Abstract
Background
Pediatric acute lymphoblastic leukemia (ALL) therapy is accompanied by treatment-related toxicities (TRTs) and impaired quality of life. In Australia and New Zealand, children with ALL are treated with either Children’s Oncology Group (COG) or international Berlin-Frankfurt-Munster (iBFM) Study Group-based therapy. We conducted a prospective registry study to document symptomatic TRTs (venous thrombosis, neurotoxicity, pancreatitis and bone toxicity), compare TRT outcomes to retrospective TRT data, and measure the impact of TRTs on children’s general and cancer-related health-related quality of life (HRQoL) and parents’ emotional well-being.
Methods
Parents of children with newly diagnosed ALL were invited to participate in the ASSET (Acute Lymphoblastic Leukaemia Subtypes and Side Effects from Treatment) study and a prospective, longitudinal HRQoL study. TRTs were reported prospectively and families completed questionnaires for general (Healthy Utility Index Mark 3) and cancer specific (Pediatric Quality of Life Inventory (PedsQL)-Cancer Module) health related quality of life as well the Emotion Thermometer to assess emotional well-being.
Results
Beginning in 2016, 260 pediatric patients with ALL were enrolled on the TRT registry with a median age at diagnosis of 59 months (range 1–213 months), 144 males (55.4%), majority with Pre-B cell immunophenotype, n = 226 (86.9%), 173 patients (66.5%) treated according to COG platform with relatively equal distribution across risk classification sub-groups. From 2018, 79 families participated in the HRQoL study through the first year of treatment. There were 74 TRT recorded, reflecting a 28.5% risk of developing a TRT. Individual TRT incidence was consistent with previous studies, being 7.7% for symptomatic VTE, 11.9% neurotoxicity, 5.4% bone toxicity and 5.0% pancreatitis. Children’s HRQoL was significantly lower than population norms throughout the first year of treatment. An improvement in general HRQoL, measured by the HUI3, contrasted with the lack of improvement in cancer-related HRQoL measured by the PedsQL Cancer Module over the first 12 months. There were no persisting differences in the HRQoL impact of COG compared to iBFM therapy.
Conclusions
It is feasible to prospectively monitor TRT incidence and longitudinal HRQoL impacts during ALL therapy. Early phases of ALL therapy, regardless of treatment platform, result in prolonged reductions in cancer-related HRQoL.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-10072-x.
Keywords: ALL, Health related quality of life, Treatment related toxicity, Quality of life, Psychosocial, Child, Registries
Introduction
Survival for pediatric acute lymphoblastic leukaemia (ALL) has made excellent gains with five-year overall survival ≥90% [1, 2]. ALL treatment is prolonged, lasting up to 3 years, with many treatment-related toxicities (TRTs) [1, 2]. Nearly 40% of ALL patients experience ≥1 TRT. The incidence of venous thromboembolism being ≈5% [3–6], neurotoxicity (central and/or peripheral) 3–28% [7–10], osteonecrosis 6–17%, fractures 8–28% [11] and pancreatitis 5–10% [12–15]. Some TRTs are life-threatening and many contribute to late effects that negatively impact health related quality of life (HRQoL) [14, 16–20].
While advances in ALL risk classification and treatment have occurred [2], TRT research is an emerging discipline [14, 18]. Survival achieved by different ALL consortia are equivalent [2], but with limited comparative data on TRT incidence and HRQoL impacts across different protocols. Tentative evidence suggests that HRQoL is worst early in treatment, when increased risk of toxicity is more likely, with improvement over time [19–22]. Children on treatment report poorer HRQoL than those who have completed treatment [21] and compared to healthy children [23]. Few studies have taken a longitudinal approach to monitoring HRQoL in ALL [23–30]. Two studies used a cancer-related HRQoL measure, making it difficult to draw conclusions regarding treatment-specific impacts, such as pain or nausea, on HRQoL [23, 27].
In an era where it is possible to achieve excellent survival, we suggest that TRT incidence, patient and parent HRQoL, and financial cost of therapy should be considered when evaluating the overall risks and benefits of alternative treatments. In considering HRQoL and parents’ emotional wellbeing during ALL treatment, we hypothesise that it is possible that the HRQoL impact across different treatment platforms is likely equivalent during early more intensive treatment, due to the broad similarities in the early treatment. However, later on, HRQoL differences may emerge, particularly for more intensive or prolonged maintenance protocols eg containing steroid pulses, vincristine and intrathecal chemotherapy. Recent Dutch HRQoL data during maintenance therapy, has shown that more intensive maintenance therapy, including intermittent dexamethasone, is associated with impaired HRQoL and higher distress compared to less intensive maintenance [31].
We previously undertook a retrospective analysis of TRT in ALL, the ERASE (Evaluation of Risk of ALL Treatment-Related Side-Effects) study, a multi-center study of 1251 children (1–18 years), consecutively diagnosed with ALL or lymphoblastic lymphoma, at six Australian Centers between 1998 and 2013 [6, 10, 32]. ERASE focused on developing clinical and/or genetic risk prediction models for TRTs [6, 10, 32]. Symptomatic TRTs of interest were venous thromboembolism, neurotoxicity, bone toxicity and pancreatitis. ERASE did not involve HRQoL monitoring. The prospective ASSET (Acute Lymphoblastic Leukaemia Subtypes and Side Effects from Treatment) study was designed to validate risk prediction models from ERASE and prospectively document longitudinal HRQoL. The research goals are to prospectively identify ALL patients at increased TRT risk, develop interventions to ameliorate TRT risk, capture whole of life impacts from ALL and its treatment, and to identify the costs of managing ALL and associated TRTs.
The ASSET study provides an opportunity to compare HRQoL between different treatment protocols within a broadly uniform healthcare system. Here, we aim to confirm the capacity of the ASSET registry to prospectively capture TRTs; compare rates of TRTs with the retrospective ERASE study; confirm the feasibility to prospectively capture children’s HRQoL and parents’ emotional well-being, examine whether children’s HRQoL and parents’ emotional well-being differed significantly across COG and iBFM treatment protocols and compare ASSET HRQoL outcomes to those documented in previous literature.
Methods
Participants
ASSET treatment related toxicity study
In January 2016, the ASSET study opened to recruitment at 9 Australian and New Zealand centers, the HRQoL substudy opened to recruitment in October 2018. Inclusion criteria included: newly diagnosed ALL or mixed phenotype acute leukaemia (MPAL), age ≤ 18 years and enrolment within 90 days of starting treatment. Patients with relapsed ALL/MPAL were excluded. Patients and their parents were identified by their treating clinicians and invited to participate by a research coordinator. The majority centers (7 of 9) used COG-based therapy with iBFM-based therapy in the others 2 centers (Table 1). The study was approved by the HNE HREC (2019/ETH00693) and was conducted according to the Australian National Statement on Ethical Conduct in Human Research (2007) [33]. Informed consent was obtained from participants or their parents and/or legal guardians. Data, including clinical features, treatment, ALL outcomes and symptomatic TRT incidence and management, was collected prospectively by the local clinicians and entered into a web-based registry (WebSpirit). Targeted symptomatic TRTs including venous thromboembolism, neurotoxicity (central and peripheral), bone toxicity and pancreatitis were prospectively documented to compare to the ERASE study. Follow-up data collection including 6-monthly updates of symptomatic TRTs, clinical progress and survival including relapse, second malignancy and/or death. TRTs were prospectively identified, graded and reported by treating clinicians at each center using international definitions and the National Cancer Institute Common Toxicology Criteria for Adverse Events (NCI-CTCAE) version 4.03 [17, 34].
Table 1.
