Abstract
Background
In the CheckMate 9ER trial, advanced renal cell carcinoma (aRCC) patients received first-line nivolumab+cabozantinib or sunitinib. The effect of treatment was evaluated via patient-reported outcomes (PROs).
Methods
In this phase 3, randomised, open-label trial, patients 18 years or older with previously untreated aRCC with a clear cell component and Karnofsky performance status ≥70% were randomised 1:1 via interactive response technology at 125 sites in 18 countries to nivolumab 240 mg intravenously every 2 weeks plus oral cabozantinib 40 mg/day (n=323), or oral sunitinib 50 mg/day monotherapy for 4 weeks in 6-week cycles (n=328). Patients were stratified by International Metastatic Renal-Cell Carcinoma Database Consortium prognostic risk score, geographic region, and tumour programmed-death ligand 1 expression. The primary endpoint of progression-free survival was reported previously. PROs were analysed as exploratory endpoints at common timepoints (at baseline and every 6 weeks) through week 115. Median (IQR) follow-up was 23·5 (21·0–26·5) months. Disease-related symptoms (DRS) were evaluated using the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI-19) and global health status with the EQ-5D-3L visual analogue scale (VAS) and UK utility index. PRO analyses were completed in the intention-to-treat population. Change from baseline was assessed using mixed-model repeated measures. Time to deterioration (TTD) analysis was conducted for first (TTFD) and confirmed (TTCD) deterioration events. This study is registered with ClinicalTrials.gov (NCT03141177) and is closed to recruitment.
Findings
Between September 11, 2017, and May 14, 2019, 323 patients were randomised to nivolumab+cabozantinib and 328 to sunitinib. At baseline, patients in both arms reported low symptom burden. Change from baseline in PRO scores indicated that nivolumab+cabozantinib was associated with more favourable outcomes versus sunitinib (treatment difference was 2·38 [95% CI 1·20–3·56] for FKSI-19 total score, 1·33 [0·84–1·83] for FKSI-19 DRS-v1, 3·48 [1·58–5·39] for EQ-5D-3L VAS, and 0·04 [0·01–0·07] for EQ-5D-3L UK utility index), reaching significance at most timepoints, with small to moderate effect sizes (0·2–0·5). Nivolumab+cabozantinib was associated with decreased risk of clinically meaningful deterioration (95% CI) for FKSI-19 total score (TTFD HR 0·70 [0·56–0·86]; nominal p=0·0007; TTCD HR 0·63 [0·50–0·80]; nominal p=0·0001).
Interpretation
PROs were maintained or improved with nivolumab+cabozantinib versus sunitinib. Compared with sunitinib, nivolumab+cabozantinib significantly delayed TTD (with and without confirmation). These results suggest a benefit for nivolumab+cabozantinib compared with sunitinib in the treatment of patients with aRCC.
Funding
Supported by Bristol Myers Squibb in collaboration with Ono Pharmaceutical and with Exelixis, Ipsen Pharma, and Takeda Pharmaceutical.
Keywords: patient-reported outcomes, health-related quality of life, renal cell carcinoma
Introduction
Renal cell carcinoma (RCC) is first diagnosed in the advanced or metastatic state in over 25% of patients, with prognosis and associated treatment strategy dependent on risk factors.1,2 Over the past decade, the RCC treatment landscape has evolved quickly as new and more effective mono- and combination therapies have emerged.3,4 First-line monotherapies for advanced or metastatic RCC (aRCC) often include multi-targeted tyrosine kinase inhibitors (TKIs) such as sunitinib, pazopanib, and cabozantinib. Data from newer treatments, including combination therapies such as nivolumab plus cabozantinib, nivolumab plus ipilimumab, and pembrolizumab plus axitinib, suggest more robust and durable improvements to survival outcomes.5,6 While novel treatments are effective in delaying disease progression and prolonging overall survival, they do not always have a demonstrable benefit on patient health-related quality of life (HRQoL); in some cases, treatments associated with greater toxicity may negatively impact HRQoL.6,7
As RCC metastasises to sites such as bone and soft tissue, patients may experience a substantially negative impact to all domains of HRQoL, including physical and mental function.2,8 It is therefore important to assess the risks and benefits of treatment options for aRCC, with the aim of identifying effective therapies with improved tolerability and substantial associated HRQoL benefits.
