Abstract
Objective
The aim of this article is to present examples of patients in whom piriformis muscle (PM) and sciatic nerve (SN) variations were detected by a diagnostic ultrasound (US)–guided examination and were successfully diagnosed and treated for piriformis syndrome (PS) by US-guided injections.
Clinical Features
This series includes 4 cases. In case 1, a 30-year-old woman with a 7 numeric rating scale (NRS) score for pain (on a 0-10 scale) was evaluated for complaints of left gluteal pain radiating to the thigh for 5 years and right gluteal pain for 2 years. Case 2 involves a 32-year-old man with a 7 NRS score presenting with left gluteal pain radiating to his leg for 1 year. The third case presents a 37-year-old man who had pain (6 NRS score), numbness, and discomfort radiating from the right hip to the leg that lasted for 7 years. Finally, in case 4, a 23-year-old male patient was examined with deep gluteal paresthesia and discomfort on the left side for 1 year.
Intervention and Outcome
Diagnostic US evaluation of the gluteal region for each patient revealed anatomical variations of the SN. In 3 of the cases, corresponding contralateral US images demonstrated similar anatomic variations. Diagnosis of PS in each patient was made by US-guided injection. After the injection of 4 cm3 of lidocaine 2% into the PM, the patients’ complaints resolved almost fully.
Conclusion
The anatomical variations of the SN and PM might be a facilitating factor for myofascial pain syndrome in PS. These cases demonstrate that SN variations could be visualized with the help of diagnostic US.
Key Indexing Terms: Ultrasonography, Anatomic Variation, Piriformis Muscle Syndrome, Sciatic Nerve, Myofascial Pain Syndromes
Introduction
Piriformis syndrome (PS) is a constellation of symptoms that include low back or buttock pain referred to the leg and caused by the compression of the sciatic nerve (SN) and other structures at the piriformis muscle (PM).1 The prevalence of PS among patients with chronic low back pain is shown to be 17.2%.2 In 1928, W. Yeoman first described it as “sacroiliac periarthritis,” and the term “piriformis syndrome” was first introduced in 1947 by Daniel R. Robinson.1 Clinically, this syndrome presents as pain and localized tenderness around the gluteal region at the area of the PM and is usually described as a deep, aching type of pain with or without signs and symptoms of sciatica.3 Many possible factors have been reported in the etiology of PS, including gluteal traumas, PM hypertrophy and spasticity, myofascial bands, and predisposing anatomic variations.1 PS usually manifests as a myofascial pain syndrome, in which the pain originates either from the muscle itself or from a nerve entrapment syndrome or both.4
The diagnosis of PS may be difficult based on clinical grounds alone and is often difficult to treat.5 On physical examination, tenderness on deep palpation of the PM is present in most patients.6 Although their sensitivity and specificity are controversial, some physical examination maneuvers have been described.4 The dramatic and almost immediate relief of pain after infiltration of the PM with a local anesthetic (LA) has been considered a reference test for diagnosis.5
