Abstract
Background
Aortic valve sclerosis (AVS) is characterized by thickening of the valve leaflets accompanied by increased echogenicity and calcification without significant limitations in valve movements. Omentin-1 is a glycoprotein of the adiponectin family released from visceral adipose tissue, and it can be used as a biomarker of atherosclerosis, obesity, and metabolic syndrome. No studies have demonstrated any relationship between AVS and omentin-1 in the literature. We aimed to explore the association of serum omentin-1 levels with AVS.
Methods
Eighty-six patients with AVS and 92 age- and sex-matched controls were enrolled into the study. The baseline clinical characteristics of the patients were recorded. Conventional 2-dimensional echocardiography was performed. Omentin-1 levels were measured.
Results
The mean omentin-1 level was significantly lower in the AVS (+) group compared to the control group (78.16 ± 44.95 vs. 163.57 ± 59.84 ng/mL, p < 0.001). Omentin-1 [odds ratio (OR) = 3.45, 95% confidence interval (CI) = 1.88-5.39, p < 0.001,] and LDL-C (OR = 1.82, 95% CI = 1.33-2.16, p = 0.015) were found to be independent predictors of AVS in multivariate logistic regression analysis. An omentin-1 level of < 92.45 ng/mL had 90.5% sensitivity and 71.4% specificity for the prediction of AVS (area under curve: 0.697, p < 0.001).
Conclusions
Our results indicated that a lower omentin-1 level was associated with an increased risk of AVS. We suggest that omentin-1 could be used as a treatment target as well as to predict AVS.
Keywords: Aortic valve sclerosis, Biomarker, Omentin-1
INTRODUCTION
Calcific aortic valve disease (CAVD) is a slow, progressive, multifactorial disease characterized by dystrophic calcification of the aortic valve.1 The initial phase, known as aortic valve sclerosis (AVS), is characterized by thickening of the valve leaflets accompanied by increased echogenicity and calcification without significant limitations in valve movements.2 Besides being a common echocardiographic finding with aging, AVS is associated with an increased risk of acute coronary syndrome, atherosclerotic heart disease, heart failure, cerebrovascular disease, and cardiovascular mortality.3,4 Pathophysiological processes such as inflammation, oxidation, endothelial damage, lipid accumulation, and calcification are involved in the formation and progression of AVS.5 Thus, AVS is considered to be an atherosclerosis-like process.6 The early identification of AVS at its subclinical stage can improve risk stratification and enable timely intervention and disease control in clinical practice. However, few biomarkers are available regarding the identification of AVS.
Omentin-1, also known as intelectin-1, is a glycoprotein of the adiponectin family released from visceral adipose tissue, endothelial cells, and visceral fat stromal-vascular cells.7 Omentin-1 has anti-inflammatory effects, and its circulating concentration has been negatively correlated with blood pressure, waist circumference, insulin resistance, and body mass index. Patients with coronary artery disease (CAD) have been observed to have lower circulating omentin-1 levels compared with those without CAD.8 Previous studies have shown that omentin-1 can be used as a biomarker of atherosclerosis, obesity, metabolic syndrome, and diabetes mellitus.9-11
Although previous studies have evaluated the association between CAD and omentin-1, no studies have demonstrated any relationship between AVS, which has a similar pathogenesis to CAD, and omentin-1. Investigations of pathophysiological processes and novel biomarkers in the initial stage of CAVD before the occurrence of severe clinical conditions are required. To address this, in the present study, we aimed to explore the association of serum omentin-1 levels with AVS.