Participating centers in the ASSET study and COG, iBFM and Interfant based ALL treatment programs
| Center | ALL treatment approach & treatment programs |
|---|---|
| Sydney Children’s Hospital | iBFM |
| Children’s Hospital at Westmead | iBFM |
| John Hunter Children’s Hospital | COG |
| Perth Children’s Hospital | COG |
| Monash Children’s Hospital | COG |
| The Royal Children’s Hospital | COG |
| Women’s and Children’s Hospital | COG |
| Christchurch Hospital | COG |
| Starship Children’s Hospital | COG |
| COG, iBFM and Interfant based treatment programs used in Australian and New Zealand Centers | COG A5971, ANZCCSG Study VII, ANZCHOG Study 8, AIEOP-BFM ALL 2009-Study 9, BFM-95, COG AALL0031, COG AALL0232, COG AALL0331, COG ALL0434, COG AALL08P1, COG ALL0932, COG AALL1131, CCG1882, CCG1952, CCG1961, CCG1991, Interfant-99 and Interfant-06 |
ASSET health related quality of life study
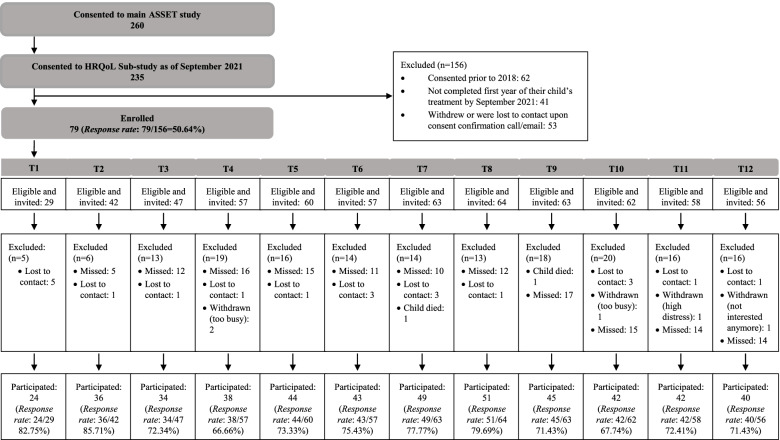
Patients enrolled on ASSET, except at one site (due to staffing requirements), were invited to participate in the HRQoL study. Following consent, eligible parents and children/young people were sent an invitation email to complete their first study questionnaire via Research Electronic Data Capture (REDCap) [35, 36]. The first questionnaire was sent within 100 days of the child or young person’s diagnosis and participants were given up to 14 days to complete it (Fig. 1). Due to delayed study staff recruitment, ASSET HRQoL study commenced data collection in October 2018. HRQoL was assessed monthly during the child or young person’s first year of treatment. Response rates were tracked for the first 12 questionnaires, which cover the first 12 months of treatment: T1 = 1 month post-diagnosis, T2 = 2 months post-diagnosis, etc. Participants were contacted by phone and/or email if they missed two consecutive questionnaires. A follow-up log was maintained to track reasons for missed questionnaires.
Fig. 1.
ASSET HRQoL Sub-Study Recruitment and Participation. Sevety-nine families consented and provided at least one questionnaire response across the first year of treatment. Sample size at each time point post diagnosis is shown. Response rate at each time point is calculated by considering the number of participants in the total sample that were within the specified time frame for a particular questionnaire, and who were invited and provided data at that time point. For example, at T3, 47 participants of the total 79 were within 2 weeks of 3 months post diagnosis and were invited to complete a survey. Of these 47, 34 provided data, 12 missed completing the questionnaire within the allocated time frame (2 weeks) and one was lost to contact
Patients’ ALL treatment was determined by the treating clinical team following local institutional practices and could include enrolment on current COG, iBFM or Interfant therapeutic clinical trials or treatment according to published trials (Table 1) [37–52].
HRQoL measures
Demographics and clinical information were collected from the medical records and from both parents and young people through purpose-designed questions asking about the child or young person’s sex, date of birth, and time since diagnosis.
Cancer-related HRQoL was assessed using The Pediatric Quality of Life Inventory (PedsQL)-Cancer Module (child, youth, and parent proxy versions) [53]. The PedsQL-Cancer Module assesses cancer-related HRQoL and has 23 items with eight scales, scored from 0 to 4. The scales assess children, youth, and parents’ perceptions of young people’s levels of pain, nausea, procedural anxiety, treatment anxiety, worry, cognitive problems, changed physical appearance and communication. Individual item scores were transformed (0 = 100, 1 = 75, 2 = 50, 3 = 25, 4 = 0), and subscale mean scores were calculated. An overall HRQoL score was generated by calculating the mean of the subscale scores. Higher scores indicate higher HRQoL.
General HRQoL was assessed using the Healthy Utility Index Mark 3 (HUI3) [54]. The HUI3 assesses general HRQoL across domains of vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain. HRQoL across those domains is scored from 1 to 6 and an overall health utility score is generated: a utility score of zero indicate worst possible HRQoL (death) and a score of one indicates best possible HRQoL (perfect health). We report here on outcomes from the parent-proxy versions of the PedsQL and HUI3 measures.
We assessed parents’ emotional well-being using the Emotion Thermometer (ET) [55, 56]. The ET has been used for monitoring emotional well-being in the context of cancer, assessing anxiety, depression, anger, and need for help on scales from 0 to 10 [57]. A score of ≥5 indicates significant distress. Additional questions ask whether an individual is currently receiving help, and if not, whether they would like additional help. For this study, parents requesting additional help were offered: (i) for the research team to flag their concerns with their treating team, (ii) to receive an email outlining recommended steps for accessing care for their concerns, or (iii) the option to speak with a psychologist on the research team for further assistance.
Analysis
To confirm the capacity of the ASSET registry to prospectively capture TRTs and HRQoL data in the first 12 months of treatment, we analyzed TRT incidence and HRQoL outcomes. TRT frequencies were calculated to determine the incidence of TRTs in the ASSET study for comparison to the ERASE study [6, 10, 32]. Changes in children’s HRQoL and parents’ emotional well-being throughout the first year of treatment were documented. This included calculation of descriptive statistics for the parent proxy PedsQL subscale scores, the ET subscale scores, and the parent proxy PedsQL and HUI overall scores, at each time point.
To address whether the HRQoL study provided a feasible method of data collection for researchers and participants, the overall parent response rate at T1 and retention rates at each HRQoL data collection time point were calculated. Time required of the study coordinator to manage the data collection was considered. To compare HRQoL across COG and iBFM protocols, independent samples t-tests were calculated to compare the mean HRQoL or parents’ emotional well-being over time. To compare ASSET HRQoL outcomes to the literature, independent samples t-tests were conducted to compare HRQoL outcome scores across three shared timepoints.
We used minimum clinically important difference (MCID) to determine whether general HRQoL between treatment platforms is broadly equivalent or meaningfully different. To compare ASSET participant outcomes to population norms for the HUI, the MCID of 0.03 was subtracted from the age-specific population norm means to generate a threshold score [58]. We then calculated the percentage of participants scoring below that threshold at each time point.
Results
Patient demographics, outcome and TRT incidence
Between January 2016–September 2020, 260 pediatric ALL patients aged ≤18 years were enrolled on ASSET. Of these, 108 (41.5%) were enrolled on therapeutic trials through COG (n = 90), iBFM (n = 12) or Interfant (n = 6). The remaining 152 participants were treated according to published protocols from COG (n = 83), iBFM (n = 68) or Interfant (n = 1).
Seventy-four symptomatic TRT episodes were recorded, and three participants had ≥2 TRTs (Table 2). The TRT incidence in ASSET was 28.5%. The incidence of symptomatic venous thromboembolism, neurotoxicity, bone-related toxicity, and pancreatitis were 7.7, 11.9, 5.4 and 5.0% respectively (Table 2). Most TRTs were Grade 2–3 in severity, and other than bone toxicity, occurred within the first few months of treatment.
