Abstract
Ethnopharmacological relevance: Serpylli herba extract (SHE), composed of the aerial parts of wild thyme (Thymus serpyllum L.) (Lamiaceae family), is traditionally used in Europe and North Africa to treat diarrhea, gastric ulcers, intestinal parasites and upper respiratory tract infections. Recently, SHE has generated a great interest for irritable bowel syndrome (IBS) management, probably due to its intestinal anti-inflammatory properties shown in experimental colitis and the fact that its active components could preserve the intestinal barrier integrity, which is altered in patients with IBS.
Aim of study: We aimed to test the effects of a SHE in a rat experimental model resembling human IBS.
Materials and methods: IBS was provoked by deoxycholic acid (DCA). Rats were then treated with SHE (100 mg/kg) or gabapentin (70 mg/kg) and different inflammatory and gut barrier integrity markers were evaluated. Moreover, several gut hypersensitivity and hyperalgesia determinations were performed.
Results: SHE improved referred pain and visceral hypersensitivity. Additionally, SHE enhanced immune status by downregulating of the expression of the pro-inflammatory mediators Il-1β, Il-6, Ifn-γ, Tlr-4, and the inducible enzyme Cox-2, thus inducing visceral analgesia, and promoting the restore of the gut barrier function by upregulating the mucins Muc-2 and Muc-3. These anti-inflammatory effects could be related to its action on mast cells since it significantly inhibited the β-Hexosaminidase production in RBL-2H3 cells. Lastly, SHE also seems to modulate the serotonin pathway by restoring the altered expression of the 5-HT receptors Htr-3 and Htr-4.
Conclusion: SHE could be considered a potential new treatment for IBS, since it ameliorates hypersensitivity, visceral hyperalgesia, and inflammation. These beneficial effects may be due to the inhibition of mast cells degranulation and serotonin pathway.
Keywords: Serpylli herba, T. serpyllum L., intestine anti inflammatory activity, visceral analgesia, IBS rat model
Introduction
IBS is a very prevalent functional digestive disease, being its worldwide prevalence of 11% (Mearin et al., 2016). It is distinguished by recurrent abdominal pain, bloating, cramping, gas and altered bowel habits as per the diagnostic Rome IV criteria (Mearin et al., 2016; Weaver et al., 2017). Moreover, 50%–90% of the patients present psychiatric comorbidities, including anxiety, social phobia, somatization disorder, major depressive disorder, or posttraumatic stress (Banerjee et al., 2017). Its etiology and pathophysiology are not properly understood, but gastrointestinal dysmotility, compromised gut barrier function, altered intestinal immune activation and visceral hypersensitivity are associated with its development (Lee and Park, 2014). Indeed, it has been described that a persistently augmented presence of intraepithelial lymphocytes and enteroendocrine and mast cells in the intestinal mucosa unbalance the network that links the enteric immune defense and the nervous system and leads to the onset of IBS symptoms (Yoo and Mazmanian, 2017; Uranga et al., 2020). Particularly, the sensitization of the immune cells triggers the release of cytokines and mediators that can affect different motor and neural functions, and produce pain, irritability, and hypersensitivity (Lee and Park, 2014; Uranga et al., 2020).
To date, the management of this disorder is limited because the drugs that are used are not well-tolerated and do not relieve the whole set of symptoms. Consequently, the development of safe, efficacious and cost effective therapeutic approaches is a pressing challenge that would improve patient quality of life and alleviate the socioeconomic burden associated with the disease. The current pharmacological treatments include antibiotics, polyethylene glycol (PEG), bile salt sequestrants, antispasmodics, and antidepressants (Ford et al., 2014). However, as commented before, they often fail to optimally alleviate the symptoms. In this sense, natural products could be used to develop effective and safe therapeutic approaches (Bordbar et al., 2020).
T. serpyllum L. (known as wild thyme) is a perennial shrub in the Lamiaceae family native to Northern and Central Europe. Wild thyme has been broadly applied in traditional medicine for centuries and the pharmacopeias recognize its medicinal properties. T. serpyllum is a significant source of active ingredients with antioxidant, antimicrobial, antitumor and cytotoxic properties including essential oil, tannins, condensed tannins, basically polymers of proanthocyanidins, and gallotannins; total hydroxycinnamic acids; flavonoids; flavonones; and triterpenes (Feistel et al., 2021) which could explain its therapeutic effects. In this sense, it has been reported that T. serpyllum is used to treat headaches caused by colds, laryngitis, cough, upper respiratory tract infections, digestive diseases including spastic colon, meteorism, diarrhea, intestinal parasites and gastric ulcer and loss of appetite (Jarić et al., 2014). Specifically, the aerial parts of T. serpyllum, Serpylli herba, have had a great traditional use all over the world (Quave et al., 2012) as anthelmintic, antiseptic, deodorant, diaphoretic, antispasmodic, carminative, tonic, sedative and expectorant. Its beneficial health effects could be related to its content in flavonoids, phenolic acids and other active compounds, among which rosmarinic acid, a well described antioxidant, stands out (Zheng and Wang, 2001). Moreover, Serpylli herba extract (SHE) has recently generated a great interest for irritable bowel syndrome (IBS) management, probably due to the fact that its active compounds have also shown to preserve the intestinal barrier integrity, which is altered in patients with IBS (Barbara, 2006) and to produce intestinal anti-inflammatory effects in a variety of experimental colitis in rodents (Algieri et al., 2014).
Considering all the above, the objective of the current study was to test the beneficial effect of SHE in the experimental model of deoxycholic acid (DCA)-induced visceral pain in rats. The repetitive colorectal instillation of DCA leads to transient colonic inflammation, persistent visceral and referred mechanical hypersensitivity. That is why this model mimic the clinical manifestations human IBS (Traub et al., 2008).
Materials and methods
Reagents
Chemicals were obtained from Sigma Chemical (Madrid, Spain), except when mentioned specifically.
Plant extract and drug
Serpylli herba aqueous extract was prepared by Finzelberg GmbH and Co. KG (Andernach, Germany) as described before (Algieri et al., 2014; Feistel et al., 2021) and supplied by PhytoLab GmbH and Co. KG (Vestenbergsgreuth, Germany). The final extract (batch TPA 47-08) consisted of 70% native extract (DERnative 4-8:1), 15% Arabic gum and 15% maltodextrin. It contained 1.8% rosmarinic acid (evaluated by HPLC), and less than 0.1% of essential oil (assessed by Clevenger apparatus), as well as less than 0.01% of thymol/carvacrol (measured by gas chromatography) (See more detailed analysis in Supplementary Material).