Once a diagnosis of PS is made, the mainstay approach is nonsurgical multidisciplinary care,7 including physical therapy, pharmacological agents, manual overpressure, massage, acupuncture, chiropractic care, and dry needling.7, 8, 9, 10 As in other patients with trigger point problems, one of the therapy options is the injection of LA into the focal point of hyperirritability deep in the belly of the muscle.11 For trigger point injection, LA with or without corticosteroid may be used.5 Botulinum toxin injections have also been shown to be beneficial in PS treatment in recent years.12 When conservative treatments are ineffective, and symptoms become persistent and disabling, surgical intervention should be considered.4 Several studies have shown that after failure of conservative care, surgical intervention alleviated the symptoms of patients with PS, especially those who had anatomical variations.13
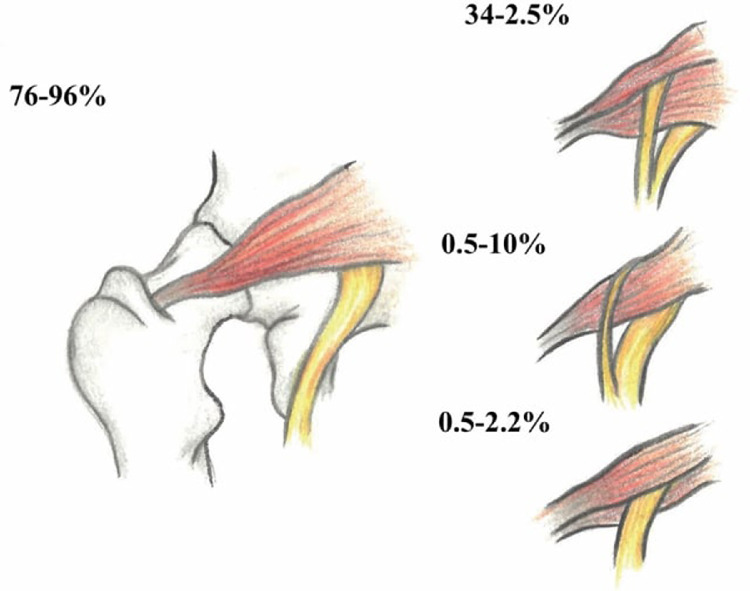
It has been suggested that variants of the SN may pose a risk for the development of the deep gluteal syndrome, but the prevalence of the anomaly in patients with PS is not significantly different from that in what is thought to be an average population.14 In 80% to 90% of individuals, the SN exits from the greater sciatic foramen deep along the inferior surface of the PM.14 In greater than 10% of the population, the SN courses through or above the piriformis (Fig 1).15 Although this anomaly may not be as crucial in PS's pathogenesis as was previously thought, awareness of SN variants is essential to avoid iatrogenic nerve injury during injections, dry needling, or surgeries.9,14,15 The purpose of this article is to present examples of patients in whom PM and SN variations were detected by diagnostic ultrasound (US)–guided examination and who were successfully diagnosed and treated for PS by diagnostic US-guided injections.
Fig 1.
The most common anatomical variations of the sciatic nerve in relation to the piriformis muscle. Drawing reproduced with permission from Gökçe Bayat. (Adapted from Beaton LE, Anson BJ. The sciatic nerve and the piriformis muscle: their interrelation as possible cause of coccygodynia. J Bone J Surg. 1938;20:686-688.)
Case 1
A 30-year-old woman was evaluated for left gluteal pain radiating to the thigh for 5 years. Her pain numeric rating scale (NRS) was 7 (on a 0-10 scale).16 She had no history of back pain, and her pain was particularly worse after exercise and prolonged sitting. On physical examination, the gluteal region was tender on deep palpation. There were no limitation and pain in the low back, hip, and sacroiliac region. Straight leg raise (SLR) tests were negative bilaterally, as well as Frieberg's sign, Pace's sign, and Beatty maneuver.11,17,18 In contrast, flexion, adduction, and internal rotation and heel contralateral knee (HCLK) maneuvers were positive on the left side.19,20 Magnetic resonance (MR) imaging (MRI) of the lumbar region demonstrated minimal bulging of the L2-L3 disc. History and physical exam findings were supportive of a diagnosis of PS, whereas lumbar MRI excluded root compression. To confirm the diagnosis, a US-guided diagnostic injection was planned.