The CheckMate 9ER trial investigated the use of the programmed cell death protein 1 (PD-1) immune checkpoint inhibitor antibody nivolumab in combination with the TKI cabozantinib versus sunitinib in previously untreated patients with aRCC.9 At a median follow-up of 18·1 months, nivolumab plus cabozantinib demonstrated greater efficacy (as indicated by overall and progression-free survival), as well as confirmed objective response compared with sunitinib monotherapy; efficacy was maintained at 16 months minimum follow-up.9,10 Cabozantinib monotherapy previously showed a longer quality-adjusted survival versus sunitinib in a similar setting.11 In addition to safety and efficacy outcomes, exploratory objectives CheckMate 9ER included evaluation of patients’ disease-related symptoms, based on the National Comprehensive Cancer Network Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI-19), and health status, based on the three-level version of the EQ-5D (EQ-5D-3L). In order to provide further insight into the benefit-risk profile of nivolumab plus cabozantinib in previously untreated patients with aRCC, the current study aimed to describe the patient-reported outcome (PRO) results for CheckMate 9ER from a database lock date of September 10, 2020 (median [IQR] follow-up 23·5 [21·0–26·5] months).12 PROs from the previous database lock (March 30, 2020; median follow-up 18·1 months) have been presented elsewhere.13
Methods
Study design and participants
CheckMate 9ER (ClinicalTrials.gov, number NCT03141177) was a randomised, open-label, phase 3 trial conducted in patients with previously untreated aRCC. Patients were randomised at 125 sites in 18 countries. Eligible patients were 18 years of age or older with measurable (according to Response Evaluation Criteria in Solid Tumors version 1.1) aRCC with a clear-cell component falling within any International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk group, Karnofsky performance status score ≥70, and available tumour tissue. Patients with active central nervous system metastases (unless treated and stable according to predefined criteria), active autoimmune disease (except for predefined conditions), conditions requiring systemic treatment with corticosteroids or immunosuppressive medications within 14 days of randomisation, prior malignancy within the previous 3 years (except locally curable cancers that appear to have been cured), tumours invading the superior vena cava (or other major blood vessels) or gastrointestinal tract (including endotracheal or endobronchial), and certain predefined gastrointestinal and vascular disorders were excluded (complete exclusion criteria are detailed in the protocol, which is available in the appendix). Patients must have had adequate organ function based on laboratory testing requirements as detailed in the protocol. Methods and selection criteria have been reported previously.9 All patients provided written informed consent as per the Declaration of Helsinki. This trial was approved by the institutional review board at each site and conducted according to Good Clinical Practice guidelines defined by the International Conference on Harmonisation. During the study, protocol amendments on December 18, 2017 and May 6, 2019 were made which affected the design of the study and recruitment. These amendments are detailed in the protocol, which is available in the appendix.
Randomisation and masking
Patients were randomly assigned 1:1 by use of an interactive response technology to treatment with nivolumab plus cabozantinib combination therapy or sunitinib monotherapy. The allocation sequence was generated by the Bristol Myers Squibb interactive response technology team. This allocation sequence was transferred to a third-party vendor for enrolment of patients and assignment to trial groups in collaboration with the investigators at the study sites. Because the trial was open-label, no masking occurred. Progression-free survival was assessed by blinded independent central review. Randomisation was stratified by IMDC prognostic score (0 vs 1–2 vs 3–6), programmed cell death ligand-1 (PD-L1) tumour expression (≥1% vs <1% or indeterminate), and geographic region (United States or Europe vs rest of the world).9 Randomisation procedures were carried out via permuted blocks within each stratum.
