Abstract
Hashimoto thyroiditis (HT) is a common autoimmune disorder, affecting women 7–10 times more often than men, that develops because of genetic susceptibility, X chromosome inactivation patterns modulated by environmental factors as well as microbiome composition, and leads to an imbalance in self-tolerance mechanisms. The consequential thyroid infiltration by lymphocytes, potentiated by antibody-mediated autoimmune response through the antibodies against thyroid peroxidase (TPOAbs), leads to a destruction of thyrocytes. The presence of TPOAbs is associated with a 2 to 4-fold increase in the risk of recurrent miscarriages and preterm birth in pregnant women. The clinical presentation of HT includes: (A) thyrotoxicosis, when stored thyroid hormones are released to circulation from destroyed thyroid follicles; (B) euthyroidism, when preserved thyroid tissue compensates for destroyed thyrocytes; and (C) hypothyroidism, when thyroid hormone production by the affected thyroid gland is insufficient. The management of Hashitoxicosis is based on symptoms control usually with β-blockers, euthyroidism requires periodical thyroid stimulating hormone measurements to assess for progression to hypothyroidism, and hypothyroidism is treated with thyroid hormone replacement therapy. The dose of levothyroxine (LT4) used for treatment is based on the degree of preserved thyroid functionality and lean body mass, and usually ranges from 1.4 to 1.8 mcg/kg/day. There is insufficient evidence to recommend for or against therapy with triiodothyronine (T3), apart from in pregnancy when only levothyroxine is indicated, as T3 does not sufficiently cross fetal blood-brain barrier. HT is associated with 1.6 times higher risk of papillary thyroid cancer and 60 times higher risk of thyroid lymphoma than in general the population.
Keywords: Hashimoto thyroiditis, overt hypothyroidism, subclinical hypothyroidism, thyroid peroxidase antibodies
Hashimoto thyroiditis: definition and epidemiology
Hashimoto thyroiditis (HT) is an eponym based on the description from 1912 by Haraku Hashimoto, and characterized as “struma lymphomatosa” that is, an enlarged thyroid gland infiltrated with lymphocytes.1 The incidence of HT is estimated to be 0.3–1.5 cases per 1000 people, with female to male predominance of 7–10:1.2,3 HT has an ethnic preponderance, with the white race characterized by a higher incidence than black or Asian, and Pacific Islanders being rarely affected.4 Prevalence increases with age,3 especially in patients diagnosed with other autoimmune conditions, such as myasthenia gravis,5 systemic sclerosis6 and other connective tissue diseases,7 Sjögren’s syndrome,8,9 pernicious anemia,8,9 autoimmune liver disease, and celiac disease.7–9 The expression of this poly-autoimmunity is likely due to an interplay between immune defects, hormones, genetic and environmental factors.10 More rarely, HT is accompanied by other endo-crinopathies of autoimmune origin thereby constituting autoimmune polyendocrine syndromes (APS): type 1 (HT with Addison’s disease, hypoparathyroidism, chronic mucocutaneous candidiasis), type 2 (HT with Addison’s disease and type 1 diabetes mellitus), or IPEX syndrome (HT with neonatal type 1 diabetes mellitus, autoimmune enteropathy and eczema).9,11 APSs are associated with certain genetic background, such as autoimmune regulator (AIRE) mutations for type 1 APS or X-linked forehead box P3 (FOXO3) pathogenic variants for IPEX syndrome.9,11
Pathomechanisms of Hashimoto thyroiditis
The autoimmune presentation of HT is based on the interplay between environmental factors and genetic background, such as polymorphisms in human leukocyte antigen (HLA), T lymphocyte-associated 4 (CTLA-4), protein tyrosine phosphatase, non-receptor type 22 (PTPN22) genes, and X chromosome inactivation patterns, leading to an imbalance between self-tolerance mechanisms sustained by regulatory T and B lymphocytes.9,12–14 In addition, genetic polymorphisms in self-antigens, cytokines and their receptors (eg, interleukin 2 receptor [IL2R]), estrogen receptors, adhesion molecules (CD14, CD40), the promoter region of selenoprotein S, and gene products associated with apoptosis have been linked to thyroid autoimmunity.9,15,16–18 These genetic susceptibilities could be epigenetically modified through methylation, histone modifications, and RNA interference of non coding RNAs (FIGURE 1).19
FIGURE 1.
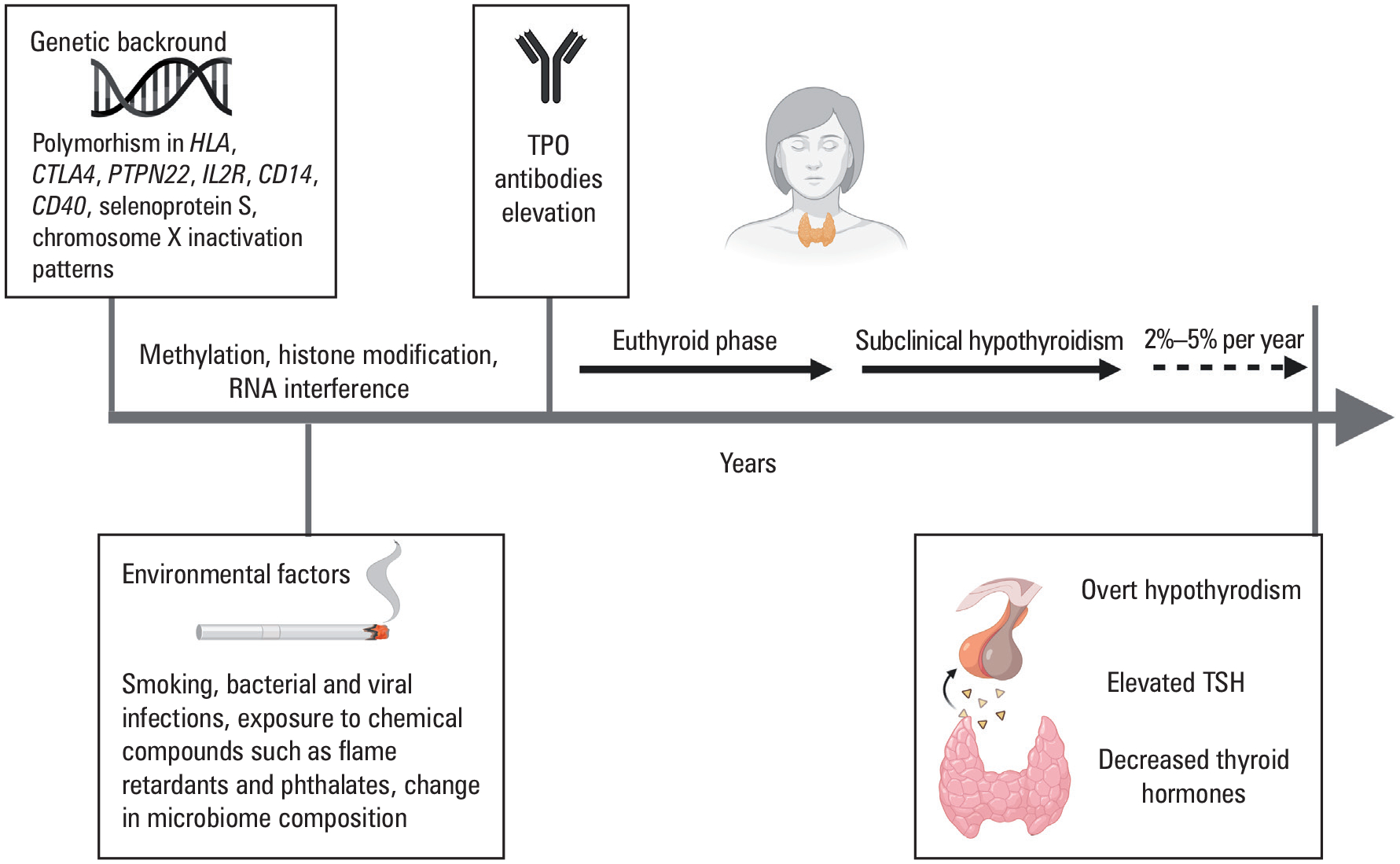
Background and natural history of Hashimoto thyroiditis
Abbreviations: TPO, thyroid peroxidase; TSH, thyroid-stimulating hormone
Several environmental factors may trigger autoimmune diseases in genetically predisposed patients. These triggers include, but are not limited to, bacterial and viral infections, cigarette smoking, maternal-fetal microchimerism, and exposure to chemical compounds, such as flame retardants and phthalates.20,21 On the other hand, limited exposure to environmental factors, for example, living in almost sterile conditions, has also been associated with a high incidence of allergic and autoimmune diseases, including HT.22 Microbiome composition has been associated with autoimmune thyroid disease with Bifidobacterium and Lactobacillus significantly decreased, and harmful microbiota such as Bacteroides fragilis significantly increased in HT as compared with control individuals without autoimmunity.23