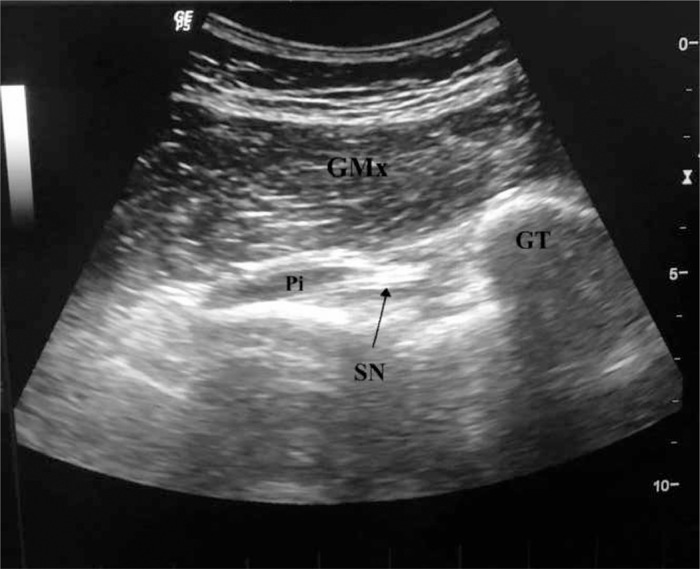
The patient was placed in a prone position, and then the buttock was prepared by appropriately cleaning the area. A sterile US transducer cover and sterile US gel were used. The buttock region was scanned with a 6- to 15-MHz curvilinear array transducer, with one of its edges placed on the greater trochanter and the other directed through the lateral border of the sacrum. In this position, the transducer was parallel to the fibers of the PM. The PM was visualized under the gluteus maximus muscle as a pear-shaped muscle pierced by the SN (Fig 2). Shortly after being injected with 4 cm3 of lidocaine 2% into her PM, the patient's pain decreased substantially, and given these findings, the patient was diagnosed with PS. There were no complications such as drop foot or paresthesia. After a year, the patient returned with the same complaint, but this time, her pain occurred on the contralateral side. She was reevaluated using US, which visualized the SN between PM fibers. She had bilateral anatomic variations resulting in PS. Again, LA was administered, and no complications were noted. At the 2-year follow-up, she was asymptomatic.
Fig 2.
Oblique 12-5 MHz ultrasound image obtained over the posterior aspect of the hip demonstrates the sciatic nerve running through the piriformis muscle. From top to bottom, subcutaneous fat tissue, the gluteus maximus muscle, and the pear-shaped piriformis muscle between the greater trochanter and sacroiliac bone are seen, respectively. GMx, gluteus maximus muscle; GT, great trochanter; Pi, piriformis muscle; SN, sciatic nerve.
Case 2
A 32-year-old male patient was referred from a neurosurgeon to our clinic with left gluteal pain (7 NRS score) radiating down to his leg, with a duration of 1 year. He had no history of any micro or macro traumas. His recent medication history included a short-term usage of paracetamol, NSAID, and myorelaxant. Upon physical examination, the left gluteal region was tender on deep palpation. The range of motion of the hip, low back, and sacroiliac joints were normal. SLR test was negative. While Frieberg, Pace, and Beatty tests were negative, HCLK and flexion, adduction, and internal rotation maneuvers were positive on the affected side. Lumbar MRI results were unremarkable. The nerve conduction study and electromyography findings were compatible with PS. Then, US-guided examination and diagnostic injection procedures were planned. US examination revealed a PM pierced by a left SN with a hyperintense signal. The patient was informed about the probable PS diagnosis, and an injection of 4 cm3 of lidocaine 2% into the PM was performed. Following the injection, his pain decreased dramatically (2 NRS score), and no complications occurred. Physical examination, US imaging findings, and diagnostic injection response were all consistent with a typical PS diagnosis. The patient reported complete alleviation of symptoms at the 3- and 6-month follow-ups.