Procedures
Nivolumab 240 mg was administered as an intravenous infusion every 2 weeks, to be continued until disease progression or unacceptable toxicity with maximum treatment of 2 years from the first dose in cycle 1. Oral cabozantinib 40 mg was given daily until disease progression or unacceptable toxicity. Oral sunitinib 50 mg was administered daily for 4 weeks, followed by 2 weeks off per cycle, and continued until disease progression or unacceptable toxicity. Protocol-defined dose reductions were allowed for cabozantinib and sunitinib, but none were permitted for nivolumab. According to the protocol, patients discontinuing either nivolumab or cabozantinib could continue treatment with the other drug that was not related to the observed toxicity.9
Disease assessments were done using CT or MRI at baseline, week 12 (±7 days), followed by every 6 weeks (±7 days) until week 60, and then every 12 weeks (±14 days) until confirmed radiographic progression. Adverse events were assessed at cycle 1 day 1, each subsequent treatment visit, and at follow-up visit 1. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Laboratory monitoring was completed at screening, cycle 1 day 1, subsequent treatment visits, at follow-up visit 1, and at follow-up visit 2 if toxicities were present.
PRO measures were administered using the paper method at study visits before the start of each treatment cycle and at follow-up visits (appendix page 5). The EQ-5D-3L only was administered by phone during survival follow-up visits (every 3 months from follow-up visit 2). Owing to differences in administration frequency inherent to the study treatment schedules and the fact that the study was designed to collect as much PRO data as operationally feasible, PRO data were collected more frequently for patients in the nivolumab plus cabozantinib arm (every 2 weeks) compared with those receiving sunitinib monotherapy (every 6 weeks). PRO data were available for both arms at common timepoints at baseline and every 6 weeks—ie, at visits occurring at weeks 7, 13, and 19, etc., up until week 115; PRO analyses were restricted to these common timepoints.
The FKSI-1914 is a multidimensional, self-administered 19-item questionnaire that measures disease-related symptoms in patients with kidney cancer. The 19 items cover symptoms (ie, lack of energy, fatigue, decreased appetite, coughing, shortness-of-breath, pain, and nausea), ability to work, and HRQoL. Patients respond by selecting one of five response categories ranging from “not at all” to “very much.” Patients are asked to select the response category that best characterises their response over the past 7 days. Higher scores indicate fewer disease-related symptoms (ie, better HRQoL). Several scores can be estimated; our study reports on the FKSI-19 total score (19 items; score range 0–76) and related scales, namely the FKSI disease-related symptoms v.1 (FKSI-DRS-v1; 9 items relating to fatigue, pain, weight loss, dyspnoea, cough, fever, and haematuria; score range 0–36), FKSI disease-related symptoms physical (FKSI-DRS-P; 12 items; score range 0–48), FKSI functional wellbeing (FKSI-FWB; three items; score range 0–12), and the single-item GP5 (FKSI-19 item 16; score range 0–4), which assess bother associated with the side effects of treatment.
The EQ-5D-3L15 is an international, standardised, generic instrument for describing and valuing global health status and includes the EQ-5D-3L descriptive system and a visual analogue rating scale. The EQ-5D-3L descriptive system includes five items that assess current problems (with three levels; none, moderate, extreme) with mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Responses can be weighted and aggregated to generate a utility index score measuring the societal value of a respondent’s health state with values ranging from 1 (full health) to 0 (dead); values less than 0 indicate a state worse than dead.16 For this study, utility index scores were generated using UK population preferences. The EQ-5D-3L also includes a visual analogue scale (VAS), which asks respondents to rate their current health on a scale ranging from “best imaginable” (100) to “worst imaginable” (0), using the recall period of today. Sample estimates based on EQ-5D-3L data were compared against established population norms.15,17
Outcomes
The primary endpoint in CheckMate 9ER was progression-free survival by blinded independent central review (BICR) in the intention-to-treat population.9 Progression-free survival was defined as the time between the date of randomisation and the first date of documented progression or death due to any cause, whichever occurs first. Secondary endpoints consisted of OS and objective response rate per BICR in intention-to-treat patients, and safety in patients who received at least one dose of treatment. Overall survival was defined as the time between the date of randomisation and the date of death due to any cause. Objective response rate was defined as the proportion of patients with a best response of complete response or partial response per RECIST version 1.1. Here we report the exploratory endpoints of PROs evaluating patient HRQoL as measured by FKSI-19 and global health status as measured by EQ-5D-3L.