Table 2.
Demographics, treatment characteristics and incidence of treatment-related toxicities in ERASE and ASSET cohorts
| Baseline Information | ERASE (n = 1251) | ASSET (N = 260) | |||
|---|---|---|---|---|---|
| N | % | N | % | P value | |
| Median age at diagnosis + range (months) | 59 (9–227) | 59 (1–213) | 0.4457* | ||
| Median duration of follow up + range (months) | 79 (3–186) | 28 (1–62) | < 0.001* | ||
| OS at 3 years | 95.5 ± 0.6 | 98.3 ± 0.8 | 0.1511 | ||
| LFS at 3 years | 90.7 ± 0.8 | 97.4 ± 1.2 | 0.0016 | ||
| EFS at 3 years | 87.2 ± 1.0 | 96.3 ± 1.4 | 0.0031 | ||
| Gender | |||||
| Male | 671 | 53.6 | 144 | 55.4 |
0.6071† (Χ2 0.26) |
| Immunophenotype | |||||
| Pre B-cell | 1082 | 86.5 | 226 | 86.9 |
0.5740† (Χ2 0.3) |
| T-cell | 151 | 12.1 | 33 | 12.7 | |
| MPAL/Other | 18 | 1.4 | 1 | 0.4 | |
| Treatment Platform | |||||
| COG | 218 | 17.4 | 173 | 66.5 |
< 0.0001† (Χ2 233.3) |
| BFM | 1033 | 82.6 | 80 | 30.8 | |
| Interfant | 0 | 0.0 | 7 | 2.7 | |
| Risk Classification | |||||
| Low/Standard Risk | 515 | 41.2 | 82 | 31.5 |
< 0.0001† (Χ2 20.8) |
| Medium/Average/ Intermediate Risk | 446 | 35.7 | 83 | 31.9 | |
| High/Very High Risk | 264 | 21.1 | 81 | 31.2 | |
| Unknown | 26 | 2.1 | 14 | 5.4 | |
| Toxicity | |||||
| Symptomatic venous thromboembolism | 68 | 5.4 | 20 | 7.7 | 0.1488 |
| Neurotoxicity (≥ grade 3) | 111 | 8.9 | 31 | 11.9 | 0.1319 |
| Bone | 239 | 19.1 | 14 | 5.4 | 0.0001 |
| Pancreatitis | 48 | 3.8 | 13 | 5.0 | 0.3695 |
OS Overall survival, LFS Leukaemia Free Survival, EFS Event Free Survival
*Mann-Whitney Test (independent samples)
†Chi-squared test
The baseline demographic, treatment characteristics, and toxicity data from this prospective study were broadly comparable. The major differences were the shorter follow-up, the inclusion of patients with infant ALL and a higher proportion of patients with high/very high risk ALL in ASSET (Table 2). In ERASE, the incidence of symptomatic TRTs was in keeping with previous published reports, with venous thromboembolism in 5.4% [3, 6, 32, 59], ≥grade 3 neurotoxicity (central and/or peripheral) in 8.9% [8, 10, 60, 61], bone toxicity (avascular necrosis and/or fractures) in 19.1% [62, 63] and pancreatitis in 3.8% of patients [64, 65]. There was no significant difference in the incidence of symptomatic venous thromboembolism, neurotoxicity and pancreatitis between ERASE and ASSET (Table 2). There was a significantly lower incidence of bone toxicity in the ASSET study (Table 2) which most likely reflected the shorter duration of follow-up.
Longitudinal HRQoL and emotional wellbeing in the first 12 months of ALL treatment
Recruitment to the ASSET HRQoL study commenced in October 2018. Figure 1 summarises participation in the HRQoL study. Eighty ASSET participants, enrolled prior to October 2018, did not participate in the HRQoL study and were excluded leaving 156 participants eligible for the HRQoL study (Fig. 1). Of these, 79 parents (overall response rate 50.6%) consented and provided HRQoL data on at least one survey in the first 12 months of their child’s treatment. Parents represented children whose average age at diagnosis was 5.7 years (Table 3).
Table 3.
Demographics, treatment characteristics and questionnaire completion of the HRQoL cohort (N = 79 parents)
| Demographics | Child/young person’s Age at Diagnosis | M = 5.74(SD = 3.81), R = 1.03–17.20 |
| Child/young person’s Gender |
25 females (31.6%) 54 males (68.4%) |
|
| Distance from Treatment Center | M = 191.9 km (SD = 479.6), R = 4.4–3922 | |
| Immunophenotype | Pre B-cell | 64 (81.0%) |
| T-cell | 15 (19.0%) | |
| MPAL/Other | 0 (0%) | |
| Treatment Platform | BFM | 26 (32.9%) |
| COG | 53 (67.1%) | |
| Risk Classification | Low/Standard Risk | 33 (41.8%) |
| Medium/Average/ Intermediate Risk | 22 (27.8%) | |
| High/Very High Risk | 14 (17.7%) | |
| Total Number of Questionnaires out of a possible 12 Completed by Participants | Number of questionnaires | Number of participants |
| 1 | 5 (6.3%) | |
| 2 | 4 (5.1%) | |
| 3 | 7 (8.9%) | |
| 4 | 7 (8.9%) | |
| 5 | 7 (8.9%) | |
| 6 | 9 (11.4%) | |
| 7 | 9 (11.4%) | |
| 8 | 10 (12.7%) | |
| 9 | 8 (10.1%) | |
| 10 | 9 (11.4%) | |
| 11 | 2 (2.5%) | |
| 12 | 1 (1.3%) |
Response rates across each HRQoL assessment varied from 66.7–85.7% (Fig. 1, Supplementary Table 1). Response rates were lowest at T4 (66.7%, M = 4.4 months post-diagnosis). On an individual response level, only one parent completed all 12 questionnaires with most parents missing at least two time points and completing fewer than 10/12 questionnaires (Table 3). The main reported reason for missed surveys was forgetting/not checking emails. Following enrolment, five families withdrew due to being too busy or distressed and 21 families were lost to contact. Two children died during the study. At least 7 h per week were required for the HRQoL study coordinator to manage the study.
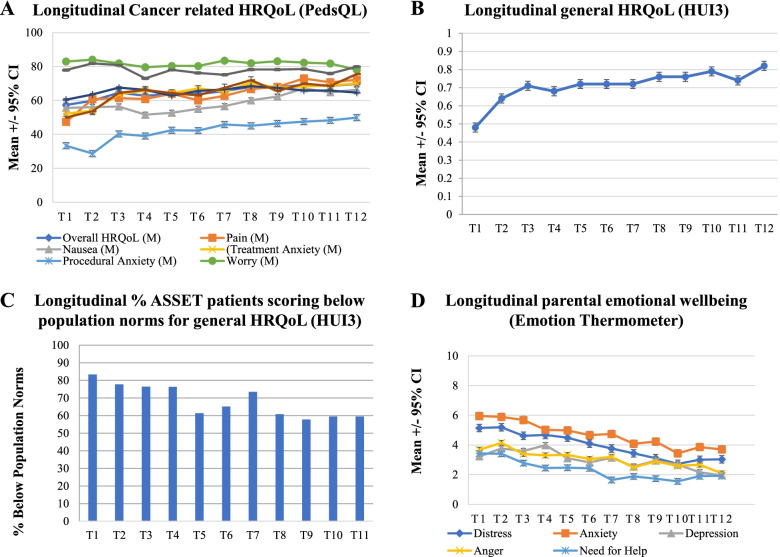
Analysis of parent-proxy reports demonstrated that cancer-related HRQoL (PedsQL) was poor over time, remaining ≤70/100 (Fig. 2A, Supplementary Table 1). Procedural anxiety had a consistently negative impact on HRQoL, with average scores ≤50/100 across all time points (Fig. 2A). Nausea was a consistent contributor to poor HRQoL. Average general HUI3 scores increased over time (Fig. 2B). More than 50% of parent proxy reports of children’s general HRQoL remained below the minimally clinically important difference (MCID) threshold in comparison to population norms across all time points (Fig. 2C). Parents’ emotional well-being was poorest in the first 6 months post-diagnosis. Anxiety was the most highly rated concern and need for help was greatest in the earlier months of treatment. Generally, we observed that parents’ average scores on the emotion thermometers decreased, indicating improvement in mental health over time, as did their need for help (Fig. 2D, Supplementary Table 2). Parents most frequently requested further help for emotional concerns in the first 6 months post-diagnosis (Supplementary Table 2). Seventeen parents (21.5%) requested help once from T1-T12, and three parents (3.8%) requested help twice. Regarding preferred support, 16 parents requested email support, three requested emotional concerns to be flagged with their child’s treating team, and six requested further help but not via any of the options listed.