Case 3
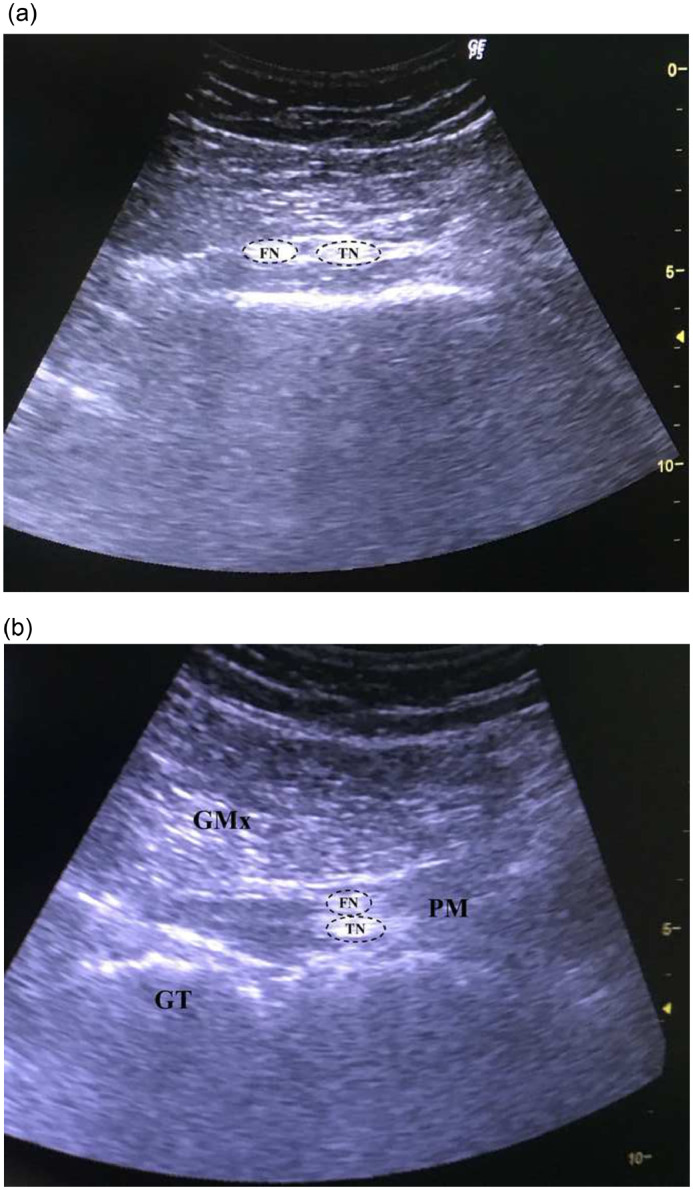
A 37-year-old male patient presented with complaints of pain (6 NRS score), numbness, and discomfort radiating from the right hip to the leg, which lasted for 7 years. The patient had no history of chronic illness or surgery. He had consulted several doctors, including an orthopedist and a neurosurgeon, before being admitted to our clinic. In these consultations, there was absence of any pathological finding that could explain his symptoms. He had attended several physical therapy sessions for the lumbar and hip regions, but these interventions did not address his problems. The patient worked as a baker and had to stand for long periods of time, and he stated that his pain increased by the end of the day. On physical examination, the gluteal region was sensitive to deep palpation. Piriformis stretching tests and SLR test were not evaluated objectively due to peripheral sensitization; the patient experienced pain during the tests but was unsure if the tests increased the pain. There was no limitation in the range of motion of the lumbar region, sacroiliac joint, and hip joint. Moreover, he had no neurological deficits. MRI of the lumbar region demonstrated minimal disc bulging. With a preliminary diagnosis of PS, a US-guided diagnostic injection was planned. During the ultrasonographic evaluation, the branches of the SN were observed as hyperintense structures between the fibers of the PM on transverse (Fig 3A) and longitudinal (Fig 3B) images. The patient was informed about possible complications. After receiving consent, an injection of 4 cm3 of lidocaine 2% into the PM was performed, and his pain decreased dramatically (2 NRS score). No complication occurred after the procedure. He had no complaints except for minimal dysesthesia at the 3- and 6-month follow-ups.
Fig 3.
(A) Transverse and (B) longitudinal 12-5 MHz ultrasound images obtained over the posterior aspect of the hip in a 37-year-old male patient with piriformis syndrome demonstrate the early branching of the sciatic nerve in the gluteal region. GMx, gluteus maximus muscle; GT, great trochanter; FN, fibular nerve; TN, tibial nerve.
