Abstract
The novel ZrIV-based perfluorinated metal–organic framework (PF-MOF) [Zr6O4(OH)4(TFS)6] (ZrTFS) was prepared under solvent-free conditions using the commercially available tetrafluorosuccinic acid (H2TFS) as a bridging ditopic linker. Since H2TFS can be seen as the fully aliphatic and perfluorinated C4 analogue of fumaric acid, ZrTFS was found to be isoreticular to zirconium fumarate (MOF-801). The structure of ZrTFS was solved and refined from X-ray powder diffraction data. Despite this analogy, the gas adsorption capacity of ZrTFS is much lower than that of MOF-801; in the former, the presence of bulky fluorine atoms causes a considerable window size reduction. To have PF-MOFs with more accessible porosity, postsynthetic exchange (PSE) reactions on (defective) MOF-801 suspended in H2TFS aqueous solutions were carried out. Despite the different H2TFS concentrations used in the PSE process, the exchanges yielded two mixed-linker materials of similar minimal formulae [Zr6O4(μ3-OH)4(μ1-OH)2.08(H2O)2.08(FUM)4.04(HTFS)1.84] (PF-MOF1) and [Zr6O4(μ3-OH)4(μ1-OH)1.83(H2O)1.83(FUM)4.04(HTFS)2.09] (PF-MOF2) (FUM2– = fumarate), where the perfluorinated linker was found to fully replace the capping acetate in the defective sites of pristine MOF-801. CO2 and N2 adsorption isotherms collected on all samples reveal that both CO2 thermodynamic affinity (isosteric heat of adsorption at zero coverage, Qst) and CO2/N2 adsorption selectivity increase with the amount of incorporated TFS2–, reaching the maximum values of 30 kJ mol–1 and 41 (IAST), respectively, in PF-MOF2. This confirms the beneficial effect coming from the introduction of fluorinated linkers in MOFs on their CO2 adsorption ability. Finally, solid-state density functional theory calculations were carried out to cast light on the structural features and on the thermodynamics of CO2 adsorption in MOF-801 and ZrTFS. Due to the difficulties in modeling a defective MOF, an intermediate structure containing both linkers in the framework was also designed. In this structure, the preferential CO2 adsorption site is the tetrahedral pore in the “UiO-66-like” structure. The extra energy stabilization stems from a hydrogen bond interaction between CO2 and a hydroxyl group on the inorganic cluster.
Keywords: metal−organic frameworks (MOFs), carbon dioxide capture, zirconium, fluorinated linkers, DFT calculations
Introduction
The urgent need to reduce CO2 emissions is pushing scientists and engineers toward the development of technologies that should prevent further buildup of this greenhouse gas in the atmosphere. Among the technologies under scrutiny, carbon capture and storage (CCS)1 from large point sources and direct air capture (DAC)2 are seen as viable options for rapid large-scale deployment. The state-of-the-art sorbents for CCS and DAC are typically amine-based: aqueous methanolamine solutions for CCS3 and solid-supported amine adsorbents for DAC.4 In both cases, the working principle exploits the formation of covalent bonds between the amine groups and CO2 to afford carbamates (chemical adsorption or chemisorption). The formation of strong covalent bonds is associated with a high heat of absorption or adsorption, which is convenient to achieve high capture loading at low partial pressures but poses challenges in terms of regeneration of the sorbent and achievable working capacity.5,6 While DAC demands a sorbent with a high enthalpy of ab/adsorption, due to the large entropic loss associated with CO2 capture from a feed that contains only ∼415 ppm of CO2, CCS deals with CO2 concentrations in the range between 4 and 30% and could greatly benefit from a sorbent with a mild heat of adsorption, i.e., a physisorbent.7 To this end, adsorbents such as porous carbons,8 zeolites,9 and metal–organic frameworks (MOFs)5−7 are intensely investigated.
The outstanding versatility of MOFs in terms of pore size, shape, and chemistry has led to the development of a large number of sorbents that display remarkable CO2 capture performance,10−14 recently culminated with the deployment of CALF-20 for capture in the cement industry.15 Fine tuning of pore size and shape is crucial to maximize the framework–adsorbate contact.16 This has recently led ultramicroporous MOFs—i.e., with pores smaller than 8 Å—to gain a privileged spot as promising sorbents for CO2 capture.17−19 Pore chemistry plays a key role at the low partial pressures relevant for CCS, where specific interactions with the framework dominate the adsorption process. Typical CO2 adsorption sites in MOFs include coordinatively unsaturated metal ions and functional groups with a basic character on the organic linker.7,11,12 A promising approach that is gaining momentum is to introduce fluorine atoms in the backbone of ultramicroporous MOFs to prepare new materials labeled as perfluorinated MOFs (PF-MOFs).20,21 Besides increasing the thermodynamic affinity for CO2 (as evidenced by a high isosteric heat of adsorption, Qst), the presence of (per)fluorinated groups can also render the framework hydrophobic, thus preserving the CO2 capture performance in humid conditions, which is crucial for real-life applications. The family of hybrid ultramicroporous materials containing fluorinated anions such as SiF62–, NbOF52–, and TiF62– represents an excellent example of how the presence of fluorine atoms exposed in narrow pores provides superior affinity for CO2 while preserving a physisorption-based mechanism and minimizing competitive water adsorption.20,22−24 We have recently reported on the water-based synthesis of two perfluorinated CeIV analogues of the well-known UiO-66 and MIL-140A framework types.21,25 The ultramicroporous F4-MIL-140A(Ce) displays an S-shaped isotherm and outstanding CO2/N2 selectivity, and it was also found to display inverse CO2/C2H2 selectivity.26
As a result of our continued effort in developing highly stable (per)fluorinated MOFs based on tetravalent metals for CCS applications,27 we report herein on the preparation and solid-state characterization of a perfluorinated analogue of the well-known MOF-801, namely, ZrIV tetrafluorosuccinate (ZrTFS), and of the two mixed-linker perfluorinated MOFs (PF-MOFs) PF-MOF1 and PF-MOF2 through postsynthetic tetrafluorosuccinate/fumarate (Scheme 1) linker exchange starting from pure zirconium fumarate MOF-801. The relationship between CO2 adsorption capacity/selectivity and the extent of fluorination in solid adsorbents has been systematically studied.
Scheme 1. Linkers Used in This Study for the Construction of PF-MOFs.
Experimental Section
Materials and Methods
All chemicals are commercially available and used as received from the specified vendors without further purification. Zirconium chloride (ZrCl4) was purchased from Alfa Aesar. Tetrafluorosuccinic acid (H2TFS) was purchased from Fluorochem. Fumaric acid (H2FUM) was purchased from Merck. Acetic acid (AA) was purchased from Carlo Erba. Powder X-ray diffraction (PXRD) patterns were collected in reflection geometry in the 4–40° 2θ range, with a 40 s step–1 counting time and with a step size of 0.016° on a PANalytical X’PERT PRO diffractometer, a PW3050 goniometer, equipped with an X’Celerator detector and using a Cu Kα radiation source. The long fine focus (LFF) ceramic tube operated at 40 kV and 40 mA. Quantitative 1H NMR and 19F NMR analyses were performed at an ambient temperature on either a Bruker Ascend 400 MHz spectrometer (MOF-801) or a Jeol JNM-ECZ500S instrument equipped with a RoyalProbe Broadband probe (PF-MOF1 and PF-MOF2): 1H: acquisition time 4.36 s, relaxation delay τ = 25 s, 4 scans; 19F: acquisition time 0.27 s, relaxation delay τ = 4 s, 16 scans. The solids (ca. 20 mg) were held in an oven at 120 °C for 2 h before being digested for 24 h in 1.5 mL of 1 M NaOH in D2O (MOF-801) or in 1.0 mL of 1 M NaOH in D2O (PF-MOF1 and PF-MOF2). The NMR tubes were then loaded with the solution, which was filtered through cotton wool to avoid the presence of solid particles in suspension. 3-Fluorobenzoic acid (0.029 M) was used as the 1H internal standard for the fumarate and acetate quantification in MOF-801. 2,6-Difluorobenzoic acid (0.1 M) was used as both 1H and 19F internal standards for the fumarate and tetrafluorosuccinate quantification in the mixed-linker MOFs (see the Supporting Information for details on the calculations). Thermogravimetric analysis (TGA) was performed using a Netzsch STA490C thermoanalyzer under a 20 mL min–1 air flux with a heating rate of 10 °C min–1. Transmission Fourier transform infrared spectroscopy (FT-IR) spectra (KBr pellets) were recorded on a Perkin-Elmer Spectrum BX Series FT-IR spectrometer, in the 4000–400 cm–1 range, with a 2 cm–1 resolution. Inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis was carried out using a Varian 700-ES series. A calibration curve was obtained with four standard zirconium solutions (0.5, 1, 2.5, and 5 mg L–1, respectively). The analyses were performed on the supernatant after dissolving the solids in an HNO3 (2%) aqueous solution. Scanning electron microscopy (SEM) images were acquired with an FEI Quanta 450 ESEM FEG, working at a 15.00 kV acceleration voltage. The samples were sputtered with graphite prior to the analysis.
