Abstract
Herein, we present the formation of acyclic frameworks bearing two consecutive stereocenters of either tertiary or quaternary nature starting from easily accessible cyclopropenes. This holistic approach involves a regio- and diastereoselective hydro- or carboborylation of substituted cyclopropenyl esters. Formation of boronate complexes of the latter via the addition of nucleophiles and subsequent stereospecific 1,2-migration with carbon–carbon bond cleavage delivered the title compounds.
The growing demand for complex molecular architectures, especially acyclic fragments bearing multiple stereocenters, is contrasted by the challenging nature of their stereoselective synthesis generated by rotational freedom as well as distorted geometries.1 Thus, the discrimination between two seemingly similar alkyl side chains is still complex to achieve in acyclic systems. Even though various strategies have appeared over the years,2 protocols for the selective formation of vicinal stereocenters of either tertiary or quaternary nature are still rather limited.3 In this context, the carbon–carbon bond cleavage of densely functionalized cyclopropanes as a new approach of molecular editing has gained increased momentum over the past few years,4 as it provides an innovative solution for the creation of well-defined stereocenters along acyclic hydrocarbon architectures from an easily accessible cyclopropyl platform.5 We have recently reported a nucleophilic substitution reaction at the quaternary carbon center of cyclopropyl carbinol derivatives, with a pure inversion of configuration, to access tertiary alkyl halides and esters as a single diastereomer (Scheme 1, top).6 Furthermore, organoaluminum species could be successfully implemented as nucleophiles when using polysubstituted cyclopropyl methyl phosphates, again with a complete inversion of configuration at the most substituted carbon center (Scheme 1, top).7 Although our previous reports showed a high degree of stereoselectivity and a broad scope with respect to the cyclopropyl unit, the nature of the nucleophilic counterpart was mainly restricted to halogens and Me3Al.8 Considering this serious limitation, we were interested in developing an alternative approach to broaden the scope of the resulting acyclic fragments. From all considered possibilities, the Matteson 1,2-metalate rearrangement seemed to be a promising starting point to overcome this restriction, as it allows the use of various organolithium reagents and their stereospecific transfer to the α-position next to the boron moiety (Scheme 1, middle).9 In this respect, Aggarwal and co-workers showed recently the application of this chemistry to small rings by accessing either stereodefined cyclobutane systems by breaking the central C–C σ-bond of bicyclo[1.1.0]butyl boronic esters10 or by using the strain release of doubly activated donor–acceptor cyclopropyl boronate complexes (Scheme 1, middle).11
Scheme 1. Stereoselective Synthesis of Acyclic Compounds Bearing Vicinal Stereocenters.
Inspired by these reports, we surmised that polysubstituted cyclopropyl boronic esters might undergo a stereospecific ring-opening when decorated with a proper leaving group to deliver acyclic fragments bearing up to two adjacent fully substituted stereocenters (Scheme 1, bottom).12 Although several diastereo- and enantioselective protocols for the formation of borylated cyclopropanes have been reported,13 the formation of their polysubstituted analogues was still missing. Therefore, we first focused on devising a convergent approach to polysubstituted cyclopropyl boronic ester 2 through the copper-catalyzed hydro- and carboboration of cyclopropenyl esters 1. At the outset of our studies, we used the original conditions reported by Tortosa [B2pin2 (1.2 equiv), CuCl (10 mol %), tBuONa (30 mol %) in THF]14 on our model substrate, namely, cyclopropenyl ester 1a (R1 = Bu, R2 = H),15 with MeOH as electrophile, and we screened various phosphine ligands to obtain 2 with high regio- and diastereoselectivity. We were delighted to observe that when SPhos was used as ligand (12 mol %), from the two possible constitutional isomers (2a and 3a), the product 2a was formed in a 99:01 ratio in excellent yield as a single diastereomer (Scheme 2; for all ligands tested and variation of experimental conditions, see the Supporting Information).
Scheme 2. Cu-Catalyzed Hydro- and Carboborylation of Substituted Cyclopropenyl Esters.
