Abstract
Introduction:
Little is known about how repeated exposure to direct-to-consumer prescription drug promotion can impact consumers’ retention and perceptions of drug information. The study described here tested effects of varied ad exposure frequency on these outcomes.
Methods:
In an in-person experiment, participants with seasonal allergies (n = 616) were randomized to view a mock prescription drug television ad either once, twice, or four times within one hour of television programming, embedded with six commercial breaks. Respondents then answered a 20-minute survey administered via computer.
Results:
Those who viewed the ad more frequently were better able to recall both risk (X2 = 20.93, p < 0.001) and benefit information (X2 = 9.34, p = 0.009) and to recognize risk (F(2,597) = 11.89, p = 0.001) and benefit information (F(2,597) = 3.17, p = 0.043) than those who viewed the ad one time. Ad exposure frequency was not associated with perceptions about the magnitude or likelihood of risks or benefits. In general, risk information seemed to require more repetitions than benefit information to be accurately remembered. Recall was mediated by elaborate processing.
Discussion:
Effects on memory were small; retention of both risks and benefits remained low overall even after four exposures.
Keywords: direct-to-consumer prescription drug advertising, prescription drugs, risk communication, major statement, Food and Drug Administration
Introduction
Research on direct-to-consumer (DTC) prescription drug promotion often reports effects of a single advertisement (ad) exposure (e.g., see Rollins & Bhutada, 2014). This may miss capturing the reality of ad exposure, in which consumers may see the same prescription drug ad many times as they form opinions about the product. In fact, advertising literature suggests repetition is one of the keys to successful advertising (Chen, Yang, & Smith, 2016; Mack, 2005; Southwell, 2005); the number of times an ad is available in one’s environment positively predicts likelihood of subsequent recognition of the ad (Southwell, 2005).
Although a significant amount of research has been conducted on the effects of DTC prescription drug television promotion (e.g., Aikin et al., 2017; Callaghan, Laraway, Snycerski, & McGee, 2013; Norris, Bailey, Bolls, & Wise, 2012; O’Donoghue, Sullivan, Aikin, Chowdhury, Moultrie, & Rupert, 2014; Wogalter, Shaver, & Kalsher, 2014), we currently lack empirical evidence as to how the frequency of prescription drug ad exposures affects cognitive processes such as perception, memory, and judgment. We know relatively little about how repetition affects processing of information about prescription drug risks and benefits in DTC ads specifically, for example. How does consumer retention of benefit and risk information vary across different levels of exposure? How many viewings are needed to fully grasp the risk information presented in a DTC television ad?
Another important question involves comparative recall for benefit information versus risk information, especially when ads include information about those two dimensions through different modalities. As Ventola (2011) writes, ads often pair verbal messages with discordant or at least unrelated visual imagery when risk information is presented. The visual elements of an ad may depict a person enjoying a walk outside while a narrator verbally lists serious side effects. When visual and verbal messages are discordant, audience members often engage visual messages, which can result in insufficient processing of the verbally-presented risk information (Frosch, Grande, Tarn, & Kravitz, 2010; Ventola, 2011). If benefit information is presented in a more appealing format than risk information, it may dominate consumer attention and memory upon first ad exposure, requiring several repetitions to achieve risk information retention comparable to that for benefit information.
Although we do not yet know empirically how repeated exposure to prescription drug ads might affect risk or benefit engagement by audiences, advertising literature provides some insights on effects of ad exposure frequency in general. Vuokko (1997) describes audience advertising response in three stages that are helpful for our discussion: exposure, attention, and processing. Repetition of exposure increases the probability that recipients focus on the stimulus at least once and pay attention to the target message when they do, particularly in an environment crowded with distractors and other ads. In the processing stage, repetition offers the opportunity for reinforced learning, or a chance to develop deep memory traces that can affect a consumer’s decision making. Research to date suggests repeated exposure leads to brand recognition, belief and attitude change, and reinforcement. In general, studies have found that mental processing (e.g., judgments or attitudes) improves with increased ad exposures up to a point and then declines as repetition continues (Cacioppo & Petty, 1979, 1985; Eaton, 1996; Hitchon & Thorson, 1995; Hughes, 1992; Pechmann & Stewart, 1988; Southwell, 2005; Southwell, Barmada, Hornik, & Maklan, 2002). The pattern of this relationship, initially posited by Berlyne (1970), has been described as a nonmonotonic, curvilinear relationship; or more simply, as an inverted-U curve (Anand & Sternthal, 1990; Batra & Ray, 1986; Berlyne, 1970; Cacioppo & Petty, 1979; Pechmann & Stewart, 1988; Stang, 1975; Vakratsas & Ambler, 1999). As ad repetition increases, attention and learning also increase up to a certain level, at which time increased repetition can lead to negative effects such as irritation, counterargument, satiation or fatigue; or even provoke avoidance of the ad message (Anand & Sternthal, 1990; Cacioppo & Petty, 1979; Calder & Sternthal, 1980; Cox & Cox, 1988; Vuokko, 1997).
Crucially, we do not yet know exactly where the inflection point in this U-curve typically occurs. Published literature has provided mixed evidence and it is possible that the shape of the relationship varies for different outcomes. For example, the relationship could be relatively linear for memory, while effects on other outcomes such as product and brand attitudes may tend to be more U-shaped. We have evidence that television advertising effects on memory are monotonically increasing and positive, e.g., Southwell (2005). In other words, information may remain fairly ingrained in memory at high levels of exposure, even though positive attitudes and interest begin to decrease at these high levels; theoretically, we are unlikely to see reduced memory, per se, with greater repetition. It is unclear whether this finding would extend to specific components of an ad, however, such as retention of benefit versus risk information in a DTC prescription drug ad.
