Standard fidaxomicin cost | $3613 | $2890–$4335 | [24] | |
| Extended-pulsed fidaxomicin cost | $3613 | $2890–$4335 | [24] | |
| Bezlotoxumab cost | $3896 | $3117–$4675 | [15,23,24] | |
|
| ||||
| Bezlotoxumab infusion time (h) | 1 | 0.8–1.2 | Gamma | [15] |
| CDI outpatient visit time (h) | 2 | 1.6–2.4 | Gamma | N/A a |
|
| ||||
| Initial attributable cost | $28 952 | $28 032–$29 919 | Gamma | [25] |
| Initial cumulative hospitalization (days) | 5.2 | 5.01–5.39 | Gamma | [25] |
|
| ||||
| Recurrent attributable cost | $12 655 | $10 584– $14,887 | Gamma | [25] |
| Recurrent cumulative hospitalization (days) | 1.95 | 1.48–2.43 | Gamma | [25] |
|
| ||||
| Colectomy cost | $43 156 | $24 548–$49 095 | Gamma | [17] |
| Post-colectomy annual cost | $10 883 | $0–$24 464 | Gamma | [26] |
|
| ||||
| Baseline utility | 0.88 | 0.7–1.00 | Beta | [3,4,28] |
| Disease utility | 0.76 | 0.61 –0.91 | Beta | [10,28] |
| Colectomy utility | 0.72 | 0.58–0.86 | Beta | [10,28] |
| Post-colectomy utility | 0.39 | 0.31–0.46 | Beta | [12,29,30] |
Abbreviations: BEZ, bezlotoxumab plus vancomycin; EPFX, extended-pulsed fidaxomicin; FDX, standard fidaxomicin; VAN, vancomycin.
Clinician Estimates.
Assumed to be the same as vancomycin.
Assumed to be the same as standard fidaxomicin.
Not included in probabilistic sensitivity analyses.
Further are shown in Technical Appendix Table 1.
All costs were adjusted to 2020 US dollars according to the medical care consumer price index [23]. Direct drug costs, procedure costs and disease management costs were included in the model. Drug costs were derived from the 2020 Veterans Affairs Federal Supply Schedule to reflect the true cost to society, rather than specific payers [24]. All direct costs attributed to CDI hospitalization were derived from the CDI-attributable cost reported in Zhang et al. and multiplied by the number of CDI episodes [25]. Post-colectomy direct costs were estimated from 120-day long-term care costs of stoma management as an approximation due to lack of data [26]. Time-loss-associated indirect costs were calculated using the Bureau of Labor Statistics civilian compensation rate, time loss due to CDI episodes and proportion of inpatients in the baseline population [15,27]. Finally, health utility measurement data were derived and adjusted from previous CDI cost-effectiveness studies [10,12,28–30]. See Technical Appendix for more details of input parameters.
Sensitivity analysis
One-way sensitivity analyses were conducted in this study to evaluate the effect of uncertainty in parameters. Ranges for parameters were derived from the same sources as base values or assumed to be ±20% from base values when 95% statistical confidence intervals were not reported [31]. Specifically, the assumed ranges for clinical success probabilities were ±10% from base values according to results of randomized trials [3–5,8], and the assumed upper bound for any utility measurement was set to be 1 if base value plus 20% was greater than 1.
Probabilistic sensitivity analyses were computed using 10 000 Monte Carlo simulations to further evaluate the robustness of model results. Probabilities, utilities and population characteristics were assumed to have β distributions, whereas costs and indirect cost-associated time losses were assumed to have γ distributions [31]. Drug costs were assumed to be constant from the societal perspective and were not included in probabilistic sensitivity analyses.
The incremental net monetary benefit and its 80% uncertainty interval were estimated based on a willingness-to-pay (WTP) threshold of $150 000 per quality-adjusted life-year (QALY) and the probabilistic sensitivity analyses simulation results. This threshold is based on the World Health Organization’s recommendation that a threshold of three times the gross domestic product of the country, c.$150 000 per QALY in the USA, should be used [32].