METHODS
Study population
A total of 178 subjects who were admitted for routine check-up and were examined at cardiology outpatient clinics were enrolled into this cross-sectional, case-control study between June 2019 and August 2020. The patient group [AVS (+) group] consisted of 86 patients with AVS, and the control group included 92 age- and sex-matched healthy subjects without AVS. AVS was identified according to the criteria described by Otto et al.5 as non-uniform thickening or spotty calcified areas of the aortic valve leaflets without a significant transvalvular aortic gradient (maximum aortic velocity < 2.5 m/s). Patients with a history of ischemic heart diseases (myocardial infarction, percutaneous coronary intervention, and/or coronary artery by-pass graft), rheumatic heart disease, bicuspid aortic valve, severe aortic stenosis, severe non-aortic valvular disease, history of valvular surgery, advanced heart failure, cardiac pacemaker, left ventricular ejection fraction (LVEF) < 50%, inflammatory and/or infectious diseases, malignancy, chronic renal and hepatic insufficiency, uncontrolled hypertension, thyroid and parathyroid dysfunction were excluded from the study. Chronic renal insufficiency was diagnosed when the estimated glomerular filtration rate (eGFR) was < 60 mL/min/1.73 m2 for 3 consecutive months, or in cases of abnormal renal structure or function other than decreased eGFR for over 3 months. The diagnosis of hyperparathyroidism was made according to a high serum parathyroid hormone (PTH) concentration and overt or mild hypercalcemia, with or without clinical manifestations of hypercalcemia. Hypoparathyroidism was defined as hypocalcemia and an inappropriately low PTH level. The local ethics committee approved the current study, which was carried out in compliance with the ethical guidelines of the Declaration of Helsinki. An informed and signed consent form was obtained from all patients.
Demographic and clinical evaluations
The baseline clinical characteristics of the study population were recorded. Information regarding risk factors, including age, sex, hypertension, diabetes mellitus (DM), hyperlipidemia, and smoking status was obtained. Hypertension (HT) was defined by a previous diagnosis of HT or the presence of systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg. DM was defined as fasting plasma glucose ≥ 126 mg/dl or plasma glucose level ≥ 200 mg/dl 2 hours after the 75 mg oral glucose tolerance test or glycated hemoglobin ≥ 6.5% or current treatment with antidiabetic medications. Hyperlipidemia (HLP) was defined as a baseline total cholesterol level > 200 mg/dL or current treatment with statins and/or lipid-lowering agents. Body mass index (BMI) was calculated as body weight (kg) divided by height squared (m2). Cigarette smoking was defined as smoking ≥ 1 packet of cigarettes a day. Venous blood samples were collected for blood count, routine biochemistry parameters, and lipid profiles after at least a 12-h fasting period from all of the subjects.
Measurement of serum omentin-1 levels
The blood samples were centrifuged, and serum was separated from the cells by centrifugation at 3000 × g for 10 min and stored at -80 °C until analysis. The serum concentration of omentin-1 was measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Sunred Technology Co., China) in accordance with the manufacturer’s instructions. All blood samples were routinely tested by ELISA in duplicate. Preliminary data obtained in our laboratory showed that the intra-assay and inter-assay coefficients of variation for omentin-1 were 4.1% and 4.5%, respectively.
Echocardiographic assessments
Measurements and images were performed in accordance with the American Society of Echocardiography (ASE) criteria from the parasternal long-axis, parasternal short-axis and apical four-chamber sections in the left lateral position, and subcostal section in the supine position with one-lead electrocardiography monitoring.12 All patients underwent 2-dimensional transthoracic echocardiographic evaluations using an ultrasound system (HD11 XE, Philips, Canada) equipped with a 1.5-4.0 MHz transducer. Standard 2-dimensional, M-mode, pulsed-Doppler measurements were done according to the updated recommendations for cardiac chamber quantification by echocardiography in adults. In the present study, 14 patients who had poor 2D image quality were excluded. Left atrial (LA) volume and LVEF were assessed using the modified Simpson biplane method. LA volume was measured from standard apical four-chamber views at end systole just before mitral valve opening. LA borders were traced using planimetry. The borders consisted of the walls of the LA excluding pulmonary veins and left atrial appendage. The biplane method of disks was used to calculate the LA volume. The left atrial volume index (LAVI) was calculated by dividing the LA volume by body surface area of the subjects.13 Mitral flow waves (E and A) were obtained via pulsed-wave Doppler in the apical four-chamber view, and their peak velocity were measured according to the recommendations of the ASE.