Studies also have not universally supported a curvilinear relationship between ad repetition and message effectiveness. Some have shown mixed results (e.g., Calder & Sternthal, 1980) and others, no support (e.g., Belch, 1982; Cannon & Riordan, 1994; Ginter, 1974; Mitchell & Olson, 1977; Rethans, Swasy, & Marks, 1986). Pechmann and Stewart (1988) explain this ambiguity by pointing to differences in methods used, phenomena studied, and characteristics of the ads and media environment. Further, consumers may not pay attention to every ad exposure nor all parts of an ad, and wearout may simply be the result of ignoring a repetitive ad rather than generating counterarguments or negative thoughts about it, per se.
One possible mechanism for this range of effects lies in Cacioppo and Petty’s (1985) Elaboration Likelihood Model (ELM). According to this model, conditions that foster people’s motivation and ability to engage a message predict high elaboration (or processing) likelihood. In “central” processing, there is considerable allocation of cognitive resources to process the persuasive message and information. Alternatively, “peripheral” processing is more likely when people’s motivation and ability to engage is limited. Although a variety of factors influence which pathway is activated, in the two-stage attitude modification process, repeated exposure also can affect processing, as it provides recipients with the opportunity to consider and elaborate on the content; moderate repetitions facilitate relatively objective considerations compared with infrequent exposure—meaning they allow for more elaborate processing of information; whereas excessive exposure may result in tedium and reactance, and consequently bias the cognitive response, resulting in less processing (Cacioppo & Petty, 1985).
Based on this literature, one might posit two alternative hypotheses regarding mediation of effects: 1. That increased ad exposure increases elaborate processing, leading to better recall and recognition. 2. Alternatively, that higher levels of tedium or annoyance lead to more counterarguing at higher levels of ad exposure frequency, which may, in turn, enhance recall and recognition.
There are some important literature gaps worth noting. Many of the studies in this area are from the 1970s and 1980s, and the media environment has changed dramatically since, particularly with regard to online viewing, with more multi-tasking and distracting ads occurring in the periphery. In addition, we did not identify any research that has examined the effects of repeated exposures to DTC prescription drug ads; the majority of studies have focused on advertising for common consumer products such as toothpaste, clothing, and other household goods. DTC ads are unique in that, unlike most commercial ads, they are required by the FDA to give a “fair balance” of information about both the drugs’ risks and benefits. It is unclear whether repeated ad exposures would result in elaboration of both types of information or how the effects on each might differ. There is plenty of literature to suggest that people have difficulty grasping risk information (Tierney, 1999) yet risk perceptions are important motivators of health decisions (Fischoff, 2009; Reyna, 2012) so understanding factors influencing how people process risk information in drug ads is particularly important.
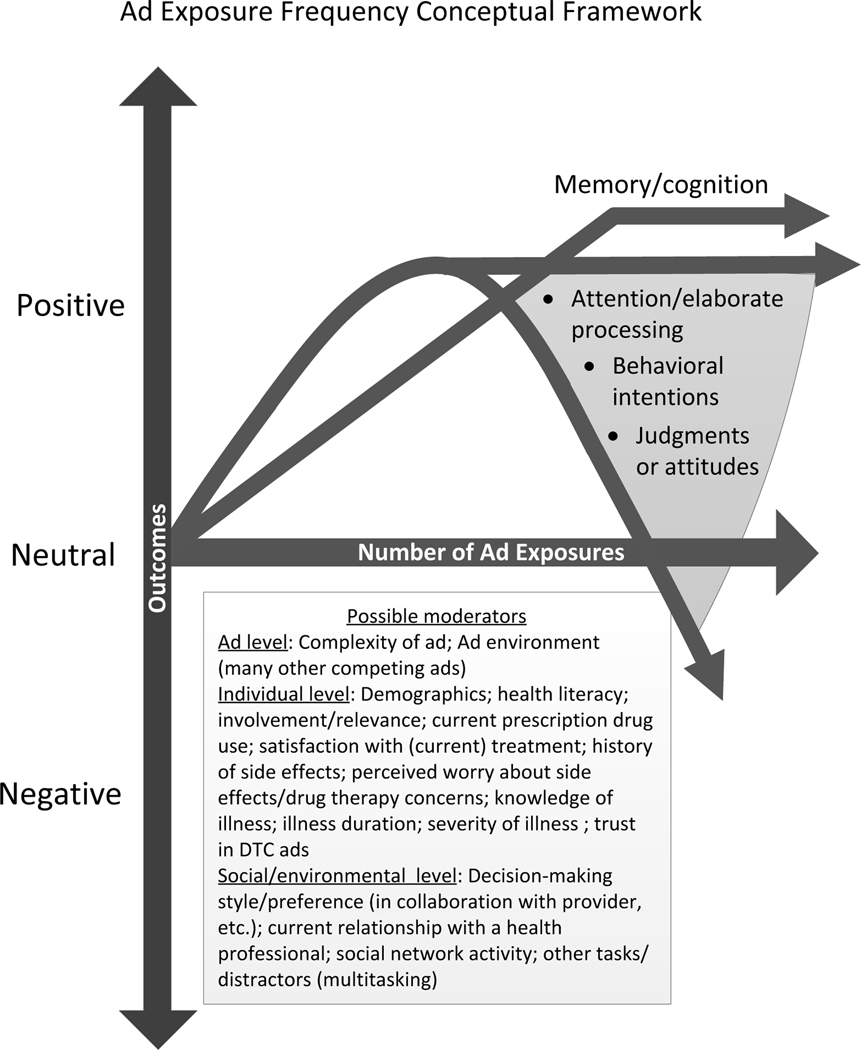
We developed a conceptual framework (see Figure 1) explaining the potential effects of DTC ad exposure frequency. This framework posits a hypothesis and a set of associated research questions regarding ad exposure effects. To test these, we designed an experiment in which participants viewed a mock DTC ad either once, twice or four times within the context of one hour of television programming and then answered a computer-based survey. The hypothesis and associated research questions were as follows:
Figure 1.
