Abstract
Cardiovascular disease is a major cause of mortality in patients with sickle cell disease (SCD). Niss et al previously reported that cardiac magnetic resonance in 25 patients showed universal myocardial fibrosis, which they correlated with increased extracellular volume fraction (ECV). In the current study, they compared patients with SCD who were treated with hydroxyurea or transfusion at age <6 years to a group of patients with SCD without therapy. They documented that patients treated early had ECV levels comparable to normal controls, potentially preventing subsequent myocardial fibrosis.
TO THE EDITOR:
The cardiomyopathy of sickle cell anemia (SCA) is characterized by ventricular diastolic dysfunction that is superimposed on an anemia-related hyperdynamic state.1-3 Diastolic dysfunction has been recognized before and shown to portend early mortality in SCA,4 but the underlying causes of diastolic dysfunction were not understood.5 We recently showed that diffuse myocardial fibrosis is a common, if not defining, feature of the cardiomyopathy of SCA that is strongly associated with diastolic dysfunction.6,7 Every participant with SCA (N = 25) who underwent cardiac magnetic resonance imaging (CMR) had marked diffuse myocardial fibrosis.6 Three quarters were receiving hydroxyurea at the time of their imaging,6 which had been first prescribed at a mean age of 11.3 years (range, 4.1-25.6 years). By design, individuals receiving chronic transfusions were excluded.
Given that fibrosis was universally present in this population and could be severe by 6 years of age,6 we hypothesized that diffuse myocardial fibrosis begins in early childhood and that early and uninterrupted use of SCA-directed disease-modifying therapy could prevent myocardial fibrosis.
We studied individuals with SCA (HbSS genotype) who had been treated with either hydroxyurea or chronic transfusions since early childhood (initiated < 6 years) for at least 5 years. Interruption in either therapy for >6 months was exclusionary, but transition from 1 therapy to the other was allowed. None of the participants received concomitant cardiovascular medications. Patients on hydroxyurea had laboratory evidence of treatment adherence. Participants were studied at 2 sites: Cincinnati Children’s Hospital Medical Center (CCHMC) and Children’s Hospital Los Angeles (CHLA). CCHMC participants were enrolled in a locally institutional review board (IRB)-approved, longitudinal study of SCA cardiomyopathy (clinicaltrials.gov #NCT02410811) and provided informed consent. CHLA participants had CMR for standard clinical care. A waiver of informed consent was granted by the CHLA IRB for use of de-identified data.
Details of CMR acquisition and echocardiography are provided in the supplemental Methods available on the Blood Web site. Briefly, CMR was performed on 1.5-T scanners. Late gadolinium enhancement (LGE) imaging was performed 10 minutes after injection with gadolinium-diethylenetriamine pentaacetate (DTPA). Extracellular volume fraction (ECV) was measured from T1 maps before and 10 minutes after contrast injection. Normal ECV and ECV of patients with SCA who had not received early therapy were taken from Niss et al6 for comparison. Diastolic function was assessed by echocardiography. Peak mitral inflow velocities at early (E) and late filling (A), the TDI annular velocities é and á, and peak tricuspid regurgitant jet velocity (TRV) were measured according to standard techniques. We classified diastolic function (normal, abnormal, inconclusive) according to the most recent guidelines, modified for patients with SCA as we previously reported (supplemental Table 1).6,8
Comparisons across groups were made by Kruskal-Wallis analysis of variance with a Dunn’s multiple comparisons posttest; P < .05 was considered statistically significant.