Statistical analysis
SPSS version 25.0 (IBM Corp., Armonk, NY, USA) software was used for all analyses. Normally distributed continuous data were expressed as mean ± standard deviation. Continuous variables that were not normally distributed were expressed as median, and categorical variables were expressed as n and percentage. Normal distribution of the data was evaluated using the Kolmogorov-Smirnov test and Shapiro-Wilk test, and variance homogeneity was evaluated using the Levene test. Data with normal distribution were compared using the Student’s t-test, and data with non-normal distribution were compared using the Mann-Whitney U-test. Pearson chi-square and Fisher’s exact tests were used to compare categorical variables. Receiver operating characteristic (ROC) curve analysis was used to determine the cut-off value of omentin-1 to predict AVS. The variables with a significant p value in univariate analysis were included in the multivariate analysis. Multivariate logistic regression analysis was performed to identify the independent predictors of AVS. Variables were examined at 95% confidence level. A p value < 0.05 was considered statistically significant.
RESULTS
A total of 178 subjects were enrolled into the present study [86 patients in the AVS (+) group and 92 subjects in the control group]. The baseline clinical characteristics and laboratory results of the study population are shown in Table 1. The mean age of the study population was 51.3 ± 7.9 years, and 54.6% of the patients were male. The mean BMI was significantly higher in the AVS (+) group than in the control group (29.8 ± 4.9 vs. 26.6 ± 4.2, p = 0.011). The frequencies of HT and HLP were higher in the AVS (+) group than in the control group (p = 0.024 and p = 0.041, respectively). Systolic BP was significantly higher in the AVS (+) group than in the control group (p = 0.019). The mean LDL-C level was significantly higher in the AVS (+) group than in the control group (p = 0.008). The mean omentin-1 level was significantly lower in the AVS (+) group compared to the control group (78.16 ± 44.95 vs. 163.57 ± 59.84 ng/mL, p < 0.001). There were no significant differences between the two groups in terms of age, sex, DBP, smoking, fasting glucose, creatinine, total cholesterol (TC), triglyceride (TG), high density lipoprotein-cholesterol (HDL-C), and history of DM (Table 1).
Table 1. The clinical characteristics and biochemical results of the study population.
| Control group (n = 92) | AVS (+) group (n = 86) | p value | |
| Age | 49.5 ± 7.2 | 52.3 ± 8.5 | 0.183 |
| Male gender, n (%) | 51 (55.4) | 46 (53.4) | 0.769 |
| BMI (kg/m2) | 26.6 ± 4.2 | 29.8 ± 4.9 | 0.011 |
| Hypertension, n (%) | 48 (52.1) | 51 (59.3) | 0.024 |
| Diabetes mellitus, n (%) | 11 (11.9) | 10 (12.1) | 0.655 |
| Hyperlipidemia, n (%) | 15 (16.3) | 19 (23.1) | 0.041 |
| Smoking, n (%) | 16 (17.3) | 17 (19.7) | 0.482 |
| Systolic BP (mmHg) | 124.7 ± 9.5 | 133.9 ± 10.6 | 0.019 |
| Diastolic BP (mmHg) | 77.6 ± 8.1 | 76.8 ± 9.2 | 0.540 |
| Fasting glucose (mg/dL) | 94.6 ± 18.5 | 98.1 ± 22.7 | 0.125 |
| Creatinine (mg/dL) | 0.89 ± 0.3 | 0.92 ± 0.4 | 0.268 |
| C-reactive protein (mg/dL) | 0.36 ± 0.25 | 0.43 ± 0.29 | 0.081 |
| TC (mg/dL) | 183.9 ± 32.2 | 185.6 ± 31.5 | 0.207 |
| TG (mg/dL) | 143.4 ± 40.2 | 152.7 ± 44.9 | 0.075 |
| LDL-C (mg/dL) | 96.5 ± 24.8 | 138.2 ± 29.5 | 0.008 |
| HDL-C (mg/dL) | 43.7 ± 9.5 | 46.2 ± 10.4 | 0.352 |
| Hemoglobin (g/dL) | 13.8 ± 1.4 | 14.1 ± 1.5 | 0.509 |
| Platelet (K/uL) | 296000 ± 102000 | 294000 ± 113000 | 0.748 |
| MPV (fL) | 9.27 ± 0.8 | 9.48 ± 1.1 | 0.699 |
| Omentin-1 (ng/mL) | 163.57 ± 59.84 | 78.16 ± 44.95 | < 0.001 |
AVS, aortic valve sclerosis; BMI, body mass index; BP, blood pressure; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; MPV, mean platelet volume; TC, total cholesterol; TG, triglyceride.