Conceptual framework depicting potential effects of repeated DTC ad exposure. As described further in the main text, outcomes such as attention and attitudes are expected to follow an inverted-U curve, though the exact pattern may vary up to a leveling off. Memory, in contrast, is expected to rise and then level off with repeated exposures.
Hypothesis: As ad exposure frequency increases, recall and recognition of risks and benefits will increase.
Research Question 1: Will risk and benefit information be recalled or recognized differently (e.g., benefits recalled/recognized more easily than risks)?
Research Question 2: Will ad exposure frequency affect risk and efficacy perceptions?
Research Question 3: Will ad exposure frequency affect behavioral intentions (specifically intentions to seek information about the drug or to take the drug if prescribed)?
Research Question 4: Are effects of ad exposure frequency on recall and recognition mediated by increased elaborate processing or counterarguing?
Research Question 5: Will attention be affected by ad exposure frequency?
Methods
We conducted a large-scale, in-person experimental study. The sample consisted of adults with seasonal allergies identified through opinion research recruitment firm databases. Staff called potential participants and screened them by telephone for eligibility. Individuals with healthcare or marketing backgrounds, or who had participated in the study’s pretest were excluded from participation. All participants could read and speak English. The study took place at two market research facilities: L&E Research in Raleigh, NC (n = 308) and Shugoll Research in Bethesda, MD (n = 308). Institutional Review Boards at author institutions approved all procedures.
There were 20 data collection sessions over five days in each data collection city. The firms scheduled up to 19 participants per session. Upon arrival at the facility, individuals reviewed a consent form and then received instructions about viewing the stimuli and answering an online survey via laptop. We randomized participants to one of three experimental conditions: low (exposure to prescription drug ad once), medium (twice) or high (four times) ad exposure frequency. All participants saw the ad embedded within 60 minutes of television programming, as described below.
The stimulus in which we embedded ads was two back-to-back episodes of a 30-minute public television program entitled, “Growing a Greener World”. One episode of this general interest program was about rooftop gardening and the other about backyard beekeeping. We counterbalanced the episodes such that roughly half of participants saw one episode first and the other saw the other first. We placed six reels or commercial breaks (each with 3–6 commercials) into the TV programming. Embedded within those advertising reels was a fictional but realistic DTC ad for a prescription drug for seasonal allergies called Trinase. The placement of this ad varied across commercial breaks, but all participants saw it at the very end of the last commercial break to control for any recency or placement effects. Prior cognitive testing with 18 individuals confirmed that consumers viewed the ad as being indistinguishable from an actual DTC ad for a real drug in terms of production quality. Other ads shown within the commercial breaks were for computer software, cars, soft drinks, and other consumer products.
After viewing the assigned stimulus, each participant completed the survey, which included 42 items and lasted approximately 20 minutes. Prior to conducting the main study, we pretested study procedures in both locations: Raleigh (n = 57) and Bethesda (n = 58). The pretests resulted in improvements in instructions for logging into the survey and minor revisions to some survey response options.
Measures
Measures are described in Supplemental Table 1.
Analysis
Of the 616 participants completing the study, 601 were included in the analysis; 15 were excluded due to randomization error. We conducted analyses using SPSS Version 23.0, SAS Version 9.4 and Mplus Version 8.
We used analysis of variance (ANOVA) models to test the effects of ad exposure on continuous outcomes (risk recognition, benefit recognition). When models showed a main effect of ad exposure on an outcome, (p < 0.05), we conducted planned contrasts to identify differences between experimental groups. To control for inflation of error rates, we applied a Holm-Bonferroni correction to the set of planned contrasts for each outcome which involved comparing the three contrasts from smallest to largest p-value against three thresholds (0.05/3 = 0.0167), (0.05/2 = 0.025), and (0.05/1 = 0.05).
For categorical outcomes, we conducted ordinal logistic regressions. If the overall effect of ad exposure on the outcome was significant at p < 0.05, we examined differences between experimental groups through odds ratios and Wald chi-square statistics. Again, multiple comparisons were adjusted using Holm-Bonferroni corrections.
To examine whether participants recalled risk and benefit information differently at various ad exposure frequencies we compared regression coefficients using z-tests for the relationships between ad exposure and recall/recognition of risk and benefit information as shown in Table 1. We employed this step-wise approach due to the curvilinear relationship between ad exposure and benefit recall and to be consistent across all of our comparisons. We judged significance based on a Bonferroni corrected p-value of <0.025 to account for two comparisons (one vs. two exposures and two vs. four exposures).
Table 1.
Analysis approach for testing differences in regression coefficients using z-tests
| Regression Model | Outcome | Ad Exposure Frequency Comparison | Coefficient Comparison Using Z-Test |
|---|---|---|---|
| Model 1a | Risk recall | 1 vs. 2 | Model 1a vs. Model 1b |
| Model 1b | Benefit recall | 1 vs. 2 | |
|
| |||
| Model 2a | Risk recall | 2 vs. 4 | Model 2a vs. Model 2b |
| Model 2b | Benefit recall | 2 vs. 4 | |
|
| |||
| Model 3a | Risk recognition | 1 vs. 2 | Model 3a vs. Model 3b |
| Model 3b | Benefit recognition | 1 vs. 2 | |
|
| |||
| Model 4a | Risk recognition | 2 vs. 4 | Model 4a vs. Model 4b |
| Model 4b | Benefit recognition | 2 vs. 4 | |
For mediation testing, we used the Mplus software (Version 8) which uses maximum likelihood functions to estimate direct and indirect effects and incorporates bootstrapping for calculating standard errors and confidence intervals for indirect effects. Specifically, we examined the indirect effects of the independent variable – ad exposure (IV) on the dependent variables - recall and recognition of risk and benefit information (DV) through the mediating variable - total number of thoughts, counterarguing (MV) as outlined in Hayes & Preacher (2014). We selected one exposure as the reference category in the mediation analysis and judged significance based on a Bonferroni corrected p-value of <0.025 to account for two comparisons (one vs. two exposures and one vs. four exposures).