Rat model of chronic post-inflammatory visceral pain induced by DCA
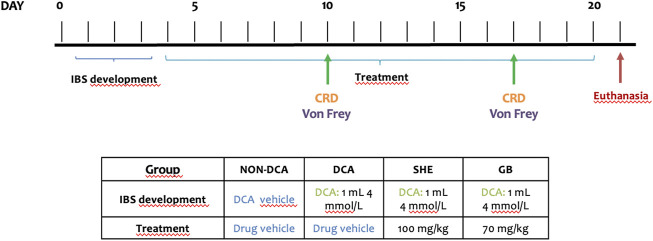
Male Sprague Dawley rats (10 weeks old) with average weight of 250 ± 15 g (Janvier Labs, St Berthevin Cedex, France) were kept in a controlled environment and had ad libitum supply of food and water. The study was performed following the “Guide for the Care and Use of Laboratory Animals” as declared by the National Institute of Health and the protocols certified by the Ethic Committee of Laboratory Animals of the University of Granada (Granada, Spain) (17/07/2020/084). All studies concerning animals are described in agreement with the ARRIVE guidelines for reporting experiments concerning animals (Kilkenny et al., 2010; McGrath et al., 2010). Animals were in makrolon cages (5 rats/cage), maintained with a 12 h light–dark cycle and provided with ad libitum food and tap water in an air-conditioned atmosphere (temperature 20°C–22°C, relative humidity 45%–55%). The rats were acclimated and then randomly divided into four groups (n = 8). Two of them were treated with SHE (100 mg/kg) and gabapentin (70 mg/kg), respectively. Doses were chosen based on previous studies (Algieri et al., 2014). Drugs were prepared in 1 ml of carboxymethylcellulose (0.2%) and daily administered by oral gavage. An untreated DCA control group and a non-DCA control group were incorporated for reference. They were similarly administered the drug vehicle. After fasting overnight, rats were anesthetized with isoflurane and inserted a gavage needle through the anus about 6 cm to inject 1 ml of 4 mmol/L DCA in Krebs solution (in mmoles: NaCl, 122; KCl, 3.5; NaHCO3, 25; KH2PO4, 1.2; MgCl2, 1.2; pH 7.4) whilst slowly withdrawing the needle. To prevent leakage, the rats were put on a mound of bedding in a head-down position. This protocol was carried out three consecutive days. Rats in the control group underwent the same procedure but were administered 1 ml of vehicle. The drug treatments started the next day of the last DCA injection and continued for 17 days until rats were euthanized with an overdose of halothane (Figure 1). Animal body weight, water and food intake, and occurrence of diarrhea was measured every day. After euthanasia, the colon was aseptically removed, longitudinally opened and cleaned with cold saline before being minced, aliquoted and stored at −80°C till biochemical analysis.
FIGURE 1.
Experimental procedures. Rats were anesthetized and 1 ml of 4 mmol/L DCA was administered through the anus for three consecutive days. The drug treatments Serpylli herba extract (SHE) (100 mg/kg) or gabapentin (GB) (70 mg/kg) started the next day of the last DCA injection and continued for 17 days until rats were euthanized with an overdose of halothane.
Gene expression analysis
Colon total RNA was isolated with NucleoZOL® (Macherey-Nagel GmbH and Co., Dueren, Germany) and retrotranscribed to cDNA utilizing oligo (dT) primers (Promega, Southampton, UK). Amplification was performed using specific primers described in Table 1 mRNA relative quantitation was assessed by the 2−ΔΔCt method, using glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as a gene to normalize the data.
TABLE 1.
qPCR primer sequences.
| Gene | Sequence 5′–3′ | Annealing Temperature (°C) | Access number |
|---|---|---|---|
| Gapdh | FW: CCATCACCATCTTCCAGGAG | 60 | NM_017008 |
| RV: CCTGCTTCACCACCTTCTTG | |||
| Ifn-γ | FW: GCCCTCTCTGGCTGTTACTG | 60 | NM_138880.2 |
| RV: CCAAGAGGAGGCTCTTTCCT | |||
| Il-1β | FW: GATCTTTGAAGAAGAGCCCG | 59 | M98820 |
| RV: AACTATGTCCCGACCATTGC | |||
| Il-6 | FW: GATGGATGCTTCCAAACTGG | 60 | M26744 |
| RV: AGGAGAGCATTGGAAGTTGG | |||
| Muc-2 | FW: ACCACCATTACCACCACCTCAG | 60 | U07615 |
| RV: CGATCACCACCATTGCCACTG | |||
| Muc-3 | FW: CACAAAGGCAAGAGTCCAGA | 60 | NP_113984.1 |
| RV: ACTGTCCTTGGTGCTGCTGAATG | |||
| Cox-2 | FW: TGATGACTGCCCAACTCCCATG | 60 | S74342 |
| RV: AATGTTGAAGGTGTCCGGCAGC | |||
| Tlr-3 | FW: GATTGGCAAGTTATTCGTC | 60 | XP_008769488.1 |
| RV: GCGGAGGCTGTTGTAGG | |||
| Tlr-4 | FW: AGCTTTGGTCAGTTGGCTCT | 60 | NP_062051.1 |
| RV: CAGGATGACACCATTGAAGC | |||
| Htr-3 | FW: CCGGCGGCCCCTCTTCTAT | 60 | NP_077370.2 |
| RV: GCAAAGTAGCCAGGCGATTCTCT | |||
| Htr-4 | FW: CAGTTGAAGTTGCCATCAGC | 60 | NP_036985.1 |
| RV: CGGCGAATTGGAGATGAACT |
Evaluation of response to colorectal distension
The noxious visceral stimulus employed was distension with air by a flexible latex balloon inserted intra-anally (Rodríguez-Nogales et al., 2019). The response of rats to colorectal distension (CRD) was evaluated one and 2 weeks after DCA last injection. Behavioral responses to CRD were assessed in triplicate by a blinded observer after measuring the abdominal withdrawal reflex and assigning a score considering the criteria described in Table 2 (Keszthelyi et al., 2012; Zhang. et al., 2014).
TABLE 2.
Abdominal withdrawal reflex scores.
| Behavioral response | Score |
|---|---|
| Normal behavior without response | 0 |
| Brief head movement at the onset of the stimulus followed by immobility | 1 |
| Contraction of abdominal muscles | 2 |
| Lifting of the abdomen off the platform | 3 |
| Body arching and lifting of pelvic structures | 4 |
Determination of referred pain
Referred hyperalgesia was tested one and 2 weeks after the last injection of DCA. Von Frey filaments (Stoelting Co., Wood Dale, IL, United States) ranging from 8 g down to 1 g were applied perpendicularly to the lower abdomen. Each filament was tested by a blinded observer five times for 1-2 s and one escape response was considered as a positive reaction. The filament of the next lower force was applied till two filaments did not give a positive response.