Dietary habits may also affect the natural history of HT, as iodine excess has been associated with up to a 4-fold increment in HT incidence.24 The mechanism behind this phenomenon could be related to an increased immunogenicity of thyroglobulin by iodine in genetically predisposed individuals.25 While excessive iodine supplementation in HT should be discouraged, an appropriate supplementation is recommended in pregnancy and lactation up to a total intake of 250 μg/day.26 There are data suggesting that decreased selenium intake may activate HT, but selenium administration has not shown any improvement in the disease course although a reduction in thyroid peroxidase (TPO) autoantibody titers was observed.27,28 Because of an association between HT and celiac disease, a low gluten diet has been suggested as potentially modulating HT. In a prospective study of patients with celiac disease compared with controls without celiac disease, a low-gluten diet was associated with decreased thyroid volume only in the patients with celiac disease, although antibodies against thyroid peroxidase (TPOAbs) were unaffected.29 However, a reduction in TPOAbs was seen in another study of patients on a low-gluten diet and characterized by the presence of both TPOAbs and transglutaminase Abs, compared with an Abs-positive group on a gluten-containing diet.30 The significance of this TPOAbs reduction in the context of modulating HT course is unknown.
Mechanistically, HT is characterized by a direct T-cell attack on the thyroid gland, as evidenced histologically by the presence of lympho-plasmacytic infiltration, fibrosis, lymphatic follicular formation, and parenchymal atrophy.31 Several different variants of HT have been identified based on clinical and histological features, such as fibrotic and atrophic,32 Riedel thyroiditis,33 and IG4 thyroiditis.34 Zhang et al,35 using single cell RNA sequencing technology, showed that the thyroid microenvironment plays an important role in the disease pathogenesis, as 3 stromal cell subtypes are associated with the recruitment of infiltrating inflammatory immune cells. Subsequent hypothyroidism is due to thyroid follicular cell destruction by infiltrating immune cells, leading to an exposure of thyroid antigens (TPO and thyroglobulin [Tg]) further enhancing antibody production (TgAbs, TPOAbs), and aggravating destruction of thyroid follicles. However, the 15%–25% prevalence of seropositivity for TPOAbs and TgAbs is much higher than the clinical expression of Hashimoto disease (hypothyroidism), particularly in iodine-sufficient populations, women, and older individuals.36
Natural history of Hashimoto thyroiditis and symptomatology
The natural history of HT, evolving from genetic predisposition, through the environmental modifiers, presence of detectable TPOAbs in the euthyroid individual, subclinical and clinical disease, is depicted in FIGURE 1. Notably, in some patients with particularly pronounced thyroid destruction in the initial phase, Hashitoxicosis (see below) may be present as a consequence of the release of preformed thyroid hormones from destroyed follicles to the circulation. Primary hypothyroidism is generally considered “overt” when the thyroid stimulating hormone (TSH) level is elevated and free thyroxine (FT4) is low. Subclinical hypothyroidism is defined biochemically as an elevated TSH, accompanied by normal FT4 and free triiodothyronine (FT3) concentrations.37, 38 However, precise definition of the TSH reference range can be challenging due to adjusted normal ranges with age, gender, pregnancy, and in certain populations. There are formulas for adjusting the TSH reference interval for age, ethnicity, and sex within the US populations, but they are neither standardized nor consistently implemented.39
The presence of symptoms in HT is linked to its evolution into hypothyroidism. Signs and symptoms of hypothyroidism are consequences of thyroid hormone deficiency in target tissues and exhibit a wide spectrum of severity that include, but are not limited to, cool and dry skin, coarse hair, loss of body hair, hoarse voice, coarse facial features, facial edema, generalized edema, bradycardia, and delayed relaxation phase of the deep tendon reflexes (FIGURE 2). Systemically, the gastroin-testinal system is affected by decreased peristalsis leading to constipation or even ileus. Hypotonia of the gallbladder and altered bile composition may lead to biliary stone formation. The skin is typically dry, cold, yellowish and thickened due to accumulation of hyaluronic acid and dry due to atrophy of the sweat glands. Cardiovascular system effects include bradycardia and decreased amplitude of cardiac waves on electrocardiography. In addition, decreased ventricular contractility and increased peripheral vascular resistance are responsible for decreased cardiac output. Mucopoly-saccharide deposition in the pericardial sac can lead to pericardial effusion, and hyperlipidemia can cause the fluid on aspiration to have a colloidal gold appearance due to cholesterol crystals. The hyperlipidemia also contributes to development or worsening of ischemic heart disease. Typically, the voice is hoarse due to myxedema of the vocal cords, and the respiratory system is significantly affected with the presence of bradypnea and hypoxia due to obstruction of the upper airways by the enlarged thyroid gland, respiratory muscle weakness, or pleural effusions. The skeletal musculature is characterized by decreased contractility and reflexes as well as painful cramps, and can be shown to be infiltrated with myxedematous fluid. Manifestations of hypothyroidism on the hematopoietic system include a normocytic anemia due to reduced renal erythropoietin synthesis, microcytic anemia due to defects in iron absorption, or megaloblastic anemia due to associated autoimmune gastritis leading to vitamin B12 deficiency. Patients with overt hypothyroidism are characterized by a co-agulopathic picture that could present as menorrhagia in women. Other effects on the reproductive system in women include altered anovulatory cycles due to impaired conversion of estrogen precursors. Consequences of hypothyroidism on the urinary system include a decreased glomerular filtration rate, particularly free water excretion that may lead to hyponatremia. Finally, effects on the central nervous system often include fatigue, depression, memory loss, and inability to concentrate. The most extreme presentation of pro-found hypothyroidism is designated myxedema coma.40 An extremely rare entity characterized as Hashimoto encephalopathy was described that is marked by positive TPOAbs, elevated spinal fluid protein, nonspecific cortical changes on magnetic resonance imaging (MRI), and variable response to steroid therapy.41
FIGURE 2.