Statistical analyses
Details regarding the statistical analyses for the primary and secondary endpoints of CheckMate 9ER have been previously reported.9 Here, we report PRO analyses based on the intention-to-treat population, which included all randomly assigned patients, classified according to their assigned treatment group regardless of the actual treatment received. Time-to-event analyses were performed on the intention-to-treat population. The longitudinal change from baseline analyses were done on the intention-to-treat population with patients who had a baseline PRO assessment and at least one PRO assessment after baseline. Completion rates for PRO instruments were defined as the proportion of patients who completed evaluable forms (ie, >50% of the items completed according to the scoring algorithms for FKSI-19 and all five items of the descriptive system or the VAS for EQ-5D-3L) among those who were expected to complete them (ie, who were alive and still on study), according to the schedule of assessments (appendix page 5).
Statistical comparisons were made at the α=0·05 significance level unless stated otherwise. Two-sided nominal p-values were calculated, with no adjustments made for multiple comparisons. The nominal p-value calculates the observed significance. Clinically meaningful thresholds for patient-level improvement or deterioration were prespecified (appendix page 4) and based on previously reported thresholds for EQ-5D-3L and FKSI-19 DRS-v1.18,19 Sensitivity analyses assessed different thresholds.
Longitudinal change from baseline was evaluated via mixed-model repeated measures (MMRM) analysis, which assumed that missing observations were missing at random. In addition, a sensitivity analysis using a pattern-mixture model (PMM) with sequential modelling with multiple imputation and delta adjustment (ie, assuming missing not at random) was also performed (details available on page 3 in the appendix).20 Analyses included all visits with at least 10 patients in each arm. Follow-up visits and unscheduled visits were excluded from MMRM analyses. The dependent variable was change from baseline for each PRO score. The model included the treatment arm, timepoint (study week), and randomisation factors (IMDC prognostic score, PD-L1 tumour expression, and region) as fixed-effect categorical factors, the baseline PRO score as a continuous parameter, and the interactions between baseline and timepoint and between treatment and timepoint. An unstructured covariance matrix was first used for model fitting, and upon a failure of the iterative procedure to converge, a heterogeneous Toeplitz covariance structure was used. Effect sizes (ES), expressed as Hedges’ g, were also calculated.
Time to first deterioration (TTFD) and time to confirmed deterioration (TTCD) were assessed for FKSI-19 and EQ-5D-3L. TTFD was defined as the time from randomisation to the first date that a patient had a change from baseline meeting or exceeding the prespecified primary meaningful change threshold for the scale (table 1). TTCD was defined as the time from randomisation to the date of first deterioration in PRO scores that was either confirmed at the next consecutive scheduled visit common for both arms (at least 6 weeks apart), or followed by dropout, resulting in missing data. Patients with no baseline assessment were censored at the randomisation date. Patients without an assessment after baseline were censored at the date of the baseline assessment. Patients who did not experience deterioration before the time of the data cutoff or patients whose baseline scores do not allow for further deterioration were censored at the date of the last available PRO assessment (i.e. date of the last non-missing value). Death or progression were not considered deterioration events. Both time to deterioration (TTD) analyses were performed by means of the Kaplan-Meier product limit method. Inferences for time-to-event endpoints were assessed by a log-rank test stratified by the factors at randomisation. Hazard ratios (HRs) and associated 95% confidence intervals (CIs) were ascertained with a stratified Cox proportional hazards model, using the same stratification factors as above.