The peak transaortic velocity was higher in the AVS (+) group compared to the control group (p = 0.013). However, there were no significant differences between the two groups in the other 2D-echocardiographic results (Table 2).
Table 2. Two-dimensional and Doppler echocardiographic results of the study population.
| Control group (n = 92) | AVS (+) group (n = 86) | p value | |
| LVEF (%) | 65.3 ± 4.2 | 64.7 ± 5.8 | 0.394 |
| Ascending aorta diameter (mm) | 34.7 ± 3.6 | 35.8 ± 4.4 | 0.192 |
| Peak transaortic velocity (m/s) | 1.22 ± 0.15 | 1.68 ± 0.21 | 0.013 |
| Left atrium diameter (mm) | 36.5 ± 3.4 | 37.2 ± 3.8 | 0.477 |
| LAVI (ml/m2) | 28.6 ± 7.5 | 29.8 ± 6.8 | 0.123 |
| LVMI (g/m2) | 52 ± 18 | 55 ± 16 | 0.108 |
| LVSWT (mm) | 9.2 ± 1.2 | 9.4 ± 1.1 | 0.562 |
| PWT (mm) | 8.7 ± 1.1 | 8.5 ± 1.3 | 0.414 |
| LVEDD (mm) | 47.7 ± 4.2 | 48.2 ± 3.9 | 0.375 |
| LVESD (mm) | 32.6 ± 4.4 | 33.5 ± 4.1 | 0.188 |
| E/A | 1.24 ± 0.4 | 1.27 ± 0.2 | 0.221 |
| Lateral e′ (cm/s) | 11.7 ± 3.4 | 12.4 ± 3.2 | 0.307 |
| Septal e′ (cm/s) | 9.2 ± 1.5 | 9.5 ± 1.8 | 0.535 |
| dT (ms) | 211.6 ± 42.5 | 207.9 ± 43.4 | 0.184 |
| TAPSE (mm) | 22.9 ± 3.7 | 21.5 ± 3.1 | 0.784 |
AVS, aortic valve sclerosis; dT, deceleration time; LAVI, left atrial volume index; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVMI, left ventricular mass index; LVSWT, left ventricular septal wall thickness; PWT, posterior wall thickness; TAPSE, tricuspid annular plane systolic excursion.
Omentin-1 [odds ratio (OR) = 3.45, 95% confidence interval (CI) = 1.88-5.39, p < 0.001], and LDL-C (OR = 1.82, 95% CI = 1.33-2.16, p = 0.015) were found to be independent predictors of AVS in multivariate logistic regression analysis (Table 3).
Table 3. The univariate and multivariate analysis for AVS.
| Univariate | Multivariate | |||
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| BMI | 1.13 (0.92-1.28) | 0.061 | ||
| Hypertension | 1.02 (0.68-1.25) | 0.085 | ||
| Hyperlipidemia | 0.99 (0.75-1.16) | 0.106 | ||
| Omentin-1 | 2.96 (1.52-3.72) | < 0.001 | 3.45 (1.88-5.39) | < 0.001 |
| LDL-C | 1.44 (1.27-1.95) | 0.028 | 1.82 (1.33-2.16) | 0.015 |
| Systolic BP | 1.09 (0.74-1.31) | 0.072 | ||
| TG | 1.01 (0.83-1.36) | 0.208 | ||
| C-reactive protein | 0.88 (0.64-1.04) | 0.316 | ||
| Peak transaortic velocity | 1.12 (0.86-1.45) | 0.057 |
AVS, aortic valve sclerosis; BMI, body mass index; BP, blood pressure; CI, confidence interval; LDL-C, low density lipoprotein cholesterol; TG, triglyceride.