Ethics
Consent was not provided for the study because it did not involve any human participants.
Results
Base case
The result for the base-case population is shown in Table 3. The total QALY gain per patient was 11.64 for SOC vancomycin. Compared with vancomycin, extended-pulsed fidaxomicin, fidaxomicin and bezlotoxumab-vancomycin led to 0.01, 0.30 and 0.13 more QALYs, respectively. Estimated total cost per patient was $39 178 for vancomycin, $37 613 for extended-pulsed fidaxomicin, $39 325 for fidaxomicin and $41 461 for bezlotoxumab-vancomycin. Extended-pulsed fidaxomicin had a lower cost and a slightly higher QALY gain than vancomycin, suggesting its dominance over vancomycin. Fidaxomicin had about the same cost as vancomycin and relatively higher QALY gained, which led to an incremental cost-effectiveness ratio (ICER) of $495 per QALY gained and an incremental net monetary benefit of $44 308. Despite being the costliest treatment, bezlotoxumab-vancomycin resulted in an ICER of $17 746 per QALY and was dominated by fidaxomicin if used over vancomycin. In addition, fidaxomicin had an ICER of $6004 per QALY gained and an incremental net monetary benefit of $41 060 when compared with extended-pulsed fidaxomicin.
Table 3.
Base-case result of total cost, QALY, ICER and INMB
| Treatment | Cost | QALY | ||
|---|---|---|---|---|
|
| ||||
| EP fidaxomicin | $37 613 | ($35 795–$38 196)b | 11.65 | (13.42–14.22)b |
| Vancomycin | $39 178 | ($36 715–$39 951)b | 11.64 | (13.40–14.19)b |
| Fidaxomicin | $39 325 | ($37 113–$39 690)b | 11.94 | (13.69–4.50)b |
| Bezlotoxumab-vancomycin | $41 461 | ($39 079–$41 864)b | 11.77 | (13.52–14.32)b |
|
| ||||
| Treatment | ICER (vs Vancomycin) | INMB (vs Vancomycin)a | ||
|
| ||||
| EP fidaxomicin | Dominant | (−$127 102 to −$16 844)b | $3,248 | ($1817–$9230)b |
| Fidaxomicin | $495 | (−$1384 to $1957)b | $44 308 | ($41 378–$49 478)b |
| Bezlotoxumab-vancomycin | $17 746 | ($12 117–$23 360)b | $17 011 | ($13 136–$21 164)b |
|
| ||||
| Treatment | ICER (vs EP fidaxomicin) | INMB (vs EP fidaxomicin)a | ||
|
| ||||
| Fidaxomicin | $6004 | ($4222–$6162)b | $41 060 | ($36 493–$43 389)b |
At willingness-to-pay threshold of $150 000 per QALY.
Reported ranges are 80% uncertainty interval based on probabilistic sensitivity analysis.Abbreviations: EP, extended-pulsed; ICER, incremental cost-effectiveness ratio; INMB, incremental net monetary benefit; QALY, quality-adjusted life-year.
Sensitivity analysis
Results of one-way sensitivity analyses are reported as tornado diagrams (see Technical Appendix Fig. 1–4). In all four one-way sensitivity analyses, the probabilities of sustained cure from initial episode for the corresponding treatments were the most sensitive inputs. Other inputs that dramatically shifted incremental net monetary benefits included first recurrence rates, baseline utility, average age of the patient population and attributable CDI mortality. Results were not particularly sensitive to drug costs.