Results
Sample characteristics
Table 2 presents participant demographic information. Almost all participants (99%) were able to recognize the name of the advertised drug, Trinase; recognition of brand name was similar across experimental conditions. Most (74%) were also able to accurately recall the number of times they had seen the ad. Overall, participants reported positive drug opinions. Specifically, 65% said the benefits of Trinase (slightly, mostly or completely) outweigh the risks and 72% reported positive attitudes toward the drug (a score of 5 or higher on a scale ranging from 1-bad to 7-good).
Table 2.
Demographic characteristics of participants overall and by ad exposure frequency
| Overall | 1 ad exposure | 2 ad exposures | 4 ad exposures | |
|---|---|---|---|---|
| % | % | % | % | |
| Gender | ||||
| Male | 252 (43.5%) | 91 (47.4%) | 81 (40.9%) | 80 (42.3%) |
| Female | 327 (56.5%) | 101 (52.6%) | 117 (59.1%) | 109 (57.7%) |
| Age | ||||
| Mean Age (SD) | 47 (13.7) | 49 (13.2) | 47 (13.9) | 46 (13.8) |
| Race/Ethnicity a | ||||
| Non-Hispanic White | 394 (73.4%) | 137 (77.4%) | 135 (72.2%) | 122 (70.5%) |
| Non-Hispanic Black | 105 (19.6%) | 29 (16.4%) | 42 (22.5%) | 34 (19.7%) |
| Hispanic | 15 (2.8%) | 3 (1.7%) | 6 (3.2%) | 6 (3.5%) |
| Other | 23 (4.3%) | 8 (4.5%) | 4 (2.1%) | 11 (6.4%) |
| Education | ||||
| Less than high school | 6 (1.0%) | 4 (2.0%) | 2 (1.0%) | 0 (0%) |
| High school | 95 (16.0%) | 26 (13.1%) | 36 (18.0%) | 33 (16.8%) |
| Some college | 104 (17.5%) | 36 (18.2%) | 34 (17.0%) | 34 (17.3%) |
| College degree | 389 (65.5%) | 132 (66.7%) | 128 (64.0%) | 129 (65.8%) |
Respondents who reported their race but not ethnicity were coded as missing on the race/ethnicity variable and were therefore excluded from this table.
Most participants (80%) had been diagnosed with seasonal allergies more than five years ago and reported their symptoms to be of at least moderate severity. Roughly 40 percent of respondents were currently taking a prescription drug for seasonal allergies, and 50 percent had taken a drug for seasonal allergies in the past but were not currently taking one. Of those who were taking a medication, 88 percent were moderately, very, or extremely satisfied with that medication.
Descriptive results show that recall of risks and benefits was low overall. Participants on average recalled 1.09 risks (SD = 1.10) (out of 7) and 1.44 benefits (SD = 1.03) (out of 8). About 30% of participants recalled two or more risks and about 41% of participants recalled two or more benefits. On average, participants correctly recognized 4.61 risk claims as real or bogus (SD = 1.27) (out of 7 claims) and 5.07 benefit claims as real or bogus (SD = 1.17) (out of 7 claims).
Hypothesis testing
Our hypothesis was partially supported (See Table 3). Specifically, the results of the ordinal logistic regression showed that ad exposure frequency was significantly related to risk recall (X2 = 20.93, p < 0.001) such that those who saw the ad four times were more likely to recall more risks compared to those who saw the ad only once (Odds Ratio [OR] = 2.36, p < 0.001) or twice (OR = 1.59, p = 0.012). Ad exposure frequency was also significantly related to benefit recall (X2 = 9.34, p = 0.009). Those who saw the ad twice (OR = 1.70, p = 0.005) and four times (OR = 1.59, p = 0.014) were more likely to recall more benefits compared to those who saw the ad only once.
Table 3.
Results of ordinal logistic regression and ANOVA testing ad exposure frequency on risk and benefit recall and recognition
| Outcome | Overall | Experimental Condition | |||
|---|---|---|---|---|---|
| 1 Ad Exposure | 2 Ad Exposures | 4 Ad Exposures | X2-value, p-value | ||
| n (%) | n (%) | n (%) | n (%) | ||
| Recall of risk information | |||||
| 0 | 218 (36.3) | 92 (46.2) | 73 (36.1) | 53 (26.5) | 20.93, p < 0.001 |
| 1 | 202 (33.6) | 63 (31.7) | 71 (35.2) | 68 (34.0) | |
| 2+ | 181 (30.1) | 44 (22.1) | 58 (28.7) | 79 (39.5) | |
| Recall of benefit information | |||||
| 0 | 97 (16.1) | 37 (18.6) | 30 (14.9) | 30 (15.0) | 9.34, p = 0.009 |
| 1 | 256 (42.6) | 99 (49.8) | 77 (38.1) | 80 (40.0) | |
| 2+ | 248 (41.3) | 63 (32.2) | 95 (47.0) | 90 (46.0) | |
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | F-value, p-value | |
| Recognition of risk information | 4.61 (0.09) | 4.30 (0.09) | 4.61 (0.09) | 4.91 (0.09) | 11.89, p < 0.001 |
| Recognition of benefit information | 5.07 (0.08) | 4.95 (0.08) | 5.01 (0.08) | 5.23 (0.08) | 3.17, p = 0.043 |
Significant differences for recall of risk information were between 4 vs. 1 and 4 vs. 2 exposures. Significant differences for recall of benefit information were between 2 vs. 1 and 4 vs. 1 exposures. Significant differences for recognition of risk information were between 2 vs. 1 and 4 vs. 1 exposures. For recognition of benefit information, significant differences were between 4 vs. 1 exposures. To be considered significant, p-values had to be below the Bonferroni adjusted p-value < 0.0167.
