Dear Editor,
While the world continues to face an ongoing coronavirus disease 2019 (COVID-19) pandemic, we are witnessing the re-emergence of two zoonotic viruses: monkeypox (MPX) and more recently Marburg virus (MARV). The MPX outbreak has infected more than 27 814 patients in 89 countries and territories as of Aug 2022 and was declared a public health emergency of international concern on July 23, 2022. The MARV outbreak involved only two cases to date in the southern Ashanti region of Ghana as of July 17th, 2022 [1].
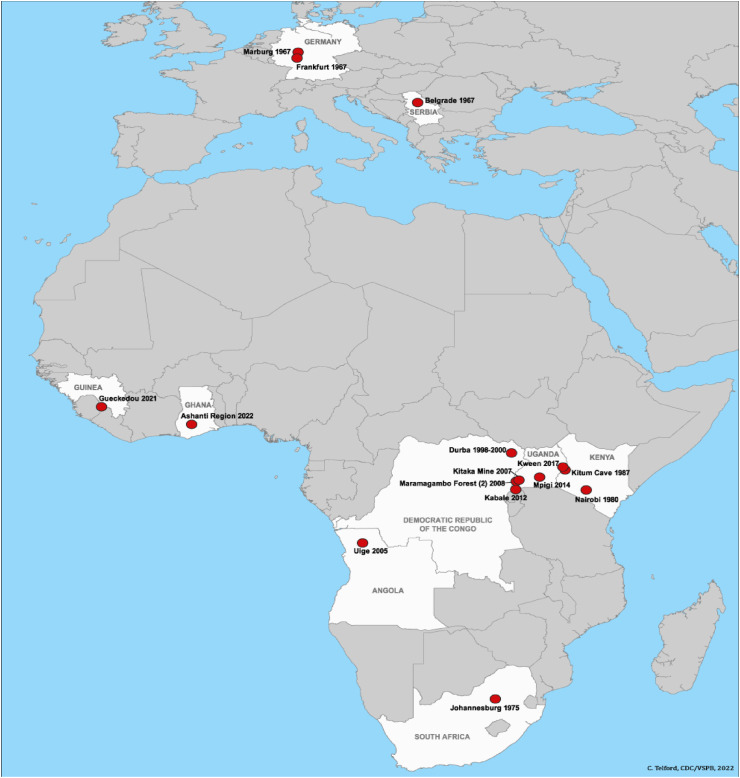
Historically, MARV, which causes the deadly hemorrhagic Marburg virus disease (MVD), was first detected in 1967 in laboratories during the two large outbreaks in Marburg (hence the virus name) and Frankfurt in Germany, and Belgrade in Serbia, respectively. 32 cases (1 case was diagnosed retrospectively) were infected with MARV by laboratory workers, followed by other medical personnel, then their family members, and 7 of whom died. The outbreak was linked to exposure of the first infected case to African green monkeys (Cercopithecus aethiops) or their tissues imported from Uganda during laboratory research work on development of poliomyelitis vaccine [[2], [3], [4]]. Sporadic cases and outbreaks have subsequently been reported in Kenya, the Democratic Republic of the Congo (DRC), Angola, South Africa, and Uganda. In 2008, two independent travelers who visited a cave of Rousettus bat colonies in Uganda were infected with MARV [4]. In September 2021, Guinea confirmed a MARV case during an outbreak and amid the COVID-19 pandemic [1] (Fig. 1 ). In 2020, Steffen et al. performed a retrospective preliminary serological analysis on 2430 serum samples that were collected during the period 1997–2012 from five African countries (Uganda, Ghana, Cameroon, Republic of Congo, DRC), and reported a seroprevalence of 6.3 and 7.5% of MARV in Ghana and Cameroon, respectively. This might indicate the presence of filoviruses or related viruses beyond the known endemic territories but undetected due to inadequate surveillance efforts and lack of validated assays [5].
Fig. 1.
Outbreak of Marburg virus with location and year [2]. This image is attributed by C. Telford, CDC/VSPB (https://www.cdc.gov/vhf/marburg/outbreaks/distribution-map.html).
MARV is a zoonotic single-stranded enveloped RNA virus of the Filovirus genus under the umbrella of the Filoviridae family, which is the same family as the sister Ebola virus causing Ebola virus disease (EVD) [2]. These two deadly diseases (MVD and EVD) have usually alerted national and regional agencies for urgent responses and preparedness. MARV infects both human and non-human primates [6]. African fruit bat (Rousettus aegyptiacus) is thought to be the main host and reservoir for MARV, which can transmit it to humans through direct contact with bats' saliva, feces, and contaminated fruits or materials (i.e., bedding). More importantly, prolonged exposure to mines and caves of bat colonies are considered a way of transmission [2,4]. Additionally, MARV can be transmitted from human to human via close contact with broken skin or mucous membranes in eyes, nose, or mouth or blood and bodily fluids. Interestingly, MARV can remain in the semen of MVD-recovered patients. However, no established evidence on whether MARV can be transmitted via semen or vaginal fluids or not [2]. Further research is needed in this area. Historically, groups at high risk for exposure to MARV include family members of infected MARV cases, medical personnel, or veterinarians handling non-human primates infected with MARV [2].