Under the optimized set of conditions, the scope of the regio- and diastereoselective copper-catalyzed hydro- and carboboration was examined using a broad range of cyclopropyl esters 1, which are easily accessible by the well-known Rh-catalyzed decomposition of diazoacetates in the presence of an alkyne (Scheme 2). It should be noted that cyclopropenyl esters 1 could easily be prepared enantiomerically enriched by using dirhodium tris(diphenyltriflylimidazolidinone)(acetate).16 Besides a primary alkyl group on the cyclopropenyl core, the reaction proceeds smoothly in the presence of a cyclohexyl substituent (2b), whereas a bulky tert-butyl group completely inhibited the reaction (not shown in Scheme 2). In all cases, the boracupration proceeds anti to the ester group and could be rationalized by the steric hindrance of the boracopper species ligated to SPhos combined with the polar nature of the solvent that should prevent intramolecular chelation.17 Functional groups on the side chain, such as chloride or silyl ether (2c and 2e, respectively), do not hamper the transformation, nor does a phenyl group tethered to the three-membered ring (2d). To further test the generality of our protocol, we subsequently investigated the reactivity of sp2-disubstituted cyclopropenyl esters 1f–m. When symmetrical cyclopropene 1f (R1 = R2 = Me) was treated under the same experimental condition, 2f was obtained as a single diastereomer in 77% yield. When the cyclopropene has now two different sp2-substituents (i.e., 1g–m, R1 = alkyl, R2 = aryl), the constitutional selectivity leaned on inherently two different substituents on the two sp2 carbon centers of the double bond, leading, after boracupration, to the formation of the electronically stabilized cyclopropyl copper species.18 Indeed, in all these cases, only the isomers 2g–m were obtained with an outstanding selectivity as a single diastereomer. The relative configuration was determined by X-ray analysis of 2f, 2g, 2h, 2j, 2k, and 2p, unambiguously confirming the anti-addition relative to the ester. All other relative configurations were assigned by analogy and eventually further corroborated by analysis of the coupling constant of the two vicinal hydrogen atoms of the cyclopropyl unit (3JHH = 8–9 Hz for cis-coupling).19 Variation of the electronic nature of the aromatic core with either electron-donating or electron-withdrawing groups had no effect on the selectivity of addition, nor did the use of an extended π-system. Next, we were interested in further extending our approach to more substituted cyclopropyl boronic esters and in situ trapping the resulting cyclopropyl copper species with a carbon electrophile. To our delight, allyl phosphate (1.2 equiv) proved to be an excellent coupling partner, as it reacted smoothly with the parent cyclopropenyl ester 1a to furnish the allylated compound 2n in 68% as a single diastereomer (accompanied with minor amounts of the opposite constitutional isomer). Similar results were observed when using branched allyl phosphate, delivering compound 2o in 55% yield. When 1p was treated similarly, 2p was obtained in excellent diastereomeric ratio but in lower yield (28%). However, by using slightly modified experimental conditions with PhPCy2 as ligand instead of SPhos, the formation of 2p was successfully achieved (71% yield) albeit with a lower diastereoselectivity. As both diastereomers could be independently isolated by purification by column chromatography, we have opted for this modified condition for this particular case. Finally, we prepared geraniol-derived cyclopropenyl ester 1q and subjected it to our standard reaction conditions. Gratifyingly, desired product 2q was obtained in 87% yield without any interference of the additional double bonds in the reaction process. Therefore, through this unified strategy, an assortment of bench-stable polysubstituted cyclopropyl boronic esters was prepared expeditiously by a predictable route in high stereoisomeric purity in only two catalytic steps from alkynes. The reaction could also be scaled up, as 1 g of 1g could be transformed into 2g with an identical selectivity. The next step for the 1,2-metalate rearrangement is based on our previous research on molecular editing by carbon–carbon bond cleavage.7 To promote this ring-fragmentation, the ester moiety had to be transformed into a good leaving group. Thus, polysubstituted cyclopropyl boronic esters 2a, 2g, 2h, 2k, 2n, and 2p were transformed in an efficient two-step procedure to phosphates 4 as shown in Scheme 3 (for all details, see the Supporting Information). Having various boronic ester-substituted cyclopropyl phosphates in hand, we then investigated the anticipated 1,2-migration reaction with subsequent carbon–carbon bond cleavage.
Scheme 3. Sequence of Reduction–Phosphorylation.