Quantification of β-Hexosaminidase release in vitro
The cell degranulation response was evaluated by quantifying released β-Hexosaminidase in culture supernatants from rat basophilic leukemia-2H3 basophils (RBL-2H3) supplied by the Cell Culture Unit of the University of Granada (Granada, Spain), which are extensively used as a mast cell model. RBL-2H3 cells (45 × 107 cells/ml) were cultured for 24 h in 96-well plates at 37°C and 5% CO2. Next day, after washing, cells were treated with SHE at 25, 50, or 100 μg/ml or gabapentin at 5, 10, or 25 μM diluted in Tyrode’s buffer for 30 min. Then, Compound 48/80 at 50 μg/ml diluted in 100 μl of Tyrode’s buffer or 100 μl Tyrode’s buffer were added for 2 h. Tyrode’s buffer and Compound 48/80 were employed as negative and positive controls, correspondingly. Amount of β-Hexosaminidase in the supernatants was quantified after 90 min incubation with substrate solution (2 mM p-nitrophenyl-N-acetyl-β-D-glucosaminide in 40 mM citric acid, pH 4.5) at 37°C. The reaction was terminated by adding glycine 400 mM, pH 10.7, and optical density was determined in a microplate reader (Tecan, Männedorf, Switzerland) at 405 nm.
Data were expressed as a percentage (%) of the total β-Hexosaminidase, measured after lysing cells with 0.1% Triton X100 (Okochi et al., 1979).
Statistics
In the in vivo and in vitro studies the data are expressed as the mean ± standard error of the mean (SEM) and are representative of three independent experiments. Non-parametric data were analyzed by the Kruskal–Wallis test. The von Frey data were registered as areas under the curve. For the rest of the data, multiple comparisons between groups were performed using the one-way ANOVA, followed by the Bonferroni post hoc test.
Results and discussion
Nowadays the currently used drugs for IBS present variable efficacy and some of them are associated with long-term side effects (Lucak et al., 2017; Patel et al., 2021). Thus, the development of new therapeutic strategies could be of great social and clinical relevance. In this regard, alternative and complementary treatments, including herbal therapies, employed to alleviate inflammation and pain may provide some benefits (Grundmann and Yoon, 2014; Larussa et al., 2019; Kim et al., 2020). This study shows the beneficial activity of SHE in DCA-induced post-inflammatory IBS in rats.
Serpylli herba extract improves visceral hypersensitivity in rats
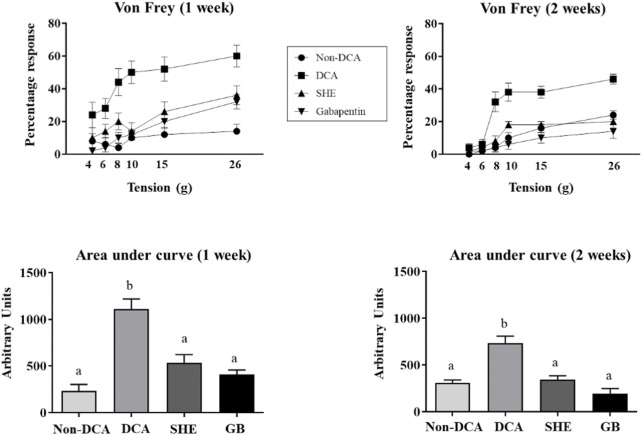
Visceral hypersensitivity is the most incapacitating symptom in human IBS. Different protocols have been used for decades to determine this visceral hypersensitivity. One of them is the CRD, with some resemblance to the visceral pain in the human condition (Guthrie et al., 2004). In the DCA model, this unconjugated secondary bile acid is described to increase colorectal sensitivity (Traub et al., 2008). Accordingly, our results showed higher visceral hypersensitivity to CRD (60 mm Hg), 7 and 14 days after installation of DCA, when compared to non-DCA rats (Figure 2). Interestingly, oral administration of SHE significantly lessened the CRD scores in comparison with the untreated DCA group. Of note, SHE diminished CRD scores to the same extent as gabapentin, one of the most frequently prescribed drugs against chronic neuropathic pain and for visceral hyperalgesia in human IBS (Trinkley and Nahata, 2014) (Figure 2). Indeed, other plant extracts or plant-derived natural compounds, like quercetin, have ameliorated visceral hypersensitivity in other experimental models of IBS as well as in vitro assays (Qin et al., 2019; Lingpeng et al., 2021). This data could support the potential future use of SHE in visceral hyperalgesia in human IBS.
FIGURE 2.
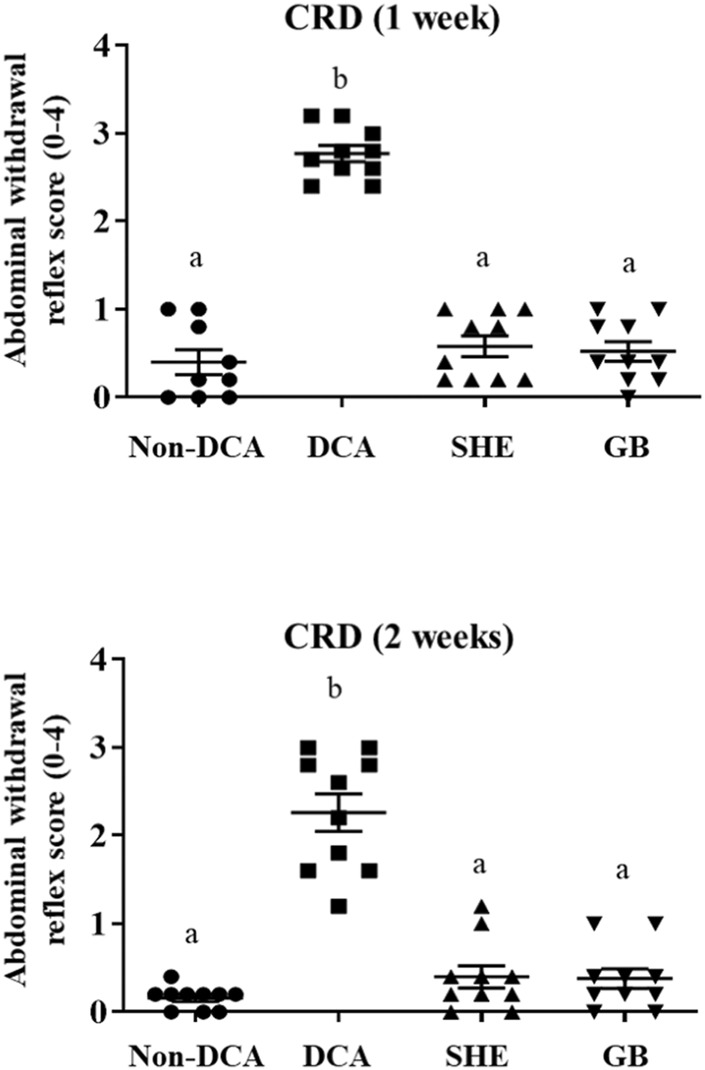
Response to colorectal distension (CRD) with a balloon catheter inflated to 60 mm Hg (20 s at 5 min intervals) was measured in triplicate one and 2 weeks after DCA intracolonic instillation in Serpylli herba extract (SHE) (100 mg/kg) or gabapentin (GB) (70 mg/kg) treated rats. Data are expressed as means (triplicate measurements) ± SEM (n = 10). Groups with different letters are statistically different (p < 0.05).