MVD causes a rare but deadly hemorrhagic fever with a case fatality rate of up to 88% [4]. MVD has an incubation period of 2–21 days with sudden initial symptoms of headache, chills, fever, and myalgia. A maculopapular rash starts to occur around the 5th day of initial symptoms with main prominence on the trunk including chest, back, and abdomen. Nausea, vomiting, diarrhea, chest or abdominal pain, and sore throat frequently occur. Severe symptoms i.e., pancreatitis, jaundice, severe weight loss, severe hemorrhage, hepatic failure, delirium, shock, and multi-organ dysfunction may occur [2]. Some other diseases including malaria, typhoid or Lassa fever, and Ebola, may be misdiagnosed with MVD, making the clinical diagnosis difficult. As a result, laboratory testing and confirmation are recommended in all cases [2].
Essential medical measures should be given with suspected cases who have early MVD symptoms. Public health authorities should be notified once finding a suspected case after isolating the case. Further, serum samples should be taken from the case to either reject or confirm MVD infection in the first few days of symptom onset. Confirmation methods include polymerase chain reaction (PCR), antigen-capture enzyme-linked immunosorbent assay (ELISA), and IgM-capture ELISA. The IgG-capture ELISA is the main method for follow-up [2].
No specific antivirals or vaccines are licensed against MARV. However, supportive care (i.e., rehydration with oral or intravenous fluids, maintaining oxygen status and blood pressure, and treatment of specific symptoms and co-infections) should be given. A group of antivirals and vaccines are being evaluated for use against MARV but they are still in phase 1 clinical trials. Further research is warranted and paramount to developing effective antivirals and vaccines against this deadly MVD [1,2].
Although this disease is still confined now to Ghana, we cannot determine the burden and extent of this MVD re-emerging with current globalization, climate changes, and international travel to and from endemic countries which may spread to multiple countries, thus posing global public health concerns. Early containment of MVD in Ghana is warranted. After reporting the two cases, the World Health Organization (WHO) has formed a team of experts to provide better disease surveillance, coordination, and infection control measures, including personal protective equipments, testing, tracing contacts and raising public awareness on the risks and dangers of MVD [1]. Moreover, health authorities in Ghana have responded quickly by starting to prepare for the possible outbreak by providing required resources to curb MARV spread early and prevent it from getting out of hand [1].
Although preventive measures against the MARV infection have not yet been well defined, it is paramount to follow the common preventive methods against its transmission, including avoidance of close contact with fruit bats (Rousettus aegyptiacus), sick non-human primates, or suspected human cases. Efficient use of diagnostic tools and prevention of making a wrong diagnosis should be established and prioritized, especially in remote areas with limited access to healthcare services, and to screen all international travelers, particularly to or from endemic countries [2].
To conclude, in the era of multiple global outbreaks, we should be fully equipped and ready for any MARV quick and sudden spread on a wide scale, especially during the ongoing COVID-19 pandemic and multi-country MPX outbreak for early containment of MARV or MVD outbreaks. Preparedness of healthcare systems and medical personnel play main and fundamental roles. Also, increasing public awareness on different aspects of the COVID-19 pandemic, MPX, and MARV outbreaks is of paramount importance [7,8].
Provenance and peer review
Not commissioned, internally peer-reviewed.
Sources of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
Not applicable.
Author contribution
RAF: designed the study. RAF: made the first draft. RAF and ZAM: updated the manuscript. RAF and ZAM: reviewed the final draft and edited final. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Research registration Unique Identifying number (UIN)
-
1.
Name of the registry:
-
2.
Unique Identifying number or registration ID:
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked):
Guarantor
All authors.
Declaration of competing interest
No conflicts to declare.
References
- 1.Ghana declares first-ever outbreak of Marburg virus disease. https://www.afro.who.int/countries/ghana/news/ghana-declares-first-ever-outbreak-marburg-virus-disease-0 n.d. August 20, 2022. [DOI] [PubMed]
- 2.Marburg (Marburg virus disease) https://www.cdc.gov/vhf/marburg/index.html n.d. August 20, 2022.
- 3.Siegert R., Shu H.-L., Slenczka W. Isolierung und Identifizierung des „Marburg-Virus. DMW - Dtsch. Medizinische Wochenschrift. 1968;93:604–612. doi: 10.1055/s-0028-1105103. [DOI] [PubMed] [Google Scholar]
- 4.Marburg virus disease. https://www.who.int/health-topics/marburg-virus-disease#tab=tab_1 n.d. August 20, 2022.
- 5.Steffen I., Lu K., Hoff N.A., Mulembakani P., Okitolonda Wemakoy E., Muyembe-Tamfum J.-J., Ndembi N., Brennan C.A., Hackett J., Switzer W.M., Saragosti S., Mbensa G.O., Laperche S., Rimoin A.W., Simmons G. Seroreactivity against Marburg or related filoviruses in west and central Africa. Emerg. Microb. Infect. 2020;9:124–128. doi: 10.1080/22221751.2019.1709563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adepoju P. West Africa on alert for haemorrhagic fevers. Lancet. 2021;398:653. doi: 10.1016/S0140-6736(21)01863-8. [DOI] [PubMed] [Google Scholar]
- 7.Hospital preparedness for epidemics. https://apps.who.int/iris/bitstream/handle/10665/151281/9789241548939_eng.pdf n.d. August 20, 2022.
- 8.Increasing preparedness and prevention measures for monkeypox. https://www.who.int/indonesia/news/detail/05-06-2022-increasing-preparedness-and-prevention-measures-for-monkeypox n.d. August 20, 2022.