Table 1:
Select demographic and baseline characteristics and baseline PRO scores (intention-to-treat population)a
| Parameter/category | Nivolumab plus cabozantinib (n=323) | Sunitinib (n=328) |
|---|---|---|
| Age, years | ||
| Mean (SD) | 61·4 (10·2) | 60·4 (10·6) |
| Median (range) | 62·0 (29–90) | 61·0 (28–86) |
|
| ||
| Sex, n (%) | ||
| Male | 249 (77·1) | 232 (70·7) |
| Female | 74 (22·9) | 96 (29·3) |
|
| ||
| Geographic region, n (%) | ||
| United States or Europe | 158 (48·9) | 161 (49·1) |
| Rest of the world | 165 (51·1) | 167 (50·9) |
|
| ||
| Baseline IMDC prognostic risk score, n (%) | ||
| Favourable: 0 | 74 (22·9) | 72 (22·0) |
| Intermediate: 1–2 | 188 (58·2) | 188 (57·3) |
| Poor: 3–6 | 61 (18·9) | 68 (20·7) |
|
| ||
| Tumour PD-L1 expression, n (%) | ||
| ≥1% | 83 (25·7) | 83 (25·3) |
| <1% or indeterminate | 240 (74·3) | 245 (74·7) |
|
| ||
| Prior nephrectomy, n (%) | ||
| Yes | 222 (68·7) | 233 (71·0) |
| No | 101 (31·3) | 95 (29·0) |
|
| ||
| Prior radiotherapy, n (%) | ||
| Yes | 46 (14·2) | 45 (13·7) |
| No | 277 (85·8) | 283 (86·3) |
|
| ||
| Time from study diagnosis to randomisation, years, n (%) | ||
| <1 year | 210 (65·0) | 214 (65·2) |
| ≥1 year | 112 (34·7) | 111 (33·8) |
| Not reported | 1 (0·3) | 3 (0·9) |
|
| ||
| FSKI-19, mean (SD) | ||
| Total score (range 0–76) | 58·74 (10·57) | 58·39 (9·92) |
| DRS-v1 (range 0–36) | 30·24 (5·19) | 30·06 (5·03) |
| DRS-P (range 0–48) | 38·19 (6·96) | 38·14 (6·47) |
| FWB (range 0–12) | 7·17 (3·49) | 7·08 (3·48) |
|
| ||
| EQ-5D-3L, mean (SD) | ||
| VAS (range 0–100) | 74·23 (22·23) | 75·68 (20·92) |
| Utility index (range 0–1) | 0·78 (0·25) | 0·73 (0·29) |
The intention-to-treat population included all the patients who underwent randomisation. IMDC prognostic risk score, PD-L1 status, and geographic region (stratification factors) were recorded at screening by means of interactive response technology.
IMDC=International Metastatic Renal Cell Carcinoma Database Consortium. PD-L1=programmed cell death ligand-1. SD=standard deviation.
In an effort to identify which symptoms may drive the observed difference in time-to-event analysis, a post-hoc analysis was conducted to plot the change from baseline responses in the individual items at the time when the deterioration event (either first or confirmed) was observed.
Descriptive analyses through week 115 were also conducted for observed scale scores for each treatment group and timepoint. In addition, we assessed the responses to the FKSI-19 GP5, item 16 “I am bothered by side effects of treatment” by treatment group21–24 by calculating the proportions of patients who felt “not at all”, “a little bit”, “somewhat”, “quite a bit”, and “very much” bothered by side-effects among those who were expected to complete the PRO assessment at each timepoint.
We analysed data using SAS version 9.4.
Role of the funding source
The funders contributed to the study design, data analysis, and data interpretation in collaboration with the authors. The funders did not have a role in data collection. Financial support for editorial and writing assistance was provided by the funders. All authors had complete access to the data in the study.
Results
Between September 11, 2017, and May 14, 2019, 651 patients were randomly assigned to nivolumab plus cabozantinib (n=323) or sunitinib (n=328). Median (IQR) follow-up was 23·5 (21·0–26·5) months. Demographic and baseline characteristics of all patients were balanced between the treatment arms (table 1).