Serpylli herba extract supplementation ameliorates the hyperalgesia and allodynia in rats
It has been previously reported that intracolonic injection of DCA (4 mM) causes mild inflammation in rats, typified by visceral pain for at least 28 days (McGrath et al., 2010). Accordingly, evaluation of the referred pain generated in the lower abdomen with von Frey filaments one and 2 weeks after DCA instillation showed a lower threshold for the response in the untreated DCA control group than the non-DCA control group, indicating an elevated sensitivity (Figure 3). Moreover, the percentages of responses were significantly increased at all the pressures tested, being more intense at week one than at week two (Figure 3). The administration of SHE or gabapentin significantly reduced the nociceptive score after the mechanical stimulation with von Frey filaments (from 6 to 26 g) (Figure 3). It is important to highlight that, although other plant extracts have been described as potential adjuvant therapies for allodynia and hyperalgesia in experimentally-induced neuropathic pain (Amoateng et al., 2015; Hosea Azi Iliya et al., 2016; Shahid et al., 2017; Xie et al., 2018), this is the first study that shows the ability of SHE to improve mechanical somatic hyperalgesia and allodynia caused by DCA in rats, at the same extent as gabapentin, a widely used drug for visceral pain in human IBS (Trinkley and Nahata, 2014) that has also supported by clinical studies, in which symptoms of abdominal pain, hyperalgesis, urgency, and bloating were significantly improved (Lee et al., 2005; Houghton et al., 2007). In this sense, gabapentin treatment has shown ability to reduce rectal sensory thresholds through attenuating rectal sensitivity to distension and enhancing rectal compliance in diarrhoea-predominant IBS patients (Lee et al., 2005; Zhang, et al., 2014; Moloney et al., 2015). Studies have indicated that the pain-relieving effect of gabapentin is through binding with α2δ-1, an auxiliary subunit of voltage gated calcium channels (Houghton et al., 2007).
FIGURE 3.
Assessment of referred hyperalgesia one and 2 weeks after DCA instillation. Von Frey filaments (1–8 g) were applied to the lower abdomen (five times for 1-2 s) of Serpylli herba extract (SHE) (100 mg/kg) or gabapentin (GB) (70 mg/kg) treated rats. Referred hyperalgesia was estimated considering percentage of response to von Frey filaments and area under the curve (AUC) was calculated. Data are expressed as means ± SEM (n = 10). Groups with different letters are statistically different (p < 0.05).
Serpylli herba extract diminishes the IBS-associated gut inflammatory process
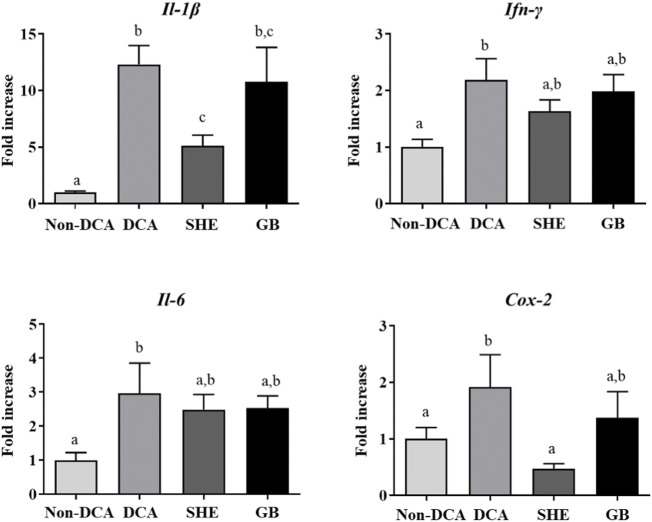
The colonic installation of DCA to rats also induced a short-lasting low-grade inflammatory response in the large intestine (Traub et al., 2008). However, an alteration of the immune response was still obvious at the end of the experiment, 3 weeks after the first administration of DCA. The expression of the different mediators, including Il-1β, Il-6, Ifn-γ and Cox-2, was higher in the IBS control group without treatment compared to non-DCA control (Figure 4), the same as it has been previously reported, both in experimental models and in humans (Algieri et al., 2014). The expression of Il-1β and Cox-2 was significantly reduced after the administration of SHE, when compared to the IBS control rats (Figure 4), similarly to effect obtained with gabapentin (Figure 4). The inflammatory implications in IBS are controversial (Martin-Viñas and Quigley, 2016) but it has been reported that the activation of the immune cells is involved in the pathology of IBS (Barbara et al., 2011). Stimulated mast cells appear to be key for the release of various biologically active compounds, such as those involved in the degranulation pathway, cytokines (IL-1β, IL-6, and IFN-γ), and metabolites derived from membrane arachidonic acid, including prostaglandins derived from COX-2 induction (Gwee et al., 2003). The ability of SHE treatment to reduce the colonic expression of Cox-2 might downregulate prostaglandin production and secretion, typically associated with these eicosanoids. This could ameliorate the hyperalgesia, given the ability of these mediators to lower the activation level of sensory afferents via activation of EP1 receptors (Sarkar et al., 2003). As commented above, it is still unknown why the expression of these cytokines is upregulated, but it could be associated with a disproportionate immune activation by the gut microorganisms, involving a mucosal barrier dysfunction, and/or stress-induced stimulation of the immune response. Moreover, SHE administration also reduced the colonic expression of Il-1β, Il-6, and Ifn-γ, effects previously seen in experimental colitis in rodents (Algieri et al., 2014). Therefore, our findings support that the immunomodulatory abilities of SHE participate in its beneficial effect.
FIGURE 4.
Impact of Serpylli herba extract (SHE) (100 mg/kg) or gabapentin (GB) (70 mg/kg) treatment on inflammatory status: gene expression of Il-1β, Ifn-γ, Il-6, and Cox-2. Data are expressed as means ± SEM (n = 10). Groups with different letters are statistically different (p < 0.05).