Synthesis of [Zr6O4(OH)4(TFS)6] (Zirconium Tetrafluorosuccinate, ZrTFS)
ZrCl4 (1 mmol, 233 mg), H2TFS (4 mmol, 773 mg), and 1 mL of deionized water were put in a ball-mill vessel. After 15 min of milling, the mixture was put in a 5 mL hydrothermal bomb and was heated to 120 °C for 72 h. The obtained white solid was recovered by centrifugation and washed with ethanol (2 × 5 mL), water (1 × 5 mL), and acetone (2 × 5 mL) and finally dried in an oven at 80 °C. Yield: 267 mg (88%, based on zirconium). IR (KBr, cm–1; Figure S1): 1663(s) [ν(COO–)], 1145/1128 (m) [ν(C–F)sym/asym].
Synthesis of [Zr6O4(μ3-OH)4(μ1-OH)3.12(H2O)3.12(FUM)4.04(AA)0.80] (Zirconium Fumarate, MOF-801)
ZrCl4 (1 mmol, 233 mg), H2FUM (3 mmol, 348 mg), and acetic acid (100 mmol, 5.7 mL) were placed together in a hydrothermal bomb with 20 mL of deionized water, and the solution was sonicated until complete reagent dissolution. Then, the reactor was held in a thermostatic oven at 120 °C for 24 h. The obtained white solid was recovered by centrifugation, washed with water (2 × 10 mL) and acetone (1 × 10 mL), and finally dried in an oven at 80 °C. Yield: 200 mg (87%, based on zirconium). The phase identity and purity were checked through PXRD. The amount of AA present in the material was estimated through 1H NMR (see the Supporting Information). IR (KBr, cm–1; Figure S1): 1576(s) [ν(C=C)FUM]. The [ν(COO–)FUM] and [ν(COO–)AA] bands are not visible, falling underneath that of [ν(C=C)FUM].
Postsynthetic Exchange on MOF-801: Preparation of [Zr6O4(μ3-OH)4(μ1-OH)2.08(H2O)2.08(FUM)4.04(HTFS)1.84] (PF-MOF1) and [Zr6O4(μ3-OH)4(μ1-OH)1.83(H2O)1.83(FUM)4.04(HTFS)2.09] (PF-MOF2)
MOF-801 (200 mg, 0.14 mmol) and H2TFS (0.28 mmol, 68 mg for the synthesis of PF-MOF1 and 0.84 mmol, 160 mg for the synthesis of PF-MOF2, respectively) were placed together in a reaction flask with 2.5 mL of deionized water. The mixture was left under stirring at 60 °C for 4 h. The obtained solids were recovered by centrifugation, washed with ethanol (2 × 5 mL), water (1 × 5 mL), and acetone (1 × 5 mL), and finally dried in an oven at 80 °C. Yield: 224 mg and 237 mg (99.99 and 99.93%, based on zirconium present in the pristine MOF-801 and using the results from ICP measurements for quantifying the metal leaching after the exchange; Table S1) for PF-MOF1 and PF-MOF2, respectively. The proposed minimal formulae reported above come from quantitative 1H and 19F NMR spectroscopies after digestion and TGA carried out to quantify the number of defects and the amount of exchanged TFS2– ligands. PF-MOF1. IR (KBr, cm–1; Figure S1): 1645(vs) [ν(COO–)TFS], 1576 (vs) [ν(C=C)FUM], 1135/1118 (s) [ν(C–F)sym/asym]. PF-MOF2. IR (KBr, cm–1; Figure S1): 1653(vs) [ν(COO–)TFS], 1576(vs) [ν(C=C)FUM], 1136/1118 (s) [ν(C–F)sym/asym]. As found in MOF-801, the [ν(COO–)FUM] bands are not visible, falling underneath that of [ν(C=C)FUM].
N2 and CO2 Adsorptions
All of the samples were activated at 120 °C under a high vacuum (10–6 Torr) for 12 h before any measurement. The Brunauer–Emmett–Teller (BET) specific surface area (SSA) and porosity were estimated by volumetric adsorption with an ASAP 2020 Micromeritics instrument, using N2 as the adsorbate at −196 °C and an equilibration time of 30 s (an optimal value for surface area measurements when using N2 as a probe with ultramicroporous materials).28 A typical measurement used 40 mg of sample. For the BET specific surface area calculation, the 0.01–0.1 p/p0 pressure range was used to fit the data. Within this range, all of the Rouquerol consistency criteria29,30 are satisfied. The material (micro)porosity was determined from the CO2 adsorption isotherms collected at T = 0 °C, using a 2D nonlocal density functional theory (2D-NLDFT) method successfully employed for carbonaceous materials with heterogeneous surfaces.31 The same kind of pore size distribution (PSD) can also be obtained from N2 isotherms at T = −196 °C.32 CO2 adsorption isotherms were measured at 0 and 25 °C up to the maximum pressure of 1.2 bar. The isosteric heat of adsorption (Qst) was calculated working on the CO2 isotherms measured at 0 and 25 °C, by applying a variant of the Clausius–Clapeyron equation (eq 1):33,34
| 1 |
where pn (n = 1 or 2) denotes the pressure value for the nth isotherm, Tn (n = 1 or 2) denotes the temperature value for the nth isotherm, and R is the gas constant expressed in the appropriate units (8.314 J K–1 mol–1). In order to validate the calculated Qst values on two temperatures, an additional isotherm was also collected at T = −20 °C for PF-MOF1 and ZrTFS and the calculation was repeated on three temperature points using the differential form of the Clausius-Clapeyron equation (see the Supporting Information). The isotherms were also fitted with the Virial equation following the guidelines of a recent paper35
| 2 |
where p is the pressure at which loading n is reached, ai (m = 7) is −Qi/R, and bj (m′ = 2) is a constant. Qst was then derived using the following equation
| 3 |
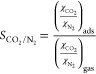
To evaluate the CO2/N2 selectivity at 25 °C, the N2 adsorption isotherms were acquired at 25 °C and up to 1.2 bar. The CO2/N2 Henry selectivity was calculated as the ratio of the initial slopes of the two adsorption isotherms in the 0.01 ≤ p ≤ 0.1 bar range. The CO2/N2 ideal adsorbed solution theory (IAST) selectivity of binary mixtures at a total pressure of 1 bar and at T = 25 °C was determined as the ratio of the adsorbed molar fractions of the two gases divided by the ratio of the gas-phase initial molar fractions36
 |
4 |
The (χ)ads values were derived from the application of the free software pyIAST37 (https://github.com/CorySimon/pyIAST) to the experimental single-component isotherms collected at the chosen temperature. The initial composition (%) for the calculation was [15:85] for the [CO2/N2] pair. This relative ratio was selected to mimic the general feed composition of a coal-fired power station.38 A Henry or a BET model was employed for the isotherm fitting. For a detailed explanation of these models and the related parameters, see the pyIAST webpage and documentation.
Computational Details
All calculations were carried out with the CP2K code.39 Atom-centered Gaussian-type basis functions are used to describe the orbitals. The MOLOPT40 basis set for Zr, O, C, F, and H was employed, with a cut-off of 500 Ha for the plane waves along with the PBE functional.41 Core–valence interactions are described by Goedecker–Teter–Hutter pseudopotentials.42 In this simulation, the experimental structures derived from PXRD data were taken as the initial geometry guess and a joint atomic position and cell parameter optimization was carried out, keeping the three axes orthogonal and symmetric. The Brillouin zone was sampled at the Γ point. Mulliken atomic charges on a reference model (SiF62–) and on the H2TFS organic linker at comparison were calculated by performing a Gaussian 0943 geometry optimization using the B3LYP exchange–correlation functional44 and a 6-31++g** basis set on all atoms.
