Calcified aortic valve disease (CAVD) is one of the most common and serious valve disease problems in the ageing population worldwide.1 Once CAVD develops, it can rapidly progress to aortic valve stenosis (AS), eventually leading to insufficient cardiac output and left ventricular dysfunction. There are no effective medical therapies to prevent or slow disease progression, and the only treatment for symptomatic severe AS is surgery or transcatheter aortic valve replacement (SAVR/TAVR). However, not all patients are suitable for invasive or costly surgery. Long-term use of anticoagulants after surgery and the risk of reoperation become an increasing economic and health burden of an aging population.2 The prevalence and mortality of CAVD have continued to rise over the past 20 years, especially in high-income countries,3 so developing new treatment strategies to effectively control AS progression is imperative.
Calcified valve with AS is a progressive disease that progresses from early local lesions to late cusps stiff and ultimately leads to the severe obstruction of left ventricular outflow and death from cardiovascular causes.4 Most AS subjects do not experience noticeable symptoms until they greatly reduced blood flow, and the average overall survival rate is reduced in symptomatic persons without aortic valve replacement.5 Early diagnosis and therapeutic intervention can greatly improve prognosis. Aortic valve sclerosis (AVS), the abnormal and heterogeneous thickening of the aortic valve leaflets in the absence of obstruction of ventricular outflow6 and the velocity through the aortic valve ≤ 2.5 m/s,7 is primarily considered a precursor to aortic stenosis. Echocardiography is the most common imaging technique for evaluating patients with aortic stenosis. Whereas the technique provides detailed information about the severity of aortic stenosis, the current era of full examination of the natural history of asymptomatic patients with severe AS has continued to challenge clinicians. Large prospective studies have demonstrated a strong positive association between AVS and coronary artery disease (CAD), stroke, cardiovascular and all-cause mortality.8 AVS is detected in nearly 50% of high-risk CAD patients, and high-risk CAD patients with signs of AVS have an approximately 30% increased risk of long-term mortality.9 It also becomes a predictor of atrial arrhythmias,10 CAD.
Omentin-1, also known as intelectin-1, is a novel adipocytokine widely expressed in many cells, including mesothelial cells, vascular cells, airway goblet cells as well as in visceral fat.11 It has been clearly established that adipose tissue releases a large number of bioactive factors to regulate different metabolic and inflammatory responses. Clinical studies have indicated the relation between adipose tissue-derived Omentin-1 and atherosclerosis by macrophage differentiation, inflammation, arterial calcification, and plaque formation.12 The pathophysiology of AS and atherosclerosis is similar, and Omentin-1 is identified as a biomarker in patients with suspected CAD, heart failure, and subclinical atherosclerosis.
Thus, it is reasonable to hypothesize that Omentin-1 may involve in the process of AS.
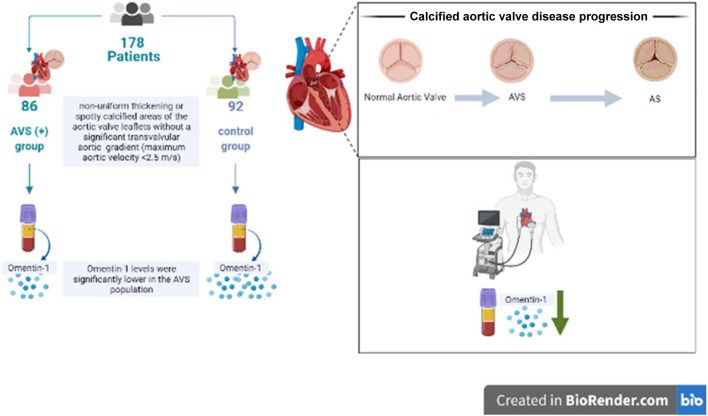
In this issue of the Journal, Mustafa Dogdus et al. first reported the relationship between serum Omentin-1 levels and AVS.13 A total of 178 subjects were included in this cross-sectional case-control study. Of these, 86 patients constituted AVS (+), and 92 age- and sex-matched healthy subjects without AVS constituted the control group. In the study population, the items with significant differences between the AVS (+) group and the control group were BMI, hypertension, hyperlipidemia, systolic blood pressure, low-density lipoprotein-cholesterol, transaortic velocity, and Omentin-1. There were no significant differences between groups in terms of blood pressure, smoking, fasting blood glucose, creatinine, total cholesterol, triglyceride, high-density lipoprotein-cholesterol, or diabetes mellitus history. Only the peak transaortic velocity was significantly different in 2-dimensional echocardiography analysis. The main finding of the study was that in the AVS population, mean Omentin-1 levels were significantly lower, suggesting that lowering Omentin-1 levels increases the risk of AVS (Figure 1).
Figure 1.
Flow charts of cross-sectional case-control study in blood marker analysis of aortic valve stenosis. AS, aortic stenosis; AVS, aortic valve sclerosis.
In summary, new evidence has been provided to show that Omentin-1 could be a possible treatment target and a biomarker predicting AVS. An earlier intervention of AS patients with/without symptoms to be closely followed up in a heart valve clinic is thus warranted.
DECLARATION OF CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005–1011. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 2.Otto CM, Prendergast B. Aortic-valve stenosis--from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744–756. doi: 10.1056/NEJMra1313875. [DOI] [PubMed] [Google Scholar]
- 3.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 Study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindman BR, Clavel MA, Mathieu P, et al. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimard BH, Larson JM. Aortic stenosis: diagnosis and treatment. Am Fam Physician. 2008;78:717–724. [PubMed] [Google Scholar]
- 6.Khilla P, Sanad O, Azm T, Ramzy A. Relationship between aortic valve sclerosis and the severity of coronary artery disease in patients undergoing diagnostic coronary angiography. J Cardiol Curr Res. 2018;11:00367. [Google Scholar]
- 7.Nightingale AK, Horowitz JD. Aortic sclerosis: not an innocent murmur but a marker of increased cardiovascular risk. Heart. 2005;91:1389–1393. doi: 10.1136/hrt.2004.057117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Owens DS, Katz R, Takasu J, et al. Incidence and progression of aortic valve calcium in the Multi-ethnic Study of Atherosclerosis (MESA). Am J Cardiol. 2010;105:701–708. doi: 10.1016/j.amjcard.2009.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myasoedova VA, Genovese S, Cavallotti L, et al. Aortic valve sclerosis in high-risk coronary artery disease patients. Front Cardiovasc Med. 2021;8:711899. doi: 10.3389/fcvm.2021.711899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akyuz AR, Ozderya A, Sahin S, et al. Relation of p-wave dispersion with presystolic a-wave and aortic valve sclerosis in asymptomatic subjects. Echocardiography. 2021;38:386–393. doi: 10.1111/echo.15011. [DOI] [PubMed] [Google Scholar]
- 11.Zhao A, Xiao H, Zhu Y, et al. Omentin-1: a newly discovered warrior against metabolic related diseases. Expert Opin Ther Targets. 2022:1–15. doi: 10.1080/14728222.2022.2037556. [DOI] [PubMed] [Google Scholar]
- 12.De Jager SC, Pasterkamp G. Atheroprotective properties of human Omentin-1 in experimental atherosclerosis. Cardiovasc Res. 2016;110:1–3. doi: 10.1093/cvr/cvw040. [DOI] [PubMed] [Google Scholar]
- 13.Dogdus M, Dindas F, Cekici Y, et al. Association between circulating omentin-1 levels and aortic valve sclerosis. Acta Cardiol Sin. 2022;38:584–590. doi: 10.6515/ACS.202209_38(5).20220314A. [DOI] [PMC free article] [PubMed] [Google Scholar]