Fig. 2.
Longitudinal HRQoL and emotional wellbeing in ASSET participants. A Pediatric Quality of Life Inventory – Cancer Module: cancer-specific health-related quality of life in the first year of treatment. Mean PedsQL score +/− 95% CI; increased score = increased HRQoL B Health Utilities Index 3: health-related quality of life in the first year of treatment. Mean HUI3 score +/− 95% CI; increased score = increased HRQoL. C Percentage of ASSET patients with general HRQoL below the population at each time point in the first 12 months of ALL therapy. D Parental emotional well-being over time measured by emotion thermometer. Mean Emotional Well-being score +/− 95% CI; increased score = increased emotional concern
Comparison of longitudinal HRQoL and emotional wellbeing between COG and iBFM treatment platforms in the first 12 months of ALL treatment
Parents of children treated according to a COG protocol reported their child experienced significantly poorer cancer-specific HRQoL than the iBFM group at some discrete time points (Supplementary Table 3). At T3, COG parents reported their child experienced significantly poorer HRQoL due to physical appearance (M = 76.92, SD = 26.70, M = 3.36 months post-diagnosis) than iBFM parents (M = 93.75, SD = 11.57, t28 = − 2.53, p = 0.02). At T9 and T10, COG parents reported their child experienced significantly poorer HRQoL due to nausea (T9 M = 54.63, SD = 26.20, M = 9.46 months post-diagnosis; T10 M = 59.20, SD = 27.07, M = 10.49 months post-diagnosis) than iBFM parents (T9 M = 73.89, SD = 19.97, t43 = − 2.65). Similarly, parents with a child treated according to a COG protocol reported their child experienced significantly poorer general HRQoL on the HUI3 compared to the iBFM group at some discrete time points: T5 (COG M = 0.67, iBFM M = 0.83, t40.87 = − 2.51. p = 0.02), T9 (COG M = 0.70, iBFM M = 0.88, t40.35 = − 2.86, p = 0.007), and T10 (COG M = 0.74, iBFM M = 0.89, t39.63 = − 2.66, p = 0.01) (Supplementary Tables 3 and 4). However, there was no evidence of persisting significant differences in the measured cancer specific or general HRQoL of the different treatment platforms over multiple consecutive time points. Some differences in the emotional well-being of parents of patients treated on each of the two protocols were identified (Supplementary Table 5). At T2, iBFM parents reported significantly more depression than COG parents (iBFM M = 5.18, COG M = 3.15, t35 = − 2.02, p = 0.05). At T3 iBFM parents also reported significantly more need for help than COG parents (iBFM M = 4.00, COG M = 2.37, t34 = 2.00, p = 0.05). At T5, T8, and T11 COG parents reported significantly more anger than iBFM parents (T5 COG M = 3.87, iBFM M = 2.14, t35.56 = 2.32, p = 0.03, T8 COG M = 3.03, iBFM M = 1.50, t48.21 = 2.36, p = 0.02, T11 COG M = 3.21, iBFM M = 1.67, t37.04 = 2.16, p = 0.04). Lastly, at T10, COG parents reported significantly more distress than iBFM parents (COG M = 3.39, iBFM M = 1.56, t42 = 2.17, p = 0.04). However, there was no evidence of persisting significant differences in the measured parental emotional wellbeing of the different treatment platforms over multiple consecutive time points.
Discussion
The results support the capacity of a prospective registry to document TRT, longitudinal HRQoL, and parents’ emotional well-being for children and adolescents treated for ALL across Australia and New Zealand. In ASSET, the incidence of individual TRTs is consistent with the available literature and the retrospective ERASE cohort [3–15, 32]. One discrepancy relates to bone toxicity, with 5.4% reported in ASSET and 19.1% in the ERASE cohort. Given that most bone toxicity occurs > 1 year into ALL treatment, the apparent low incidence of bone toxicity in ASSET is likely due to the shorter duration of follow-up.
We demonstrated the capacity to capture longitudinal HRQoL and emotional well-being data, providing a clear picture of change in HRQoL and well-being over time. Improvement in general HRQoL (HUI3) was contrasted by a lack of improvement in cancer-related HRQoL (PedsQL). Although there is improvement in general HRQoL with time, most families report general HRQoL below population norms throughout the entire first year of ALL therapy highlighting the sustained impact of ALL therapy. Parents’ emotional well-being also demonstrated improvement over time, although anxiety remained a consistent concern.
Although we observed discrete differences between protocol groups at some timepoints for HRQoL and emotional wellbeing, this needs to be interpreted with caution. First, we did not observe a consistent pattern of persisting difference in measures of HRQoL over multiple consecutive time points. Second we did not control for any potential confounders such as child sex and other sociodemographic factors previously shown to impact HRQoL due to sample size [20]. Furthermore, the confidence intervals for the differences in general HRQoL (HUI3) include plausible values larger than the MCID of 0.03 in both directions, suggesting that the sample size may not be sufficient to reject the possibility that there exist small but meaningful differences between protocols. On the PedsQL, the significant differences we observed at T9 and T10 seemed inconsistent with other time points and therefore could be attributed as type I errors. Taken together this may suggest that the impact of COG or iBFM based therapy on HRQoL and parental emotional wellbeing during the first 12 months of ALL treatment is no different. It is reassuring that we did not observe persistent longitudinal HRQoL and emotional wellbeing differences between these different treatment programs in the first 12 months of ALL therapy as there is substantial similarity in the intensity of treatment during this period. However, analysis of HRQoL and emotional wellbeing in the second year of treatment and beyond is likely to be informative, as this is the time period where the substantial differences in the duration of treatment (total treatment duration 2 years versus 3.5 years) and the intensity of maintenance chemotherapy (pulsed dexamethasone, intravenous vincristine and lumbar punctures versus oral chemotherapy only) may result in differing HRQoL impacts. Dutch HRQoL data suggests that prolonged and intensive maintenance therapy results in reduced HRQoL [31].