Results and Discussion
Synthesis and Structural Characterization of ZrTFS
ZrTFS was prepared with a “Shake ‘n Bake” procedure that we have previously employed for the synthesis of several UiO-66 MOF analogues.45 In this procedure, a dense slurry of reagents in a small amount of a liquid is obtained instead of a clear solution upon a preliminary treatment using a ball mill. This probably induces a partial MOF crystallization before the heating stage, which provides a crystalline material at the end of the synthesis. If the milling stage is bypassed, a much less crystalline product is obtained (Figure S2) and the reaction yield decreases from 88 to 69%. No solid product was obtained when larger volumes of water were used, suggesting that a high concentration of reagents is necessary to induce the crystallization of ZrTFS. This is likely due to the high solubility of H2TFS in water, a result of its highly acidic character (pKa values of 1.64 and 3.68, determined using Chemicalize, https://chemicalize.com/, developed by ChemAxon). Notably, to the best of our knowledge, ZrTFS is only the second material with an extended three-dimensional framework built with tetrafluorosuccinic acid reported in the Cambridge Structural Database, the other one being a lithium derivative.46
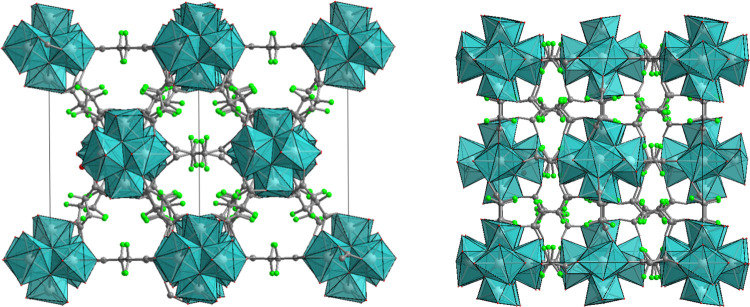
The structure of ZrTFS was solved ab initio from PXRD data using the parallel tempering algorithm implemented in the FOX program47 and refined with the Rietveld method using TOPAS.48 Details of structure solution and refinement are reported in the Supporting Information (Figure S3). A polyhedral representation of the structure as obtained from the Rietveld refinement is reported in Figure 1. The structure was solved in the same cubic space group as MOF-801 (Pn3̅), and it is fully consistent with the optimized DFT structure (see below). The lattice parameter is 18.0690 Å, slightly larger than that reported for MOF-801 (17.8469 Å).49 The framework topology is fcu with 12-connected [Zr6O4(OH)4]12+ hexanuclear clusters bridged by the TFS2– linkers. The geometry of one crystallographically independent linker recalls that of fumaric acid in MOF-801 with the O–C–C–O carbon–oxygen chain torsion angle close to 180° (i.e., O11–C11–C12–C12#1 176.9° #1 = −x, y + 1/2, z + 1/2), whereas the second TFS2– displays a bent geometry between the carboxylic plane and the C–F chain (O21–C21–C22–C22#1 146.5° #1 = −x, y + 1/2, z + 1/2). Fluorine atoms linked to internal carbon atoms (C–F distance 1.37 Å) are pointing toward the cavities, thus reducing the window size with respect to those of MOF-801 from 4.8 to 3.5 Å (average distances measured from atomic centers). These small window sizes strongly limit the gas diffusion into the cavities, as observed in its gas adsorption isotherms (vide infra). SEM analysis (Figure S4) reveals a peculiar truncated octahedral morphology, with the crystallite size in the range of 500 nm.
Figure 1.
Polyhedral representation of ZrTFS viewed along the (110) and (100) directions. Carbon atoms are depicted in gray, fluorine atoms are depicted in green, and zirconium atoms are depicted in light blue.
Synthesis of Mixed-Linker MOFs via Postsynthetic Exchange
The lack of accessible porosity in the framework of ZrTFS limits its ability to adsorb CO2. This prompted us to prepare alternative materials where the (flexible) fully aliphatic TFS2– linker and its (more rigid) unsaturated analogue FUM2– could coexist in the same framework. Mixed-linker tetrafluorosuccinate/fumarate MOFs should feature both satisfactory accessible surface area and high fluorine content. To achieve this result, we carried out postsynthetic exchange (PSE) reactions50−52 starting from pure MOF-801.53MOF-801 was synthesized from fumaric acid and ZrIV chloride according to the literature procedure of Zahn et al., using acetic acid as the crystallinity modulator.54 Its PXRD pattern is reported in Figure 2, confirming the phase purity and the high crystallinity degree. The use of AA as a modulator leads to the obtainment of a defective material where the bridging fumarate linkers are partially replaced by terminal acetates and water/hydroxide couples. The 1H NMR signal integration of the digested sample using 3-fluorobenzoic acid as the internal standard (Figure S5) allowed us to quantify the ratio of fumarate (FUM) and acetate (AA) in the structure (calculation details are provided in the Supporting Information). The general formula found is [Zr6O4(μ3-OH)4(μ1-OH)3.12(H2O)3.12(FUM)4.04(AA)0.80]. From MOF-801, fumarate/tetrafluorosuccinate partial exchange performed in water with a (1:2) or (1:6) [Zr6]:H2TFS stoichiometric ratio gave the two mixed-linker MOFs labeled as PF-MOF1 and PF-MOF2. Their PXRD patterns are also shown in Figure 2, whereas their thermogravimetric (TG) profiles are shown in Figure 3. PSE did not seem to affect the structural integrity of the pristine fumarate; the diffractograms are nearly identical, and they clearly show that both PF-MOFs are isostructural to MOF-801. The only difference is in the intensity of a small peak at 2θ of ∼ 13° (already present in MOF-801) that slightly increases with the increasing amount of H2TFS used for the exchange. To gain additional information on the structural features of the presented PF-MOFs, an ab initio indexing using the N-TREOR suite55 was carried out. In all cases, cubic cells were found: a = 17.8469 Å, V = 5684 Å3 (MOF-801); a = 17.8498 Å, V = 5687 Å3 (PF-MOF1); a = 17.8621 Å, V = 5699 Å3 (PF-MOF2); a = 18.0690 Å, V = 5899 Å3 (ZrTFS). The results indicate that the unit cell size remains almost unchanged when passing from MOF-801 to PF-MOF1 to PF-MOF2, thus suggesting that very small differences exist between these three materials, whereas ZrTFS displays a larger unit cell. Integration of 19F and 1H NMR peaks of the digested samples allowed us to quantify the relative amounts of FUM2–, AA, and TFS2– present in the lattice. In the framework structure of MOF-801, the externally added TFS2– linkers may be incorporated through the replacement of either the pristine fumarates or the capping ligands (either acetates or water/hydroxide) placed in its defective sites, given the hypothesis that AA induced missing cluster defects.56 In the exchanged samples, after treatment with H2TFS, the signal of AA disappears. This suggests that the tetrafluorosuccinate replaces AA at defective sites in its monoprotonated form (HTFS–). Based on the combination of NMR analysis (Figures S6 and S7) and TGA (Figure 3), the proposed formulae for PF-MOF1 and PF-MOF2 are [Zr6O4(μ3-OH)4(μ1-OH)2.08(H2O)2.08(FUM)4.04(HTFS)1.84] and [Zr6O4(μ3-OH)4(μ1-OH)1.83(H2O)1.83(FUM)4.04(HTFS)2.09], respectively. Based on these formulae, we can conclude that no TFS2–/FUM2– exchange has occurred and that HTFS– has been grafted at defective sites, affording compounds with similar stoichiometry. Apparently, the fluorination degree is not appreciably increased by the use of more concentrated H2TFS solutions (1:2–1:6 MOF-801:H2TFS stoichiometric ratios) to foster a larger fluorinated linker uptake. TGA analysis of the four MOFs (Figure 3) shows a different thermal behavior depending on their fluorination extent. MOF-801 contains almost 30 wt % of water, and it starts to decompose at temperatures higher than 380 °C. ZrTFS contains a smaller amount of solvent than MOF-801 (about 7%), indicative of its lower porosity. In addition, the thermal stability is much lower (Tdec ≈ 260 °C), as expected for the lower Zr–O coordination bond strength of the (less basic) TFS2– compared to that of FUM2–. The two PF-MOFs have a thermal behavior, which is intermediate between ZrTFS and MOF-801. The only difference is the water content, higher for PF-MOF2 (23%, with a higher tetrafluorosuccinate loading) with respect to PF-MOF1 (15%); the thermal stabilities though are comparable (Tdec ≈ 280 °C).
Figure 2.
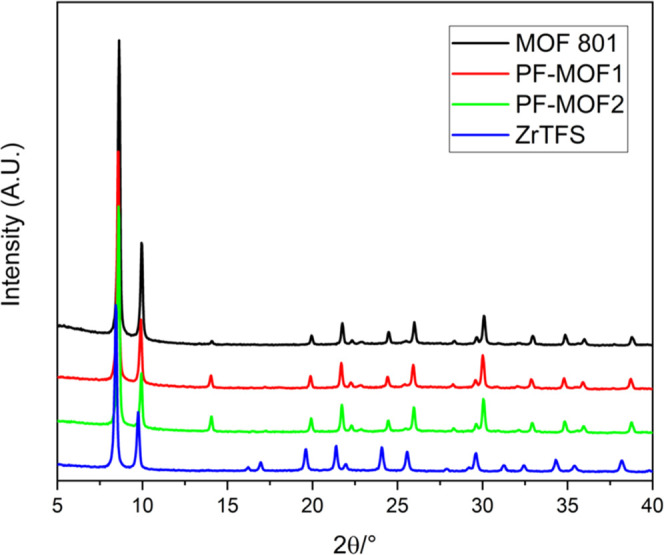
PXRD patterns of MOF-801 (black), PF-MOF1 (red), PF-MOF2 (green), and ZrTFS (blue) in comparison.
Figure 3.

TGA profiles of MOF-801 (black), PF-MOF1 (red), PF-MOF2 (green), and ZrTFS (blue) in comparison.