Case 4
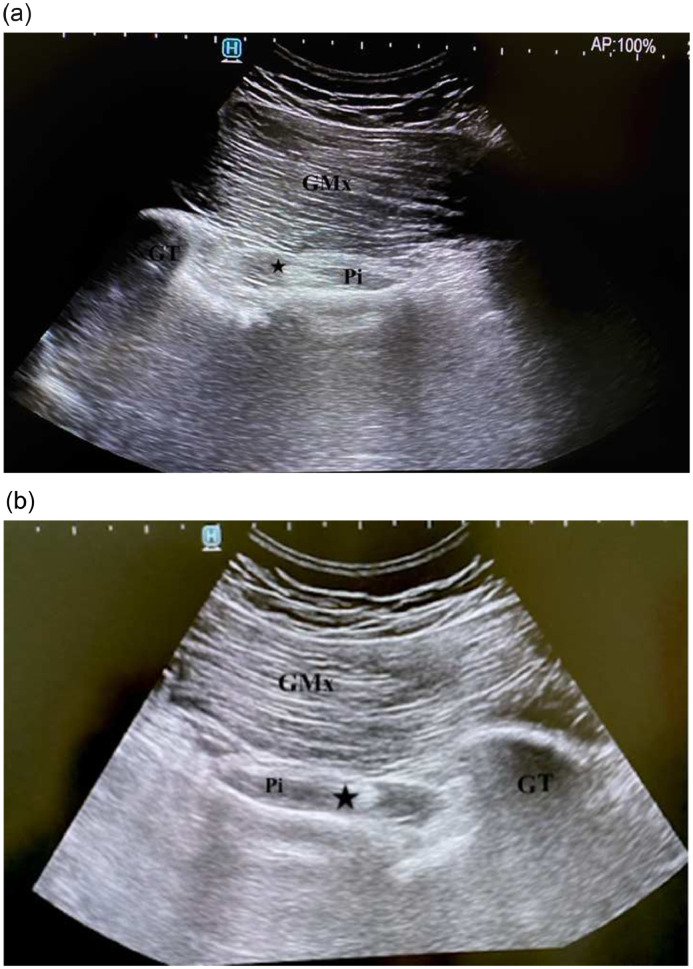
A 23-year-old male patient with left-sided deep gluteal paresthesia and discomfort for 1 year was evaluated. He had no record of chronic illness and surgery. Physical examination demonstrated no limitation in the range of motion of the hip, sacroiliac joint, and lumbar joint. SLR test, hip and sacroiliac mobility, and stretch tests were negative. Furthermore, he had no neurological deficits. The gluteal region was sensitive to deep palpation. Beatty, Pace, and HCLK tests were positive on the left side. PS was ruled in as the probable cause, and US-guided LA injection was planned to confirm the diagnosis. During the US-guided evaluation, nerve fibers with a honeycomb appearance were observed between PM fibers (Fig 4A). The injection was administered with informed consent. The patient's complaints regressed following the injection. During the US-guided evaluation, the nerve between the PM was also observed on the asymptomatic right hip (Fig 4B). He had bilateral anatomic variants of the SN with regard to the PM. During the 3-month follow-up, the patient was free of complaints.
Fig 4.
Oblique 12-5 MHz ultrasound images demonstrate (A) the left sciatic nerve (star) running through the piriformis muscle in a 23-year-old male patient with piriformis syndrome. (B) Corresponding contralateral ultrasound image demonstrates the similar anatomic variation of the sciatic nerve (star). GMx, gluteus maximus muscle; GT, great trochanter; Pi, piriformis muscle.
All 4 patients provided consent for their health information to be published in this article.