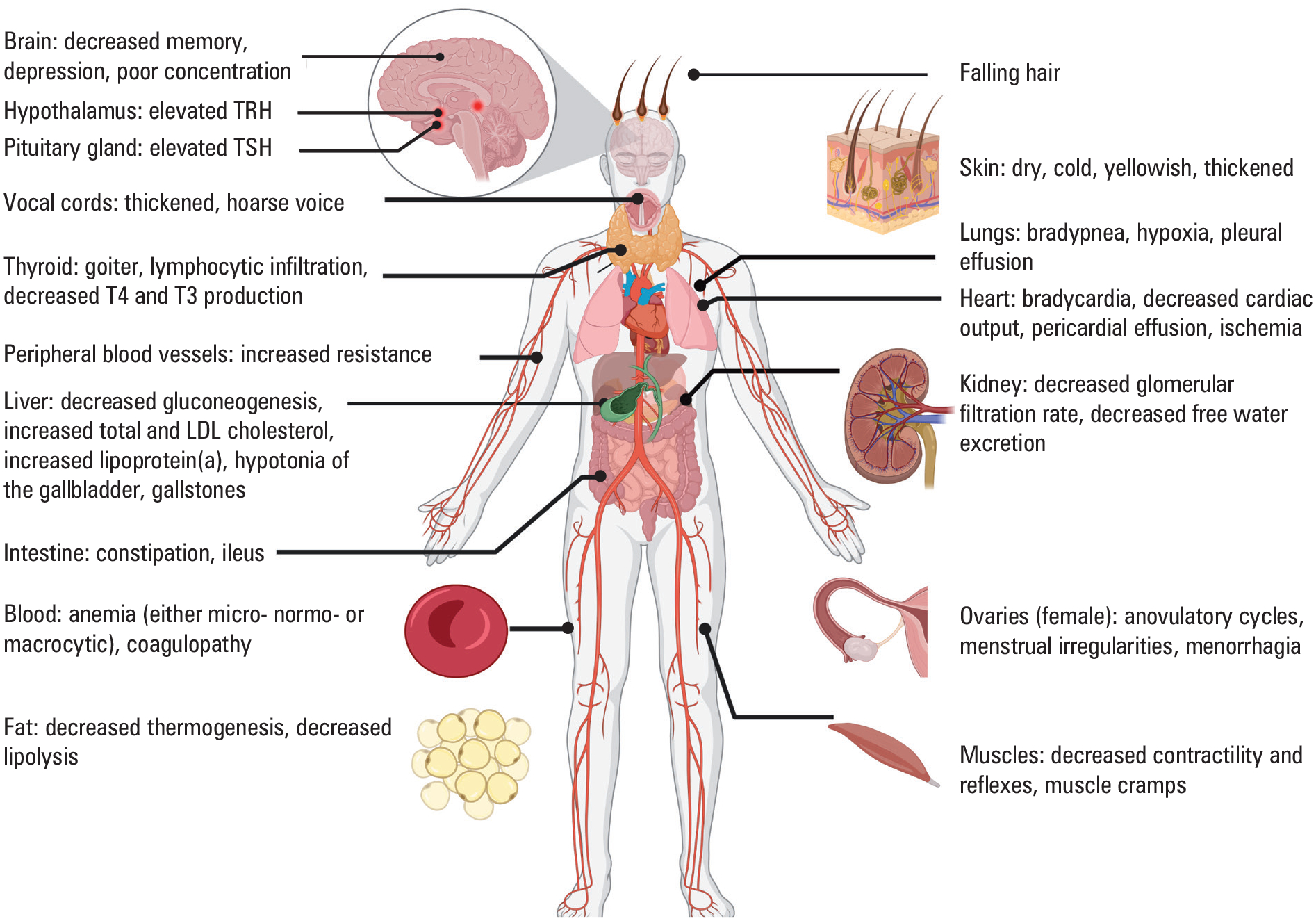
Signs and symptoms of thyroid hormone deficiency
Abbreviations: LDL, low-density lipoprotein; TRH, thyrotropin-releasing hormone; others, see FIGURE 1
Diagnosis of Hashimoto thyroiditis
The diagnosis of HT is based on clinical symptoms of hypothyroidism and presence of TPOAbs, although seronegative HT can be seen in 5%–10% of cases. The ultrasound appearance of the thyroid gland may help with differential diagnosis, particularly in patients with TPOAbs-negative HT.37 The ultrasound features of HT include decreased echogenicity, heterogeneity, hypervascularity, and presence of small cysts (FIGURE 3).42
FIGURE 3.
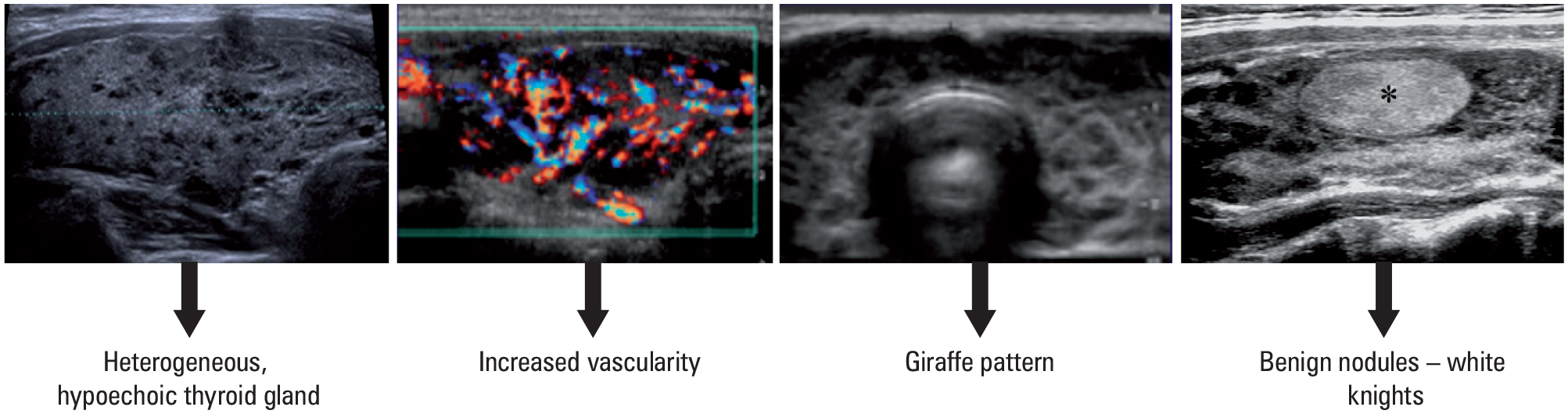
Sonographic appearance of Hashimoto thyroiditis
Serum anti-TPOAbs are present in about 95% of patients, with positive anti-TgAbs in 60%–80%.41,43 TPOAbs are recognized as risk factors for progression into overt hypothyroidism over time in the general population as well as in patients developing hypothyroidism after exposure to amiodarone, lithium, or interferon-α. TPOAbs are associated with the risk of hypothyroidism during pregnancy, increased likelihood of miscarriage, and failure of in vitro fertilization.44
Treatment of Hashimoto thyroiditis
Hashitoxicosis
Hashitoxicosis was first described by Fatourechi in 1971 in patients with clinical presentation of thyrotoxicosis associated with lymphocytic infiltration of the thyroid gland, typical for HT.45 The release of stored thyroxine (T4) and triiodothyronine (T3) to the circulation results in transient signs and symptoms of thyroid hormone excess. As a result of thyroiditis rather than thyroid hyperfunction, thyrotoxicosis usually requires only supportive care and implementation of β-blockers rather than therapy with thyreostatics, and it subsides within 3–24 months, giving rise to usually permanent hypothyroidism.41
This clinical picture is distinguished from the variants of HT associated with thyrotoxicosis including silent thyroiditis and postpartum thyroiditis that are characterized by transient thyrotoxicosis lasting for about a month, also not warranting therapy with thyreostatics, followed by hypothyroid phase, lasting about 2–6 months but leading to recovery to euthyroidism. However, there are some patients, particularly women with multiple pregnancies associated with postpartum thyroiditis, who may develop permanent hypothyroidism.41
Hashimoto thyroiditis associated with euthyroidism
The diagnosis of euthyroid HT is based on the presence of TPO Abs and / or typical sonographic appearance of the thyroid gland (FIGURE 3), but normal serum TSH and T4 levels. These patients require periodical, that is, annual TSH measurements to screen for hypothyroidism or guide management before conception and during pregnancy.26 This population of patients might be vulnerable to other autoimmune disorders, and a low threshold for further diagnostic work-up should be considered, if any symptoms occur.