Gas Adsorption Properties
N2 Adsorption
The porous nature of the four samples was investigated through N2 adsorption at −196 °C on desolvated samples. As shown in Figure 4, all compounds display type I isotherm, typical of microporous materials. MOF-801 has the highest BET specific surface area (SSA) value (948 m2 g–1), and this result is in line with that obtained by other groups in the literature working with the same compound (833 m2 g–1 found by Serre and co-workers;57 990 m2 g–1 reported by Yaghi and co-workers).49 The accessible surface area then decreases with an increasing content of the aliphatic fluorinated linker TFS2–, passing from 649 and 626 m2 g–1 for PF-MOF1 and PF-MOF2, respectively, to only 46 m2 g–1 for ZrTFS. This drastic reduction of the surface area was also found in the hydrogenated counterpart of ZrTFS, zirconium succinate MIP-203-S.58 In there, it is claimed that this MOF does not show any accessible porosity to N2 at 77 K, presumably because after thermal activation under vacuum to remove the guest molecules, it tends to stay in a closely packed form that is not accessible to nitrogen. Even in the presence of bipyridyl-based auxiliary ligands, the framework empty volume is not accessible to N2, as observed in a family of cadmium succinate mixed-linker MOFs.59 Despite the different crystal topologies, it is reasonable to draw the same kind of conclusion for ZrTFS as well. This is further confirmed by the fact that the surface area of ZrTFS does not change when the equilibration time during the isotherm collection is doubled from 30 to 60 s. To gain additional insights into the textural properties of ZrTFS, the theoretical MOF surface area was estimated using a Monte Carlo procedure, which randomly places spheres with a given diameter in the free space (N2 to match experiments—sphere diameter = 3.681 Å)60 and calculates the interface between the spheres and the atoms (for further details and explanations, see the webpage: https://mausdin.github.io/MOFsite/mofPage.html). This is a commonly used procedure, and it normally gives reasonable results.61 By applying this procedure on ZrTFS, we have obtained a surface area of 226.8 m2 g–1. This value is reasonable if the pore size is taken into consideration, but it is much higher than the experimentally measured surface area. This discrepancy may be justified by a window size limitation for N2 free diffusion through the MOF pores. The micropore size distribution of the four samples retrieved from the NLDFT analysis (Figure S8) does not show a significant change when passing from the homolinker to the mixed-linker materials. The main contribution to the total pore volume comes from pores in the 10 ≤ w ≤ 12 Å range, in line with the Zr···Zr distance found in the crystal structure between adjacent [Zr6] nodes (∼13 Å).49 The percentage of the ultramicropore volume decreases with an increasing extent of fluorinated linker insertion, passing from 77% in MOF-801 to 72 and 62% in PF-MOF1 and PF-MOF2, respectively. Degradation coming from PSE cannot be considered the main cause of the observed microporosity reduction since the small amount of zirconium found in solution after the exchange indicates a very small degree of degradation (Table S2). This is also supported by the SEM analysis, which shows that the morphology and crystallite size seen in the parent MOF-801 are preserved upon PSE (Figures S9–S11). Thus, PSE does not seem to affect the framework stability. The total pore volume derived from the N2 isotherm at −196 °C, measured at p/p0 = 0.98, equals 0.57, 0.45, 0.36, and 0.17 cm3 g–1 for MOF-801, PF-MOF1, PF-MOF2, and ZrTFS, respectively.
Figure 4.
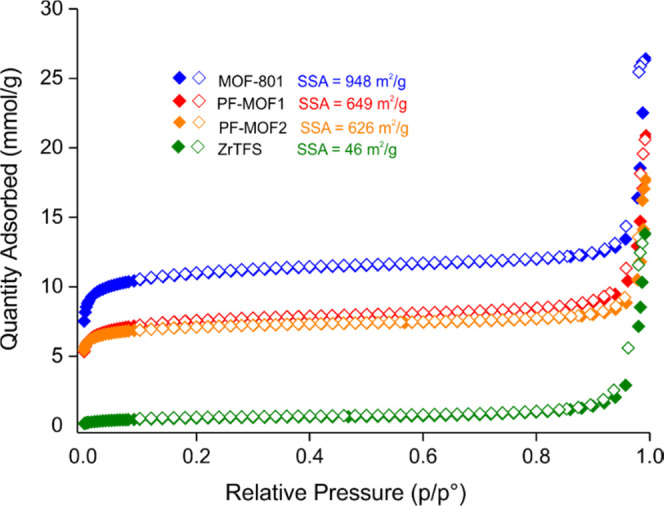
N2 adsorption isotherms measured at −196 °C on the four MOFs discussed in this work. Empty symbols denote desorption branches.
CO2 Adsorption
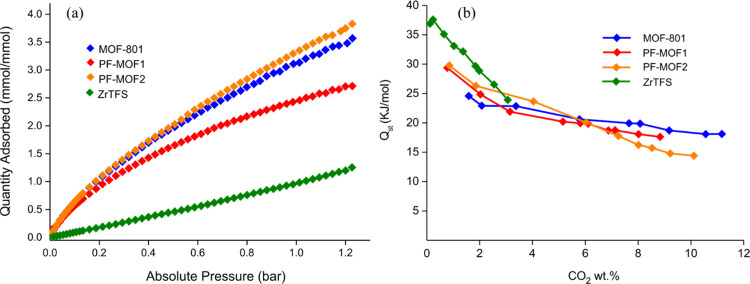
The four MOFs were tested as CO2 adsorbents at T = 0 and 25 °C and pCO2 up to 1.2 bar. The corresponding isotherms at an ambient temperature are reported in Figure 5a, where the amounts of CO2 adsorbed are reported as mmol(CO2) adsorbed per mmol (MOF) to account for the material density variation upon the fluorinated linker insertion. The more conventional mmol g–1 unit is reported in Table 1 and Figure S12. To quantify the strength of the host–guest interactions, the isosteric heat of adsorption (Qst) of CO2 was evaluated from the isotherms recorded at T = 0 and 25 °C, applying a variant of the Clausius–Clapeyron equation (Figure 5b). In order to validate the calculation made on two temperature points, for ZrTFS and PF-MOF1Qst of CO2 was also re-calculated using three temperatures (T = −20, 0 and 25 °C; Figures S13 and S20). An alternative approach for the extrapolation of the Qst values at zero coverage is the virial fitting of the adsorption isotherms.35 The absolute values and the general trends calculated in our samples are identical to those obtained through the Clausius–Clapeyron equation (Figures S14–S18 and Tables S3–S6). The isosteric heat of adsorption reflects the interaction strength between CO2 and the inner pore walls of the MOFs. Finally, we estimated the CO2/N2 selectivity using the ratio of the initial slopes in the Henry region of the (CO2 and N2) adsorption isotherms measured at 25 °C (Figure S19). From the critical and comparative analysis of the results, we can state that the good performance in CO2 adsorption depends on two factors: BET area and fluorinated linker content. The former is predominant in MOF-801, where the absence of F atoms is compensated by the high SSA value. Consequently, MOF-801 shows the best performance among the MOFs considered in this study in terms of CO2 loading on a gravimetric basis: 2.42 (10.6 wt %) and 3.51 (15.4 wt %) mmol g–1 at T = 25 and 0 °C, respectively. On the other hand, the introduction of TFS2– partially reduces the accessible surface area (and the related CO2 loading) but considerably improves the thermodynamic affinity of the material for CO2 and its CO2/N2 selectivity. The latter is particularly important in the purification of postcombustion industrial flue gases, where the amount of CO2 is very low (4–30%). The high Henry and IAST selectivity achieved by PF-MOF2 and ZrTFS, in particular, may be ascribed to the presence of fluorine-decorated ultramicropores that hamper N2 diffusion (because of its large kinetic diameter) but favor CO2 adsorption through the beneficial gas–fluorine interaction. On this basis, the best compromise can be found in the PF-MOF2, with a good CO2 loading of 2.1 mmol g–1 (9.3 wt %) at an ambient temperature and 1 bar, a Qst value of almost 30 kJ mol–1, and the good CO2/N2 selectivity of 95 (Henry)/41 (IAST). The same conclusion can be drawn from the isotherms reported in Figure 5a, where PF-MOF2 shows the highest CO2 uptake: 3.7 mmolCO2 mmolMOF–1 at p = 1 bar. In comparison with other PF-MOFs reported in the literature (Table S7), at ambient (T,p) conditions (25 °C, 1 bar), PF-MOF2 outperforms F4-UiO-66(Ce) (1.5 mmol g–1 at 293 K),21 but it is less efficient than F4-MIL-140A(Ce) (2.4 mmol g–1)25 and AlFFIVE-1-Ni/NbOFFIVE-1-Ni (2.7/2.2 mmol g–1, respectively) published by the team of Eddaoudi in 201623,24,62 or than SIFSIX-18-Ni (3.0 mmol g–1), reported by Zaworotko and co-workers in 2019.20
Figure 5.
(a) CO2 adsorption isotherms measured at 25 °C on the four MOFs. (b) CO2 isosteric heat of adsorption as a function of surface coverage.
Table 1. Main CO2 Adsorption Data for the Four MOFs in This Study.
| CO2 quantity adsorbed (p = 1 bar) [mmol g–1] |
||||||
|---|---|---|---|---|---|---|
| BET area [m2 g–1] | Qst [kJ mol–1] | CO2/N2 selectivity (Henry) | CO2/N2 selectivity (IAST) | T = 25 °C | T = 0 °C | |
| MOF-801 | 948 | 24.6 | 23 | 25 | 2.42 (10.6 wt %) | 3.51 (15.4 wt %) |
| PF-MOF1 | 649 | 29.4 | 30 | 34 | 1.58 (6.9 wt %) | 2.22 (9.8 wt %) |
| PF-MOF2 | 626 | 29.8 | 95 | 41 | 2.10 (9.3 wt %) | 2.78 (12.2 wt %) |
| Zr_TFS | 46 | 37.6 | ∞a | ∞a | 0.56 (2.5 wt %) | 1.24 (5.4 wt %) |
The N2 adsorption in ZrTFS at T = 25 °C is practically zero at low coverage.