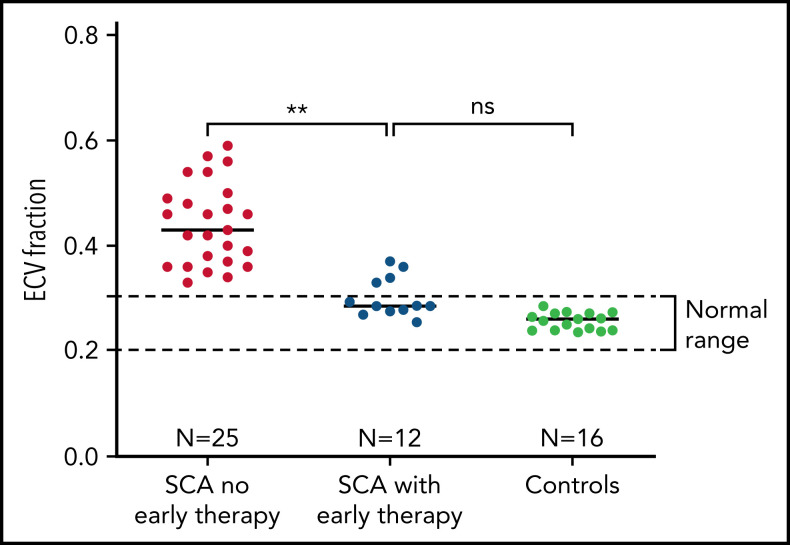
We studied 12 individuals (Table 1) with a mean age of 16.9 years (range, 7-28 years), all of whom had SCA. Mean age at the start of hydroxyurea or chronic transfusions was 3.1 years (range, 1.1-5.5 years). At the time of CMR, disease-modifying therapy had been administered for a mean of 13.7 years. There was no evidence of diffuse myocardial fibrosis in 8 of 12 individuals (67%), all of whom had normal ECV values (Figure 1). Mean ECV for all 12 early therapy patients was 0.30, which was significantly lower than the ECV of individuals with SCA who had not received early therapy (0.44, P = .002) and not different from normal controls (0.26; P = .11). None had macroscopic fibrosis by LGE imaging or myocardial hemosiderosis by T2* imaging. We found a trend in association between N-terminal pro-brain natriuretic peptide (NT-ProBNP) and ECV (Spearman ρ = 0.64, P = .05), but neither hemoglobin concentration nor therapy duration was significantly associated with ECV in this small cohort.
Table 1.
Characteristics of patients
| ID | Age at start of therapy (y) | Age at evaluation (y) | Sex | Therapy | Duration of therapy (y) | ECV* | Diastolic classification |
|---|---|---|---|---|---|---|---|
| CCH01 | 2.3 | 11 | Male | Chronic transfusions, hydroxyurea | 8.9 | 0.29 | No diastolic dysfunction |
| CCH02 | 2.7 | 17 | Female | Chronic transfusions | 14.6 | 0.27 | No diastolic dysfunction |
| CCH03 | 1.7 | 8 | Male | Chronic transfusions, hydroxyurea | 6.6 | 0.26 | Inconclusive |
| CCH04 | 1.9 | 7 | Female | Chronic transfusions, hydroxyurea | 5.9 | 0.29 | No diastolic dysfunction |
| CCH05 | 1.1 | 7 | Male | Chronic transfusions | 6.5 | 0.34 | No diastolic dysfunction |
| CHL01 | 3 | 28 | Female | Chronic transfusions | 24.9 | 0.37 | No diastolic dysfunction |
| CHL02 | 3.4 | 24 | Female | Hydroxyurea | 20.9 | 0.28 | No diastolic dysfunction |
| CHL03 | 3.2 | 19 | Female | Chronic transfusions | 15.8 | 0.28 | No diastolic dysfunction |
| CHL04 | 4.5 | 22 | Female | Hydroxyurea | 17.4 | 0.28 | No diastolic dysfunction |
| CHL05 | 5.4 | 21 | Female | Chronic transfusions | 11.7 | 0.36 | Inconclusive |
| CHL06 | 5.5 | 23 | Female | Hydroxyurea | 17 | 0.33 | No diastolic dysfunction |
| CHL07 | 2.4 | 16 | Male | Hydroxyurea | 13.7 | 0.29 | No diastolic dysfunction |
ECV for normal controls: 0.26 ± 0.02; ECV for patients with untreated SCA: 0.44 ± 0.08.
Figure 1.
Prevention of diffuse myocardial fibrosis in SCA with early initiation of disease-modifying therapy. Patients who had early initiation and uninterrupted use of hydroxyurea or chronic transfusions had significantly less myocardial fibrosis than patients with no early therapy. Mean ECV was 0.30, which was significantly lower than the ECV of individuals with SCA who have not received early uninterrupted therapy (0.44, P = .002) and not different from normal controls (0.26 ± 0.02; P = .11).