Serpylli herba extract treatment restores the altered gut epithelial barrier induced by DCA in rats
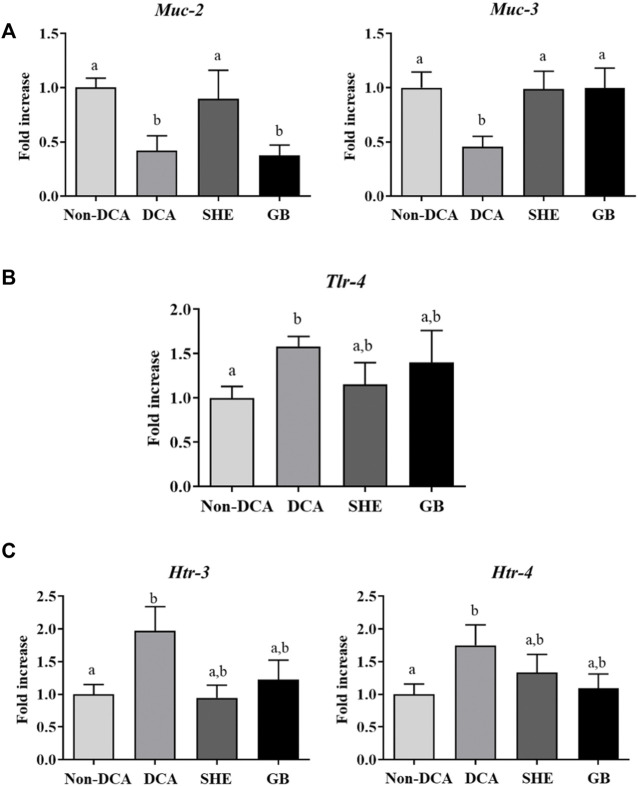
Many studies have reported the correlation between compromised epithelial barrier function and impaired immune stimulation and gut malfunction in human IBS (Piche, 2014; Shukla et al., 2018). Our study coincides with these observations since IBS rats showed a decreased expression of the mucins Muc-2 and Muc-3, the major glycoproteins in the mucus protective barrier of the gastrointestinal surface, compared to the saline group (Figure 5A). This modified expression of mucins has also been shown other experimental models of IBS in rats, including the water avoidance-stress and the neonatal maternal separation (Da Silva et al., 2014; Barouei et al., 2015). Remarkably, SHE was able to normalize the expression of both mucins, thus promoting epithelial barrier restoration. Similarly, numerous researchers have evidenced the key role of several compounds (i.e., phenolic compounds) in restoring the damaged gut barrier in vivo animal models of intestinal inflammation, however, their mechanism of action is still unknown (Bernardi et al., 2020; Sandoval-Ramírez et al., 2021). Nevertheless, different mechanisms may be involved, including: 1) inhibition of pro-inflammatory molecules; 2) increased expression of tight-junction proteins, and 3) enhancement of intracellular antioxidant activity. Consequently, summing up the current results and others obtained in various experimental models of intestinal inflammation, SHE could be considered to improve the intestinal mucosal barrier integrity through the mechanisms described above. On the contrary, gabapentin only restored Muc-3 expression which may imply that this drug might act through other mechanisms (Figure 5A).
FIGURE 5.
Effects of Serpylli herba extract (SHE) (100 mg/kg) or gabapentin (GB) (70 mg/kg) administration on (A) colonic gene expression of Muc-2 and Muc-3; (B) colonic gene expression of Tlr-4 and (C) Htr-3 and Htr-4 expression. Data are expressed as means ± SEM (n = 10). Groups with different letters are statistically different (p < 0.05).
The imbalance in the mucosal barrier and the immune response previously mentioned may arise from a dysregulation of the intestinal microbiota, which could favor aberrant immune responses, gut inflammation (Shulman et al., 2014) and expression of toll-like receptors (TLRs). These are Pattern-recognition receptors, key for the mucosal immune response in human IBS (Belmonte et al., 2012), trigger the innate immune response through the production of cytokines and chemokines (Brint et al., 2011). TLR-4 recognizes lipopolysaccharides (LPS) of Gram-negative bacteria, and it is widely known that the administration of Gram-negative bacterial LPS to human volunteers induces the secretion of pro-inflammatory mediators, including TNF and IL-6, in addition to the fact that patients with diarrhea-predominant IBS display increased LPS serum levels (Dlugosz et al., 2015). Indeed, in the present study, the DCA group manifested an increased expression of colonic Tlr-4 mRNA compared with the untreated control group, while lower expression rates were observed in the SHE-treated group (Figure 5B), which may be explained considering the effect shown in the mucosal barrier and the inflammatory status. This result reveals that the enhancement in the barrier function may be linked to a modulation of the response in the mucosa to specific microbial components of the gut lumen, preventing diverse stimuli that may be related to the visceral hyperalgesia that takes place in experimentally induced IBS.
Similarly, alterations in 5-HT homeostasis are pivotal in IBS. In fact, different 5-HT agents have been utilized therapeutically in IBS patients in whom 5-HT3 receptor antagonists have shown an improvement of the gastrointestinal symptoms, lowering stool frequency, urgency, and abdominal discomfort while augmenting stool consistency (Lacy, 2016). Thus, 5-HT receptor 4 (Htr-4) and 3 (Htr-3) expressions have been described to be determining in the pathology of visceral hypersensitivity through the release of several neurotransmitters and the progress of IBS (Yan et al., 2012; Fu et al., 2019). Consequently, when the expression of these receptors was assessed in the present study, the IBS group displayed a double-fold increase in comparison with the control group (Figure 5C), which corroborates previous mentioned observations. Notably, both SHE and gabapentin treatment managed to reestablish the expression of both 5-HT receptors (Figure 5C), which suggests that this mechanism of action could be involved in their beneficial effect in the DCA experimental model.
Serpylli herba supplementation weakens the mast cell degranulation in vitro
To better understand the effect of SHE in the IBS experimental model induced by DCA, we assessed the activity of SHE in a stimulated RBL-2H3 cell line. RBL-2H3 cell line shows similar features with basophils and mast cells (Passante and Frankish, 2009). Mast cells are crucial in the pathophysiology of functional gut conditions, mainly in IBS, being considered as a pharmacological target for its management. Moreover, mast cells show other functions, including modulation of permeability, secretion, peristalsis, nociception, immunity, and angiogenesis (Krystel-Whittemore et al., 2015). Recently, these cells have being paid much attention in IBS because of their sensor and effector function (Lee and Lee, 2016). In fact, a significant greater infiltration of these cells has been observed in the intestinal mucosa in IBS in comparison with non-IBS controls (Park et al., 2006; Guilarte et al., 2007; Sohn et al., 2014). Moreover, an increment in their activity has been described (Cremon et al., 2009). Correspondingly, the data obtained with C48/80-stimulated RBL-2H3 cells indicated that only the SHE supplementation (25, 50, and 100 μg/ml) significantly inhibited β-Hexosaminidase production induced by C48/80 (Figure 6) in comparison to non-stimulated cells, without affecting cell viability (data not shown). Other plant extracts containing flavonoids (such as luteolin and rutin) in their composition have been described to reduce the expression of β-Hexosaminidase in vitro, both in human and rodent mast cells (Park et al., 2008; Seelinger et al., 2008; Hagenlocher et al., 2017). These results reveal the highly relevant and novel mechanism of action of the SHE administration; its beneficial effect can be ascribed to its capacity to suppress the release of β-Hexosaminidase and consequently to modulate the mast cell degranulation.
FIGURE 6.