DFT Study of the CO2–Framework Interaction
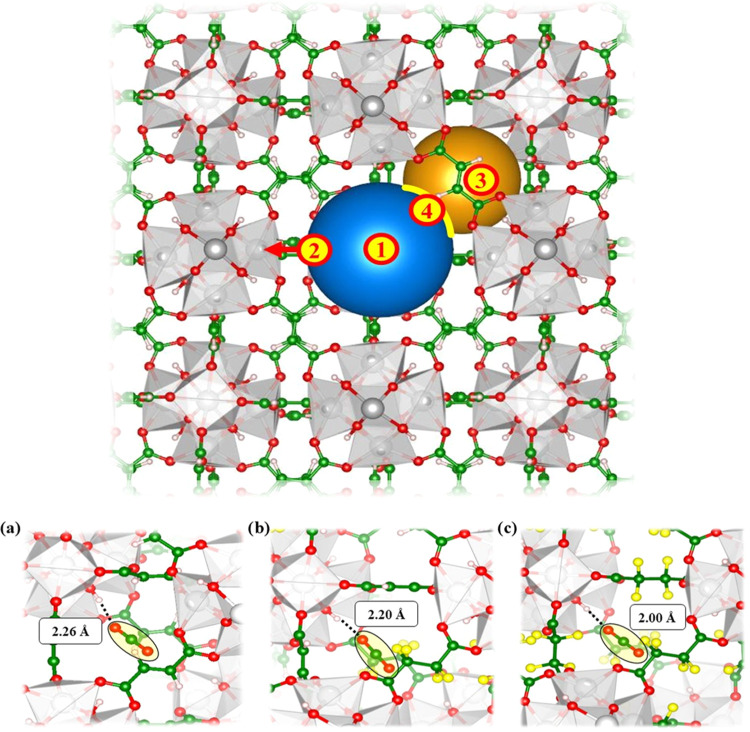
To gain insight into the effect of the fluorinated ligand on the MOF structural properties and CO2 adsorption ability, DFT simulations in the gas phase at the [PBE/MOLOPT] level of theory were carried out. As a first step in the computational analysis, the structures of MOF-801 and ZrTFS were optimized. For MOF-801, the starting geometry was the defect-free crystal lattice with formula [Zr6O4(OH)4(FUM)6], while for ZrTFS the initial guess [Zr6O4(OH)4(TFS)6] was considered. To evaluate the effect of the presence of both linkers in the same solid phase on the CO2 adsorption performance from a theoretical viewpoint, a hypothetical (simplified) structure of minimal formula [Zr6O4(OH)4(FUM)4(TFS)2] (PF-MOF) was also built in silico and subsequently optimized. Albeit this computational model cannot be considered fully representative of the real exchanged materials PF-MOF1 and PF-MOF2, it is a useful reference for a comparison between a mixed-linker MOF and its homolinker counterparts. Indeed, the design of computationally representative defective structures is too challenging, as the linker is placed onto the defects randomly. On these models, a joint variable-cell and atomic position optimization was performed (see the Computational Details section). As shown in Table 2, the optimized lattice parameters are in perfect agreement with the experimentally determined values, with the reproduction of the slight unit cell size increment moving from pure fumarate to the fluorinated systems. These MOFs feature tetrahedral and octahedral pores interconnected by triangular windows, with a lattice structure similar to that of UiO-66.63 The windows are smaller than the pores (Figure 6 and Table 2), and they could be responsible for the percolation of the gas inside the MOF. The gas adsorption process is driven by two main factors: the host–guest chemical affinity and the (high) accessible SSA. Another interesting factor that deeply influences the adsorption performance is the gas percolation and diffusion inside the material.64 Literature evidence suggests that MOFs featuring high CO2 chemical affinity but with very small pore windows are poor adsorbents.65,66 To compare the window and pores of the different models, the surface of the window is defined as the area of the triangle defined by the three hydrogen (or fluorine) atoms, as indicated in Figure S21a, while the pore dimension is evaluated as the distance between opposite hydrogen or fluorine atoms in Figure S21b. The average values of the cavities and window size are summarized in Table 2. Moving from MOF-801 to ZrTFS, the octahedral pore size does not change significantly, while the window area and the tetrahedral pore size are considerably reduced. Moreover, the mixed-linker PF-MOF shows a variable pore width (between 6 and 9 Å; Table 2) that depends on the presence of either TFS2– or FUM2–. To gain deeper insights into the preferential CO2 adsorption sites in these materials, CO2 was introduced in the computational model and located in four different lattice positions (Figure 6): in the center of the octahedral cavity (1), close to the zirconium ions (2), in the center of the tetrahedral pore (3), and in the middle of the pore windows (4). Afterward, the ensemble was reoptimized. The CO2 adsorption energy (ΔEads) is then calculated as the energy difference between the optimized [MOF + CO2] ensemble and the separated components; a negative value indicates a favorable interaction, while the opposite holds for positive ΔEads values. The most favorable adsorption site is the tetrahedral pore, even if it is slightly smaller than the octahedral one. This energy stabilization comes from a hydrogen bond interaction between CO2 and a hydroxyl group on the inorganic cluster (Figure 6), which is known to be the most favorable adsorption site for polar and quadrupolar species in UiO-66.67 Apparently, there is no simple correlation between F-functionalization and MOF–CO2 interaction. We can only state that the interaction of CO2 with the linker F atoms is not particularly strong, at least at the computational level used here. To better understand the role of fluorine atoms in increasing the affinity with CO2, the Mulliken partial charges on F species were evaluated as already reported in the literature for the SIFSIX family MOFs.68 In particular, the SiF62– anion was taken as the benchmark model, obtaining a partial F charge of −0.76e, in agreement with the values found for similar systems.68 Subsequently, the partial charge on the fluorine atoms of the H2TFS linker was calculated at the same level of theory, obtaining an average value of −0.25e. This clearly indicates a reduced C–F bond polarization in H2TFS if compared to the Si–F bond polarization in SiF62–. Consequently, these results suggest a lower CO2 affinity of ZrTFS with respect to a general SIFSIX system. If the (ΔEads)T values found for the different materials are compared, an increase in the interaction energy is found when moving from MOF-801 to ZrTFS, in perfect agreement with the Qst trend reported in Table 1. It is to be noted that the window site in ZrTFS is associated with a positive (ΔEads) value, which suggests that CO2 diffusion through the windows is unfavorable, explaining the low adsorption capacity of this MOF.
Table 2. Main Calculated Structural Parameters and CO2 Adsorption Enthalpies for MOF-801, PF-MOF, and ZrTFS.
| structural
parameters |
CO2 ΔEads (eV) |
||||||
|---|---|---|---|---|---|---|---|
| cell (Å) | window area (Å2) | pore (Å)a | pore Oct. center (1) | pore Zr (2) | pore Tetr. center (3) | window (4) | |
| MOF-801 | 18.00 | 9.3 | 9.6/8.6 | –0.19 | –0.29 | –0.34 | –0.29 |
| PF-MOF | 18.04 | 9.1 | 10.0/6.3–9.0 | –0.18 | –0.27 | –0.47 | –0.29 |
| ZrTFS | 18.17 | 5.0 | 10.1/6.6 | –0.18 | –0.31 | –0.49 | 0.32 |
We report both octahedral and tetrahedral pore sizes, respectively.
Figure 6.
Top: optimized DFT structure of ZrTFS. The blue and the orange spheres represent the octahedral and tetragonal pores, respectively; the yellow curved line represents the window between the two pores; the numbers indicate the different sites taken into account for the evaluation of CO2 adsorption enthalpies (ΔEads) in reference to Table 2. Bottom: detail of the hydrogen bonds between CO2 and the OH group for (a) MOF-801, (b) PF-MOF, and (c) ZrTFS.
Conclusions
The novel perfluorinated MOF ZrTFS containing tetrafluorosuccinic acid and isostructural to MOF-801 has been synthesized and its crystal structure was solved and refined from PXRD data. The presence of fluorine atoms on the alkyl chains hinders gas adsorption; consequently, the measured surface area of this MOF is very low. To obtain a fluorinated material with an acceptable surface area, MOF-801 was taken as the starting point to carry out PSE in aqueous solutions with variable amounts of H2TFS. In the two exchanged materials, the amount of a fluorinated linker is about the same despite the different concentrations used in the synthesis, and it is most likely placed in the precursor defective sites. This suggests that the postsynthetic exchange involves only acetate and not fumarate. The as-obtained PF-MOFs show lower CO2 adsorption capacity with respect to MOF-801 (most likely because of the lower surface area) but higher CO2/N2 selectivity and CO2 heat of adsorption. These parameters are directly proportional to the extent of a fluorinated linker present in the lattice, proving the beneficial effect of the presence of fluorinated groups on the CO2 affinity of the resulting material. DFT-optimized structures of ZrTFS account for the low diffusion of gases into the framework due to the reduction of the window size. The theoretical model of [CO2@PF-MOF] ensembles shows the formation of strong hydrogen-bonding interactions between CO2 and hydroxyl groups of the [Zr6] clusters dangling in the tetrahedral cavities and favored by the exposed fluorine atoms. Although a real linker exchange did not occur in the present work, being a defect functionalization, the PSE methodology is very efficient for the preparation of mixed-linker MOFs with tunable properties and opens new horizons for selected applications such as gas mixture separation and purification. Current work is undergoing in our laboratories in this direction.