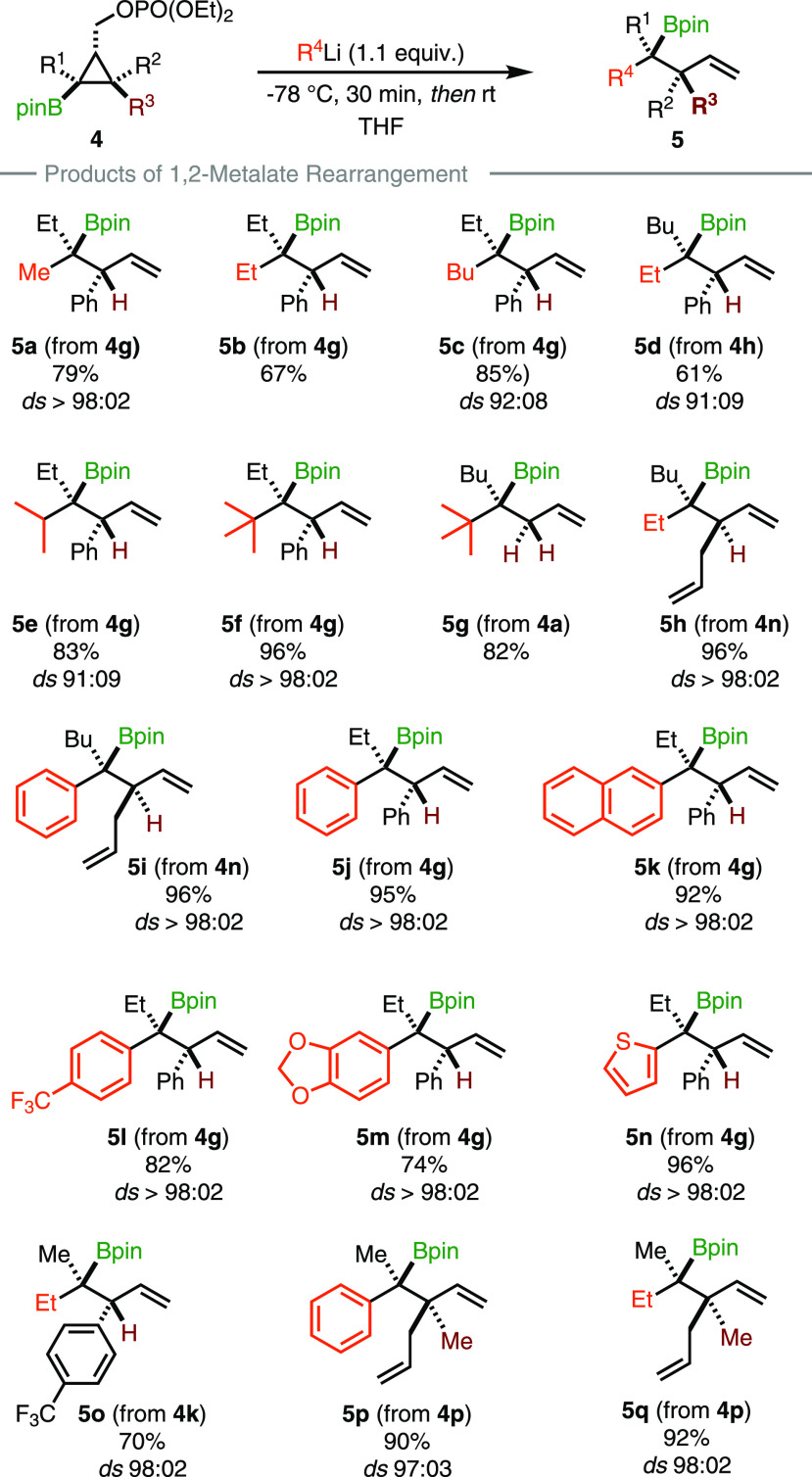
When phosphate 4g (R1 = Et, R2 = Ph, R3 = H) was subjected to the addition of methyllithium in THF at −78 °C, the formation of the ate-complex was obtained. Simply warming the reaction mixture to room temperature triggered the rearrangement to occur and provided the acyclic product 5a in good yield as a single diastereomer (Scheme 4). Pleasingly, the transformation proceeded with a complete stereospecificity. Based on this promising result, we then explored the scope of the potential nucleophile for the 1,2-migration. Adding commercially available primary alkyllithium reagents of various lengths gave the desired acyclic fragments in good yields (5a–c, Scheme 4), although a slight erosion of stereospecificity for 5c was observed. Both diastereomers at the tertiary carbon center holding the pinacol boronic ester could be prepared by permuting the nature of the alkyl group on the original cyclopropene and of the alkyllithium reagent (compare 5c and 5d, Scheme 4). Similar results were also observed when secondary and tertiary alkyllithium reagents, such as isopropyl- and tert-butyllithium (5e and 5f) were employed. In the latter case, the transformation proceeds in quantitative yield with excellent diastereospecificity. The stereochemistry of the allylic substituent has no effect on the overall process (compare 5a–f with 5h, Scheme 4). Indeed, when we applied our procedure to the phosphate 4n bearing an allyl group in a cis fashion to the pinacol boronic ester (R1 = Bu, R2 = H, R3 = allyl), ethyllithium furnished the desired acyclic fragment 5h in excellent yield as a single diastereomer. We then focused on the use of sp2-carbon nucleophiles starting with phenyllithium, which was found to be an excellent coupling partner, delivering boronic ester 5i and 5j in nearly quantitative yield, irrespective of the stereochemistry of the adjacent stereocenter. Extending the π-system by introducing a naphthyl group (5k) does not change the stereochemical outcome neither by decorating the aromatic core with either electron-withdrawing or electron-rich groups (5l and 5m, Scheme 4). In all cases, a smooth transformation was observed. In addition, a heteroaromatic ring as nucleophile, such as a thienyl derivative, performed extremely well to produce 5n with an excellent selectivity. The same holds when the electronic nature of the allylic substituent is different (5o, Scheme 4).