There are limitations to the HRQoL sub-study. Response rates across monthly assessments of HRQoL remained ≥60%, consistent with that of previous studies such as the UKALL2003 clinical trial (63%) [29, 30]. However, follow-up logs indicated that parents frequently missed surveys due to forgetting or not checking emails. These findings suggest room to improve the feasibility of data collection. The early months of treatment can be a busy, overwhelming time for families, when many TRTs occur. While frequent assessment can provide useful indicators of HRQoL, it creates additional burden for parents that may affect participation. Time and staff costs for study management should be considered as well. Bi-monthly or less frequent assessment may improve the feasibility of longitudinal HRQoL data collection, providing similarly useful information, with reduced burdens. One previous longitudinal study of children’s HRQoL on active treatment for ALL included only four assessment time points and reported higher retention rates across similar time points (90% at T1 to 63% at T4) to our study [29]. There is also the possibility that the thematic information for HRQoL might be different at T1 than at T6 and hence, missing some of the earlier time points may skew the results. Utilization of iBFM versus COG protocols is site specific and there are likely systematic differences in the support and resources available to families at each site, which could affect HRQoL outcomes. There is a relative deficiency in data pertaining to site-specific resource differences which has the potential to influence and skew results, with the relatively small sample size not amenable to cluster analysis. Despite these limitations, there are several strengths of this HRQoL sub-study. It is prospective in nature and although potentially burdensome, the frequent, robust questionnaires provide an ideal opportunity to thoroughly track HRQoL across the early phase of treatment. This patient population also provides a unique opportunity to prospectively compare two contemporary treatment platforms, with respect to TRT and HRQoL.
Three longitudinal studies have assessed cancer-related HRQoL in pediatric ALL using the PedsQL cancer module [23, 27, 29, 30], and two (COG [27, 29] and UKALL [30]) reported outcomes more frequently than pre- and post-maintenance [27, 30]. The cancer-related HRQoL outcomes in ASSET differed significantly from COG and UKALL findings across the 1 month, 6 months and 12 months post-diagnosis, particularly in the domains of nausea, pain, and overall HRQoL [27, 30]. There are notable differences between the ASSET, COG, and UKALL studies, including participant age (ASSET = 0–18, COG = 2–10, UKALL = 4–18), treatment risk groups (ASSET all risk groups, COG standard risk only, UKALL all risk groups), and clinical trial involvement (ASSET a combination of clinical trial (41.5%) and standard treatment compared to patients enrolled on clinical trials for the COG and UKALL).
Compared to the COG study, in the first month post-diagnosis and 6 months post-diagnosis, our sample reported similar pain, treatment anxiety, and procedural anxiety, but significant nausea [27]. At 12 months post-diagnosis, our sample reported similar pain and treatment anxiety, but poorer HRQoL due to nausea and procedural anxiety [27]. While the UKALL study did not report on cancer-related HRQoL subscales, worse overall cancer-related HRQoL at 4 weeks post-diagnosis was reported compared to our T1 (1 month post-diagnosis) results. Additionally, in the UKALL study, overall cancer-related HRQoL at T3 (24–47 weeks = 5–12 months) was similar to our T5-T8 results but poorer than our T9-T12 results.
ALL therapy impacts HRQoL and therefore collecting HRQoL data throughout treatment is important to improve care for patients and families. The finding regarding the lack of improvement in procedural anxiety may indicate a lack of intervention or support for these concerns in early treatment which could be addressed with existing, well-established, non-pharmacological interventions, such as music therapy or distraction [66, 67]. The finding regarding parents’ need for help during the first year of ALL treatment also suggests that healthcare providers have an opportunity to address parents’ emotional concerns and foster improved well-being, given it is a time when they have frequent contact with children and families. Improving our understanding of ‘real-time’ needs for this population could assist in implementing targeted support strategies. Future research priorities for the ASSET study include examining HRQoL in the 2nd and subsequent years after a diagnosis of ALL; a comparison of the short, medium and long term treatment related toxicity incidence and patterns in patients treated on iBFM and COG platforms with a specific focus of the HRQoL experience related to the duration and intensity of maintenance therapy; the impact of an ALL diagnosis, treatment and toxicity on educational achievement; a health economic analysis of the cost of ALL therapy including the additional costs of managing treatment related toxicities arising from ALL therapy. Health economic and educational outcome analyses will be undertaken by data linkage between the ASSET study with population linked administrative data sets.
With long-term survival rates for pediatric ALL reaching new highs, it is important to understand and improve the treatment experience for children and their families, to optimize both immediate and long-term well-being. Our findings (i) validate the capacity of the ASSET study to capture TRTs, children’s HRQoL, and parents’ emotional well-being, (ii) demonstrate poor ongoing cancer-related HRQoL and increased anxiety among parents in the first 12 months for children on ALL treatment, (iii) suggest there are no major differences in early HRQoL impact between different treatment platforms in Australia and New Zealand, and (iv) suggest the HRQoL study feasibility can be improved to increase response and retention rates. We aim to continue recruitment but to refine the HRQoL study. It will be important to optimize the timing of HRQoL assessment such that it can be both clinically informative and less burdensome for families and researchers alike.
Supplementary Information
Additional file 1: Supplementary Table 1. ASSET Health-Related Quality of Life Outcomes (PedsQL Cancer Module and HUI3). Supplementary Table 2. Parents’ emotional well-being (ET). Supplementary Table 3. COG vs. iBFM Protocol Group Health-Related Quality of Life Comparisons (HUI3, PedsQL & PedsQL Nausea, Pain & Procedural Anxiety subscales). Supplementary Table 4. COG vs. iBFM Protocol Group Health Related Quality of Life Comparisons (PedsQL Anxiety, Worry, Cognitive Functioning, Physical appearance & Communication subscales). Supplementary Table 5. COG vs. iBFM Protocol group parental emotional well-being comparisons (ET).
Additional file 2: Data Table 1. HUI3 Data. Data Table 2. PedsQL Cancer Data. Data Table 3. Emotion Thermometer Data.
Acknowledgements
We would like to thank the participants and their parents and/or guardians. We acknowledge that this work was supported by the Australian and New Zealand Children’s Haematology/Oncology Group.
Abbreviations
- AIEOP-BFM
Associazione Italiana Ematologia Oncologia Pediatrica – Berlin-Franklin-Munster protocol
- iBFM
International Berlin-Franklin-Munster study group
- ALL
Acute Lymphoblastic Leukemia
- ANZCCSG
Australian and New Zealand Children’s Cancer Study Group
- ANZCHOG
Australian and New Zealand Children’s Haematology Oncology Group
- ASSET
Acute Lymphoblastic Leukemia Sub-types and Side Effects from Treatment Study
- COG
Children’s Oncology Group
- ERASE
Evaluation of Risk of ALL Treatment-Related Side Effects
- HNE HREC
Hunter New England Human Research Ethics Committee
- HRQoL
Health-related quality of life
- HUI3
Health Utility Index 3
- MPAL
Mixed Phenotype Acute Leukaemia
- PedsQL
Pediatric Quality of Life Inventory
- REDCap
Research Electronic Data Capture
- TRT
Treatment-related toxicity
Authors’ contributions
Study concept and design: Joanna E Fardell, Richard De Abreu Lourenco, Claire E Wakefield, Glenn M Marshall, Marion K Mateos & Toby N Trahair. Obtaining funding support: Joanna E Fardell, Claire E Wakefield, Glenn M Marshall, Marion K Mateos & Toby N Trahair. Database design & central data collection: Clarissa E Schilstra, Karen McCleary, Joanna E Fardell, Emma McCormack, Marion K Mateos & Toby N Trahair. Statistical support: Mark W Donoghoe. Patient recruitment & local data collection: Karen McCleary, Rishi S Kotecha, Shanti Ramachandran, Ruelleyn Cockcroft, Rachel Conyers, Siobhan Cross, Luciano Dalla-Pozza, Peter Downie, Tamas Revesz, Michael Osborn, Frank Alvaro, Claire E Wakefield, Glenn M Marshall, Marion K Mateos & Toby N Trahair. Data analysis & interpretation: Clarissa E Schilstra, Karen McCleary, Joanna E Fardell, Mark W Donoghoe, Marion K Mateos & Toby N Trahair. Manuscript writing and editing: Clarissa E Schilstra, Karen McCleary, Joanna E Fardell, Glenn Marshall, Marion K Mateos & Toby N Trahair. Manuscript review and approval: all authors.