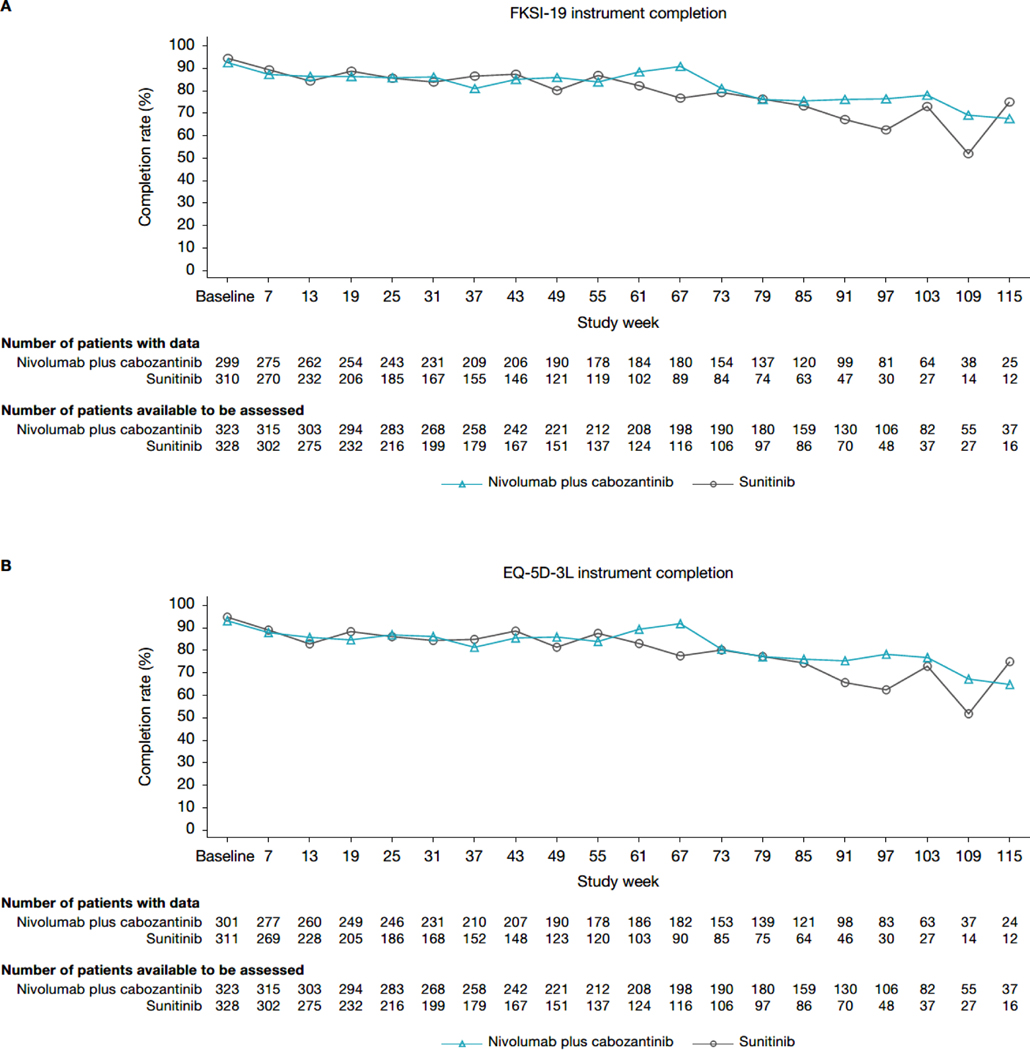
PRO completion rates were comparable across groups (figure 1). The percentage of patients completing the FKSI-19 and EQ-5D-3L instruments was high at baseline (≥93·0% for both treatment arms). The completion rate for FKSI-19 and EQ-5D-3L remained high (≥75%) at most timepoints for the nivolumab plus cabozantinib arm through week 103, after which the completion rate decreased below 65%. For the sunitinib arm, the completion rate was high (≥75%) at most timepoints through week 79, decreasing thereafter. Patient disposition is illustrated in appendix page 6.