Discussion
Diagnostic US is increasingly becoming a part of the armory of the musculoskeletal practitioner. It helps in diagnosis, treatment, and assessing response to interventions. The importance of US in PS is that it provides optimal imaging of the deep gluteal space, including neurovascular structures. Regarding that more than 10% of individuals have anatomical variation of the SN, US imaging during piriformis muscle interventions is essential for preventing complications.6,15
Our patients did not have any history of trauma and surgery. Moreover, lumbar MRI findings showed no root compression that could explain sciatica. In most cases, PS is believed to be myofascial in origin.4 As in other patients with trigger point problems, PS is treated by the injection of LA into the focal point of hyperirritability deep in the belly of the muscle.5,11,21 In all patients, it was found that the complaints and disability were resolved following LA injection, and anatomical variations of the SN were observed during their ultrasonographic evaluation. Anatomical variations as an etiological factor for PS is a controversial issue in the literature.14,22,23 Most recent data shows that the prevalence of SN anomaly in patients with PS is not significantly different from that in the normal population.23 As a result, anatomical variation of the SN may not be as important in the pathogenesis of PS as previously thought.14 However, an awareness of SN variants is essential to avoid iatrogenic nerve injury during medical or surgical interventions.15,24
US is a frequently used imaging method for musculoskeletal system evaluation due to its low cost, easy accessibility, dynamic imaging, ability to show vascular structures without using contrast, and utility in guiding injections.25,26 During US-guided evaluation, the PM can be seen as a deep, triangular, hypoechoic structure beneath the gluteus maximus muscle. The SN can be detected as fascicles surrounded by hyperechoic connective tissue.27 In our cases, hyperechoic SN fascicles were visualized between the fascicles of the PM. Broadhurst et al28 reported that scar, fibrosis, enlargement of the PM, thickening of the sacrotuberous-sacrospinous ligament complex, and anatomical variations of the SN could be demonstrated with US-guided examination. With an appropriate imaging protocol, anatomic variations can also be visualized with MRI.13 Zhang et al29 stated that US and MRI show similar muscle changes in PS. MR neurography is another imaging method that is helpful in the diagnosis of PS by directly visualizing the nerve anatomy. However, it is not available in many institutions. Here, aberrant SNs were identified by US with careful assessment by an expert physiatrist with particular interest in musculoskeletal US.
Owing to the depth and small size of the PM and its close proximity to important neurovascular structures, image guidance has been recommended to improve the safety and accuracy of PM injections. Piriformis injections have been described using fluoroscopy, US, computed tomography scan, electromyography, and MRI guidance. Among these techniques, US-guided PM injection is considered to be superior.30 The accuracy of needle placement with US was recently validated in a cadaver study, suggesting an accuracy of 95%.31 The most crucial advantage of ultrasonic evaluation in our cases is that it provides the opportunity for dynamic imaging and increases the reliability and safety of the injections. Furthermore, US guidance may also allow the detection of twitch response.9,10 While visualizing the gluteal region, the LA injection was performed into PM fibers away from the aberrant SNs. No complication, such as paresthesia, tingling, numbness, muscle weakness, or foot drop, occurred following the procedures.
Limitations
We selected 4 example patients who responded positively to the diagnostic and treatment procedures; therefore, the findings cannot necessarily be generalized to other patients. This case series included a small, homogeneous group of patients with PS who had similar clinical presentations and treatment methods; thus, variants may have resulted in different outcomes. Additional studies including more patients are needed to support the findings.
Conclusion
This report describes that with careful examination and imaging with diagnostic US, SN variations could be visualized in patients with PS. The use of musculoskeletal diagnostic US provides an opportunity for dynamic imaging during injection, which could increase the accuracy of the procedure and reduce its complications; however, studies need to be done to test this hypothesis.
Funding Sources and Conflicts of Interest
No funding sources or conflicts of interest were reported for this study.
Contributorship Information
Concept development (provided idea for the research): G.G.G., K.N.K.O., B.Y.
Design (planned the methods to generate the results): G.G.G., K.N.K.O., İ.A.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): G.G.G., I.A., B.Y.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): B.Y., K.N.K.O.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): G.G.G., İ.A.
Literature search (performed the literature search): G.G.G., K.N.K.O.
Writing (responsible for writing a substantive part of the manuscript): G.G.G., K.N.K.O., İ.A.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): İ.A., B.Y.
Practical Applications.
-
•
We provided examples showing that with careful examination and imaging with diagnostic ultrasound, sciatic nerve variations could be visualized in patients with piriformis syndrome.
-
•
The use of musculoskeletal diagnostic ultrasound may allow for dynamic imaging during injection.
-
•
This could increase the accuracy of the procedure and reduce its complications; however, studies need to be done to test this hypothesis.