Results of probabilistic sensitivity analyses are reported as cost-effectiveness acceptability curves and ICER scatterplots, respectively (see Technical Appendix Fig. 5–12). The acceptability curves show that at a low WTP threshold, vancomycin may be favoured over fidaxomicin and bezlotoxumab-vancomycin, but that fidaxomicin quickly becomes more favoured as the WTP threshold increases towards $3500 per QALY, where fidaxomicin has a 100% probability of being cost-effective. Bezlotoxumab-vancomycin becomes more favoured than vancomycin as the threshold passes $20 000 per QALY. At any WTP threshold lower than $150 000 per QALY, extended-pulsed fidaxomicin is more favoured than vancomycin; but compared with fidaxomicin, extended-pulsed fidaxomicin is only more favoured at a WTP threshold lower than $5000 per QALY.
Discussion
Among key cost-effectiveness studies, our study is the first to compare the cost-effectiveness of extended-pulsed fidaxomicin versus bezlotoxumab-vancomycin as well as fidaxomicin, and the first to evaluate the cost-effectiveness of CDI treatments based on lifetime horizon and from the US societal perspective (Table 4). Based on our results, fidaxomicin led to higher QALYs gained at a cost below any typical WTP threshold, and therefore was the most cost-effective treatment. Although bezlotoxumab-vancomycin is more cost-effective than vancomycin at our WTP threshold of $150 000 per QALY, the higher costs and lower QALY gained suggest that it is dominated by fidaxomicin. Extended-pulsed fidaxomicin is associated with lowest cost and dominates over vancomycin, but is less cost-effective than fidaxomicin.
Table 4.
Characteristics of key cost-effectiveness studies on CDI
| Study | Chen et al. | Prabhu et al. [10] | Lam et al. [12] | Cornely et al. [9] |
|---|---|---|---|---|
|
| ||||
| Model design | Markov transition | Markov transition | Decision tree | Markov transition |
| Time horizon | Lifetime | Lifetime | 1 year | 1 year |
| Perspective | Societal perspective | Payer’s perspective | Payer’s perspective | Health-care system perspective |
| Inclusion of indirect costs | Yes | No | No | No |
| Base case | Patients with any CDI episode | Patients with any CDI episode | Patients with first CDI recurrence | Patients with any CDI episode |
| Treatment arms | Vancomycin, Fidaxomicin, Bezlotoxumab + SOC, Extended-pulsed fidaxomicin | Bezlotoxumab + SOC, Placebo + SOC | Vancomycin, Fidaxomicin, Bezlotoxumab + SOC | Vancomycin, Extended-pulsed fidaxomicin |
| Most cost-effective treatment | Fidaxomicin | Bezlotoxumab + SOC | Vancomycin | Extended-pulsed fidaxomicin |
Abbreviations: CDI, Clostridioides difficile infection; SOC, standard of care.
Our conclusions are partly consistent with Lam et al., which supports the cost-effectiveness of fidaxomicin versus bezlotoxumab-vancomycin [12]. Considering the lower recurrence rates of bezlotoxumab-vancomycin versus vancomycin in clinical trials and the similarly high prices of fidaxomicin and bezlotoxumab-vancomycin, it is likely that lower clinical treatment success rates of bezlotoxumab-vancomycin led to decreased QALYs gained and consequently made bezlotoxumab-vancomycin less cost-effective compared with fidaxomicin. Quantitatively, our results are similar to those of Prabhu et al. (ICER for bezlotoxumab-vancomycin of $17 746 per QALY gained versus $19 824 per QALY gained) and different from those of Lam et al. (ICER for fidaxomicin of $495 per QALY gained versus $500 975 per QALY gained) [10,12]. This is likely due to the similarity of base case populations, model structures and time horizons between this study and Prabhu et al. [10,12].
Our conclusions on extended-pulsed fidaxomicin versus vancomycin are also similar to those of Cornely et al. [9]. Extended-pulsed fidaxomicin probably benefited from lower recurrence rates and, consequently, from lower total costs, despite its price being far higher than that of vancomycin. On the other hand, extended-pulsed fidaxomicin failed to outperform fidaxomicin in our model, probably because of its extended course of treatment. Patients who took extended-pulsed fidaxomicin stayed in diseased stages for a longer period, which reduced total QALY gained compared with fidaxomicin and therefore diminished the overall performance of extended-pulsed fidaxomicin in the model.