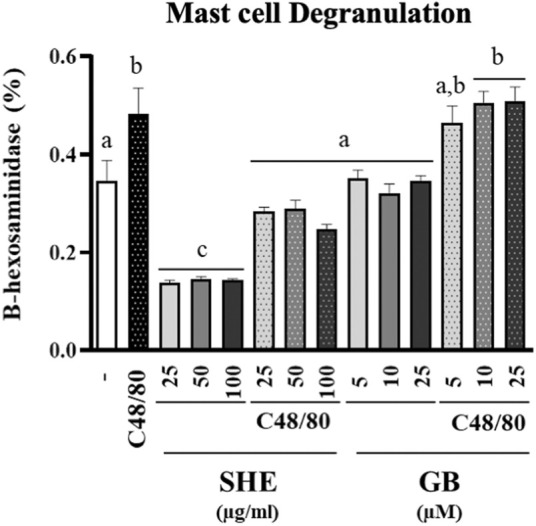
Determination of β-Hexosaminidase production in RBL-2H3 cells. Cells were incubated with Serpylli herba extract (SHE) (25, 50, and 100 μg/ml) or gabapentin (GB) (5, 10, and 25 μM) for 30 min and then stimulated with compound C48/80 or vehicle. Experiment was performed in triplicate. Data are expressed as means ± SEM. Groups with different letters are statistically different (p < 0.05).
Conclusion
This study showed the beneficial effects of SHE on an experimental IBS model. SHE lessened DCA-induced visceral hyperalgia and referred pain. Additionally, SHE improved the immune status of the rats, lowering the expression of pro-inflammatory mediators that could play a part in the visceral analgesia achieved, and repairing the gut epithelial barrier integrity. One of the mechanisms of action implicated in the beneficial effect of SHE in the DCA experimental model seems to be mediated by the serotonin pathway. Furthermore, this study suggests, for the first time, novel mechanisms of action that might be involved, including inhibition of mast cell degranulation. Consequently, it would be worthy to deeper explore the administration of SHE in the prevention and/or treatment of IBS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by 17/07/2020/084.
Author contributions
AR-M, MR, MR-S, and TV collected and analyzed the data; FA and MR-C also analyzed and interpretated the data; IP participated in the elaboration and description of the SHE and analysis of data; IP, AR-N, and JG participated in the design of the study, analysis and interpretation of data, and preparation of the article. All authors have read and approved the content and the final version of the manuscript.
Funding
This study was supported by the Junta de Andalucia (AGR-6826 and CTS 164), the Spanish Ministry of Economy and Competitivity (SAF 2011-29648) and by the Instituto de Salud Carlos III (ISCIII) (PI19/01058, PI20/01447; CIBER-EHD).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.967644/full#supplementary-material
Abbreviations
CR, colorectal distension; DCA, deoxycholic acid; Gapdh, glyceraldehyde-3-phosphate dehydrogenase; IBS, irritable bowel syndrome; LPS, lipopolysaccharide; PEG, polyethylene glycol; RBL-2H3, rat basophilic leukemia-2H3 basophils; SHE, Serpylli herba extract; Ta, annealing temperature; TLRs, toll-like receptors.
References
- Algieri F., Rodriguez-Nogales A., Garrido-Mesa N., Zorrilla P., Burkard N., Pischel I., et al. (2014). Intestinal anti-inflammatory activity of the Serpylli herba extract in experimental models of rodent colitis. J. Crohns Colitis 8 (8), 775–788. 10.1016/j.crohns.2013.12.012 [DOI] [PubMed] [Google Scholar]
- Amoateng P., Adjei S., Osei-Safo D., Ameyaw E. O., Ahedor B., N'Guessan B B., et al. (2015). A hydro-ethanolic extract of Synedrella nodiflora (L.) Gaertn ameliorates hyperalgesia and allodynia in vincristine-induced neuropathic pain in rats. J. Basic Clin. Physiol. Pharmacol. 26 (4), 383–394. 10.1515/jbcpp-2014-0084 [DOI] [PubMed] [Google Scholar]
- Banerjee A., Sarkhel S., Sarkar R., Dhali G. K. (2017). Anxiety and depression in irritable bowel syndrome. Indian J. Psychol. Med. 39 (6), 741–745. 10.4103/IJPSYM.IJPSYM_46_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara G., Cremon C., Carini G., Bellacosa L., Zecchi L., De Giorgio R., et al. (2011). The immune system in irritable bowel syndrome. J. Neurogastroenterol. Motil. 17 (4), 349–359. 10.5056/jnm.2011.17.4.349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara G. (2006). Mucosal barrier defects in irritable bowel syndrome. Who left the door open? Am. J. Gastroenterol. 101 (6), 1295–1298. 10.1111/j.1572-0241.2006.00667.x [DOI] [PubMed] [Google Scholar]
- Barouei J., Moussavi M., Hodgson D. M. (2015). Perinatal maternal probiotic intervention impacts immune responses and ileal mucin gene expression in a rat model of irritable bowel syndrome. Benef. Microbes 6 (1), 83–95. 10.3920/BM2013.0011 [DOI] [PubMed] [Google Scholar]
- Belmonte L., Beutheu Youmba S., Bertiaux-Vandaële N., Antonietti M., Lecleire S., Zalar A., et al. (2012). Role of toll like receptors in irritable bowel syndrome: Differential mucosal immune activation according to the disease subtype. PloS one 7 (8), e42777. 10.1371/journal.pone.0042777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi S., Del Bo C., Marino M., Gargari G., Cherubini A., Andrés-Lacueva C., et al. (2020). Polyphenols and intestinal permeability: Rationale and future perspectives. J. Agric. Food Chem. 68 (7), 1816–1829. 10.1021/acs.jafc.9b02283 [DOI] [PubMed] [Google Scholar]
- Bordbar G., Miri M. B., Omidi M., Shoja S., Akhavan M. (2020). Efficacy and safety of a novel herbal medicine in the treatment of irritable bowel syndrome: A randomized double-blinded clinical trial. Gastroenterol. Res. Pract. 2020, 8213082. 10.1155/2020/8213082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brint E. K., MacSharry J., Fanning A., Shanahan F., Quigley E. M. (2011). Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am. J. Gastroenterol. 106 (2), 329–336. 10.1038/ajg.2010.438 [DOI] [PubMed] [Google Scholar]
- Cremon C., Gargano L., Morselli-Labate A. M., Santini D., Cogliandro R. F., De Giorgio R., et al. (2009). Mucosal immune activation in irritable bowel syndrome: Gender-dependence and association with digestive symptoms. Am. J. Gastroenterol. 104 (2), 392–400. 10.1038/ajg.2008.94 [DOI] [PubMed] [Google Scholar]
- Da Silva S., Robbe-Masselot C., Ait-Belgnaoui A., Mancuso A., Mercade-Loubière M., Salvador-Cartier C., et al. (2014). Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: Prevention by a probiotic treatment. Am. J. Physiol. Gastrointest. Liver Physiol. 307 (4), G420–G429. 10.1152/ajpgi.00290.2013 [DOI] [PubMed] [Google Scholar]
- Dlugosz A., Nowak P., D'Amato M., Mohammadian Kermani G., Nyström J., Abdurahman S., et al. (2015). Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 27 (12), 1747–1754. 10.1111/nmo.12670 [DOI] [PubMed] [Google Scholar]
- Feistel B., Suarez-Rizzo C. G., Pischel I. (2021). Herba Thymi serpylli (Wild Thyme) – traditional herbal plant for digestive health. Z. für Phytother. 42 (01), 3. 10.1055/s-0041-1731495 [DOI] [Google Scholar]
- Ford A. C., Moayyedi P., Lacy B. E., Lembo A. J., Saito Y. A., Schiller L. R., et al. (2014). American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol. 109 (1), S2–S26. 10.1038/ajg.2014.187 [DOI] [PubMed] [Google Scholar]