Acknowledgments
F.C. and M.T. thank the Italian MIUR through the Project PRIN 2020 doMino (ref 2020P9KBKZ) "“(Per-)fluorinateD metal-Organic frameworks-based Mixed matrIx membraNes for large-scale carbon diOxide separation. G.G. and A.R. thank the Italian MIUR through the PRIN 2017 project MULTI-e (ref 20179337R7) “Multielectron transfer for the conversion of small molecules: an enabling technology for the chemical use of renewable energy” and the TRAINER project “Catalysts for Transition to Renewable Energy Future” (ref ANR-17-MPGA-0017) for financial support. M.T. thanks the University of Pisa for the provision of funding through the Progetto di Ricerca di Ateneo (PRA) “New horizons in CO2 chemistry: from capture to fine chemicals and metal based drugs” (PRA_2020_39).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.2c07640.
IR spectra, PXRD patterns of ZrTFS obtained through different synthetic methods, PXRD structure determination of ZrTFS, its Rietveld refinement plot and crystallographic details, 1H NMR and 19F NMR spectra of the digested samples of MOF-801, PF-MOF1, and PF-MOF2 and related linker quantification, ICP-OES analysis of the reaction supernatant after PSE on MOF-801, MOF pore size distribution, calculation of the CO2/N2 Henry selectivity, CO2 adsorption isotherm plot in [mmol g–1] units at 25 °C, further Qst calculations using three temperatures and geometrical considerations on the DFT-optimized structures (PDF)
Accession Codes
CCDC-2160377 contains the crystallographic data for ZrTFS that can be obtained free of charge from the Cambridge Crystallographic Data Centre (http://www.ccdc.cam.ac.uk/data_request/cif).
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Bui M.; Adjiman C. S.; Bardow A.; Anthony E. J.; Boston A.; Brown S.; Fennell P. S.; Fuss S.; Galindo A.; Hackett L. A.; Hallett J. P.; Herzog H. J.; Jackson G.; Kemper J.; Krevor S.; Maitland G. C.; Matuszewski M.; Metcalfe I. S.; Petit P.; Puxty G.; Reimer J.; Reiner D. M.; Rubin E. S.; Scott S. A.; Shah N.; Smit B.; Trusler J. P. M.; Webley P.; Wilcoxx J.; Mac Dowell N. Carbon Capture and Storage (CCS): the Way Forward. Energy Environ. Sci. 2018, 11, 1062–1176. 10.1039/C7EE02342A. [DOI] [Google Scholar]
- Erans M.; Sanz-Pérez E. S.; Hanak D. P.; Clulow Z.; Reiner D. M.; Mutch G. A. Direct Air Capture: Process Technology, Technoeconomic and Socio-Political Challenges. Energy Environ. Sci. 2022, 15, 1360–1405. 10.1039/D1EE03523A. [DOI] [Google Scholar]
- Rochelle G. T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. 10.1126/science.1176731. [DOI] [PubMed] [Google Scholar]
- McQueen N.; Vaz Gomes K.; McCormick C.; Blumanthal K.; Pisciotta M.; Wilcox J. A Review of Direct Air Capture (DAC): Scaling Up Commercial Technologies and Innovating for the Future. Prog. Energy 2021, 3, 032001 10.1088/2516-1083/abf1ce. [DOI] [Google Scholar]
- Patel H. A.; Byun J.; Yavuz C. T. Carbon Dioxide Capture Adsorbents: Chemistry and Methods. ChemSusChem 2017, 10, 1303–1317. 10.1002/cssc.201601545. [DOI] [PubMed] [Google Scholar]
- Sumida K.; Rogow D. L.; Mason J. A.; McDonald T. M.; Bloch E. D.; Herm Z. R.; Bae T.-H.; Long J. R. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 724–781. 10.1021/cr2003272. [DOI] [PubMed] [Google Scholar]
- Taddei M.; Petit C. Engineering Metal–Organic Frameworks for Adsorption-Based Gas Separations: from Process to Atomic Scale. Mol. Syst. Des. Eng. 2021, 6, 841–875. 10.1039/D1ME00085C. [DOI] [Google Scholar]
- Chen J.; Jiang L.; Wang W.; Wang P.; Li X.; Ren H.; Wang Y. Facile Construction of Highly Porous Carbon Materials Derived from Porous Aromatic Frameworks for Greenhouse Gas Adsorption and Separation. Microporous Mesoporous Mater. 2021, 326, 111385 10.1016/j.micromeso.2021.111385. [DOI] [Google Scholar]
- Zagho M. M.; Hassan M. K.; Khraisheh M.; Al Ali Al-Maadeed M.; Nazarenko S. A Review on Recent Advances in CO2 Separation Using Zeolite and Zeolite-like Materials as Adsorbents and Fillers in Mixed Matrix Membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091 10.1016/j.ceja.2021.100091. [DOI] [Google Scholar]
- Mercuri G.; Moroni M.; Galli S.; Tuci G.; Giambastiani G.; Yan T.; Liu D.; Rossin A. Temperature-Dependent Nitrous Oxide/Carbon Dioxide Preferential Adsorption in a Thiazolium-Functionalized NU-1000 Metal–Organic Framework. ACS Appl. Mater. Interfaces 2021, 13, 58982–58993. 10.1021/acsami.1c21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri G.; Moroni M.; Domasevitch K. V.; Di Nicola C.; Campitelli P.; Pettinari C.; Giambastiani G.; Galli S.; Rossin A. Carbon Dioxide Capture and Utilization with Isomeric Forms of Bis(amino)-Tagged Zinc Bipyrazolate Metal–Organic Frameworks. Chem. - Eur. J. 2021, 27, 4746–4754. 10.1002/chem.202005216. [DOI] [PubMed] [Google Scholar]
- Vismara R.; Tuci G.; Tombesi A.; Domasevitch K. V.; Di Nicola C.; Giambastiani G.; Chierotti M. R.; Bordignon S.; Gobetto R.; Pettinari C.; Rossin A.; Galli S. Tuning Carbon Dioxide Adsorption Affinity of Zinc(II) MOFs by Mixing Bis(pyrazolate) Ligands with N-Containing Tags. ACS Appl. Mater. Interfaces 2019, 11, 26956–26969. 10.1021/acsami.9b08015. [DOI] [PubMed] [Google Scholar]
- Müller P.; Bucior B.; Tuci G.; Luconi L.; Getzschmann J.; Kaskel S.; Snurr R. Q.; Giambastiani G.; Rossin A. Computational Screening, Synthesis and Testing of Metal–Organic Frameworks with a Bithiazole Linker for Carbon Dioxide Capture and its Green Conversion into Cyclic Carbonates. Mol. Syst. Des. Eng. 2019, 4, 1000–1013. 10.1039/C9ME00062C. [DOI] [Google Scholar]
- Yu J.; Xie L.-H.; Li J.-R.; Ma Y.; Seminario J. M.; Balbuena P. B. CO2 Capture and Separations Using MOFs: Computational and Experimental Studies. Chem. Rev. 2017, 117, 9674–9754. 10.1021/acs.chemrev.6b00626. [DOI] [PubMed] [Google Scholar]
- Lin J.-B.; Nguyen T. T. T.; Vaidhyanathan R.; Burner J.; Taylor J. M.; Durekova H.; Akhtar F.; Mah R. K.; Ghaffari-Nik O.; Marx S.; Fylstra N.; Iremonger S. S.; Dawson K. W.; Sarkar P.; Hovington P.; Rajendran A.; Woo T. K.; Shimizu G. K. H. A Scalable Metal-Organic Framework as a Durable Physisorbent for Carbon Dioxide Capture. Science 2021, 374, 1464–1469. 10.1126/science.abi7281. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Rasel Mian M.; Lee S.-J.; Chen H.; Zhang X.; Kirlikovali K. O.; Shulda S.; Melix P.; Rosen A. S.; Parilla P. A.; Gennett T.; Snurr R. Q.; Islamoglu T.; Yildirim T.; Farha O. K. Fine-Tuning a Robust Metal–Organic Framework toward Enhanced Clean Energy Gas Storage. J. Am. Chem. Soc. 2021, 143, 18838–18843. 10.1021/jacs.1c08749. [DOI] [PubMed] [Google Scholar]
- Wang T.; Lin E.; Peng Y.-L.; Chen Y.; Cheng P.; Zhang Z. Rational Design and Synthesis of Ultramicroporous Metal-Organic Frameworks for Gas Separation. Coord. Chem. Rev. 2020, 423, 213485 10.1016/j.ccr.2020.213485. [DOI] [Google Scholar]
- Shalini S.; Nandi S.; Justin A.; Maity R.; Vaidhyanathan R. Potential of Ultramicroporous Metal–Organic Frameworks in CO2 Clean-Up. Chem. Commun. 2018, 54, 13472–13490. 10.1039/C8CC03233E. [DOI] [PubMed] [Google Scholar]
- Adil K.; Belmabkhout Y.; Pillai R. S.; Cadiau A.; Bhatt P. M.; Assen A. H.; Maurin G.; Eddaoudi M. Gas/Vapour Separation using Ultra-Microporous Metal–Organic Frameworks: Insights into the Structure/Separation Relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. 10.1039/C7CS00153C. [DOI] [PubMed] [Google Scholar]