We also found that ad exposure was significantly related to risk recognition (F(2,597)= 11.89, p = .001); those who saw the ad four times (M = 4.91, SE = 0.09, p < 0.001) and those who saw the ad two times (M = 4.61, SE = 0.09, p = 0.012) had higher risk recognition than those who saw it only once (M = 4.30, SE = 0.09). We also found a significant relationship between ad exposure frequency and benefit recognition (F(2,597) = 3.17, p = 0.043); planned comparisons show that those who saw the ad four times (M = 5.23, SE = 0.08, p = 0.0166) had higher benefit recognition than those who saw the ad only once (M = 4.95, SE = 0.08).
We found that overall, 46.1% of participants accurately recalled that Trinase treats seasonal allergies. This was the first benefit presented, but it is also the main indication for the drug, which likely explains the high recall. While recall of this drug indication was most frequent compared to other benefits overall, it did not differ significantly with increased ad exposure (p = 0.551).
Our first research question asked whether ad exposure frequency would have a different effect on recall/recognition of benefits versus risks. When comparing those exposed to the ad two and four times, the p-value for the z-test comparing the slope of the relationship between ad exposure frequency and recall of risk information with the slope of the relationship between ad exposure frequency and recall of benefit information was just above our Bonferroni corrected threshold of 0.025 (BRisk = 0.47, SERisk = 0.18, BBenefit = −0.07, SEBenefit = 0.19, z = 2.01, p = 0.044). While not statistically significant, the slopes seem to indicate that when going from two to four exposures, we should expect an increase in risk recall (i.e., 0.47 increase in the outcome in the ordered logit scale), but essentially no increase in benefit recall (i.e., −0.07 decrease in the outcome in the ordered logit scale). We did not find significant differences between the slopes comparing those with one exposure to those with two exposures BRisk = 0.39, SERisk = 0.19, BBenefit = 0.53, SEBenefit = 0.19, z = −0.52, p = 0.602.). The difference in slopes for the relationship between ad exposure frequency and recognition of risks and ad exposure frequency and recognition of benefits was, similarly, not significant when comparing one vs. two exposures (BRisk = 0.32, SERisk = 0.13, BBenefit = 0.07, SEBenefit = 0.12, z = 1.47, p = 0.143) or two vs. four exposures (BRisk = 0.30, SERisk = 0.12, BBenefit = 0.22, SEBenefit = 0.12, z = 0.47, p = 0.635).
Research question 2 was not supported as there were no significant effects of ad exposure frequency on risk or benefit perceptions--either likelihood or magnitude (See Table 4).
Table 4.
Results of Logistic Regression and ANOVA testing effects of ad exposure frequency on risk and benefit perceptions
| Outcome | Overall | Experimental Condition | |||
|---|---|---|---|---|---|
| 1 Ad Exposure | 2 Ad Exposures | 4 Ad Exposures | X2-value, p-value | ||
| n (%) | n (%) | n (%) | n (%) | ||
| Perceived risk likelihood (% >median = slightly likely-extremely likely) | 158 (26.3%) | 49 (24.6%) | 61 (30.2%) | 48 (24.0%) | 2.41, .230 |
| Perceived risk magnitude (% >median = somewhat serious-extremely serious) | 125 (20.8%) | 38 (19.1%) | 48 (23.8%) | 39 (19.5%) | 1.63, .443 |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F-value, p-value | |
| Perceived efficacy likelihood | 3.41 (.84) | 3.39 (.83) | 3.42 (.87) | 3.43 (.84) | .11, .896 |
| Perceived efficacy magnitude | 3.45 (.84) | 3.40 (.85) | 3.49 (.82) | 3.48 (.84) | .71, .490 |
There are no significant differences between any of the ad exposure groups for any of the four risk or efficacy perception measures.
Research question 3 was not supported. We did not find any significant association between ad exposure frequency and intentions to look for information about Trinase or to talk with family members or friends about Trinase; intentions to ask a doctor for more information about Trinase or to prescribe Trinase; or intentions to take the drug if prescribed by a doctor.
Research question 4 was partially supported. We found that ad exposure frequency was significantly (F(2,396.1) = 4.20, p = 0.016) related to the total number of thoughts, the indicator of elaborate processing; participants reported a higher number of thoughts with each additional ad exposure (one exposure: mean = 3.58, SD = 1.50; two exposures: mean = 3.75, SD = 1.35; four exposures: mean = 3.97, SD = 1.24), but this difference was significant only with four exposures versus one exposure (p = 0.004). The mediation analysis indicated that the number of thoughts, i.e., elaborate processing, mediated the relationship between ad exposure frequency and risk recall. Compared to those who saw the ad only one time, participants who saw the ad four times were more likely to recall more risks as a result of the positive effect of ad exposure frequency on elaborate processing (indirect effect coefficient = 0.031, SE = 0.015, p = 0.033); however, this mediation effect was just above our Bonferroni corrected threshold p-value of 0.025. Examining benefit recall, participants who saw the ad four times were more likely than those who saw the ad once to recall more benefits (indirect effect coefficient = 0.034, SE = 0.015, p = 0.024); this mediation effect was below our threshold p-value of 0.025. Finally, we did not observe significant indirect effects of two ad exposures versus one ad exposure on risk recall (indirect effect coefficient = 0.015, SE = 0.013, p = 0.242) or benefit recall (indirect effect coefficient = 0.0.016, SE = 0.013, p = 0.226) through elaborate processing. Elaborate processing did not mediate the relationship between ad exposure frequency and risk or benefit recognition.