Funding
The Kids Cancer Alliance (a Translational Cancer Research Centre of Cancer Institute NSW), Cancer Institute NSW (ECF 181430, M.K.M.), Royal Australasian College of Physicians - Kids Cancer Project Research Entry Scholarship (M.K.M.), Cancer Therapeutics CRC (CTx) PhD Clinician Researcher Top-Up Scholarship (M.K.M.), Anthony Rothe Memorial Trust (M.K.M., T.N.T.), the Kids Cancer Project (J.E.F.), a Research Training Program Scholarship from the Australian Government (C.E.S.), a PhD top-up scholarship from the Kids Cancer Alliance (C.E.S.), and a PhD top-up scholarship from the Kids to Adults (K2A) Clinical Academic Group, which is part of Maridulu Budyari Gumal, The Sydney Partnership for Health, Education, Research & Enterprise (C.E.S.). The Behavioural Sciences Unit is proudly supported by the Kids with Cancer Foundation, by the Kids Cancer Alliance and a Cancer Council New South Wales Program Grant (PG16–02) with the Estate of the Late Harry McPaul. R.S.K. is supported by a Fellowship from NHMRC Australia (APP1142627) and the Children’s Leukaemia and Cancer Research Foundation (CLCRF, Australia).
Availability of data and materials
The data that support the findings of this study are available in the Supplementary files.
Declarations
Ethics approval and consent to participate
The study was approved by the Hunter New England Human Research Ethics Committee (2019/ETH00693) and was conducted according to the Australian National Statement on Ethical Conduct in Human Research (2007) [33]. Informed consent was obtained from participants or their parents and/or legal guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clarissa E Schilstra and Karen McCleary contributed equally.
Contributor Information
Clarissa E. Schilstra, Email: c.schilstra@student.unsw.edu.au
Toby N. Trahair, Email: Toby.Trahair@health.nsw.gov.au
References
- 1.Pui C-H, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. 2013;50(3):185–196. doi: 10.1053/j.seminhematol.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui C-H, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, Vora A, Baruchel A, Silverman LB, Schmiegelow K. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33(27):2938. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caruso V, Iacoviello L, Di Castelnuovo A, Storti S, Mariani G, De Gaetano G, Donati MB. Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients. Blood. 2006;108(7):2216–2222. doi: 10.1182/blood-2006-04-015511. [DOI] [PubMed] [Google Scholar]
- 4.Athale U, Siciliano S, Thabane L, Pai N, Cox S, Lathia A, Khan A, Armstrong A, Chan AK. Epidemiology and clinical risk factors predisposing to thromboembolism in children with cancer. Pediatr Blood Cancer. 2008;51(6):792–797. doi: 10.1002/pbc.21734. [DOI] [PubMed] [Google Scholar]
- 5.Grace RF, Dahlberg SE, Neuberg D, Sallan SE, Connors JM, Neufeld EJ, DeAngelo DJ, Silverman LB. The frequency and management of asparaginase-related thrombosis in paediatric and adult patients with acute lymphoblastic leukaemia treated on Dana-Farber Cancer Institute consortium protocols. Br J Haematol. 2011;152(4):452–459. doi: 10.1111/j.1365-2141.2010.08524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mateos M, Trahair T, Mayoh C, Barbaro P, Sutton R, Revesz T, Barbaric D, Giles J, Alvaro F, Mechinaud F. Risk factors for symptomatic venous thromboembolism during therapy for childhood acute lymphoblastic leukemia. Thromb Res. 2019;178:132–138. doi: 10.1016/j.thromres.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Parasole R, Petruzziello F, Menna G, Mangione A, Cianciulli E, Buffardi S, Marchese L, Nastro A, Misuraca A, Poggi V. Central nervous system complications during treatment of acute lymphoblastic leukemia in a single pediatric institution. Leuk Lymphoma. 2010;51(6):1063–1071. doi: 10.3109/10428191003754608. [DOI] [PubMed] [Google Scholar]
- 8.Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, Krull KR, Inaba H, Rubnitz JE, Metzger ML. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32(9):949. doi: 10.1200/JCO.2013.53.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diouf B, Crews KR, Lew G, Pei D, Cheng C, Bao J, Zheng JJ, Yang W, Fan Y, Wheeler HE. Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA. 2015;313(8):815–823. doi: 10.1001/jama.2015.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mateos MK, Marshall GM, Barbaro PM, Quinn MCJ, George C, Mayoh C, et al. Methotrexate-related central neurotoxicity: clinical characteristics, risk factors and genome-wide association study in children treated for acute lymphoblastic leukemia. Haematologica. 2022;107(3):635–43. 10.3324/haematol.2020.268565. [DOI] [PMC free article] [PubMed]
- 11.Parasole R, Valsecchi MG, Silvestri D, Locatelli F, Barisone E, Petruzziello F, Putti MC, Micalizzi C, Colombini A, Mura R. Correspondence: osteonecrosis in childhood acute lymphoblastic leukemia: a retrospective cohort study of the Italian Association of Pediatric Haemato-Oncology (AIEOP) Blood Cancer J. 2018;8(12):1–6. doi: 10.1038/s41408-018-0150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez OA, Zimmerman G. Pegaspargase-induced pancreatitis. Med Pediatr Oncol. 2000;34(3):200–205. doi: 10.1002/(SICI)1096-911X(200003)34:3<200::AID-MPO7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 13.Knoderer HM, Robarge J, Flockhart DA. Predicting asparaginase-associated pancreatitis. Pediatr Blood Cancer. 2007;49(5):634–639. doi: 10.1002/pbc.21037. [DOI] [PubMed] [Google Scholar]
- 14.Schmiegelow K, Müller K, Mogensen SS, Mogensen PR, Wolthers BO, Stoltze UK, Tuckuviene R, Frandsen T. Non-infectious chemotherapy-associated acute toxicities during childhood acute lymphoblastic leukemia therapy. F1000Res. 2017;6:444. doi: 10.12688/f1000research.10768.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rank CU, Wolthers BO, Grell K, Albertsen BK, Frandsen TL, Overgaard UM, Toft N, Nielsen OJ, Wehner PS, Harila-Saari A. Asparaginase-associated pancreatitis in acute lymphoblastic leukemia: results from the NOPHO ALL2008 treatment of patients 1-45 years of age. J Clin Oncol. 2020;38(2):145–154. doi: 10.1200/JCO.19.02208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frandsen TL, Heyman M, Abrahamsson J, Vettenranta K, Åsberg A, Vaitkeviciene G, Pruunsild K, Toft N, Birgens H, Hallböök H, et al. Complying with the European clinical trials directive while surviving the administrative pressure - an alternative approach to toxicity registration in a cancer trial. Eur J Cancer. 2014;50(2):251–259. doi: 10.1016/j.ejca.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 18.Schmiegelow K, Attarbaschi A, Barzilai S, Escherich G, Frandsen TL, Halsey C, Hough R, Jeha S, Kato M, Liang DC, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016;17(6):e231–e239. doi: 10.1016/S1470-2045(16)30035-3. [DOI] [PubMed] [Google Scholar]
- 19.Vetsch J, Wakefield C, Robertson E, Trahair T, Mateos M, Grootenhuis M, Marshall G, Cohn R, Fardell J. Health-related quality of life of survivors of childhood acute lymphoblastic leukemia: a systematic review. Qual Life Res. 2018;27(6):1431–1443. doi: 10.1007/s11136-018-1788-5. [DOI] [PubMed] [Google Scholar]