- Mukherjee S.; Sikdar N.; O’Nolan D.; Franz D. M.; Gascón V.; Kumar A.; Kumar N.; Scott H. S.; Madden D. G.; Kruger P. E.; Space B.; Zaworotko M. J. Trace CO2 Capture by an Ultramicroporous Physisorbent with Low Water Affinity. Sci. Adv. 2019, 5, eaax9171 10.1126/sciadv.aax9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato R.; Donnadio A.; Carta M.; Sangregorio C.; Tiana D.; Vivani R.; Taddei M.; Costantino F. Water-Based Synthesis and Enhanced CO2 Capture Performance of Perfluorinated Cerium-Based Metal–Organic Frameworks with UiO66 and MIL-140 Topology. ACS Sustainable Chem. Eng. 2019, 7, 394–402. 10.1021/acssuschemeng.8b03765. [DOI] [Google Scholar]
- Palakkal A. S.; Pillai R. S. Tuning the Ultra-Micropore Size of Fluorinated MOFs (M′F6-Ni-L) for CO2 Capture from Flue Gases by Advanced Computational Methods. J. Phys. Chem. C 2020, 124, 16975–16989. 10.1021/acs.jpcc.0c03633. [DOI] [Google Scholar]
- Tchalala M. R.; Belmabkhout Y.; Adil K.; Chappanda K. N.; Cadiau A.; Bhatt P. M.; Salama K. N.; Eddaoudi M. Concurrent Sensing of CO2 and H2O from Air Using Ultramicroporous Fluorinated Metal–Organic Frameworks: Effect of Transduction Mechanism on the Sensing Performance. ACS Appl. Mater. Interfaces 2019, 11, 1706–1712. 10.1021/acsami.8b18327. [DOI] [PubMed] [Google Scholar]
- Belmabkhout Y.; Zhang Z.; Adil K.; Bhatt P. M.; Cadiau A.; Solovyeva V.; Xing H.; Eddaoudi M. Hydrocarbon Recovery Using Ultra-Microporous Fluorinated MOF Platform with and without Uncoordinated Metal Sites: I- Structure Properties Relationships for C2H2/C2H4 and CO2/C2H2 separation. Chem. Eng. J. 2019, 359, 32–36. 10.1016/j.cej.2018.11.113. [DOI] [Google Scholar]
- Shearan S. J. I.; Jacobsen J.; Costantino F.; D’Amato R.; Novikov D.; Stock N.; Andreoli E.; Taddei M. In Situ X-ray Diffraction Investigation of the Crystallisation of Perfluorinated CeIV-Based Metal–Organic Frameworks with UiO-66 and MIL-140 Architectures. Chem. - Eur. J. 2021, 27, 6579–6592. 10.1002/chem.202005085. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Peh S. B.; Krishna R.; Kang C.; Chai K.; Wang Y.; Shi D.; Zhao D. Optimal Pore Chemistry in an Ultramicroporous Metal–Organic Framework for Benchmark Inverse CO2/C2H2 Separation. Angew. Chem., Int. Ed. 2021, 60, 17198–17204. 10.1002/anie.202106769. [DOI] [PubMed] [Google Scholar]
- Mercuri G.; Giambastiani G.; Di Nicola C.; Pettinari C.; Galli S.; Vismara R.; Vivani R.; Costantino F.; Taddei M.; Atzori C.; Bonino F.; Bordiga S.; Civalleri B.; Rossin A. Metal–Organic Frameworks in Italy: From Synthesis and Advanced Characterization to Theoretical Modeling and Applications. Coord. Chem. Rev. 2021, 437, 213861 10.1016/j.ccr.2021.213861. [DOI] [Google Scholar]
- Islamoglu T.; Idrees K. B.; Son F. A.; Chen Z.; Lee S.-J.; Li P.; Farha O. K. Are you Using the Right Probe Molecules for Assessing the Textural Properties of Metal–Organic Frameworks?. J. Mater. Chem. A 2021, 10, 157–173. 10.1039/D1TA08021K. [DOI] [Google Scholar]
- Gómez-Gualdrón D. A.; Moghadam P. Z.; Hupp J. T.; Farha O. K.; Snurr R. Q. Application of Consistency Criteria To Calculate BET Areas of Micro-And Mesoporous Metal–Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 215–224. 10.1021/jacs.5b10266. [DOI] [PubMed] [Google Scholar]
- Rouquerol J.; Llewellyn P.; Rouquerol F.. Studies in Surface Science and Catalysis, Llewellyn P. L.; Rodriquez-Reinoso F.; Rouquerol J.; Seaton N., Eds.; Elsevier: Amsterdam, 2007; Vol. 160, p 49. [Google Scholar]
- Jagiello J.; Olivier J. P. Carbon Slit Pore Model Incorporating Surface Energetical Heterogeneity and Geometrical Corrugation. Adsorption 2013, 19, 777–783. 10.1007/s10450-013-9517-4. [DOI] [Google Scholar]
- Jagiello J.; Ania C.; Parra J. B.; Cook C. Dual Gas Analysis of Microporous Carbons using 2D-NLDFT Heterogeneous Surface Model and Combined Adsorption Data of N2 and CO2. Carbon 2015, 91, 330–337. 10.1016/j.carbon.2015.05.004. [DOI] [Google Scholar]
- Zhu X.; Tian C.; Veith G. M.; Abney C. W.; Dehaudt J.; Dai S. In Situ Doping Strategy for the Preparation of Conjugated Triazine Frameworks Displaying Efficient CO2 Capture Performance. J. Am. Chem. Soc. 2016, 138, 11497–11500. 10.1021/jacs.6b07644. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Mahurin S. M.; An S.-H.; Do-Thanh C.-L.; Tian C.; Li Y.; Gill L. W.; Hagaman E. W.; Bian Z.; Zhou J.-H.; Hu J.; Liu H.; Dai S. Efficient CO2 Capture by a Task-Specific Porous Organic Polymer Bifunctionalized with Carbazole and Triazine Groups. Chem. Commun. 2014, 50, 7933–7936. 10.1039/c4cc01588f. [DOI] [PubMed] [Google Scholar]
- Nuhnen A.; Janiak C. A Practical Guide to Calculate the Isosteric Heat/Enthalpy of Adsorption via Adsorption Isotherms in Metal–Organic Frameworks, MOFs. Dalton Trans. 2020, 49, 10295–10307. 10.1039/D0DT01784A. [DOI] [PubMed] [Google Scholar]
- Schell J.; Casas N.; Pini R.; Mazzotti M. Pure and Binary Adsorption of CO2, H2, and N2 on Activated Carbon. Adsorption 2012, 18, 49–65. 10.1007/s10450-011-9382-y. [DOI] [Google Scholar]
- Simon C. M.; Smit B.; Haranczyk M. pyIAST: Ideal Adsorbed Solution Theory (IAST) Python Package. Comput. Phys. Commun. 2016, 200, 364–380. 10.1016/j.cpc.2015.11.016. [DOI] [Google Scholar]
- Das P.; Mandal S. K. Unprecedented High Temperature CO2 Selectivity and Effective Chemical Fixation by a Copper-Based Undulated Metal-Organic Framework. ACS Appl. Mater. Interfaces 2020, 12, 37137–37146. 10.1021/acsami.0c09024. [DOI] [PubMed] [Google Scholar]
- Hutter J.; Iannuzzi M.; Schiffmann F.; VandeVondele J. cp2k: Atomistic Simulations of Condensed Matter Systems. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2014, 4, 15–25. 10.1002/wcms.1159. [DOI] [Google Scholar]
- VandeVondele J.; Hutter J. Gaussian Basis Sets for Accurate Calculations on Molecular Systems in Gas and Condensed Phases. J. Chem. Phys. 2007, 127, 114105 10.1063/1.2770708. [DOI] [PubMed] [Google Scholar]
- Peverati R.; Zhao Y.; Truhlar D. G. Generalized Gradient Approximation That Recovers the Second-Order Density-Gradient Expansion with Optimized Across-the-Board Performance. J. Phys. Chem. Lett. 2011, 2, 1991–1997. 10.1021/jz200616w. [DOI] [Google Scholar]
- Goedecker S.; Teter M.; Hutter J. Separable Dual-Space Gaussian Pseudopotentials. Phys. Rev. B 1996, 54, 1703–1710. 10.1103/PhysRevB.54.1703. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Montgomery J. J. A.; Vreven T.; Kudin K. N.; Burant J. C.; Millam J. M.; Iyengar S. S.; Tomasi J.; Barone V.; Mennucci B.; Cossi M.; Scalmani G.; Rega N.; Petersson G. A.; Nakatsuji H.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Klene M.; Li X.; Knox J. E.; Hratchian H. P.; Cross J. B.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Ayala P. Y.; Morokuma K.; Voth G. A.; Salvador P.; Dannenberg J. J.; Zakrzewski V. G.; Dapprich S.; Daniels A. D.; Strain M. C.; Farkas O.; Malick D. K.; Rabuck A. D.; Raghavachari K.; Foresman J. B.; Ortiz J. V.; Cui Q.; Baboul A. G.; Clifford S.; Cioslowski J.; Stefanov B. B.; Liu G.; Liashenko A.; Piskorz P.; Komaromi I.; Martin R. L.; Fox D. J.; Keith T.; Al-Laham M. A.; Peng C. Y.; Nanayakkara A.; Challacombe M.; Gill P. M. W.; Johnson B.; Chen W.; Wong M. W.; Gonzalez C.; Pople J. A.. Gaussian 09, revision C.01; Gaussian, Inc.: Wallingford, CT, 2009.