ROC analysis was performed to identify the ideal omentin-1 cut-off value to predict AVS. An omentin-1 value of < 92.45 ng/mL had 90.5% sensitivity and 71.4% specificity for the prediction of area under curve (AUC): 0.697, p < 0.001) (Figure 1).
Figure 1.
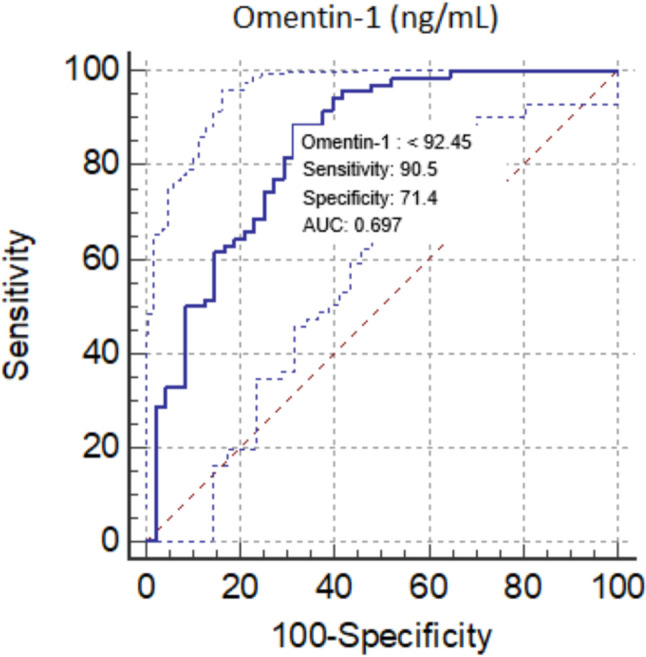
ROC curve of Omentin-1 for predicting of AVS. AVS, aortic valve sclerosis; AUC, area under curve; ROC, receiver operating characteristic.
DISCUSSION
The results of the present study demonstrated that the mean omentin-1 level was significantly lower in the AVS (+) group compared to the control group, and that the serum omentin-1 level was an independent predictor of AVS. To the best of our knowledge, this is the first study to evaluate the association between circulating omentin-1 levels and AVS.
AVS is defined as focal or diffuse thickening and calcification of the aortic valve without significant obstruction. The reported prevalence is 25-30% in subjects over 65 years of age, and it increases with age. Sclerosis of the aortic valve is an active process that involves the interaction of several pathways, including inflammation, lipid infiltration, osteogenic activation, and active mineralization within the aortic valve. Despite its wide prevalence, AVS is not a benign condition. Several recent studies have suggested that AVS may progress to aortic stenosis, and it has been associated with increased acute coronary syndrome, heart failure, cardiovascular and all-cause mortality. Therefore, biomarkers are needed to better understand the pathophysiology and disease management before CAVD progresses.
Adipokines modulate the functions of endothelial cells, macrophages, and vascular smooth muscle cells, and also play an important role in many pathophysiological processes.14,15 Omentin-1 is a novel adipocytokine which is expressed in visceral adipose tissue, and it has been shown to modulate obesity-related cardiometabolic disorders with anti-inflammatory activity.16 Several recent studies demonstrated that omentin-1 plays important roles in insulin sensitivity and body metabolism, and also that it has anti-atherosclerotic, anti-inflammatory, and cardiovascular protective effects.17 Lower circulating omentin-1 levels have been associated with metabolic syndrome, and metabolic syndrome has been associated with an increased risk of ischemic heart disease.18 In addition, metabolic syndrome has also been linked to an increased prevalence of AVS and faster progression of calcific AS.19-21 Taken together, these findings imply that there may be a relationship between serum omentin-1 level and AVS pathophysiology. We explored this relationship in this study, and found that omentin-1 level was significantly lower in the patients with AVS.