There was no association between ad exposure frequency and counterarguing (F(2,596) = 0.09, p = 0.911); The total number of counterarguments was similar across all three exposure groups: one exposure: mean = 0.22 (SD = 0.51); two exposures: mean = 0.21, (SD = 0.49); four exposures: mean = 0.24, (SD = 0.47). Thus, we did not test counterarguing as a mediator.
In response to research question 5, we found a significant effect of ad exposure frequency on self-reported attention level (F(2,567) = 34.29, p < 0.001). Specifically, ad attention decreased significantly from one exposure (M = 3.58, SE = 0.07) to two exposures (M = 3.24, SE = 0.07), p < 0.001 and from two exposures to four exposures (M = 2.77, SE = 0.07), p < 0.001.
Discussion
Most DTC prescription drug television ads include a substantial amount of information, particularly when compared to ads for other products. Limitations to human memory capacity and other information processing constraints (Jacoby, 1984; Wilson & Wolf, 2009) show the potential disadvantages to this approach. For example, studies by Betts et al. (2018) and Aikin et al. (2017) report that consumers recall only about one to two risks on average after viewing a DTC television ad. In this study, we explored whether risk and benefit retention are boosted upon multiple exposures, as is typical in the marketplace. We found that as exposures increased, participants exhibited greater retention of both risk and benefit information. In terms of how these increases occur, we found an interesting pattern such that going from one to two exposures resulted in significantly higher recall of benefits but not risks; recall of benefit information remained somewhat stable after two exposures while recall of risk information continued to increase with additional exposures. Moreover, benefit information tended to be remembered somewhat easier than risk information. Eighty-four percent of respondents recalled at least one benefit, compared to just 64% of respondents recalling one risk. This difference was more pronounced among those with one exposure and diminished among those with four exposures. Still, effects on memory were small; retention of both risks and benefits remained low overall even after four exposures.
The results partially support our proposed conceptual framework. We expected to see an increase in memory up to a certain point and then a leveling off. We did see that recall of benefit information leveled off, but risk information recall continued to increase with more exposures. It may be that we did not include sufficient exposures to reach the memory plateau for risk information.
We also expected to see an increase and then potential decrease (inverted U) in drug perceptions as exposure level increased, but observed no significant effects of exposure on perceptions of the drug’s risks and benefits. These findings are surprising, but useful in their apparent indication that increased exposure may have little impact on drug perceptions. It is possible that these types of judgments are formed upon first exposure to information about a new drug and remain unchanged after subsequent exposures, or that they are more affected by other factors, such as past experience with similar drugs.
What should we make of these findings? Impacts on memory warrant consideration from policy makers. Betts et al. (2018) have provided evidence that risks presented in DTC television ads can be limited to those that are serious and actionable, and accompanied by a disclosure indicating that not all risks were presented. Other approaches for limiting presented risks have been suggested as well (Hoek, Gendall & Feetham, 2001). Given low levels of retention in the present research, our findings lend further support to this general strategy. It appears that consumers do not perform well when asked to recall the entirety of risk statements from DTC television ads, even after multiple exposures. However, our mediation findings suggest that additional exposures may result in more elaborate processing (Cacioppo & Petty, 1985), which could enhance retention of the risks.
In this study, benefit information seemed to be recalled at lower levels of ad exposure than were required for risk information. We see a few potential explanations for this outcome. It may be that DTC prescription drug television ads present benefit information that is easier to understand, resulting in greater memory. In a content analysis, Sullivan et al. (2017) found that the major statement of risks often “included long and complex sentences…were often accompanied by competing non-risk information in the visual images, presented with moderately fast-paced music, and read at a faster pace than benefit information.” This combination of language and visuals may promote attention to benefit communications over risk communications. It may also be that consumers are inherently less motivated to process risk information. There is evidence in the literature that threatening, personally relevant health information is more likely to be defensively processed (Janis & Feshbach, 1953), as people are motivated to avoid or deny the threatening message.
There was an interesting contradiction in some of the findings. We saw a decrease in self-reported attention to the ads, while simultaneously observing a significant positive effect of ad exposure frequency on recall and recognition of risk information. So, while people perceive themselves to be paying less attention at higher ad exposure levels, the information is somehow being encoded in memory nonetheless. It is possible that the self-reported measure of attention used in this study was not exclusively measuring attention, but also picking up negative attitudes toward the ad, defensive avoidance or other constructs.
Our research benefited from several methodological strengths. First, we utilized a large sample size and well-controlled experimental design. These features allowed for detection of relatively small but important effects and allowed us to reach conclusions about causality in regard to the impact of number of exposures. Second, to promote realism, the target ad in our study was presented within the context of television programming and amidst other ads. Most research involves presentation of a target ad either in isolation or as part of a commercial break consisting of several unrelated ads, negating the full context under which such ads are viewed in the real world. Third, in-person administration of the study protocol allowed us to ensure that participants viewed the entirety of the programming, something that would have been more challenging with Internet administration of the experiment.