Hashimoto thyroiditis associated with subclinical hypothyroidism
It is controversial whether subclinical hypothyroidism in most individuals can be observed without treatment. Initiation of treatment has been suggested for patients with subclinical hypothyroidism and serum thyrotropin levels of 10 mIU/ml or higher, as this TSH level has been associated with increased rate of cardiovascular events and cardiovascular mortality.38 A lower threshold for therapy could be used for young and middle-aged individuals with subclinical hypothyroidism who are somewhat symptomatic.37,38 Based on meta-analyses documenting increased rates of fatal stroke and mortality from coronary heart disease in individuals with TSH ranging from 7 to 9.9 mIU/ml, some authors postulate lowering the therapy threshold for both younger and older individuals and treating with levothyroxine when TSH level exceeds 7 mIU/ml (TABLE 1).38,46,47 When the decision on initiating the therapy is established, levothyroxine doses ranging from 25 to 75 mcg per day are usually sufficient to achieve euthyroidism.48
TABLE 1.
Indications for levothyroxine treatment considerations in patients with subclinical hypothyroidism (adapted from Biondi et al38)
| TSH concentration, mlU/ml | Grade | Age <65 years | Age ≥65 years |
|---|---|---|---|
| 4.5–6.9 | Grade I subclinical hypothyroidism | Annual follow-up TSH measurement in asymptomatic patients Consider therapy for the following groups of patients:
|
Treatment not recommended |
| 7–9.9 | Treat with levothyroxine to reduce the risk of fatal stroke and ischemic heart disease–associated mortality | Consider treatment with levothyroxine to reduce the risk of ischemic heart disease–associated mortality | |
| ≥10 | Grade II subclinical hypothyroidism | Treat with levothyroxine to reduce the risk of progression to overt hypothyroidism, heart failure, cardiovascular events, and ischemic heart disease–associated mortality | |
Abbreviations: TPOAbs, antibodies against thyroid peroxidase; others, see FIGURE 1
Hashimoto thyroiditis associated with overt hypothyroidism
Overt hypothyroidism should be treated with thyroid hormone replacement therapy.37 Compared with untreated patients, patients treated for hypothyroidism were shown to have decreased risk of myocardial infarction, stroke, atrial fibrillation, heart failure, and cardiovascular death49 as well as lower all-cause mortality.50,51
The aim of optimal management based on T4 replacement dosage is to mimic normal physiology. Unfortunately, several studies reported that therapy with levothyroxine (LT4) leads to over- or undertreatment in a significant proportion of patients. A large study including 162 369 patients with hypothyroidism followed for up to 23 years, revealed that 11.6% of achieved TSH values were below 0.4 mIU/ml, suggesting overtreatment, and 32.4% were above 4.0 mIU/ml, suggesting under-treatment.52 A smaller study supported these observations showing 19.8% overtreated and 17.4% undertreated patients over the 5-years course of follow-up.53 Overtreatment was associated with longer duration of the therapy, undertreatment with male sex, and health disparities due to ethnical background could also play a role in achieving the appropriate level of biochemical control. Based on the National Health and Nutritional Examination Surveys (NHANES) database, suboptimal treatment was more likely in Hispanic individuals than in individuals of non-Hispanic ethnicity.54 These observations are important in view of data showing that overtreatment was associated with increased mortality in time-dependent manner, and both over- and undertreatment were associated with increased cardiovascular risk.49,51
The dose of LT4 required to normalize serum TSH depends on the amount of residual endogenous thyroid function and the patient’s weight, particularly lean body mass.37,55 Notably, the intact thyroid gland is estimated to produce around 85 to 100 mcg of T4 per day and 5 to 6.5 mcg T3 per 24 h, while the remaining daily production of 26.5 mcg/day of T3 results from peripheral conversion of T4 to T3 by type 1 and type 2 deiodinases.56 Consequently, in patients with a preserved degree of endogenous thyroid function, the required initial LT4 dose ranges between 1.4 and 1.8 mcg/kg body weight.37,57,58 Upon initiation of therapy with LT4, there is a need for on-going dose adjustments based on the TSH targets, with optimal management aiming for the age appropriate TSH reference range. Based on NHANES III data, for individuals aged 30–39 years, median TSH is 1.2 mIU/ml, with 2.5 and 97.5 percentiles being 0.42 to 3.56 mIU/ml, respectively. For older patients targeting TSH of 4–6 mIU/ml might be reasonable.59 These considerations are based on a meta-analysis of 40 studies evaluating the variability within normal TSH ranges. It showed that lower TSH levels were associated with decreased bone mineral density and increased fracture risk, whereas higher TSH levels were associated with worse cardiovascular and metabolic outcomes.60 Although this pathophysiological rationale is often utilized in clinical practice, several prospective studies revealed a lack of effect of establishing specific within-normal-range TSH goals on the quality of life, mood, body mass index, fat mass, cognitive function, heart disease, strokes, fractures, and all-cause mortality.40,52,61–64
The optimal timeframe to make decisions about LT4 dose adjustments is 6–8 weeks after the initiation of the therapy or a dose change, to provide sufficient time for the hypothalamus-pituitary-thyroid axis set point to be re-established.37,40 Although TSH levels exhibit a diurnal rhythm, the magnitude of this variability does not require testing at a particular time of day.40,65 Once optimal dosage is established, further confirmation of euthyroidism at 3 to 6 months is recommended, and thereafter only annual monitoring is appropriate for most patients.40
Several studies analyzed the effect of timing of LT4 administration, and its association with meals, on the serum TSH concentration. Despite some variability in study designs, the take home message is that postfasting as well as bedtime regimens are acceptable, as either can be associated with normalization of TSH concentration.40
Interestingly, normalization of TSH and biochemical euthyroidism do not always translate into improvement in perceived quality of life. Therefore, some investigators and clinicians postulated the necessity of objective evaluation of some end points of thyroid hormone actions on tissue levels. Potential evaluation markers include sex hormone-binding globulin, osteocalcin, cholesterol, creatine kinase, ferritin, N-telopeptides, and enzymes such as tissue plasminogen activator, angiotensin-converting enzyme, glutathione S-transferase, and glucose 6-phosphate dehydrogenase.37 Physiological parameters important in evaluation of response to therapy include heart rate, pulse wave arrival time, echo-cardiographic parameters of left ventricular function, Achilles tendon reflex time, and basal metabolic rate.37 There were also attempts at quantifying thyroid hormone responsive gene expression profiles in whole blood, but this approach requires validation in a larger sample size before it could be potentially implemented as an index of thyroid status.66
Another approach commonly brought up by patients on LT4 with persistent complaints is the use of a combination therapy including LT4 and T3. This regimen was addressed by 14 randomized trials of the combination therapy that did not demonstrate benefit,37,44 and 5 other studies67–71 that reported some benefit.40 However, the study protocols differed in terms of design, including variable use of crossover or parallel groups, blinding, the ratio of T4 to T3 dosage, treatment duration as well as definitions of primary and secondary outcomes. In addition, some studies were subject to carryover effects, overtreatment, and limited inclusion of men and older age groups, underpowered sample size, short duration and once daily T3 dosing. Consistently, 5 meta-analyses or reviews also suggested no clear advantage of the combination therapy.37,72–75 Importantly, potential long-term risks of T3 addition, such as cardiac arrhythmias, or decreased bone mineral density were not fully investigated. Therefore, Guidelines of the American Thyroid Association concluded that there is insufficient evidence to recommend the combination therapy. However, if such a therapy is chosen, it should resemble physiology, that is, the physiological molar T4 to T3 ratio of 14:1 to 15:1,37 and synthetic T4 to T3 conversion factor 3:1.76 Sustained release T3 formulations under development may help achieving physiological goals.