Alt-text: Unlabelled box
References
- 1.Kirschner JS, Foye PM, Cole JL. Piriformis syndrome, diagnosis and treatment. Muscle and Nerve. 2009;40(1):10–18. doi: 10.1002/mus.21318. [DOI] [PubMed] [Google Scholar]
- 2.Kean Chen C, Nizar AJ. Prevalence of piriformis syndrome in chronic low back pain patients. a clinical diagnosis with modified FAIR test. Pain Pract. 2013;13(4):276–281. doi: 10.1111/j.1533-2500.2012.00585.x. [DOI] [PubMed] [Google Scholar]
- 3.Hallin RP. Sciatic pain and the piriformis muscle. Postgrad Med. 1983;74(2):69–72. doi: 10.1080/00325481.1983.11698378. [DOI] [PubMed] [Google Scholar]
- 4.Jankovic D, Peng P, van Zundert A. Brief review: piriformis syndrome: etiology, diagnosis, and management. Can J Anesth. 2013;60(10):1003–1012. doi: 10.1007/s12630-013-0009-5. [DOI] [PubMed] [Google Scholar]
- 5.Misirlioglu TO, Akgun K, Palamar D, Erden MG, Erbilir T. Piriformis syndrome: comparison of the effectiveness of local anesthetic and corticosteroid injections: a double-blinded, randomized controlled study. Pain Physician. 2015;18(2):163–171. [PubMed] [Google Scholar]
- 6.Durrani Z, Winnie AP. Piriformis muscle syndrome: an underdiagnosed cause of sciatica. J Pain Symptom Manage. 1991;6(6):374–379. doi: 10.1016/0885-3924(91)90029-4. [DOI] [PubMed] [Google Scholar]
- 7.Probst D, Stout A, Hunt D. Piriformis syndrome: a narrative review of the anatomy, diagnosis, and treatment. PM R. 2019;11(suppl 1):S54–S63. doi: 10.1002/pmrj.12189. [DOI] [PubMed] [Google Scholar]
- 8.Chapman C, Bakkum BW. Chiropractic management of a US Army veteran with low back pain and piriformis syndrome complicated by an anatomical anomaly of the piriformis muscle: a case study. J Chiropr Med. 2012;11(1):24–29. doi: 10.1016/j.jcm.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fusco P, Di Carlo S, Scimia P, Degan G, Petrucci E, Marinangeli F. Ultrasound-guided dry needling treatment of myofascial trigger points for piriformis syndrome management: a case series. J Chiropr Med. 2018;17(3):198–200. doi: 10.1016/j.jcm.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagcier F, tufanoglu FH. A new treatment modality in piriformis syndrome: ultrasound guided dry needling treatment. Agri. 2020;32(3):175–176. doi: 10.14744/agri.2019.92170. [DOI] [PubMed] [Google Scholar]
- 11.Pace JB, Nagle D. Piriform syndrome. West J Med. 1976;124(6):435–439. [PMC free article] [PubMed] [Google Scholar]
- 12.Santamato A, Micello MF, Valeno G, et al. Ultrasound-guided injection of botulinum toxin type a for piriformis muscle syndrome: a case report and review of the literature. Toxins (Basel) 2015;7(8):3045–3056. doi: 10.3390/toxins7083045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ro TH, Edmonds L. Diagnosis and management of piriformis syndrome: a rare anatomic variant analyzed by magnetic resonance imaging. J Clin Imaging Sci. 2018;8:6. doi: 10.4103/jcis.JCIS_58_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smoll NR. Variations of the piriformis and sciatic nerve with clinical consequence: a review. Clin Anat. 2010;23(1):8–17. doi: 10.1002/ca.20893. [DOI] [PubMed] [Google Scholar]
- 15.Poutoglidou F, Piagkou M, Totlis T, Tzika M, Natsis K. Sciatic nerve variants and the piriformis muscle: a systematic review and meta-analysis. Cureus. 2020;12(11):e11531. doi: 10.7759/cureus.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haefeli M, Elfering A. Pain assessment. Eur Spine J. 2006;15(suppl 1):S17–S24. doi: 10.1007/s00586-005-1044-x. suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niu CC, Lai PL, Fu TS, Chen LH, Chen WJ. Ruling out Piriformis syndrome before diagnosing lumbar radiculopathy. Chang Gung Med J. 2009;32(2):182–187. [PubMed] [Google Scholar]