Scheme 4. 1,2-Metalate Rearrangement of Cyclopropyl Boronic Esters.
Spurred by these results, we then decided to evaluate the behavior of pentasubstituted cyclopropyl boronic ester 2p (R1 = R2 = CH3, R3 = allyl), which would potentially lead to the vicinal formation of tertiary and quaternary carbon stereocenters. The addition of phenyllithium proceeded smoothly to provide 5p with high stereospecificity and 90% yield. The relative configuration of 5p was determined by X-ray analysis, confirming that the intramolecular nucleophilic substitution at the most substituted carbon center proceeded with inversion of configuration. The same holds for the preparation of 5q.
In conclusion, we have developed an efficient method to construct acyclic architectures bearing up to two adjacent stereocenters of either tertiary or quaternary nature. Starting from simple cyclopropenyl esters, highly strained and densely functionalized cyclopropyl boronic esters were synthesized in a regio- and diastereoselective fashion. Formation of the corresponding boronate complexes with various carbon nucleophiles delivered, after 1,2-migration and carbon–carbon bond cleavage, the desired acyclic fragments with high degrees of stereospecificity.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c07394.
Experimental procedures, characterization data for all new compounds, optimizing tables, crystal structures along with copies of spectra (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This project has received funding from the Israel Science Foundation administrated by the Israel Academy of Sciences and Humanities (Grant No. 487/21), from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 101028628 (AUA), and from the European Research Council under the Excellence Science H2020 Program of the European Community (ERC grant agreement No. 786976).
The authors declare no competing financial interest.
Supplementary Material
References
- Gonthier J. F.; Wodrich M. D.; Steinmann S. N.; Corminboeuf C. Branched alkanes have contrasting stabilities. Org. Lett. 2010, 12, 3070–3073. 10.1021/ol1010642. [DOI] [PubMed] [Google Scholar]
- Pierrot D.; Marek I. Synthesis of enantioenriched vicinal tertiary and quaternary carbon stereogenic centers within an acyclic chain. Angew. Chem. Int. Ed. 2020, 59, 36–49. 10.1002/anie.201903188. [DOI] [PubMed] [Google Scholar]
- For selected examples of vicinal tertiary or quaternary stereocenters, see:; a Taylor M. S.; Jacobsen E. N. Enantioselective Michael additions to α,β-unsaturated imides catalyzed by a salen-Al complex. J. Am. Chem. Soc. 2003, 125, 11204–11205. 10.1021/ja037177o. [DOI] [PubMed] [Google Scholar]; b Lalonde M. P.; Chen Y.; Jacobsen E. N. A chiral primary amine thiourea catalyst for the highly enantioselective direct conjugate addition of α,α-disubstituted aldehydes to nitroalkenes. Angew. Chem. Int. Ed. 2006, 45, 6366–6370. 10.1002/anie.200602221. [DOI] [PubMed] [Google Scholar]; c Uyeda C.; Jacobsen E. N. Enantioselective Claisen rearrangements with a hydrogen-bond donor catalyst. J. Am. Chem. Soc. 2008, 130, 9228–9229. 10.1021/ja803370x. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Liu W.-B.; Reeves C. M.; Stoltz B. M. Enantio-, diastereo-, and regioselective iridium-catalyzed asymmetric allylic alkylation of acyclic β-ketoesters. J. Am. Chem. Soc. 2013, 135, 17298–17301. 10.1021/ja4097829. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Ardolino M. J.; Morken J. P. Congested C-C bonds by Pd-catalyzed enantioselective allyl-allyl cross-coupling, a mechanism-guided solution. J. Am. Chem. Soc. 2014, 136, 7092–7100. 10.1021/ja502280w. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Singh S.; Bruffaerts J.; Vasseur A.; Marek I. A unique Pd-catalysed Heck arylation as a remote trigger for cyclopropane selective ring-opening. Nat. Commun. 2017, 8, 14200. 10.1038/ncomms14200. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Kaldre D.; Klose I.; Maulide N. Stereodivergent synthesis of 1,4-dicarbonyls by traceless charge-accelerated sulfonium rearrangement. Science 2018, 361, 664–667. 10.1126/science.aat5883. [DOI] [PubMed] [Google Scholar]; h Bruffaerts J.; Pierrot D.; Marek I. Efficient and stereodivergent synthesis of unsaturated acyclic fragments bearing contiguous stereogenic elements. Nat. Chem. 2018, 10, 1164–1170. 10.1038/s41557-018-0123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Moghadam F. A.; Hicks E. F.; Sercel Z. P.; Cusumano A. Q.; Bartberger M. D.; Stoltz B. M. Ir-catalyzed asymmetric allylic alkylation of dialkyl malonates enabling the construction of enantioenriched all-carbon quaternary centers. J. Am. Chem. Soc. 2022, 144, 7983–7987. 10.1021/jacs.2c02960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected examples of molecular/skeletal editing, see:; a Roque J. B.; Kuroda Y.; Göttemann L. T.; Sarpong R. Deconstructive diversification of cyclic amines. Nature 2018, 564, 244–248. 10.1038/s41586-018-0700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Campos K. R.; Coleman P. J.; Alvarez J. C.; Dreher S. D.; Garbaccio R. M.; Terrett N. K.; Tillyer R. D.; Truppo M. D.; Parmee E. R. The importance of synthetic chemistry in the pharmaceutical industry. Science 2019, 363, eaat0805. 10.1126/science.aat0805. [DOI] [PubMed] [Google Scholar]; c Hui C.; Brieger L.; Strohmann C.; Antonchick A. P. Stereoselective synthesis of cyclobutanes by contraction of pyrrolidines. J. Am. Chem. Soc. 2021, 143, 18864–18870. 10.1021/jacs.1c10175. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Dherange B. D.; Kelly P. Q.; Liles J. P.; Sigman M. S.; Levin M. D. Carbon atom insertion into pyrroles and indoles promoted by chlorodiazirines. J. Am. Chem. Soc. 2021, 143, 11337–11344. 10.1021/jacs.1c06287. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Kennedy S. H.; Dherange B. D.; Berger K. J.; Levin M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 2021, 593, 223–227. 10.1038/s41586-021-03448-9. [DOI] [PubMed] [Google Scholar]; f Jurczyk J.; Woo J.; Kim S. F.; Dherange B. D.; Sarpong R.; Levin M. D. Single-atom logic for heterocycle editing. Nat. Synth 2022, 1, 352–364. 10.1038/s44160-022-00052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Fokin A. A.; Reshetylova O. K.; Bakhonsky V. V.; Pashenko A. E.; Kivernik A.; Zhuk T. S.; Becker J.; Dahl J. E. P.; Carlson R. M. K.; Schreiner P. R. Synthetic doping of diamondoids through skeletal editing. Org. Lett. 2022, 24, 4845–4849. 10.1021/acs.orglett.2c00982. [DOI] [PubMed] [Google Scholar]
- Cohen Y.; Cohen A.; Marek I. Creating stereocenters within acyclic systems by C-C Bond cleavage of cyclopropanes. Chem. Rev. 2021, 121, 140–161. 10.1021/acs.chemrev.0c00167. [DOI] [PubMed] [Google Scholar]
- Lanke V.; Marek I. Nucleophilic substitution at quaternary carbon stereocenters. J. Am. Chem. Soc. 2020, 142, 5543–5548. 10.1021/jacs.0c01133. [DOI] [PubMed] [Google Scholar]