We also acknowledge several limitations of our study which may pose directions for future research. First, depending on context, the number of times we exposed participants to the target ad may fall short of, match, or exceed what occurs in the marketplace. Sponsors generally desire to expose their target audience to a particular television ad multiple times (Pechmann & Stewart, 1988). In broadcast advertising contexts, this often involves multiple exposures during a brief period of time; for example, up to four exposures across back-to-back television shows. This is the situation we sought to mirror. In other contexts, however, the number of exposures may be far greater. One study has found that when television content is streamed online, the same advertisement may be presented as many as 4 times within one hour of programming and as many as 20 times during four hours of binge-watching a television program (Johnson & et al., in preparation). That number obviously exceeds the maximal number of exposures we included in this study. Thus, replications of this research with a higher maximal number of exposures, or replications across different viewing platforms (TV, online) would prove useful. In a related vein, future research should explore the differential effects of repeated exposure in short periods of time versus exposure over longer periods of time. We chose to investigate the former situation, but it is evident in the marketplace that the latter situation occurs as well.
As another potential limitation to this research, the target ad in this study was the only ad that repeated across clutter reels. While intentional from the perspective of experimental control, this particular situation may be uncommon in the marketplace, and other researchers may wish to take a different approach when balancing experimental control with realism. Of note, we have no reason to believe that this approach impacted attention and processing of the target ad.
As another limitation, our sample was restricted to patients suffering from seasonal allergies. It is possible that other patient populations may react differently to variations in ad exposure. For example, patients suffering from medical conditions involving cognitive decline or neurological issues may require additional exposures for the same effect. Similarly, the medical condition itself may have an impact on the number of exposures needed; for example, conditions that are asymptomatic or products that carry multiple serious risks could engender different attention and processing than seasonal allergies. For this reason, practitioners should be cautious when attempting to draw conclusions about patient populations not studied here, and to alleviate this concern, we encourage researchers to replicate our study using other patient populations.
Conclusion
In this study, additional ad repetitions had an effect on retention of both risks and benefits. In contrast, repeated ad exposure did not affect risk or benefit perceptions. This lack of findings could be due to an insufficient number of ad repetitions in the highest ad condition group, or it could be that perceptions about a drug’s risks and benefits formed after one exposure are resistant to change, while outcomes like memory are more affected by repetition. Both perceptions of the product’s risks and benefits and recall of specific product information are important. From a public health perspective, methods of presentation that lead to increased retention and, by extension, comprehension of important information can be considered a top priority. Therefore, strategies that increase recall, recognition and comprehension in addition to exposure, such as alterations to the major statement, should be explored.
Supplementary Material
Acknowledgments
This study was granted an exemption by FDA’s Research Involving Human Subjects Committee and RTI International’s Institutional Review Board. The data that support the findings of this study are available from the corresponding author, KB, upon reasonable request. Use of brand names in this research does not imply endorsement by FDA. This article reflects the views of the authors and should not be construed to represent FDA’s views or policies.
References
- Aikin KJ, Southwell BG, Paquin RS, Rupert DJ, O’Donoghue AC, Betts KR, & Lee PK (2017). Correction of misleading information in prescription drug television advertising: The roles of advertisement similarity and time delay. Research in Social and Administrative Pharmacy, 13(2), 378–388. 10.1016/j.sapharm.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Anand P, & Sternthal B. (1990). Ease of message processing as a moderator of repetition effects in advertising. Journal of Marketing Research, 27(3), 345–353. [Google Scholar]
- Batra R, & Ray ML (1986). Situational effects of advertising repetition: The moderating influence of motivation, ability, and opportunity to respond. Journal of Consumer Research, 12(4), 432–445. [Google Scholar]
- Belch GE (1982). The effects of television commercial repetition on cognitive response and message acceptance. Journal of Consumer Research, 9(1), 56–65. [Google Scholar]
- Berlyne DE (1970). Novelty, complexity, and hedonic value. Perception & Psychophysics, 8(5a), 279. 10.3758/Bf03212593 [DOI] [Google Scholar]
- Betts KR, Boudewyns V, Aikin KJ, Squire C, Dolina S, Hayes J, & Southwell B. (2018). Serious and actionable risks, plus disclosure: Investigating an alternative approach for presenting risk information in prescription drug television advertisements. Research in Social and Administrative Pharmacy, 14, 951–963. doi: 10.1016/j.sapharm.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, & Petty RE (1979). Effects of message repetition and position on cognitive response, recall, and persuasion. Journal of Personality and Social Psychology, 37(1), 97. [Google Scholar]
- Cacioppo JT, & Petty RE (1985). Central and peripheral routes to persuasion: The role of message repetition. In Mitchell AA & Alwitt LF (Eds.), Psychological processes and advertising effects (pp. 911–912). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Calder BJ, & Sternthal B. (1980). Television commercial wearout: An information processing view. Journal of Marketing Research, 17(2), 173–186. [Google Scholar]
- Callaghan GM, Laraway S, Snycerski S, & McGee SC (2013). Antidepressant advertising effects on drug knowledge and drug seeking. Journal of Consumer Marketing, 30(3), 267–272. [Google Scholar]