- Fu R., Chen M., Chen Y., Mao G., Liu S. (2019). Expression and clinical significance of 5-HT and 5-HT(3)R in the intestinal mucosa of patient with diarrhea-type irritable bowel syndrome. Exp. Ther. Med. 17 (4), 3077–3082. 10.3892/etm.2019.7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann O., Yoon S. L. (2014). Complementary and alternative medicines in irritable bowel syndrome: An integrative view. World J. Gastroenterol. 20 (2), 346–362. 10.3748/wjg.v20.i2.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte M., Santos J., de Torres I., Alonso C., Vicario M., Ramos L., et al. (2007). Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut 56 (2), 203–209. 10.1136/gut.2006.100594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie E., Barlow J., Fernandes L., Ratcliffe J., Read N., Thompson D. G., et al. (2004). Changes in tolerance to rectal distension correlate with changes in psychological state in patients with severe irritable bowel syndrome. Psychosom. Med. 66 (4), 578–582. 10.1097/01.psy.0000128899.22514.c0 [DOI] [PubMed] [Google Scholar]
- Gwee K. A., Collins S. M., Read N. W., Rajnakova A., Deng Y., Graham J. C., et al. (2003). Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut 52 (4), 523–526. 10.1136/gut.52.4.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenlocher Y., Feilhauer K., Schäffer M., Bischoff S. C., Lorentz A. (2017). Citrus peel polymethoxyflavones nobiletin and tangeretin suppress LPS- and IgE-mediated activation of human intestinal mast cells. Eur. J. Nutr. 56 (4), 1609–1620. 10.1007/s00394-016-1207-z [DOI] [PubMed] [Google Scholar]
- Hosea Azi Iliya W. K. M. A., Benneh C., Woode E. (2016). Maerua angolensis extract reduces allodynia and hyperalgesia in a mouse model of vincristine-induced peripheral neuropathy. J. Appl. Pharm. Sci. 6 (5), 124–130. 10.7324/japs.2016.60519 [DOI] [Google Scholar]
- Houghton L. A., Fell C., Whorwell P. J., Jones I., Sudworth D. P., Gale J. D. (2007). Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut 56 (9), 1218–1225. 10.1136/gut.2006.110858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarić S., Mitrović M., Karadžić B., Kostić O., Djurjević L., Pavlović M., et al. (2014). Plant resources used in Serbian medieval medicine. Ethnobotany and Ethnomedicine. Genet. Resour. Crop Evol. 61 (7), 1359–1379. 10.1007/s10722-014-0118-1 [DOI] [Google Scholar]
- Keszthelyi D., Troost F. J., Masclee A. A. (2012). Irritable bowel syndrome: Methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 303 (2), G141–G154. 10.1152/ajpgi.00060.2012 [DOI] [PubMed] [Google Scholar]
- Kilkenny C., Browne W., Cuthill I. C., Emerson M., Altman D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 160 (7), 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Kim J. W., Ha N. Y., Kim J., Ryu H. S. (2020). Herbal therapies in functional gastrointestinal disorders: A narrative review and clinical implication. Front. Psychiatry 11, 601. 10.3389/fpsyt.2020.00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystel-Whittemore M., Dileepan K. N., Wood J. G. (2015). Mast cell: A multi-functional master cell. Front. Immunol. 6, 620. 10.3389/fimmu.2015.00620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy B. E. (2016). Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int. J. Gen. Med. 9, 7–17. 10.2147/IJGM.S93698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larussa T., Rossi M., Suraci E., Marasco R., Imeneo M., Abenavoli L., et al. (2019). Use of complementary and alternative medicine by patients with irritable bowel syndrome according to the roma IV criteria: A single-center Italian survey. Med. Kaunas. Lith. 55 (2), E46. 10.3390/medicina55020046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. J., Kim J. H., Cho S. W. (2005). Gabapentin reduces rectal mechanosensitivity and increases rectal compliance in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 22 (10), 981–988. 10.1111/j.1365-2036.2005.02685.x [DOI] [PubMed] [Google Scholar]
- Lee K. N., Lee O. Y. (2016). The role of mast cells in irritable bowel syndrome. Gastroenterol. Res. Pract. 2016, 2031480. 10.1155/2016/2031480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. J., Park K. S. (2014). Irritable bowel syndrome: Emerging paradigm in pathophysiology. World J. Gastroenterol. 20 (10), 2456–2469. 10.3748/wjg.v20.i10.2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingpeng P., Jingzhu S., Wei L., Enqi W., Yaqin L. (2021). Effect of water extracts from Cynanchum thesioides (Freyn) K. Schum. on visceral hypersensitivity and gut microbiota profile in maternally separated rats. J. Ethnopharmacol. 264, 113352. 10.1016/j.jep.2020.113352 [DOI] [PubMed] [Google Scholar]
- Lucak S., Chang L., Halpert A., Harris L. A. (2017). Current and emergent pharmacologic treatments for irritable bowel syndrome with diarrhea: Evidence-based treatment in practice. Ther. Adv. Gastroenterol. 10 (2), 253–275. 10.1177/1756283X16663396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Viñas J. J., Quigley E. M. (2016). Immune response in irritable bowel syndrome: A systematic review of systemic and mucosal inflammatory mediators. J. Dig. Dis. 17 (9), 572–581. 10.1111/1751-2980.12379 [DOI] [PubMed] [Google Scholar]
- McGrath J. C., Drummond G. B., McLachlan E. M., Kilkenny C., Wainwright C. L. (2010). Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 160 (7), 1573–1576. 10.1111/j.1476-5381.2010.00873.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mearin F., Lacy B. E., Chang L., Chey W. D., Lembo A. J., Simren M., et al. (2016).Bowel disorders, Gastroenterology S0016-5085, 00222. 10.1053/j.gastro.2016.02.031 [DOI] [PubMed] [Google Scholar]
- Moloney R. D., O'Mahony S. M., Dinan T. G., Cryan J. F. (2015). Stress-induced visceral pain: Toward animal models of irritable-bowel syndrome and associated comorbidities. Front. Psychiatry 6, 15. 10.3389/fpsyt.2015.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi T., Seike H., Higashino K., Hada T., Watanabe S., Yamamura Y., et al. (1979). Alteration of hexosaminidase isozymes in human renal carcinoma. Cancer Res. 39, 1829–1834. [PubMed] [Google Scholar]
- Park H. H., Lee S., Son H. Y., Park S. B., Kim M. S., Choi E. J., et al. (2008). Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 31 (10), 1303–1311. 10.1007/s12272-001-2110-5 [DOI] [PubMed] [Google Scholar]