Capoulade et al. evaluated the relationship between metabolic syndrome and progression of aortic stenosis.22 They demonstrated that metabolic syndrome was an independent predictor of faster AS progression, with a more pronounced impact in younger patients. Considering the components of the metabolic syndrome, in the current study, we found that mean BMI was significantly higher in the AVS (+) group compared to the controls. Also, the frequency of HLP was higher in the AVS (+) patients than in the controls. In addition, SBP was significantly higher in the AVS (+) patients than in the controls. Our findings support the literature by showing that increased BMI and SBP are related to the pathophysiologic process of AVS.
Mustu et al. investigated the relationship between aortic sclerosis, which is the initial lesion of CAVD, and periaortic adipose tissue volume adjacent to the aortic annulus. Their results revealed a significant association between periaortic adipose tissue volume and aortic sclerosis independent of the baseline clinical and biochemical characteristics of the participants.23 They hypothesized that there may be a paracrine interaction between periaortic adipose tissue and adjacent aortic valve that regulates the structural changes in the valve tissue. Supporting their study, we found that omentin-1 secreted from adipose tissue was involved in this process.
Hypertension may play a facilitatory role in producing AVS in concert with the other factors that produce atherosclerosis. AVS has been likened to atherosclerosis because of the shared histopathological features, namely the occurrence in both conditions of inflammatory cells, macrophages and T cell infiltration, as well as lipid deposition.24 This suggests that adipocytokines play a role in the HT-AVS interaction in this atherosclerosis-like process. To illuminate this relationship, Çelik et al. investigated circulating omentin-1 levels in HT patients compared to healthy normotensive controls.25 Their results demonstrated that serum omentin-1 levels were lower in the patients with HT compared with the normotensive controls. In our study, in accordance with their study, the AVS (+) patients were more hypertensive and had lower serum omentin-1 levels than the controls.
The early identification of individuals with AVS may be clinically meaningful, as they tend to develop severe aortic stenosis and have a higher risk of all-cause mortality. We found a lower serum omentin-1 level in the subjects with AVS, and it was an independent predictor of AVS. Accordingly, we suggest that omentin-1 could be used as a treatment target and to predict AVS, and that treating patients with a low level of omentin-1 could prevent disease progression.
Limitations
Our study has several limitations. It was a cross-sectional study with a limited number of patients (178). The ability to test for omentin-1 is limited in routine clinical laboratory practice. We mentioned that AVS is an atherosclerosis-like process, however the patients were not examined for CAD. We did not evaluate calcific load in the patients with AVS using computed tomography. We could not assess other biomarkers for this process or dysmetabolic state. Because we did not evaluate any clinical outcomes, the effect of our results is not clear with regards to long-term clinical outcomes. Hence, further large-scale, multicenter studies with follow-up are needed to determine the effect of omentin-1 in clinical practice.
CONCLUSIONS
To the best of our knowledge, this is the first study to investigate the association between serum omentin-1 level and AVS. Our results indicated that a lower omentin-1 level was associated with an increased risk of AVS. This observation could extend our understanding of the pathophysiology of CAVD. We suggest that omentin-1 could be used as a treatment target as well as to predict AVS.