- Cannon HM, & Riordan EA (1994). Effective reach and frequency: Does it really make sense? Journal of Advertising Research, 34(2). [Google Scholar]
- Chen JM, Yang XJ, & Smith RE (2016). The effects of creativity on advertising wear-in and wear-out. Journal of the Academy of Marketing Science, 44(3), 334–349. 10.1007/s11747-014-0414-5 [DOI] [Google Scholar]
- Cox DS, & Cox AD (1988). What does familiarity breed? Complexity as a moderator of repetition effects in advertisement evaluation. Journal of Consumer Research, 15(1), 111–116. [Google Scholar]
- Eaton H Jr. (1996). Cognitive and affective responses to repeated exposures of television commercials. Dissertation Abstracts International: Section B: The Sciences and Engineering, 57(6-B), 40–48. [Dissertation]. [Google Scholar]
- Fischoff B. (2009). Risk perception and communication. In Detels R, Beaglehole R, Lansang MA & Guilliford M. (Eds.), Oxford textbook of public health. 5 (pp. 940–952). Oxford, UK: Oxford University Press. [Google Scholar]
- Frosch DL, Grande D, Tarn DM, & Kravitz RL (2010). A decade of controversy: Balancing policy with evidence in the regulation of prescription drug advertising. American Journal of Public Health, 100(1), 24–32. 10.2105/ajph.2008.153767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginter J. (1974). An experimental investigation of attitude change and choice of a new brand. Journal of Marketing Research, 11(1), 30–37. [Google Scholar]
- Hitchon JC, & Thorson E. (1995). Effects of emotion and product involvement on the experience of repeated commercial viewing. Journal of Broadcasting & Electronic Media, 39(3), 376–389. [Google Scholar]
- Hayes AF, & Preacher KJ (2014). Statistical mediation analysis with a multicategorical independent variable. British Journal of Mathematical and Statistical Psychology, 67. [DOI] [PubMed] [Google Scholar]
- Hoek J, Gendall P, & Feetham P. (2001). Could less be more? An analysis of direct to consumer advertising of prescription medicines. Marketing Bulletin, 12, 1–15. [Google Scholar]
- Hughes GD (1992). Realtime response measures redefine advertising wearout. Journal of Advertising Research, 32(3), 61–77. [Google Scholar]
- Jacoby J. (1984). Perspectives on information overload. Journal of Consumer Research, 10(4), 432. 10.1086/208981 [DOI] [Google Scholar]
- Janis IL, & Feshbach S. (1953). Effects of fear-arousing communications. Journal of Abnormal and Social Psychology, 48, 78–92. [DOI] [PubMed] [Google Scholar]
- Johnson M, Kelly B, Betts K, Aikin KJ, Southwell B, Williams P. This ad again? A content analysis of the frequency of Direct-to-Consumer prescription drug and other advertising repetition in broadcast television; livestreaming and on-demand programming. Journal of Broadcast and Electronic Media. (in preparation). [Google Scholar]
- Mack J. (2005). To ban or not to ban DTC: That is the question. Pharma Marketing News, 4, 1–7. [Google Scholar]
- Mitchell AA, & Olson JC (1977). Cognitive effects of advertising repetition. Advances in Consumer Research, 4(1), 213–220. [Google Scholar]
- Norris RL, Bailey RL, Bolls PD, & Wise KR (2012). Effects of emotional tone and visual complexity on processing health information in prescription drug advertising. Health Communication, 27(1), 42–48. 10.1080/10410236.2011.567450 [DOI] [PubMed] [Google Scholar]
- O’Donoghue AC, Sullivan HW, Aikin KJ, Chowdhury D, Moultrie RR, & Rupert DJ (2014). Presenting efficacy information in direct-to-consumer prescription drug advertisements. Patient Education and Counseling, 95(2), 271–280. 10.1016/j.pec.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechmann C, & Stewart DW (1988). Advertising repetition: A critical review of wearin and wearout. Current Issues and Research in Advertising, 11(1–2), 285–329. [Google Scholar]
- Rethans AJ, Swasy JL, & Marks LJ (1986). Effects of television commercial repetition, receiver knowledge, and commercial length: A test of the two-factor model. Journal of Marketing Research, 23(1), 50–61. [Google Scholar]
- Reyna VF (2012, May 28). Risk perception and communication in vaccination decisions: A fuzzy-trace theory approach. Vaccine, 30(25), 3790–3797. 10.1016/j.vaccine.2011.11.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B, & Bhutada N. (2014). Impact of celebrity endorsements in disease-specific direct-to-consumer (DTC) advertisements: An elaboration likelihood model approach. International Journal of Pharmaceutical and Healthcare Marketing, 8(2), 164–177. [Google Scholar]
- Southwell BG (2005). Between messages and people - A multilevel model of memory for television content. Communication Research, 32(1), 112–140. 10.1177/0093650204271401 [DOI] [Google Scholar]
- Southwell BG, Barmada CH, Hornik RC, & Maklan DM (2002, Oct-Dec). Can we measure encoded exposure? Validation evidence from a national campaign. Journal of Health Communication, 7(5), 445–453. 10.1080/10810730290001800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang DJ (1975). Effects of “mere exposure” on learning and affect. Journal of Personality and Social Psychology, 31(1), 7–12. [DOI] [PubMed] [Google Scholar]
- Sullivan HW, Aikin KJ, & Poehlman J. (2017). Communicating risk information in direct-to-consumer prescription drug television ads: A content analysis. Health Communication, 1–8. 10.1080/10410236.2017.1399509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney KJ (1999). Toward a critical sociology of risk. Sociological Forum, 14(2), 215–242. 10.1023/A:1021414628203 [DOI] [Google Scholar]
- Vakratsas D, & Ambler T. (1999). How advertising works: What do we really know? The Journal of Marketing, 63(1), 26–43. [Google Scholar]
- Ventola CL (2011). Direct-to-consumer pharmaceutical advertising: Therapeutic or toxic? Pharmacy and Therapeutics, 36(10), 669–684. [PMC free article] [PubMed] [Google Scholar]
- Vuokko P. (1997). The determinants of advertising repetition effects. In Wells WD (Ed.), Measuring advertising effectiveness (p. 239). Mahwah, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Wilson EA, & Wolf MS (2009). Working memory and the design of health materials: A cognitive factors perspective. Patient Education and Counseling, 74(3), 318–322. 10.1016/j.pec.2008.11.005 [DOI] [PubMed] [Google Scholar]
- Wogalter MS, Shaver EF, & Kalsher MJ (2014). Effect of presentation modality in direct-to-consumer (DTC) prescription drug television advertisements. Appled Ergonomics, 45(5), 1330–1336. 10.1016/j.apergo.2013.12.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.