- 20.Fardell JE, Vetsch J, Trahair T, Mateos M, Grootenhuis M, Touyz L, Marshall G, Wakefield C. Health-related quality of life of children on treatment for acute lymphoblastic leukemia: a systematic review. Pediatr Blood Cancer. 2017;64(9):e26489. doi: 10.1002/pbc.26489. [DOI] [PubMed] [Google Scholar]
- 21.Meeske K, Katz ER, Palmer SN, Burwinkle T, Varni JW. Parent proxy-reported health-related quality of life and fatigue in pediatric patients diagnosed with brain tumors and acute lymphoblastic leukemia. Cancer. 2004;101(9):2116–2125. doi: 10.1002/cncr.20609. [DOI] [PubMed] [Google Scholar]
- 22.Savage E, Riordan A, Hughes M. Quality of life in children with acute lymphoblastic leukaemia: a systematic review. Eur J Oncol Nurs. 2009;13(1):36–48. doi: 10.1016/j.ejon.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Sung L, Yanofsky R, Klaassen RJ, Dix D, Pritchard S, Winick N, Alexander S, Klassen A. Quality of life during active treatment for pediatric acute lymphoblastic leukemia. Int J Cancer. 2011;128(5):1213–1220. doi: 10.1002/ijc.25433. [DOI] [PubMed] [Google Scholar]
- 24.Eiser C, Davies H, Jenney M, Stride C, Glaser A. HRQOL implications of treatment with dexamethasone for children with acute lymphoblastic leukemia (ALL) Pediatr Blood Cancer. 2006;46(1):35–39. doi: 10.1002/pbc.20432. [DOI] [PubMed] [Google Scholar]
- 25.Peeters J, Meitert J, Paulides M, Wiener A, Beck J, Calaminus G, Langer T. Health-related quality of life (HRQL) in ALL-patients treated with chemotherapy only-a report from the late effects surveillance system in Germany. Klin Padiatr. 2009;221(3):156. doi: 10.1055/s-0029-1216366. [DOI] [PubMed] [Google Scholar]
- 26.van Litsenburg RR, Huisman J, Pieters R, Verhaak C, Kaspers GJ, Gemke RJ. Determinants of quality of life during induction therapy in pediatric acute lymphoblastic leukemia. Support Care Cancer. 2014;22(12):3235–3242. doi: 10.1007/s00520-014-2349-2. [DOI] [PubMed] [Google Scholar]
- 27.Dupuis LL, Lu X, Mitchell HR, Sung L, Devidas M, Mattano LA, Jr, Carroll WL, Winick N, Hunger SP, Maloney KW, et al. Anxiety, pain, and nausea during the treatment of standard-risk childhood acute lymphoblastic leukemia: a prospective, longitudinal study from the Children's oncology group. Cancer. 2016;122(7):1116–1125. doi: 10.1002/cncr.29876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng DJ, Lu X, Schore RJ, Balsamo L, Devidas M, Winick NJ, Raetz EA, Loh ML, Carroll WL, Sung L. Longitudinal analysis of quality-of-life outcomes in children during treatment for acute lymphoblastic leukemia: a report from the Children's oncology group AALL0932 trial. Cancer. 2018;124(3):571–579. doi: 10.1002/cncr.31085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell HR, Lu X, Myers RM, Sung L, Balsamo LM, Carroll WL, Raetz E, Loh ML, Mattano LA, Jr, Winick NJ. Prospective, longitudinal assessment of quality of life in children from diagnosis to 3 months off treatment for standard risk acute lymphoblastic leukemia: results of Children's oncology group study AALL0331. Int J Cancer. 2016;138(2):332–339. doi: 10.1002/ijc.29708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eiser C, Stride CB, Vora A, Goulden N, Mitchell C, Buck G, et al. Prospective evaluation of quality of life in children treated in UKALL 2003 for acute lymphoblastic leukaemia: a cohort study. Pediatr Blood Cancer. 2017;64:e26615. 10.1002/pbc.26615. [DOI] [PubMed]
- 31.Rensen N, Steur LMH, Grootenhuis MA, van Eijkelenburg NKA, van der Sluis IM, Dors N, van den Bos C, Tissing WJE, Kaspers GJL, van Litsenburg RRL. Parental functioning during maintenance treatment for childhood acute lymphoblastic leukemia: effects of treatment intensity and dexamethasone pulses. Pediatr Blood Cancer. 2020;67(11):e28697. doi: 10.1002/pbc.28697. [DOI] [PubMed] [Google Scholar]
- 32.Mateos MK, Tulstrup M, Quinn MC, Tuckuviene R, Marshall GM, Gupta R, Mayoh C, Wolthers BO, Barbaro PM, Ruud E. Genome-wide association Meta-analysis of single-nucleotide polymorphisms and symptomatic venous thromboembolism during therapy for acute lymphoblastic leukemia and lymphoma in Caucasian children. Cancers. 2020;12(5):1285. doi: 10.3390/cancers12051285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The National Health and Medical Research Council tARCaUA: National Statement on Ethical Conduct in Human Research 2007 (Updated 2018): National Health and Medical Research Council; 2007 (Updated 2018).
- 34.National Cancer Institute: DHHS . National Cancer Institute Common Terminology Criteria for Adverse Events v4. 0. NIH publication# 09–7473. 2009. [Google Scholar]
- 35.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN, Wolff L, Uckun FM, Gaynon PS. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338(23):1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 38.Matloub Y, Lindemulder S, Gaynon PS, Sather H, La M, Broxson E, Yanofsky R, Hutchinson R, Heerema NA, Nachman J, et al. Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: results of the Children's Cancer group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children's oncology group. Blood. 2006;108(4):1165–1173. doi: 10.1182/blood-2005-12-011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pieters R, Schrappe M, De Lorenzo P, Hann I, De Rossi G, Felice M, Hovi L, LeBlanc T, Szczepanski T, Ferster A. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370(9583):240–250. doi: 10.1016/S0140-6736(07)61126-X. [DOI] [PubMed] [Google Scholar]
- 40.Möricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dördelmann M, Löning L, Beier R, Ludwig WD, Ratei R, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111(9):4477–4489. doi: 10.1182/blood-2007-09-112920. [DOI] [PubMed] [Google Scholar]
- 41.Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, Freyer DR, Mattano LA, Jr, Hastings CA, Rubin CM, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's oncology group. Blood. 2008;111(5):2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schultz KR, Bowman WP, Aledo A, Slayton WB, Sather H, Devidas M, Wang C, Davies SM, Gaynon PS, Trigg M, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children's oncology group study. J Clin Oncol. 2009;27(31):5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutton R, Venn NC, Tolisano J, Bahar AY, Giles JE, Ashton LJ, Teague L, Rigutto G, Waters K, Marshall GM, et al. Clinical significance of minimal residual disease at day 15 and at the end of therapy in childhood acute lymphoblastic leukaemia. Br J Haematol. 2009;146(3):292–299. doi: 10.1111/j.1365-2141.2009.07744.x. [DOI] [PubMed] [Google Scholar]
- 44.Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grümayer R, Möricke A, Aricò M, Zimmermann M, Mann G, De Rossi G, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. doi: 10.1182/blood-2009-10-248146. [DOI] [PubMed] [Google Scholar]
- 45.Matloub Y, Bostrom BC, Hunger SP, Stork LC, Angiolillo A, Sather H, La M, Gastier-Foster JM, Heerema NA, Sailer S, et al. Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children's oncology group. Blood. 2011;118(2):243–251. doi: 10.1182/blood-2010-12-322909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall GM, Dalla Pozza L, Sutton R, Ng A, de Groot-Kruseman HA, van der Velden VH, Venn NC, van den Berg H, de Bont ES, Maarten Egeler R, et al. High-risk childhood acute lymphoblastic leukemia in first remission treated with novel intensive chemotherapy and allogeneic transplantation. Leukemia. 2013;27(7):1497–1503. doi: 10.1038/leu.2013.44. [DOI] [PubMed] [Google Scholar]