Interestingly, a benefit of a therapy containing T3 was shown in a subgroup analysis of patients who remained the most symptomatic while taking LT4. Therefore, this might be the group of patients that may need to be targeted in future, well designed and appropriately powered studies on the combination therapies.77 The subset of patients potentially benefiting from the combination therapy is likely to have a pathophysiological explanation, as it was shown that lower T3 levels during monotherapy with LT4 were associated with the presence of Thr92Ala polymorphism of deiodinase type 2 (DIO2) gene.78 Genotyping for the presence of Thr92Ala polymorphism in patients treated for hypothyroidism revealed that Ala/Ala homozygotes had worse quality of life scores while taking LT4.79 In addition, another small study showed that patients with both Thr92Ala polymorphism and a polymorphism in one of the thyroid hormone transporters (MTC10) preferred the combination therapy with both LT4 and T3.80 However, other studies did not confirm these findings.81–83 Hence, only the results from a new, prospective, well-designed, adequately powered study of the effects of DIO2 and MTC10 polymorphisms on response to therapy can assess if this genetic background could be a marker guiding either a monotherapy or the combination therapy in overtly hypothyroid patients.
The role of surgery for HT has been traditionally limited to the patients presenting with either pain or compressive symptoms due to goiter or co-existing malignant thyroid nodules.84 However, it was recently hypothesized that thyroidectomy might be a therapeutic modality used to reduce TPOAbs titers, as the presence of such antibodies is associated with lower quality of life even in euthyroid individuals. Consequently, a clinical trial addressed this concept, randomizing highly positive TPOAb patients with continued symptoms while receiving LT4 to either thyroidectomy or continued medical management. In those who underwent thyroidectomy, TPOAbs significantly declined, quality of life and fatigue improved, and the effect was sustained at 12 to 18 month landmarks.85
Hashimoto thyroiditis and thyroid nodules
Based on evaluation of pathological specimens, the average prevalence of papillary thyroid cancer in patients with HT was around 27%, with an associated increased risk ratio of 1.59, as compared with the general population.86, 87 A recent meta-analysis that combined the studies analyzing cytological and pathological specimens derived from patients with HT concluded that this association is based on low-to-moderate quality evidence.88 Apart from papillary thyroid cancer, a non-Hodgkin primary thyroid lymphoma was strongly associated with HT, with a risk of about 60 times higher than in the general population.32 Thyroid lymphoma accounts for approximately 5% of all thyroid neoplasms. Diagnosis of thyroid lymphoma is important to be established, as it changes the first line therapy from surgery, that is routinely implemented for malignant thyroid nodules, to appropriately targeted chemotherapy for lymphoproliferative disorders. Therapy of thyroid lymphoma and malignant thyroid nodules is beyond the scope of this review, but can be found in the respective guidelines.89
Hashimoto thyroiditis and pregnancy
The prevalence of TPOAbs in pregnant women is estimated to be 5%–14% and TgAbs are seen in 3%–18% of pregnant female individuals.90 The presence of these Abs indicating thyroid autoimmunity, is associated with a 2 to 4-fold increase in the risk of recurrent miscarriages91,92 and 2 to 3- fold increased risk of preterm birth.91,93,94 The mechanisms behind these adverse pregnancy outcomes in TPOAb positive euthyroid women are unclear but some authors postulate that TPOAbs might be markers for other forms of autoimmunity that target the placental-fetal unit.95 However, thyroid autoimmunity seems to have an additive or synergistic effect on miscarriage93 and prematurity96 risk in women with maternal subclinical hypothyroidism. A recent meta-analysis including 19 cohort studies enrolling 47 045 pregnant women showed almost 3-fold increased risk of preterm birth in women with subclinical hypothyroidism and 1.5-fold increased risk of preterm birth in women with isolated hypothyroxinemia.94 Another meta-analysis of 26 studies found significant associations between maternal subclinical hypothyroidism or hypothyroxinemia and lower child IQ, language delay or global developmental delay as compared with children of euthyroid women.97
Overt hypothyroidism was associated with increased rates of gestational hypertension including preeclampsia and eclampsia, gestational diabetes, placental abruption, postpartum hemorrhage, preterm delivery, low birthweight, infant intensive care unit admissions, fetal death, and neurodevelopmental delays in the offspring.98,99,100 Therefore, overt hypothyroidism should be treated to prevent adverse effects on pregnancy and child developmental outcomes and should be started before conception to achieve biochemical euthyroidism.26 Therapy with LT4 improved success rate of in vitro fertilization in TPOAbs positive women with TSH above 2.5 mIU/ml.26 Importantly, women treated for hypothyroidism typically require a 20% to 30% increase in their LT4 dose, which usually translates into addition of 2 pills per week early in the first trimester.26 The physiological explanation for increased thyroid hormone requirements is based upon several factors including increased hepatic thyroxine binding globulin synthesis and enhanced metabolism of thyroid hormone through its inactivation by the placental type 3 DIO.26,101 The use of T3 or T4+T3 combination therapy is not indicated in pregnancy, as liothyronine does not cross the blood-brain barrier to the fetal brain.102 LT4 replacement therapy should be monitored monthly, as over- and undertreatment lead to adverse pregnancy outcomes.26 The suggested target TSH is within the lower half of the trimester-specific reference range or below 2.5 mIU/ml, if the trimester-specific ranges are not available.26
Regarding maternal subclinical hypothyroidism, the 2017 American Thyroid Association guidelines recommend utilizing TPOAb status along with serum levels of TSH to guide treatment decisions (TABLE 2).26 LT4 therapy is not recommended for isolated hypothyroxinemia.26
TABLE 2.
Indications for treatment of Hashimoto thyroiditis in pregnancy (adapted from Lee and Pearce95 and Alexander et al26)
| Laboratory data | Treatment |
|---|---|
| TPOAbs negative, TSH ≥10 mIU/ml | Treat with levothyroxine |
| TPOAbs negative and TSH between 4 mIU (or pregnancy specific upper reference) and 10 mIU/ml | Consider treatment with levothyroxine |
| TPOAbs positive, TSH exceeding the pregnancy-specific range or >4 mIU/ml | Treat with levothyroxine |
| TPOAbs positive and TSH between 2.5 and 4 mIU/ml (or pregnancy-specific upper reference range) | Consider treatment with levothyroxine |
| Isolated hypothyroxinemia | Do not treat with levothyroxine |
A 2021 systematic review and meta-analysis of 6 randomized controlled trials assessing the effect of LT4 treatment in euthyroid women with thyroid autoimmunity did not find any significant differences in the relative risk of miscarriage and preterm delivery, or outcomes with live birth. Therefore, no strong recommendations regarding the therapy in such scenarios could be made, but consideration on a case-by-case basis might be implemented (TABLE 2).103
Areas of research
There are promising new models being developed to study the pathophysiology of thyroid disease, as functional thyroid follicles from embryonic or pluripotent stem cells were established in animal models.104,105 This potentially allows for studying mechanisms of autoimmunity that could guide prevention of the disease progression to overt hypothyroidism in predisposed individuals. Stem cells could be also used in regenerative medicine to replace those destroyed by the autoimmune processes in the thyroid gland. A better understanding of the response to therapy with thyroid hormones might be achieved from studies focusing on transcriptome profiling of expression of genes responsive to thyroid hormone action. This could help titrating thyroid hormone replacement therapy. New preparations of sustained release T3 have successfully passed phase 1 clinical trials and may add to our armamentarium for HT therapy once necessary efficacy trials are completed.