- Patel K.; Lanke V.; Marek I. Stereospecific construction of quaternary carbon stereocenters from quaternary carbon stereocenters. J. Am. Chem. Soc. 2022, 144, 7066–7071. 10.1021/jacs.2c01695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanke V.; Marek I. Stereospecific nucleophilic substitution at tertiary and quaternary stereocentres. Chem. Sci. 2020, 11, 9378–9385. 10.1039/D0SC02562C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. S.; Mah R. W. H. Neighboring boron in nucleophilic displacement. J. Am. Chem. Soc. 1963, 85, 2599–2603. 10.1021/ja00900a017. [DOI] [Google Scholar]
- a Fawcett A.; Biberger T.; Aggarwal V. K. Carbopalladation of C-C σ-bonds enabled by strained boronate complexes. Nat. Chem. 2019, 11, 117–122. 10.1038/s41557-018-0181-x. [DOI] [PubMed] [Google Scholar]; b Bennett S. H.; Fawcett A.; Denton E. H.; Biberger T.; Fasano V.; Winter N.; Aggarwal V. K. Difunctionalization of C-C σ-bonds enabled by the reaction of bicyclo[1.1.0]butyl boronate complexes with electrophiles: reaction development, scope, and stereochemical origins. J. Am. Chem. Soc. 2020, 142, 16766–16775. 10.1021/jacs.0c07357. [DOI] [PubMed] [Google Scholar]
- Gregson C. H. U.; Ganesh V.; Aggarwal V. K. Strain release of donor-acceptor cyclopropyl boronate complexes. Org. Lett. 2019, 21, 3412–3416. 10.1021/acs.orglett.9b01152. [DOI] [PubMed] [Google Scholar]
- For selected examples of two vicinal quaternary stereocenters, see:; a Watson C. G.; Balanta A.; Elford T. G.; Essafi S.; Harvey J. N.; Aggarwal V. K. Construction of multiple, contiguous quaternary stereocenters in acyclic molecules by lithiation-borylation. J. Am. Chem. Soc. 2014, 136, 17370–17373. 10.1021/ja509029h. [DOI] [PubMed] [Google Scholar]; b Yang H.; Cao K.-S.; Zheng W.-H. A catalytic enantioselective approach to tetrol bearing vicinal all-carbon quaternary stereogenic centers. Chem. Commun. 2017, 53, 3737–3740. 10.1039/C7CC00457E. [DOI] [PubMed] [Google Scholar]; c Hethcox J. C.; Shockley S. E.; Stoltz B. M. Enantioselective synthesis of vicinal all-carbon quaternary centers via iridium-catalyzed allylic alkylation. Angew. Chem. Int. Ed. 2018, 57, 8664–8667. 10.1002/anie.201804820. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Chang T. Y.; Dotson J. J.; Garcia-Garibay M. A. Scalable synthesis of vicinal quaternary stereocenters via the solid-state photodecarbonylation of a crystalline hexasubstituted ketone. Org. Lett. 2020, 22, 8855–8859. 10.1021/acs.orglett.0c03226. [DOI] [PubMed] [Google Scholar]; e Isomura M.; Petrone D. A.; Carreira E. M. Construction of vicinal quaternary centers via iridium-catalyzed asymmetric allenylic alkylation of racemic tertiary alcohols. J. Am. Chem. Soc. 2021, 143, 3323–3329. 10.1021/jacs.1c00609. [DOI] [PubMed] [Google Scholar]
- For selected examples of the construction of borylated cyclopropanes, see:; a Rubina M.; Rubin M.; Gevorgyan V. Catalytic enantioselective hydroboration of cyclopropenes. J. Am. Chem. Soc. 2003, 125, 7198–7199. 10.1021/ja034210y. [DOI] [PubMed] [Google Scholar]; b Ito H.; Kosaka Y.; Nonoyama K.; Sasaki Y.; Sawamura M. Synthesis of optically active boron-silicon bifunctional cyclopropane derivatives through enantioselective copper(I)-catalyzed reaction of allylic carbonates with a diboron derivative. Angew. Chem. Int. Ed. 2008, 47, 7424–7427. 