Our results were sensitive to sustained clinical cure rates from the initial CDI episode and first recurrence, which indicated that the cost-effectiveness of CDI treatments was mostly affected by patient responses during their initial episodes. In light of previous findings showing that the rate of CDI recurrence increases as the number of recurrences increases [18], the most desired strategy for CDI management is to maximize initial episode treatment response and prevent recurrences.
The new CDI treatment guidelines in preparation in the USA recommend that: (a) for patients with initial CDI episode, fidaxomicin be used versus standard course vancomycin; (b) for patients with a recurrent CDI episode, fidaxomicin or extended-pulsed fidaxomicin be used versus standard course vancomycin; (c) for patients with a CDI episode and at least one risk factor for recurrence, bezlotoxumab be used as a co-intervention along with SOC antibiotics versus SOC antibiotics alone [33]. Our model results support the recommendations of fidaxomicin or extended-pulsed fidaxomicin rather than vancomycin as the preferred therapy for treating initial and recurrent CDI episodes. However, our model does not support the recommendation to use bezlotoxumab for CDI patients with higher risk of recurrence, as it favours the use of fidaxomicin, one of the SOC antibiotics. In addition, studies have shown that adding bezlotoxumab to fidaxomicin would give a similar magnitude of reduction in recurrent CDI as bezlotoxumab plus other SOC antibiotics such as vancomycin or metronidazole, whereas very few patients have received this combination [34]. Based on the current evidence and price of bezlotoxumab, the application of this recommendation should be limited until more data can be obtained and further cost-effectiveness research can be conducted.
This cost-effectiveness analysis has several limitations. First, instead of modelling severe CDI patients and mild/moderate CDI patients differently, the analysis considered both subgroups in the same way and instead used percentage of severe CDI cases in the population at baseline to account for the potential difference, which may lead to underestimates of colectomy cost in each treatment arm. Second, the rates of sustained cure from recurrent CDI for vancomycin and fidaxomicin were calculated according to the reported value in clinical trials with assumptions. Similarly, the rates of sustained cure for bezlotoxumab-vancomycin and extended-pulsed fidaxomicin were assumed to be the same as for vancomycin or fidaxomicin because of data limitations. The sensitivity analysis illustrated that model results were not significantly sensitive to sustained cure rates from recurrent CDI; nevertheless, if these probabilities were higher in real clinical settings, fidaxomicin may not be the most cost-effective treatment. Third, this cost-effectiveness analysis chose vancomycin taper as the only second-line therapy and assumed all patients treated with vancomycin taper after experiencing clinical failure with first treatment were cured within 4 weeks. This assumption was based on clinicians’ expert opinion and findings in clinical practice guidelines for CDI in the USA [14]. If different clinical guidelines are used in different health centres, these assumptions may be violated. Fourth, the current analysis did not include different strains of C. difficile and their influence on recurrence rates. The hypervirulent BI/NAP1/027 strain of C. difficile is known to produce higher rates of severe and recurrent disease, and the trial data suggest that the main benefit of fidaxomicin, lowering recurrence rate, is limited in treatment of the BI/NAP1/027 strain [3,4]. If a patient population has a high proportion of BI/NAP1/027 clones of C. difficile, the results of this analysis may be less applicable because the recurrence rates of such population are likely to be quite different. Fifth, the only SOC drug considered in this model was vancomycin. Although metronidazole may also be used as initial therapy for CDI in settings where access to vancomycin and fidaxomicin is limited, it is no longer recommended as first-line therapy by current Infectious Diseases Society of America guidelines [14]. Sixth, though we adjusted the parameters to simulate a head-to-head comparison between bezlotoxumab-vancomycin, fidaxomicin, and extended-pulsed fidaxomicin, this analysis is not based on any real randomized controlled trials comparing them for either initial or recurrent CDI. Finally, many input parameters of the analysis were from clinical studies with small population sizes, which may not represent the general population. We aimed to account for such variability by adjusting the base-case values and testing the robustness of the model with probabilistic sensitivity analysis.