- 47.Termuhlen AM, Smith LM, Perkins SL, Lones M, Finlay JL, Weinstein H, Gross TG, Abromowitch M. Disseminated lymphoblastic lymphoma in children and adolescents: results of the COG A5971 trial: a report from the Children's oncology group. Br J Haematol. 2013;162(6):792–801. doi: 10.1111/bjh.12460. [DOI] [PubMed] [Google Scholar]
- 48.Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, Mattano LA, Jr, Cole C, Eicher A, Haugan M, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children's oncology group study AALL0232. J Clin Oncol. 2016;34(20):2380–2388. doi: 10.1200/JCO.2015.62.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez V, Kairalla J, Salzer WL, Raetz EA, Loh ML, Carroll AJ, Heerema NA, Wood BL, Borowitz MJ, Burke MJ, et al. A pilot study of intensified PEG-Asparaginase in high-risk acute lymphoblastic leukemia: Children's oncology group study AALL08P1. J Pediatr Hematol Oncol. 2016;38(6):409–417. doi: 10.1097/MPH.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pieters R, De Lorenzo P, Ancliffe P, Aversa LA, Brethon B, Biondi A, Campbell M, Escherich G, Ferster A, Gardner R. Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the Interfant-06 protocol; results from an international randomised study. Blood. 2018;132(Supplement 1):655. doi: 10.1182/blood-2018-99-112854. [DOI] [PubMed] [Google Scholar]
- 51.Winter SS, Dunsmore KP, Devidas M, Wood BL, Esiashvili N, Chen Z, Eisenberg N, Briegel N, Hayashi RJ, Gastier-Foster JM, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: results from the Children’s oncology group AALL0434 methotrexate randomization. J Clin Oncol. 2018;36(29):2926–2934. doi: 10.1200/JCO.2018.77.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maloney KW, Devidas M, Wang C, Mattano LA, Friedmann AM, Buckley P, Borowitz MJ, Carroll AJ, Gastier-Foster JM, Heerema NA, et al. Outcome in children with standard-risk B-cell acute lymphoblastic leukemia: results of Children's oncology group trial AALL0331. J Clin Oncol. 2020;38(6):602–612. doi: 10.1200/JCO.19.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL™ in pediatric cancer: reliability and validity of the pediatric quality of life inventory™ generic core scales, multidimensional fatigue scale, and cancer module. Cancer. 2002;94(7):2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 54.Horsman J, Furlong W, Feeny D, Torrance G. The health utilities index (HUI®): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1(1):54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell AJ, Baker-Glenn EA, Granger L, Symonds P. Can the distress thermometer be improved by additional mood domains? Part I. initial validation of the emotion thermometers tool. Psychooncology. 2010;19(2):125–133. doi: 10.1002/pon.1523. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell AJ, Baker-Glenn EA, Park B, Granger L, Symonds P. Can the distress thermometer be improved by additional mood domains? Part II. What is the optimal combination of emotion thermometers? Psychooncology. 2010;19(2):134–140. doi: 10.1002/pon.1557. [DOI] [PubMed] [Google Scholar]
- 57.Harju E, Michel G, Roser K. A systematic review on the use of the emotion thermometer in individuals diagnosed with cancer. Psychooncology. 2019;28(9):1803–1818. doi: 10.1002/pon.5172. [DOI] [PubMed] [Google Scholar]
- 58.Furlong W, Rae C, Feeny D, Gelber RD, Laverdiere C, Michon B, Silverman L, Sallan S, Barr R. Health-related quality of life among children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59(4):717–724. doi: 10.1002/pbc.24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santoro N, Colombini A, Silvestri D, Grassi M, Giordano P, Parasole R, Barisone E, Caruso R, Conter V, Valsecchi MG. Screening for coagulopathy and identification of children with acute lymphoblastic leukemia at a higher risk of symptomatic venous thrombosis: an AIEOP experience. J Pediatr Hematol Oncol. 2013;35(5):348–355. doi: 10.1097/MPH.0b013e31828dc614. [DOI] [PubMed] [Google Scholar]
- 60.Badke C, Fleming A, Iqbal A, Khilji O, Parhas S, Weinstein J, Morgan E, Hijiya N. Rechallenging with intrathecal methotrexate after developing subacute neurotoxicity in children with hematologic malignancies. Pediatr Blood Cancer. 2016;63(4):723–726. doi: 10.1002/pbc.25850. [DOI] [PubMed] [Google Scholar]
- 61.Rubnitz J, Relling M, Harrison P, Sandlund J, Ribeiro R, Rivera G, Thompson S, Evans W, Pui C. Transient encephalopathy following high-dose methotrexate treatment in childhood acute lymphoblastic leukemia. Leuk. 1998;12(8):1176–1181. doi: 10.1038/sj.leu.2401098. [DOI] [PubMed] [Google Scholar]
- 62.Kawedia JD, Kaste SC, Pei D, Panetta JC, Cai X, Cheng C, Neale G, Howard SC, Evans WE, Pui C-H. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117(8):2340–2347. doi: 10.1182/blood-2010-10-311969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strauss AJ, Su JT, Dalton VMK, Gelber RD, Sallan SE, Silverman LB. Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol. 2001;19(12):3066–3072. doi: 10.1200/JCO.2001.19.12.3066. [DOI] [PubMed] [Google Scholar]
- 64.Samarasinghe S, Dhir S, Slack J, Iyer P, Wade R, Clack R, Vora A, Goulden N. Incidence and outcome of pancreatitis in children and young adults with acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol. 2013;162(5):710–713. doi: 10.1111/bjh.12407. [DOI] [PubMed] [Google Scholar]
- 65.Wolthers B, Frandsen TL, Abrahamsson J, Albertsen B, Helt L, Heyman M, Jónsson ÓG, Kõrgvee L, Lund B, Raja R. Asparaginase-associated pancreatitis: a study on phenotype and genotype in the NOPHO ALL2008 protocol. Leukemia. 2017;31(2):325–332. doi: 10.1038/leu.2016.203. [DOI] [PubMed] [Google Scholar]
- 66.Nunns M, Mayhew D, Ford T, Rogers M, Curle C, Logan S, Moore D. Effectiveness of nonpharmacological interventions to reduce procedural anxiety in children and adolescents undergoing treatment for cancer: a systematic review and meta-analysis. Psychooncology. 2018;27(8):1889–1899. doi: 10.1002/pon.4749. [DOI] [PubMed] [Google Scholar]
- 67.Landier W, Alice MT. Use of complementary and alternative medical interventions for the management of procedure-related pain, anxiety, and distress in pediatric oncology: an integrative review. J Pediatr Nurs. 2010;25(6):566–579. doi: 10.1016/j.pedn.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. ASSET Health-Related Quality of Life Outcomes (PedsQL Cancer Module and HUI3). Supplementary Table 2. Parents’ emotional well-being (ET). Supplementary Table 3. COG vs. iBFM Protocol Group Health-Related Quality of Life Comparisons (HUI3, PedsQL & PedsQL Nausea, Pain & Procedural Anxiety subscales). Supplementary Table 4. COG vs. iBFM Protocol Group Health Related Quality of Life Comparisons (PedsQL Anxiety, Worry, Cognitive Functioning, Physical appearance & Communication subscales). Supplementary Table 5. COG vs. iBFM Protocol group parental emotional well-being comparisons (ET).
Additional file 2: Data Table 1. HUI3 Data. Data Table 2. PedsQL Cancer Data. Data Table 3. Emotion Thermometer Data.
Data Availability Statement
The data that support the findings of this study are available in the Supplementary files.