DECLARATION OF CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
REFERENCES
- 1.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 2.Otto CM, Lind BK, Kitzman DW, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 3.Shah SJ, Ristow B, Ali S, et al. Acute myocardial infarction in patients with versus without aortic valve sclerosis and effect of statin therapy (from the Heart and Soul Study). Am J Cardiol. 2007;99:1128–1133. doi: 10.1016/j.amjcard.2006.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aronow WS, Ahn C, Shirani J, et al. Comparison of frequency of new coronary events in older subjects with and without valvular aortic sclerosis. Am J Cardiol. 1999;83:599–600. doi: 10.1016/s0002-9149(98)00922-9. [DOI] [PubMed] [Google Scholar]
- 5.Otto CM, Kuusisto J, Reichenbach DD, et al. Characterization of the early lesion of ‘degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 6.Gharacholou SM, Karon BL, Shub C, et al. Aortic valve sclerosis and clinical outcomes: moving toward a definition. Am J Med. 2011;124:103–110. doi: 10.1016/j.amjmed.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Yang RZ, Lee MJ, Hu H, et al. Identification of omentin as a novel depot specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:1253–1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 8.Shibata R, Ouchi N, Kikuchi R, et al. Circulating omentin is associated with coronary artery disease in men. Atherosclerosis. 2011;219:811–814. doi: 10.1016/j.atherosclerosis.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 9.De Souza Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56:1655–1661. doi: 10.2337/db06-1506. [DOI] [PubMed] [Google Scholar]
- 10.Onur I, Oz F, Yildiz S, et al. A decreased serum omentin-1 level may be an independent risk factor for peripheral arterial disease. Int Angiol. 2014;33:455–460. [PubMed] [Google Scholar]
- 11.Kocijancic M, Vujicic B, Racki S, et al. Serum omentin-1 levels as a possible risk factor of mortality in patients with diabetes on haemodialysis. Diabetes Res Clin Pract. 2015;110:44–50. doi: 10.1016/j.diabres.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 13.Stefano GT, Zhao H, Schluchter M, et al. Assessment of echocardiographic left atrial size: accuracy of M-mode and two-dimensional methods and prediction of diastolic dysfunction. Echocardiography. 2012;29:379–384. doi: 10.1111/j.1540-8175.2011.01643.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin TH, Lee WL, Lee WJ, et al. Dyslipidemia, not inflammatory markers or adipokines, contributes significantly to a higher SYNTAX score in stable coronary artery disease (from the Taichung CAD Study). Acta Cardiol Sin. 2021;37:232–238. doi: 10.6515/ACS.202105_37(3).20201116B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu HP, Jen HL, Yin WH, et al. Circulating adiponectin levels following treatment can predict late clinical outcomes in chronic heart failure. Acta Cardiol Sin. 2017;33:139–149. doi: 10.6515/ACS20160427B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan YL, Zheng XL, Tang CK. The protective functions of omentin in cardiovascular diseases. Clin Chim Acta. 2015;448:98–106. doi: 10.1016/j.cca.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe T, Watanabe KK, Takahashi Y, et al. Adipose tissue-derived omentin-1 function and regulation. Compr Physiol. 2017;7:765–781. doi: 10.1002/cphy.c160043. [DOI] [PubMed] [Google Scholar]
- 18.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Katz R, Wong ND, Kronmal R, et al. Features of the metabolic syndrome and diabetes mellitus as predictors of aortic valve calcification in the Multi-Ethnic Study of Atherosclerosis. Circulation. 2006;113:2113–2119. doi: 10.1161/CIRCULATIONAHA.105.598086. [DOI] [PubMed] [Google Scholar]
- 20.Duman H, Bahceci I, Hamur H, et al. The relationship between serum apelin levels and the severity of calcific aortic stenosis. Acta Cardiol Sin. 2018;34:259–266. doi: 10.6515/ACS.201805_34(3).20180207A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briand M, Lemieux I, Dumesnil JG, et al. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol. 2006;47:2229–2237. doi: 10.1016/j.jacc.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 22.Capoulade R, Clavel MA, Dumesnil JG, et al. Impact of metabolic syndrome on progression of aortic stenosis: influence of age and statin therapy. J Am Coll Cardiol. 2012;60:216–223. doi: 10.1016/j.jacc.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 23.Mustu M, Gurses KM, Alpaydin MS, et al. Periaortic adipose tissue volume is associated with sclerotic changes in the adjacent aortic valve. Int J Cardiovasc Imaging. 2020;36:1559–1565. doi: 10.1007/s10554-020-01852-2. [DOI] [PubMed] [Google Scholar]
- 24.Aronow WS, Ahn C, Kronzon I, et al. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001;88:693–695. doi: 10.1016/s0002-9149(01)01821-5. [DOI] [PubMed] [Google Scholar]
- 25.Çelik M, Nar R, Nar G, et al. Serum omentin-1 levels in hypertensive patients. J Hum Hypertens. 2021;35:290–295. doi: 10.1038/s41371-020-00420-4. [DOI] [PubMed] [Google Scholar]