In conclusion, fidaxomicin was the most cost-effective regimen for the treatment of initial episode of CDI to prevent recurrence based on our analysis. Bezlotoxumab-vancomycin was found to be dominated by fidaxomicin. Although extended-pulsed fidaxomicin was dominant over vancomycin, it was shown to be less cost-effective than fidaxomicin.
Supplementary Material
Transparency declaration
The authors received no financial support for this work and have declared that no conflicts of interests exist. MMH was previously supported by the National Institutes of Health (grants NIH T32 AI 052073-11 A1 and T32 AI 007502-22).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.04.004.
References
- [1].Soriano MM, Johnson S. Treatment of Clostridium difficile infections. Infect Dis Clin North Am 2015;29:93–108. [DOI] [PubMed] [Google Scholar]
- [2].Chapin RW, Lee T, McCoy C, Alonso CD, Mahoney MV. Bezlotoxumab: could this be the answer for Clostridium difficile recurrence. Ann Pharmacother 2017;51:804–10. [DOI] [PubMed] [Google Scholar]
- [3].Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011;364:422–31. [DOI] [PubMed] [Google Scholar]
- [4].Cornely OA, Crook DW, Esposito R, Poirier A, Somero MS, Weiss K, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012;12:281–9. [DOI] [PubMed] [Google Scholar]
- [5].Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017;376:305–17. [DOI] [PubMed] [Google Scholar]
- [6].Gallagher JC, Reilly JP, Navalkele B, Downham G, Haynes K, Trivedi M. Clinical and economic benefits of fidaxomicin compared to vancomycin for Clostridium difficile infection. Antimicrob Agents Chemother 2015;59:7007–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Burton HE, Mitchell SA, Watt M. A systematic literature review of economic evaluations of antibiotic treatments for Clostridium difficile infection. Pharmacoeconomics 2017;35:1123–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Guery B, Menichetti F, Anttila VJ, Adomakoh N, Aguado JM, Bisnauthsing K, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis 2018;18:296–307. [DOI] [PubMed] [Google Scholar]
- [9].Cornely OA, Watt M, McCrea C, Goldenberg SD, De Nigris E. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients aged ≥60 years (EXTEND): analysis of cost-effectiveness. J Antimicrob Chemother 2018;73:2529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Prabhu VS, Dubberke ER, Dorr MB, Elbasha E, Cossrow N, Jiang Y, et al. Cost-effectiveness of bezlotoxumab compared with placebo for the prevention of recurrent Clostridium difficile infection. Clin Infect Dis 2018;66:355–62. [DOI] [PubMed] [Google Scholar]
- [11].Salavert M, Cobo J, Pascual A, Aragón B, Maratia S, Jiang Y, et al. Cost-effectiveness analysis of bezlotoxumab added to standard of care versus standard of care alone for the prevention of recurrent Clostridium difficile infection in high-risk patients in Spain. Adv Ther 2018;35:1920–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lam SW, Neuner EA, Fraser TG, Delgado D, Chalfin DB. Cost-effectiveness of three different strategies for the treatment of first recurrent Clostridium difficile infection diagnosed in a community setting. Infect Control Hosp Epidemiol 2018;39:924–30. [DOI] [PubMed] [Google Scholar]
- [13].Neumann PJ. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press; 2016. [Google Scholar]
- [14].McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and society for healthcare epidemiology of America (SHEA). Clin Infect Dis 2018;66:987–94. [DOI] [PubMed] [Google Scholar]
- [15].ZINPLAVA™ (bezlotoxumab) injection, for intravenous use. Merck & CO., Inc.; 2016. Available at: https://www.merck.com/product/usa/pi_circulars/z/zinplava/zinplava_pi.pdf/. [Accessed 30 September 2020]. [Google Scholar]