10.1002/anie.200802342. [DOI] [PubMed] [Google Scholar]; c Zhong C.; Kunii S.; Kosaka Y.; Sawamura M.; Ito H. Enantioselective synthesis of trans-aryl- and -heteroaryl-substituted cyclopropylboronates by copper(I)-catalyzed reactions of allylic phosphates with a diboron derivative. J. Am. Chem. Soc. 2010, 132, 11440–11442. 10.1021/ja103783p. [DOI] [PubMed] [Google Scholar]; d Tian B.; Liu Q.; Tong X.; Tian P.; Lin G.-Q. Copper(I)-catalyzed enantioselective hydroboration of cyclopropenes: facile synthesis of optically active cyclopropylboronates. Org. Chem. Front. 2014, 1, 1116–1122. 10.1039/C4QO00157E. [DOI] [Google Scholar]; e Benoit G.; Charette A. B. Diastereoselective borocyclopropanation of allylic ethers using a boromethylzinc carbenoid. J. Am. Chem. Soc. 2017, 139, 1364–1367. 10.1021/jacs.6b09090. [DOI] [PubMed] [Google Scholar]; f Edwards A.; Rubina M.; Rubin M. Directed RhI-catalyzed asymmetric hydroboration of prochiral 1-arylcycloprop-2-ene-1-carboxylic acid derivatives. Chem.—Eur. J. 2018, 24, 1394–1403. 10.1002/chem.201704443. [DOI] [PubMed] [Google Scholar]; g Wu F.-P.; Luo X.; Radius U.; Marder T. B.; Wu X.-F. Copper-catalyzed synthesis of stereodefined cyclopropyl bis(boronates) from alkenes with CO as the C1 source. J. Am. Chem. Soc. 2020, 142, 14074–14079. 10.1021/jacs.0c06800. [DOI] [PubMed] [Google Scholar]; h Gutiérrez-Bonet Á.; Popov S.; Emmert M. H.; Hughes J. M. E.; Nolting A. F.; Ruccolo S.; Wang Y. Asymmetric synthesis of tertiary and secondary cyclopropyl boronates via cyclopropanation of enantioenriched alkenyl boronic esters. Org. Lett. 2022, 24, 3455–3460. 10.1021/acs.orglett.2c01018. [DOI] [PubMed] [Google Scholar]; i Neouchy Z.; Hullaert J.; Verhoeven J.; Meerpoel L.; Thuring J.-W.; Verniest G.; Winne J. Synthesis of cyclopropyl pinacol boronic esters from dibromocyclopropanes. Synlett 2022, 33, 759–766. 10.1055/s-0037-1610794. [DOI] [Google Scholar]; j Iwamoto H.; Ozawa Y.; Hayashi Y.; Imamoto T.; Ito H. Conformationally fixed chiral bisphosphine ligands by steric modulators on the ligand backbone: selective synthesis of strained 1,2-disubstituted chiral cis-cyclopropanes. J. Am. Chem. Soc. 2022, 144, 10483–10494. 10.1021/jacs.2c02745. [DOI] [PubMed] [Google Scholar]; k Shi Y.; Yang Y.; Xu S. Iridium-catalyzed enantioselective C(sp3)-H borylation of aminocyclopropanes. Angew. Chem. Int. Ed. 2022, 61, e202201463. 10.1002/anie.202201463. [DOI] [PubMed] [Google Scholar]; l Mali M.; Sharma G. V. M.; Ghosh S.; Roisnel T.; Carboni B.; Berrée F. Simmons-Smith cyclopropanation of alkenyl 1,2-bis(boronates): stereoselective access to functionalized cyclopropyl derivatives. J. Org. Chem. 2022, 87, 7649–7657. 10.1021/acs.joc.2c00152. [DOI] [PubMed] [Google Scholar]
- Parra A.; Amenós L.; Guisán-Ceinos M.; López A.; García Ruano J. L.; Tortosa M. Copper-catalyzed diastereo- and enantioselective desymmetrization of cyclopropenes: synthesis of cyclopropylboronates. J. Am. Chem. Soc. 2014, 136, 15833–15836. 10.1021/ja510419z. [DOI] [PubMed] [Google Scholar]
- Petiniot N.; Anciaux A. J.; Noels A. F.; Hubert A. J.; Teyssié P. Rhodium catalysed cyclopropenation of acetylenes. Tetrahedron Lett. 1978, 19, 1239–1242. 10.1016/S0040-4039(01)94511-3. [DOI] [Google Scholar]