- 18.Beatty RA. The piriformis muscle syndrome: a simple diagnostic maneuver. Neurosurgery. 1994;34(3):512–514. doi: 10.1227/00006123-199403000-00018. discussion 514. [DOI] [PubMed] [Google Scholar]
- 19.Michel F, Decavel P, Toussirot E, et al. Piriformis muscle syndrome: diagnostic criteria and treatment of a monocentric series of 250 patients. Ann Phys Rehabil Med. 2013;56(5):371–383. doi: 10.1016/j.rehab.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Solheim LF, Siewers P, Paus B. The piriformis muscle syndrome: sciatic nerve entrapment treated with section of the piriformis muscle. Acta Orthop. 1981;52(1):73–75. doi: 10.3109/17453678108991762. [DOI] [PubMed] [Google Scholar]
- 21.Travell JG, Simons DG. Philadelphia, PA: Williams & Wilkins; 1992. Myofascial Pain and Dysfunction: The Trigger Point Manual – The Lower Extremities. [Google Scholar]
- 22.Barbosa ABM, Dos Santos PV, Targino VA, et al. Sciatic nerve and its variations: is it possible to associate them with piriformis syndrome? Arq Neuropsiquiatr. 2019;77(9):646–653. doi: 10.1590/0004-282X20190093. [DOI] [PubMed] [Google Scholar]
- 23.Bartret AL, Beaulieu CF, Lutz AM. Is it painful to be different? Sciatic nerve anatomical variants on MRI and their relationship to piriformis syndrome. Eur Radiol. 2018;28(11):4681–4686. doi: 10.1007/s00330-018-5447-6. [DOI] [PubMed] [Google Scholar]
- 24.Tomaszewski KA, Graves MJ, Henry BM, et al. Surgical anatomy of the sciatic nerve: a meta-analysis. J Orthop Res. 2016;34(10):1820–1827. doi: 10.1002/jor.23186. [DOI] [PubMed] [Google Scholar]
- 25.Peng PWH. Ultrasound-guided interventional procedures in pain medicine: a review of anatomy, sonoanatomy, and procedures. Part IV: hip. Reg Anesth Pain Med. 2013;38(4):264–273. doi: 10.1097/AAP.0b013e318291c8ed. [DOI] [PubMed] [Google Scholar]
- 26.Chang KV, Wu WT, Lew HL, Özçakar L. Ultrasound imaging and guided injection for the lateral and posterior hip. Am J Phys Med Rehabil. 2018;97(4):285–291. doi: 10.1097/PHM.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 27.Bendtsen TF, Lnnqvist PA, Jepsen KV, Petersen M, Knudsen L, Børglum J. Preliminary results of a new ultrasound-guided approach to block the sacral plexus: the parasacral parallel shift. Br J Anaesth. 2011;107(2):278–280. doi: 10.1093/bja/aer216. [DOI] [PubMed] [Google Scholar]
- 28.Broadhurst NA, Simmons DN, Bond MJ. Piriformis syndrome: correlation of muscle morphology with symptoms and signs. Arch Phys Med Rehabil. 2004;85(12):2036–2039. doi: 10.1016/j.apmr.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Luo F, Sun H, Ding H. Ultrasound appears to be a reliable technique for the diagnosis of piriformis syndrome. Muscle and Nerve. 2019;59(4):411–416. doi: 10.1002/mus.26418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith J, Hurdle MF, Locketz AJ, Wisniewski SJ. Ultrasound-guided piriformis injection: technique description and verification. Arch Phys Med Rehabil. 2006;87(12):1664–1667. doi: 10.1016/j.apmr.2006.08.337. [DOI] [PubMed] [Google Scholar]
- 31.Finnoff JT, Hurdle MFB, Smith J. Accuracy of ultrasound-guided versus fluoroscopically guided contrast-controlled piriformis injections: a cadaveric study. J Ultrasound Med. 2008;27(8):1157–1163. doi: 10.7863/jum.2008.27.8.1157. [DOI] [PubMed] [Google Scholar]