Footnotes
CONFLICT OF INTEREST None declared.
REFERENCES
- 1.Hashimoto H The knowledge of the lymphomatous changes in the thyroid gland (struma lymphomatosa) [in German]. Archiv für klinische Chirurgie. 1912; 97: 219. [Google Scholar]
- 2.Caturegli P, De Remigis A, Chuang K, et al. Hashimoto’s thyroiditis: celebrating the centennial through the lens of the Johns Hopkins hospital surgical pathology records. Thyroid. 2013; 23: 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ralli M, Angeletti D, Fiore M, et al. Hashimoto’s thyroiditis: an update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev. 2020; 19: 102649. [DOI] [PubMed] [Google Scholar]
- 4.McLeod DS, Caturegli P, Cooper DS, et al. Variation in rates of autoimmune thyroid disease by race/ethnicity in US military personnel. JAMA. 2014; 311: 1563–1565. [DOI] [PubMed] [Google Scholar]
- 5.Song RH, Yao QM, Wang B, et al. Thyroid disorders in patients with myasthenia gravis: a systematic review and meta-analysis. Autoimmun Rev. 2019; 18: 102368. [DOI] [PubMed] [Google Scholar]
- 6.Yao Q, Song Z, Wang B, et al. Thyroid disorders in patients with systemic sclerosis: a systematic review and meta-analysis. Autoimmun Rev. 2019; 18: 634–636. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura H, Usa T, Motomura M, et al. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J Endocrinol Ivest. 2008; 31: 861–865. [DOI] [PubMed] [Google Scholar]
- 8.Feldt-Rasmussen U, Høier-Madsen M, Bech K, et al. Anti-thyroid peroxidase antibodies in thyroid disorders and non-thyroid autoimmune diseases. Autoimmunity. 1991; 9: 245–254. [DOI] [PubMed] [Google Scholar]
- 9.Bliddal S, Nielsen CH, Feldt-Rasmussen U. Recent advances in understanding autoimmune thyroid disease: the tallest tree in the forest of polyautoimmunity. F1000Research. 2017; 6: 1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazúrová I, Benhatchi K. Autoimmune thyroid diseases and nonorgan-specific autoimmunity. Pol Arch Med Wewn. 2012; 122 Suppl 1: 55–59. [PubMed] [Google Scholar]
- 11.Eisenbarth GS, Gottlieb PA. Autoimmune polyendocrine syndromes. N Engl J Med. 2004; 350: 2068–2079. [DOI] [PubMed] [Google Scholar]
- 12.Brix TH, Hegedüs L. Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin Endocrinol. 2012; 76: 457–464. [DOI] [PubMed] [Google Scholar]
- 13.Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimmun. 2007; 28: 1–6. [DOI] [PubMed] [Google Scholar]
- 14.Brand O, Gough S, Heward J. HLA, CTLA-4 and PTPN22: the shared genetic master-key to autoimmunity? Expert Rev Mol Med. 2005; 7: 1–15. [DOI] [PubMed] [Google Scholar]
- 15.Weetman AP. The immunopathogenesis of chronic autoimmune thyroiditis one century after hashimoto. Eur Thyroid J. 2013; 1: 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johar A, Sarmiento-Monroy JC, Rojas-Villarraga A, et al. Definition of mutations in polyautoimmunity. J Autoimmun. 2016; 72: 65–72. [DOI] [PubMed] [Google Scholar]
- 17.Santos LR, Durães C, Mendes A, et al. A polymorphism in the promoter region of the selenoprotein S gene (SEPS1) contributes to Hashimoto’s thyroiditis susceptibility. J Clin Endocrinol Metab. 2014; 99: E719–723. [DOI] [PubMed] [Google Scholar]
- 18.Jia X, Wang B, Yao Q, et al. Variations in CD14 gene are associated with autoimmune thyroid diseases in the Chinese population. Front Endocrinol. 2018; 9: 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feil R, Fraga MF. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet. 2012; 13: 97–109. [DOI] [PubMed] [Google Scholar]
- 20.Lepez T, Vandewoestyne M, Deforce D. Fetal microchimeric cells in autoimmune thyroid diseases: harmful, beneficial or innocent for the thyroid gland? Chimerism. 2013; 4: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mynster Kronborg T, Frohnert Hansen J, Nielsen CH, et al. Effects of the commercial flame retardant mixture DE-71 on cytokine production by human immune cells. PloS one. 2016; 11: e0 154621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiersinga WM. Clinical relevance of environmental factors in the pathogenesis of autoimmune thyroid disease. Endocrinol Metab (Seoul). 2016; 31: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong B, Wang C, Meng F, et al. Association between gut microbiota and autoimmune thyroid disease: a systematic review and meta-analysis. Front Endocrinol. 2021; 12: 774362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aghini Lombardi F, Fiore E, Tonacchera M, et al. The effect of voluntary iodine prophylaxis in a small rural community: the Pescopagano survey 15 years later. J Clin Endocrinol Metab. 2013; 98: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 25.Carayanniotis G Recognition of thyroglobulin by T cells: the role of iodine. Thyroid. 2007; 17: 963–973. [DOI] [PubMed] [Google Scholar]
- 26.Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017; 27: 315–389. [DOI] [PubMed] [Google Scholar]
- 27.Toulis KA, Anastasilakis AD, Tzellos TG, et al. Selenium supplementation in the treatment of Hashimoto’s thyroiditis: a systematic review and a meta-analysis. Thyroid. 2010; 20: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 28.Winther KH, Wichman JE, Bonnema SJ, et al. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine. 2017; 55: 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metso S, Hyytiä-Ilmonen H, Kaukinen K, et al. Gluten-free diet and autoimmune thyroiditis in patients with celiac disease. A prospective controlled study. Scand J Gastroenterol. 2012; 47: 43–48. [DOI] [PubMed] [Google Scholar]
- 30.Krysiak R, Szkróbka W, Okopień B. The effect of gluten-free diet on thyroid autoimmunity in drug-naïve women with hashimoto’s thyroiditis: a pilot study. Exp Clin Endocrinol. 2019; 127: 417–422. [DOI] [PubMed] [Google Scholar]
- 31.Giordano C, Stassi G, De Maria R, et al. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis. Science. 1997; 275: 960–963. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed R, Al-Shaikh S, Akhtar M. Hashimoto thyroiditis: a century later. Adv Anat Pathol. 2012; 19: 181–186. [DOI] [PubMed] [Google Scholar]
- 33.Hennessey JV. Clinical review: Riedel’s thyroiditis: a clinical review. J Clin Endocrinol Metab. 2011; 96: 3031–3041. [DOI] [PubMed] [Google Scholar]
- 34.Stone JH, Khosroshahi A, Deshpande V, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012; 64: 3061–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang QY, Ye XP, Zhou Z, et al. Lymphocyte infiltration and thyrocyte destruction are driven by stromal and immune cell components in Hashimoto’s thyroiditis. Nat Commun. 2022; 13: 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen IB, Knudsen N, Jørgensen T, et al. Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency. Clin Endocrinol. 2003; 58: 36–42. [DOI] [PubMed] [Google Scholar]
- 37.Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014; 24: 1670–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: a review. JAMA. 2019; 322: 153–160. [DOI] [PubMed] [Google Scholar]