None had diastolic dysfunction. The diastolic function of 2 was classified as “inconclusive”: 1 had elevated ECV (0.36) and 1 had normal ECV (Table 1; supplemental Table 1). Left ventricular (LV) ejection fraction was preserved in all participants.
Here we demonstrate that the early initiation and uninterrupted use of SCA-directed disease-modifying therapy, either chronic transfusions or hydroxyurea, can impede or prevent the development of diffuse myocardial fibrosis. Diffuse myocardial fibrosis is a common, if not defining, feature of the cardiomyopathy of SCA.3,6 An independent group has recently confirmed our finding that diffuse myocardial fibrosis is nearly universally present in SCA.9 Our current results are compelling, although the sample size is small, because the ECV values of patients with early disease-modifiying therapy are remarkably lower than patients with SCA who have not received early disease-modifying therapy (Figure 1). Eight of 12 participants had normal ECV, the 4 with detectable fibrosis had lower ECV than most patients with untreated SCA, and none had diastolic dysfunction.
Cardiopulmonary disease is now the leading cause of mortality in SCA and presents an unmet therapeutic need.10 The cardiomyopathy of SCA is approximated to a model of heart failure with preserved ejection fraction (HFpEF) in which interstitial fibrosis is a key cause of diastolic dysfunction leading to pulmonary hypertension.11,12 We previously showed a strong association between diffuse myocardial fibrosis and diastolic dysfunction in SCA.2,6 We hypothesized that diffuse myocardial fibrosis was at least one of the causes of diastolic dysfunction in SCA.2,6 Here we show that participants without diffuse myocardial fibrosis do not have diastolic dysfunction, supporting our hypothesis that diffuse myocardial fibrosis underlies and likely precedes diastolic dysfunction in SCA.
Myocardial fibrosis portends early mortality and sudden cardiac death in other cardiomyopathies.13-15 Most prior studies of cardiac fibrosis in SCA used LGE imaging, which detects macroscopic fibrosis only, and found few affected individuals.16-18 In contrast, ECV mapping is a robust technique that detects and quantifies microscopic myocardial fibrosis, even in areas of normal-appearing myocardium by LGE imaging. LGE imaging alone underestimates the true burden of myocardial fibrosis, so the importance of diffuse myocardial fibrosis as a mechanism of cardiac disease in SCA may have been obscured or overlooked by these studies.
The underlying mechanism of myocardial fibrosis in SCA is unknown; therefore, targeted therapy to prevent or reverse it in SCA is also unknown. However, the development of diffuse myocardial fibrosis seems to be impeded by the early (<6 years of age) and uninterrupted use of either hydroxyurea or chronic transfusions, at least in this small group. Patients who received early treatment had smaller LV volume, mass, and cardiac index, indicating an amelioration of the hyperdynamic state of SCA, although this was not statistically significant (supplemental Table 2). It is unclear whether favorable hemodynamic effects directly limits fibrosis and diastolic dysfunction or there are other protective effects of disease-modifying therapy, such as decreased profibrotic signaling. Limiting myocardial fibrosis of any proximate cause could reduce the burden of cardiac disease in adults with SCA, including the poorly understood complications of dysrhythmia and sudden death. Ongoing follow-up of these patients with serial CMR studies to document longitudinal changes in myocardial fibrosis and myocardial mass, cardiac biomarkers, and ambulatory electrocardiography are key. Likewise, study of many more such patients is necessary to conclusively establish whether diffuse myocardial fibrosis and diastolic dysfunction are preventable. In addition, studies of the mechanisms of myocardial fibrosis in SCA and therapeutic trials of antifibrotic agents and SCA-directed therapy that include ECV as a quantitative clinical end point are needed.