- Becke A. D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- D’Amato R.; Bondi R.; Moghdad I.; Marmottini F.; McPherson M.; Naïli H.; Taddei M.; Costantino F. “Shake ‘n Bake” Route to Functionalized Zr-UiO-66 Metal–Organic Frameworks. Inorg. Chem. 2021, 60, 14294–14301. 10.1021/acs.inorgchem.1c01839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung H. H.-M.; Li W.; Saines P. J.; Köster T. K. J.; Grey C. P.; Cheetham A. K. Ligand-Directed Control over Crystal Structures of Inorganic–Organic Frameworks and Formation of Solid Solutions. Angew. Chem., Int. Ed. 2013, 52, 5544–5547. 10.1002/anie.201300440. [DOI] [PubMed] [Google Scholar]
- Černý R.; Favre-Nicolin V. FOX: A Friendly Tool to Solve Nonmolecular Structures from Powder Diffraction. Powder Diffraction 2005, 20, 359–365. 10.1154/1.2135314. [DOI] [Google Scholar]
- Topas V4. 2. General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS: Karlsruhe, Germany, 2009. [Google Scholar]
- Furukawa H.; Gándara F.; Zhang Y.-B.; Jiang J.; Queen W. L.; Hudson M. R.; Yaghi O. M. Water Adsorption in Porous Metal–Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. 10.1021/ja500330a. [DOI] [PubMed] [Google Scholar]
- Bury W.; Fairen-Jimenez D.; Lalonde M. B.; Snurr R. Q.; Farha O. K.; Hupp J. T. Control over Catenation in Pillared Paddlewheel Metal–Organic Framework Materials via Solvent-Assisted Linker Exchange. Chem. Mater. 2013, 25, 739–744. 10.1021/cm303749m. [DOI] [Google Scholar]
- Li T.; Kozlowski M. T.; Doud E. A.; Blakely M. N.; Rosi N. L. Stepwise Ligand Exchange for the Preparation of a Family of Mesoporous MOFs. J. Am. Chem. Soc. 2013, 135, 11688–11691. 10.1021/ja403810k. [DOI] [PubMed] [Google Scholar]
- Kim M.; Cahill J. F.; Su Y.; Prather K. A.; Cohen S. M. Postsynthetic Ligand Exchange as a Route to Functionalization of ‘Inert’ Metal–Organic Frameworks. Chem. Sci. 2012, 3, 126–130. 10.1039/C1SC00394A. [DOI] [Google Scholar]
- Preliminary work carried out in our laboratories revealed that the same kind of MIXMOF materials can be also obtained through a direct “one-pot” synthesis starting from ZrCl4, AA (modulator), H2FUM and H2TFS (unpublished results)
- Zahn G.; Schulze H. A.; Lippke J.; König S.; Sazama U.; Fröba M.; Behrens P. A. Water-Born Zr-Based Porous Coordination Polymer: Modulated Synthesis of Zr-Fumarate MOF. Microporous Mesoporous Mater. 2015, 203, 186–194. 10.1016/j.micromeso.2014.10.034. [DOI] [Google Scholar]
- Altomare A.; Giacovazzo C.; Guagliardi A.; Moliterni A. G. G.; Rizzi R.; Werner P.-E. New Techniques for Indexing: N-TREOR in EXPO. J. Appl. Crystallogr. 2000, 33, 1180–1186. 10.1107/S0021889800006427. [DOI] [Google Scholar]
- Taddei M.; Wakeham R. J.; Koutsianos A.; Andreoli E.; Barron A. R. Post-Synthetic Ligand Exchange in Zirconium-Based Metal–Organic Frameworks: Beware of The Defects!. Angew. Chem., Int. Ed. 2018, 57, 11706–11710. 10.1002/anie.201806910. [DOI] [PubMed] [Google Scholar]
- Wang S.; Wahiduzzaman M.; Martineau-Corcos C.; Maurin G.; Serre C.; Microporous A. Zirconium Metal-Organic Framework Based on trans-Aconitic Acid for Selective Carbon Dioxide Adsorption. Eur. J. Inorg. Chem. 2019, 2674–2679. 10.1002/ejic.201801284. [DOI] [Google Scholar]
- Wang S.; Xhaferaj N.; Wahiduzzaman M.; Oyekan K.; Li X.; Wei K.; Zheng B.; Tissot A.; Marrot J.; Shepard W.; Martineau-Corcos C.; Filinchuk Y.; Tan K.; Maurin G.; Serre C. Engineering Structural Dynamics of Zirconium Metal–Organic Frameworks Based on Natural C4 Linkers. J. Am. Chem. Soc. 2019, 141, 17207–17216. 10.1021/jacs.9b07816. [DOI] [PubMed] [Google Scholar]
- Bhattacharya B.; Dey R.; Pachfule P.; Banerjee R.; Ghoshal D. Four 3D Cd(II)-Based Metal Organic Hybrids with Different N,N′-Donor Spacers: Syntheses, Characterizations, and Selective Gas Adsorption Properties. Cryst. Growth Des. 2013, 13, 731–739. 10.1021/cg3014464. [DOI] [Google Scholar]
- Molecular Theory of Gases and Liquids, Hirschfelder C. F.; Curtiss C. F.; Bird R. B., Eds.; Wiley VCH: New York, 1954. [Google Scholar]
- Walton K. S.; Snurr R. Q. Applicability of the BET Method for Determining Surface Areas of Microporous Metal-Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. 10.1021/ja071174k. [DOI] [PubMed] [Google Scholar]
- Bhatt P. M.; Belmabkhout Y.; Cadiau A.; Adil K.; Shekhah O.; Shkurenko A.; Barbour L. J.; Eddaoudi M. A Fine-Tuned Fluorinated MOF Addresses the Needs for Trace CO2 Removal and Air Capture Using Physisorption. J. Am. Chem. Soc. 2016, 138, 9301–9307. 10.1021/jacs.6b05345. [DOI] [PubMed] [Google Scholar]
- Cavka J. H.; Jakobsen S.; Olsbye U.; Guillou N.; Lamberti C.; Bordiga S.; Lillerud K. P.; New A. Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. 10.1021/ja8057953. [DOI] [PubMed] [Google Scholar]
- Qian Q.; Asinger P. A.; Lee M. J.; Han G.; Rodriguez K. M.; Lin S.; Benedetti F. M.; Wu A. X.; Chi W. S.; Smith Z. P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. 10.1021/acs.chemrev.0c00119. [DOI] [PubMed] [Google Scholar]
- Molavi H.; Eskandari A.; Shojaei A.; Mousavi S. A. Enhancing CO2/N2 Adsorption Selectivity via Post-Synthetic Modification of NH2-UiO-66(Zr). Microporous Mesoporous Mater. 2018, 257, 193–201. 10.1016/j.micromeso.2017.08.043. [DOI] [Google Scholar]
- Belmabkhout Y.; Bhatt P. M.; Adil K.; Pillai R. S.; Cadiau A.; Shkurenko A.; Maurin G.; Liu G.; Koros W. J.; Eddaoudi M. Natural Gas Upgrading Using a Fluorinated MOF with Tuned H2S and CO2 Adsorption Selectivity. Nat. Energy 2018, 3, 1059–1066. 10.1038/s41560-018-0267-0. [DOI] [Google Scholar]
- Nandy A.; Forse A. C.; Witherspoon V. J.; Reimer J. A. NMR Spectroscopy Reveals Adsorbate Binding Sites in the Metal–Organic Framework UiO-66(Zr). J. Phys. Chem. C 2018, 122, 8295–8305. 10.1021/acs.jpcc.7b12628. [DOI] [Google Scholar]
- Forrest K. A.; Pham T.; Elsaidi S. K.; Mohamed M. H.; Thallapally P. K.; Zaworotko M. J.; Space B. Investigating CO2 Sorption in SIFSIX-3-M (M = Fe, Co, Ni, Cu, Zn) through Computational Studies. Cryst. Growth Des. 2019, 19, 3732–3743. 10.1021/acs.cgd.9b00086. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.