- 39.Boucai L, Hollowell JG, Surks MI. An approach for development of age-, gender-, and ethnicity-specific thyrotropin reference limits. Thyroid. 2011; 21: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jonklaas J Optimal thyroid hormone replacement. Endocr Rev. 2022; 43: 366–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014; 13: 391–397. [DOI] [PubMed] [Google Scholar]
- 42.Anderson L, Middleton WD, Teefey SA, et al. Hashimoto thyroiditis: part 2, sonographic analysis of benign and malignant nodules in patients with diffuse Hashimoto thyroiditis. AJR Am J Roentgenol. 2010; 195: 216–222. [DOI] [PubMed] [Google Scholar]
- 43.McLachlan SM, Rapoport B. Why measure thyroglobulin autoantibodies rather than thyroid peroxidase autoantibodies? Thyroid. 2004; 14: 510–520. [DOI] [PubMed] [Google Scholar]
- 44.Jonklaas J, Bianco AC, Cappola AR, et al. Evidence-based use of levothyroxine/liothyronine combinations in treating hypothyroidism: a consensus document. Eur Thyroid J. 2021; 10: 10–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fatourechi V, McConahey WM, Woolner LB. Hyperthyroidism associated with histologic Hashimoto’s thyroiditis. Mayo Clin Proc. 1971; 46: 682–689. [PubMed] [Google Scholar]
- 46.Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010; 304: 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaker L, Baumgartner C, den Elzen WP, et al. Subclinical hypothyroidism and the risk of stroke events and fatal stroke: an individual participant data analysis. J Clin Endocrinol Metab. 2015; 100: 2181–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kabadi UM, Jackson T. Serum thyrotropin in primary hypothyroidism. A possible predictor of optimal daily levothyroxine dose in primary hypothyroidism. Arch Intern Med. 1995; 155: 1046–1048. [PubMed] [Google Scholar]
- 49.Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, et al. Duration of over- and under-treatment of hypothyroidism is associated with increased cardiovascular risk. Eur J Endocrinol. 2019; 180: 407–416. [DOI] [PubMed] [Google Scholar]
- 50.Huang HK, Wang JH, Kao SL. Association of hypothyroidism with all-cause mortality: a cohort study in an older adult population. J Clin Endocrinol Metab. 2018; 103: 3310–3318. [DOI] [PubMed] [Google Scholar]
- 51.Lillevang-Johansen M, Abrahamsen B, Jørgensen HL, et al. Over- and under-treatment of hypothyroidism is associated with excess mortality: a register-based cohort study. Thyroid. 2018; 28: 566–574. [DOI] [PubMed] [Google Scholar]
- 52.Thayakaran R, Adderley NJ, Sainsbury C, et al. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study. BMJ. 2019; 366: l4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okosieme OE, Belludi G, Spittle K, et al. Adequacy of thyroid hormone replacement in a general population. QJM - Mon J Assoc Physicians. 2011; 104: 395–401. [DOI] [PubMed] [Google Scholar]
- 54.Ettleson MD, Bianco AC, Zhu M, et al. Sociodemographic disparities in the treatment of hypothyroidism: NHANES 2007–2012. J Endocr Soc. 2021; 5: bvab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santini F, Pinchera A, Marsili A, et al. Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J Clin Endocrinol Metab. 2005; 90: 124–127. [DOI] [PubMed] [Google Scholar]
- 56.Pilo A, Iervasi G, Vitek F, et al. Thyroidal and peripheral production of 3,5,3’-triiodothyronine in humans by multicompartmental analysis. Am J Physiol. 1990; 258: E715–726. [DOI] [PubMed] [Google Scholar]
- 57.Devdhar M, Drooger R, Pehlivanova M, et al. Levothyroxine replacement doses are affected by gender and weight, but not age. Thyroid. 2011; 21: 821–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jonklaas J Sex and age differences in levothyroxine dosage requirement. Endocr Pract. 2010; 16: 71–79. [DOI] [PubMed] [Google Scholar]
- 59.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002; 87: 489–499. [DOI] [PubMed] [Google Scholar]
- 60.Taylor PN, Razvi S, Pearce SH, et al. Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range. J Clin Endocrinol Metab. 2013; 98: 3562–3571. [DOI] [PubMed] [Google Scholar]
- 61.Samuels MH, Kolobova I, Niederhausen M, et al. Effects of altering levothyroxine (L-T4) doses on quality of life, mood, and cognition in L-T4 treated subjects. J Clin Endocrinol Metab. 2018; 103: 1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samuels MH, Kolobova I, Niederhausen M, et al. Effects of altering levothyroxine dose on energy expenditure and body composition in subjects treated with LT4. J Clin Endocrinol Metab. 2018; 103: 4163–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walsh JP, Ward LC, Burke V, et al. Small changes in thyroxine dosage do not produce measurable changes in hypothyroid symptoms, well-being, or quality of life: results of a double-blind, randomized clinical trial. J Clin Endocrinol Metab. 2006; 91: 2624–2630. [DOI] [PubMed] [Google Scholar]
- 64.Boeving A, Paz-Filho G, Radominski RB, et al. Low-normal or high-normal thyrotropin target levels during treatment of hypothyroidism: a prospective, comparative study. Thyroid. 2011; 21: 355–360. [DOI] [PubMed] [Google Scholar]
- 65.Sturgess I, Thomas SH, Pennell DJ, et al. Diurnal variation in TSH and free thyroid hormones in patients on thyroxine replacement. Acta Endocrinol. 1989; 121: 674–676. [DOI] [PubMed] [Google Scholar]
- 66.Massolt ET, Meima ME, Swagemakers SMA, et al. Thyroid state regulates gene expression in human whole blood. J Clin Endocrinol Metab. 2018; 103: 169–178. [DOI] [PubMed] [Google Scholar]
- 67.Nygaard B, Jensen EW, Kvetny J, et al. Effect of combination therapy with thyroxine (T4) and 3,5,3’-triiodothyronine versus T4 monotherapy in patients with hypothyroidism, a double-blind, randomised cross-over study. Eur J Endocrinol. 2009; 161: 895–902. [DOI] [PubMed] [Google Scholar]
- 68.Bunevicius R, Kazanavicius G, Zalinkevicius R, et al. Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. N Eng J Med. 1999; 340: 424–429. [DOI] [PubMed] [Google Scholar]
- 69.Escobar-Morreale HF, Botella-Carretero JI, Gómez-Bueno M, et al. Thyroid hormone replacement therapy in primary hypothyroidism: a randomized trial comparing L-thyroxine plus liothyronine with L-thyroxine alone. Ann Intern Med. 2005; 142: 412–424. [DOI] [PubMed] [Google Scholar]
- 70.Saravanan P, Simmons DJ, Greenwood R, et al. Partial substitution of thyroxine (T4) with tri-iodothyronine in patients on T4 replacement therapy: results of a large community-based randomized controlled trial. J Clin Endocrinol Metab. 2005; 90: 805–812. [DOI] [PubMed] [Google Scholar]
- 71.Valizadeh M, Seyyed-Majidi MR, Hajibeigloo H, et al. Efficacy of combined levothyroxine and liothyronine as compared with levothyroxine monotherapy in primary hypothyroidism: a randomized controlled trial. Endocr Res. 2009; 34: 80–89. [DOI] [PubMed] [Google Scholar]
- 72.Ma C, Xie J, Huang X, et al. Thyroxine alone or thyroxine plus triiodothyronine replacement therapy for hypothyroidism. Nucl Med Commun. 2009; 30: 586–593. [DOI] [PubMed] [Google Scholar]