- [16].Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P, West Midlands Research Collaborative. Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg 2012;99:1501–13. [DOI] [PubMed] [Google Scholar]
- [17].Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health 2013;16:297–304. [DOI] [PubMed] [Google Scholar]
- [18].Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection. Clin Microbiol Infect 2012;18:21–7. [DOI] [PubMed] [Google Scholar]
- [19].Hota SS, Achonu C, Crowcroft NS, Harvey BJ, Lauwers A, Gardam MA. Determining mortality rates attributable to Clostridium difficile infection. Emerging Infect Dis 2012;18:305–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Actuarial life table 2017. Social Security Administration; 2018. Available at: https://www.ssa.gov/oact/STATS/table4c6.html/. [Accessed 30 September 2020]. [Google Scholar]
- [21].Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”). I. Validation of the method. Am J Med 1982;73:883–8. [DOI] [PubMed] [Google Scholar]
- [22].Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of lifeexpectancy (the “DEALE”). II. Use in medical decision-making. Am J Med 1982;73:889–97. [DOI] [PubMed] [Google Scholar]
- [23].Employer Costs for Employee Compensation for civilian workers by occupational and industry group. Bureau of Labor Statistics; 2020. Available at: https://www.bls.gov/news.release/ecec.t02.htm/. [Accessed 30 September 2020]. [Google Scholar]
- [24].Pharmaceutical prices. Department of Veterans Affairs, Federal Supply Schedule; 2020. Available at: https://www.va.gov/opal/docs/nac/fss/vaFssPharmPrices.xlsx/. [Accessed 30 September 2020]. [Google Scholar]
- [25].Zhang D, Prabhu VS, Marcella SW. Attributable healthcare resource utilization and costs for patients with primary and recurrent Clostridium difficile infection in the United States. Clin Infect Dis 2018;66:1326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Taneja C, Netsch D, Rolstad BS, Inglese G, Lamerato L, Oster G. Clinical and economic burden of peristomal skin complications in patients with recent ostomies. J Wound Ostomy Continence Nurs 2017;44:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Medical care CPI in U.S. city average, all urban consumers, not seasonally adjusted. Bureau of Labor Statistics; 2020. Available at: https://data.bls.gov/cgi-bin/surveymost?cu/. [Accessed 30 September 2020]. [Google Scholar]
- [28].Bartsch SM, Umscheid CA, Fishman N, Lee BY. Is fidaxomicin worth the cost? An economic analysis. Clin Infect Dis 2013;57:555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hayes JL, Hansen P. Is laparoscopic colectomy for cancer cost-effective relative to open colectomy. ANZ J Surg 2007;77:782–6. [DOI] [PubMed] [Google Scholar]
- [30].Pickard AS, Law EH, Jiang R, Pullenayegum E, Shaw JW, Xie F, et al. United States valuation of EQ-5D-5L health states using an international protocol. Value in Health 2019;22:931–41. [DOI] [PubMed] [Google Scholar]
- [31].Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. New York, NY: Oxford University Press; 2006. [Google Scholar]
- [32].Messali A, Hay JW, Villacorta R. The cost-effectiveness of temozolomide in the adjuvant treatment of newly diagnosed glioblastoma in the United States. Neuro Oncol 2013;15:1532–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Johnson S, Auwaerter P, Daley CL. IDSA case-based clinical guideline overview and update. IDWeek 2020:2020. [Google Scholar]
- [34].Dubberke ER, Gerding DN, Kelly CP, Garey KW, Rahav G, Mosley A, et al. Efficacy of Bezlotoxumab in participants receiving metronidazole, vancomycin, or fidaxomicin for treatment of Clostridioides (Clostridium) difficile infection. Open Forum Infect Dis 2020;7:ofaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