- For examples of enantioselective cyclopropenylation of alkynes, see:; a Doyle M. P.; Winchester W. R.; Hoorn J. A. A.; Lynch V.; Simonsen S. H.; Ghosh R. Dirhodium(II) tetrakis(carboxamidates) with chiral ligands. structure and selectivity in catalytic metal-carbene transformations. J. Am. Chem. Soc. 1993, 115, 9968–9978. 10.1021/ja00075a013. [DOI] [Google Scholar]; b Lou Y.; Horikawa M.; Kloster R. A.; Hawryluk N. A.; Corey E. J. A New chiral Rh(II) catalyst for enantioselective [2 + 1]-cycloaddition. Mechanistic implications and applications. J. Am. Chem. Soc. 2004, 126, 8916–8918. 10.1021/ja047064k. [DOI] [PubMed] [Google Scholar]; c Davies H. M. L.; Lee G. H. Dirhodium(II) tetra(n-(dodecylbenzenesulfonyl)prolinate) catalyzed enantioselective cyclopropenation of alkynes. Org. Lett. 2004, 6, 1233–1236. 10.1021/ol049928c. [DOI] [PubMed] [Google Scholar]; d Briones J. F.; Hansen J.; Hardcastle K. I.; Autschbach J.; Davies H. M. L. Highly enantioselective Rh2(S-DOSP)4-catalyzed cyclopropenation of alkynes with styryldiazoacetates. J. Am. Chem. Soc. 2010, 132, 17211–17215. 10.1021/ja106509b. [DOI] [PubMed] [Google Scholar]; e Goto T.; Takeda K.; Shimada N.; Nambu H.; Anada M.; Shiro M.; Ando K.; Hashimoto S. Highly enantioselective cyclopropenation reaction of 1-alkynes with α-alkyl-α-diazoesters catalyzed by dirhodium(II) carboxylates. Angew. Chem. Int. Ed. 2011, 50, 6803–6808. 10.1002/anie.201101905. [DOI] [PubMed] [Google Scholar]; f Briones J. F.; Davies H. M. Rh2(S-PTAD)4-catalyzed asymmetric cyclopropenation of aryl alkynes. Tetrahedron 2011, 67, 4313–4317. 10.1016/j.tet.2011.04.029. [DOI] [Google Scholar]; g Uehara M.; Suematsu H.; Yasutomi Y.; Katsuki T. Enantioenriched synthesis of cyclopropenes with a quaternary stereocenter, versatile building blocks. J. Am. Chem. Soc. 2011, 133, 170–171. 10.1021/ja1089217. [DOI] [PubMed] [Google Scholar]; h Cui X.; Xu X.; Lu H.; Zhu S.; Wojtas L.; Zhang X. P. Enantioselective cyclopropenation of alkynes with acceptor/acceptor-substituted diazo reagents via Co(II)-based metalloradical catalysis. J. Am. Chem. Soc. 2011, 133, 3304–3307. 10.1021/ja111334j. [DOI] [PubMed] [Google Scholar]; i Briones J. F.; Davies H. M. L. Gold(I)-catalyzed asymmetric cyclopropenation of internal alkynes. J. Am. Chem. Soc. 2012, 134, 11916–11919. 10.1021/ja304506g. [DOI] [PubMed] [Google Scholar]
- a Didier D.; Delaye P.-O.; Simaan M.; Island B.; Eppe G.; Eijsberg H.; Kleiner A.; Knochel P.; Marek I. Modulable and highly diastereoselective carbometalations of cyclopropenes. Chem.—Eur. J. 2014, 20, 1038–1048. 10.1002/chem.201303569. [DOI] [PubMed] [Google Scholar]; b Roy S. R.; Didier D.; Kleiner A.; Marek I. Diastereodivergent combined carbometalation/zinc homologation/C-C fragmentation reaction as an efficient tool to prepare acyclic allylic quaternary carbon stereocenters. Chem. Sci. 2016, 7, 5989–5994. 10.1039/C6SC02191C. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Müller D. S.; Marek I. Copper mediated carbometalation reactions. Chem. Soc. Rev. 2016, 45, 4552–4566. 10.1039/C5CS00897B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y.; Augustin A. U.; Levy L.; Jones P. G.; Werz D. B.; Marek I. Regio- and diastereoselective copper-catalyzed carbomagnesiation for the synthesis of penta- and hexa-substituted cyclopropanes. Angew. Chem. Int. Ed. 2021, 60, 11804–11808. 10.1002/anie.202102509. [DOI] [PubMed] [Google Scholar]
- Patel D. J.; Howden M. E. H.; Roberts J. D. Nuclear magnetic resonance spectroscopy. cyclopropane derivatives. J. Am. Chem. Soc. 1963, 85, 3218–3223. 10.1021/ja00903a036. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.