In summary, disease-modifying therapy for SCA, if started in early childhood and continued indefinitely thereafter, may prevent diffuse myocardial fibrosis and possibly diastolic dysfunction. Our study provides further evidence supporting the universal use of early, presymptomatic, disease-modifying therapy in SCA, rather than waiting for organ injury to occur before initiating therapy.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors thank Eileen Beckman, Courtney Little, and Amy Shova for assistance with recruitment of participants and collection of clinical data.
This study was supported by the National Institutes of Health-National Heart, Lung, and Blood Institute Excellence in Hemoglobinopathy research award program (U01HL117709) and the Cincinnati Children’s Research Foundation (Procter Scholar award).
Footnotes
For original data, please contact omar.niss@cchmc.org.
There is a Blood Commentary on this article in this issue.
Authorship
Contribution: All authors designed the study; C.T.Q., O.N., J.C.W., J.D., and T.D.C. recruited participants and collected clinical data; M.D.T., J.D., and J.C.W. acquired CMR data; C.T.Q. and O.N. analyzed the data; and C.T.Q. wrote the first draft of the manuscript. All authors critically revised, edited, and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Omar Niss, Cincinnati Children’s Hospital Medical Center, Division of Hematology, 3333 Burnet Ave, MLC 7015, Cincinnati, OH 45229; e-mail: omar.niss@cchmc.org.
REFERENCES
- 1.Niss O, Quinn CT, Lane A, et al. Cardiomyopathy with restrictive physiology in sickle cell disease. JACC Cardiovasc Imaging. 2016;9(3):243-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakeer N, James J, Roy S, et al. Sickle cell anemia mice develop a unique cardiomyopathy with restrictive physiology. Proc Natl Acad Sci USA. 2016;113(35):E5182-E5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caughey MC, Ataga KI, Hinderliter AL. Sickle cardiomyopathy: the missing forest in the trees. JACC Cardiovasc Imaging. 2016;9(3):253-254. [DOI] [PubMed] [Google Scholar]
- 4.Sachdev V, Machado RF, Shizukuda Y, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. 2007;49(4):472-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood JC. The heart in sickle cell disease, a model for heart failure with preserved ejection fraction. Proc Natl Acad Sci USA. 2016;113(35):9670-9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niss O, Fleck R, Makue F, et al. Association between diffuse myocardial fibrosis and diastolic dysfunction in sickle cell anemia. Blood. 2017; 130(2):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detterich JA. Myocardial fibrosis: the heart of diastole? Blood. 2017;130(2):104-105. [DOI] [PubMed] [Google Scholar]
- 8.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321-1360. [DOI] [PubMed] [Google Scholar]
- 9.Morin C, Sharma A, Beasley G, et al. Assessment of cardiac abnormalities in sickle cell disease patients using cardiac magnetic resonance imaging (CMR). Blood. 2021;138(suppl 1):3110-3110. [Google Scholar]
- 10.Fitzhugh CD, Lauder N, Jonassaint JC, et al. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol. 2010;85(1):36-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263-271. [DOI] [PubMed] [Google Scholar]
- 12.Guazzi M. Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail. 2014;7(2):367-377. [DOI] [PubMed] [Google Scholar]
- 13.Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309(9):896-908. [DOI] [PubMed] [Google Scholar]
- 14.Roy C, Slimani A, de Meester C, et al. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson. 2018;20(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang EY, Ghosn MG, Khan MA, et al. Myocardial extracellular volume fraction adds prognostic information beyond myocardial replacement fibrosis. Circ Cardiovasc Imaging. 2019;12(12):e009535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai AA, Patel AR, Ahmad H, et al. Mechanistic insights and characterization of sickle cell disease-associated cardiomyopathy. Circ Cardiovasc Imaging. 2014;7(3):430-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pistoia L, Meloni A, Allò M, et al. Relationship between pancreatic iron overload, glucose metabolism and cardiac complications in sickle cell disease: an Italian multicentre study. Eur J Haematol. 2022;ejh.13809. [DOI] [PubMed] [Google Scholar]
- 18.Westwood MA, Shah F, Anderson LJ, et al. Myocardial tissue characterization and the role of chronic anemia in sickle cell cardiomyopathy. J Magn Reson Imaging. 2007;26(3):564-568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.