- Park J. H., Rhee P. L., Kim H. S., Lee J. H., Kim Y. H., Kim J. J., et al. (2006). Mucosal mast cell counts correlate with visceral hypersensitivity in patients with diarrhea predominant irritable bowel syndrome. J. Gastroenterol. Hepatol. 21 (1), 71–78. 10.1111/j.1440-1746.2005.04143.x [DOI] [PubMed] [Google Scholar]
- Passante E., Frankish N. (2009). The RBL-2H3 cell line: Its provenance and suitability as a model for the mast cell. Inflamm. Res. 58 (11), 737–745. 10.1007/s00011-009-0074-y [DOI] [PubMed] [Google Scholar]
- Patel S., Doerfler B., Boutros K., Ng S., Manuel M., DeSimone E. (2021). Review of treatment options for irritable bowel syndrome with constipation and chronic idiopathic constipation. Int. J. Gen. Med. 14, 1457–1468. 10.2147/IJGM.S274568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piche T. (2014). Tight junctions and IBS--the link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroenterol. Motil. 26 (3), 296–302. 10.1111/nmo.12315 [DOI] [PubMed] [Google Scholar]
- Qin H. Y., Zang K. H., Zuo X., Wu X. A., Bian Z. X. (2019). Quercetin attenuates visceral hypersensitivity and 5-hydroxytryptamine availability in postinflammatory irritable bowel syndrome rats: Role of enterochromaffin cells in the colon. J. Med. Food 22 (7), 663–671. 10.1089/jmf.2018.4264 [DOI] [PubMed] [Google Scholar]
- Quave C. L., Pardo-de-Santayana M., Pieroni A. (2012). Medical ethnobotany in Europe: From field ethnography to a more culturally sensitive evidence-based CAM? Evidence-based complementary and alternative medicine. eCAM 2012, 156846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Nogales A., Algieri F., Vezza T., Garrido-Mesa J., Molina-Tijeras J. A., Rodríguez-Cabezas M. E., et al. (2019). Calcium pyruvate exerts beneficial effects in an experimental model of irritable bowel disease induced by DCA in rats. Nutrients 11 (1), E140. 10.3390/nu11010140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval-Ramírez B. A., Catalán Ú., Pedret A., Valls R. M., Motilva M. J., Rubió L., et al. (2021). Exploring the effects of phenolic compounds to reduce intestinal damage and improve the intestinal barrier integrity: A systematic review of in vivo animal studies. Clin. Nutr. 40 (4), 1719–1732. 10.1016/j.clnu.2020.09.027 [DOI] [PubMed] [Google Scholar]
- Sarkar S., Hobson A. R., Hughes A., Growcott J., Woolf C. J., Thompson D. G., et al. (2003). The prostaglandin E2 receptor-1 (EP-1) mediates acid-induced visceral pain hypersensitivity in humans. Gastroenterology 124 (1), 18–25. 10.1053/gast.2003.50022 [DOI] [PubMed] [Google Scholar]
- Seelinger G., Merfort I., Schempp C. M. (2008). Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 74 (14), 1667–1677. 10.1055/s-0028-1088314 [DOI] [PubMed] [Google Scholar]
- Shahid M., Subhan F., Ahmad N., Ullah I. (2017). A bacosides containing Bacopa monnieri extract alleviates allodynia and hyperalgesia in the chronic constriction injury model of neuropathic pain in rats. BMC Complement. Altern. Med. 17 (1), 293. 10.1186/s12906-017-1807-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla R., Ghoshal U., Ranjan P., Ghoshal U. C. (2018). Expression of toll-like receptors, pro-and anti-inflammatory cytokines in relation to gut microbiota in irritable bowel syndrome: The evidence for its micro-organic basis. J. Neurogastroenterol. Motil. 24 (4), 628–642. 10.5056/jnm18130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R. J., Jarrett M. E., Cain K. C., Broussard E. K., Heitkemper M. M. (2014). Associations among gut permeability, inflammatory markers, and symptoms in patients with irritable bowel syndrome. J. Gastroenterol. 49 (11), 1467–1476. 10.1007/s00535-013-0919-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn W., Lee O. Y., Lee S. P., Lee K. N., Jun D. W., Lee H. L., et al. (2014). Mast cell number, substance P and vasoactive intestinal peptide in irritable bowel syndrome with diarrhea. Scand. J. Gastroenterol. 49 (1), 43–51. 10.3109/00365521.2013.857712 [DOI] [PubMed] [Google Scholar]
- Traub R. J., Tang B., Ji Y., Pandya S., Yfantis H., Sun Y. (2008). A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology 135 (6), 2075–2083. 10.1053/j.gastro.2008.08.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkley K. E., Nahata M. C. (2014). Medication management of irritable bowel syndrome. Digestion 89 (4), 253–267. 10.1159/000362405 [DOI] [PubMed] [Google Scholar]
- Uranga J. A., Martínez V., Abalo R. (2020). Mast cell regulation and irritable bowel syndrome: Effects of food components with potential nutraceutical use. Mol. (Basel, Switz. 25 (18), E4314. 10.3390/molecules25184314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K. R., Melkus G. D., Henderson W. A. (2017). Irritable bowel syndrome. Am. J. Nurs. 117 (6), 48–55. 10.1097/01.NAJ.0000520253.57459.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H. T., Xia Z. Y., Pan X., Zhao B., Liu Z. G. (2018). Puerarin ameliorates allodynia and hyperalgesia in rats with peripheral nerve injury. Neural Regen. Res. 13 (7), 1263–1268. 10.4103/1673-5374.235074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Xin-Guang L., Hua-Hong W., Jun-Xia L., Xuan l. (2012). Effect of the 5-HT4 receptor and serotonin transporter on visceral hypersensitivity in rats. Braz. J. Med. Biol. Res. 45, 948–954. 10.1590/s0100-879x2012007500122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo B. B., Mazmanian S. K. (2017). The enteric network: Interactions between the immune and nervous systems of the gut. Immunity 46 (6), 910–926. 10.1016/j.immuni.2017.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Rui Y. Y., Zhou Y. Y., Ju Z., Zhang H. H., Hu C. Y., et al. (2014). Adrenergic β2-receptors mediates visceral hypersensitivity induced by heterotypic intermittent stress in rats. PloS one 9 (4), e94726. 10.1371/journal.pone.0094726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M. M., Liu S. B., Chen T., Koga K., Zhang T., Li Y. Q., et al. (2014). Effects of NB001 and gabapentin on irritable bowel syndrome-induced behavioral anxiety and spontaneous pain. Mol. Brain 7, 47. 10.1186/1756-6606-7-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W., Wang S. Y. (2001). Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 49 (11), 5165–5170. 10.1021/jf010697n [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.