- 73.Escobar-Morreale HF, Botella-Carretero JI, Morreale de Escobar G. Treatment of hypothyroidism with levothyroxine or a combination of levothyroxine plus L-triiodothyronine. Best Pract Res Clin Endocrinol Metab. 2015; 29: 57–75. [DOI] [PubMed] [Google Scholar]
- 74.Joffe RT, Brimacombe M, Levitt AJ, et al. Treatment of clinical hypothyroidism with thyroxine and triiodothyronine: a literature review and meta-analysis. Psychosomatics. 2007; 48: 379–384. [DOI] [PubMed] [Google Scholar]
- 75.Grozinsky-Glasberg S, Fraser A, Nahshoni E, et al. Thyroxine-triiodothyronine combination therapy versus thyroxine monotherapy for clinical hypothyroidism: meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2006; 91: 2592–2599. [DOI] [PubMed] [Google Scholar]
- 76.Celi FS, Zemskova M, Linderman JD, et al. The pharmacodynamic equivalence of levothyroxine and liothyronine: a randomized, double blind, cross-over study in thyroidectomized patients. Clin Endocrinol. 2010; 72: 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shakir MKM, Brooks DI, McAninch EA, et al. Comparative effectiveness of levothyroxine, desiccated thyroid extract, and levothyroxine+liothyronine in hypothyroidism. J Clin Endocrinol Metab. 2021; 106: e4400–e4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castagna MG, Dentice M, Cantara S, et al. DIO2 Thr92Ala reduces deiodinase-2 activity and serum-T3 levels in thyroid-deficient patients. J Clin Endocrinol Metab. 2017; 102: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 79.Panicker V, Saravanan P, Vaidya B, et al. Common variation in the DIO2 gene predicts baseline psychological well-being and response to combination thyroxine plus triiodothyronine therapy in hypothyroid patients. J Clin Endocrinol Metab. 2009; 94: 1623–1629. [DOI] [PubMed] [Google Scholar]
- 80.Carlé A, Faber J, Steffensen R, et al. Hypothyroid patients encoding combined MCT10 and DIO2 gene polymorphisms may prefer L-T3 + L-T4 combination treatment - data using a blind, randomized, clinical study. Eur Thyroid J. 2017; 6: 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wouters HJ, van Loon HC, van der Klauw MM, et al. No effect of the Thr92Ala polymorphism of deiodinase-2 on thyroid hormone parameters, health-related quality of life, and cognitive functioning in a large population-based cohort study. Thyroid. 2017; 27: 147–155. [DOI] [PubMed] [Google Scholar]
- 82.Young Cho Y, Jeong Kim H, Won Jang H, et al. The relationship of 19 functional polymorphisms in iodothyronine deiodinase and psychological well-being in hypothyroid patients. Endocrine. 2017; 57: 115–124. [DOI] [PubMed] [Google Scholar]
- 83.Appelhof BC, Fliers E, Wekking EM, et al. Combined therapy with levothyroxine and liothyronine in two ratios, compared with levothyroxine monotherapy in primary hypothyroidism: a double-blind, randomized, controlled clinical trial. J Clin Endocrinol Metab. 2005; 90: 2666–2674. [DOI] [PubMed] [Google Scholar]
- 84.Gan T, Randle RW. The role of surgery in autoimmune conditions of the thyroid. Surg Clin North Am. 2019; 99: 633–648. [DOI] [PubMed] [Google Scholar]
- 85.Guldvog I, Reitsma LC, Johnsen L, et al. Thyroidectomy versus medical management for euthyroid patients with hashimoto disease and persisting symptoms: a randomized trial. Ann Intern Med. 2019; 170: 453–464. [DOI] [PubMed] [Google Scholar]
- 86.Jankovic B, Le KT, Hershman JM. Clinical Review: Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013; 98: 474–482. [DOI] [PubMed] [Google Scholar]
- 87.Sulaieva O, Selezniov O, Shapochka D, et al. Hashimoto’s thyroiditis attenuates progression of papillary thyroid carcinoma: deciphering immunological links. Heliyon. 2020; 6: e03077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abbasgholizadeh P, Naseri A, Nasiri E, et al. Is Hashimoto thyroiditis associated with increasing risk of thyroid malignancies? A systematic review and meta-analysis. Thyroid Res. 2021; 14: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26: 1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.De Leo S, Pearce EN. Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol. 2018; 6: 575–586. [DOI] [PubMed] [Google Scholar]
- 91.Thangaratinam S, Tan A, Knox E, et al. Association between thyroid autoantibodies and miscarriage and preterm birth: meta-analysis of evidence. BMJ. 2011; 342: d2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie J, Jiang L, Sadhukhan A, et al. Effect of antithyroid antibodies on women with recurrent miscarriage: a meta-analysis. Am J Reprod Immunol. 2020; 83: e13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu H, Shan Z, Li C, et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid. 2014; 24: 1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Korevaar TIM, Derakhshan A, Taylor PN, et al. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: a systematic review and meta-analysis. JAMA. 2019; 322: 632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee SY, Pearce EN. Assessment and treatment of thyroid disorders in pregnancy and the postpartum period. Nat Rev Endocrinol. 2022; 18: 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Korevaar TI, Schalekamp-Timmermans S, de Rijke YB, et al. Hypothyroxinemia and TPO-antibody positivity are risk factors for premature delivery: the generation R study. J Clin Endocrinol Metab. 2013; 98: 4382–4390. [DOI] [PubMed] [Google Scholar]
- 97.Thompson W, Russell G, Baragwanath G, et al. Maternal thyroid hormone insufficiency during pregnancy and risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. Clin Endocrinol. 2018; 88: 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Davis LE, Leveno KJ, Cunningham FG. Hypothyroidism complicating pregnancy. Obstet Gynecol. 1988; 72: 108–112. [PubMed] [Google Scholar]
- 99.Leung AS, Millar LK, Koonings PP, et al. Perinatal outcome in hypothyroid pregnancies. Obstet Gynecol. 1993; 81: 349–353. [PubMed] [Google Scholar]
- 100.Männistö T, Mendola P, Grewal J, et al. Thyroid diseases and adverse pregnancy outcomes in a contemporary US cohort. J Clin Endocrinol Metab. 2013; 98: 2725–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Okosieme OE, Khan I, Taylor PN. Preconception management of thyroid dysfunction. Clin Endocrinol. 2018; 89: 269–279. [DOI] [PubMed] [Google Scholar]
- 102.Calvo R, Obregón MJ, Ruiz de Oña C, et al. Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3’-tri-iodothyronine in the protection of the fetal brain. J Clin Invest. 1990; 86: 889–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lau L, Benham JL, Lemieux P, et al. Impact of levothyroxine in women with positive thyroid antibodies on pregnancy outcomes: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2021; 11: e043751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ma R, Morshed SA, Latif R, et al. Thyroid cell differentiation from murine induced pluripotent stem cells. Front Endocrinol. 2015; 6: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Antonica F, Kasprzyk DF, Opitz R, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012; 491: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