Figure 1: PRO completion. (A) FKSI-19. (B) EQ-5D-3L.
EQ-5D-3L=three-level version of the EQ-5D. FKSI-19=Functional Assessment of Cancer Therapy-Kidney Symptom Index.
Baseline PRO scores were comparable between the treatment groups and showed relatively low symptom burden (table 1). FKSI-19 DRS-v1 mean scores at baseline for nivolumab plus cabozantinib and for sunitinib were 30·24 and 30·06, respectively, on a scale of 0 to 36, where higher scores indicate lower symptom burden. Baseline EQ-5D-3L scores suggested a baseline health status that was slightly lower than population norms (VAS 82·8; utility index 0·856),17 but higher than previously reported patient scores (eg, VAS 68; utility index 0·72).18
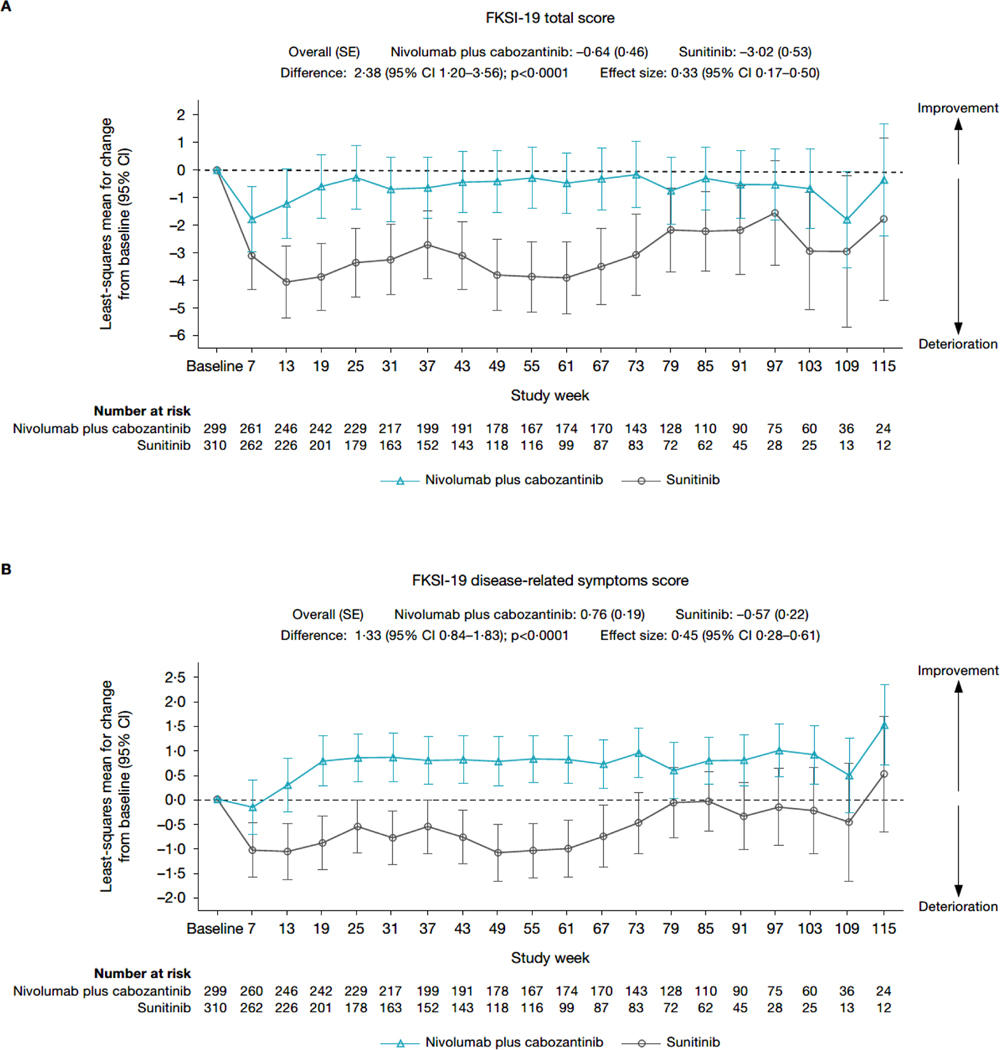
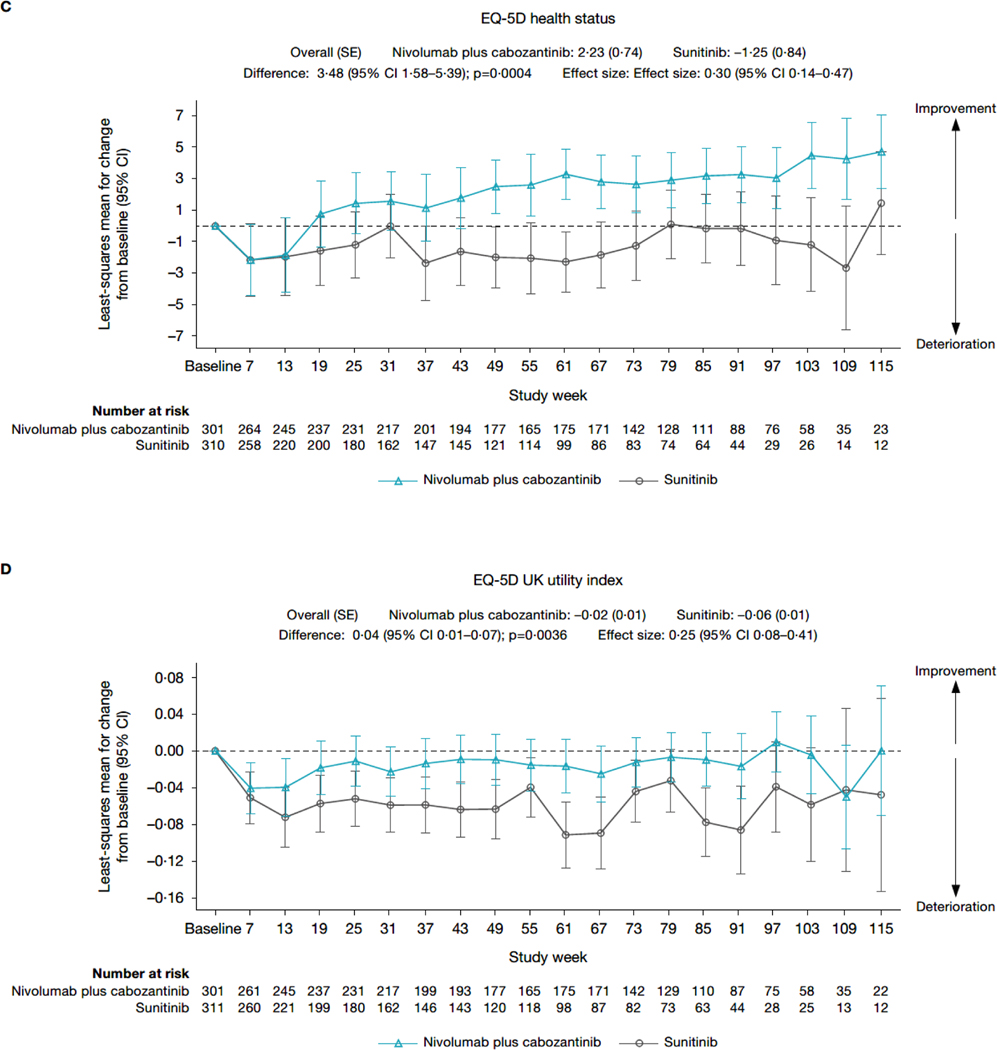
After an initial decline in most scores across treatment arms, MMRM analysis (figure 2; appendix page 7) showed that nivolumab plus cabozantinib was associated with scores that were stable (FKSI-19 total score and EQ-5D-3L UK utility index) or improved (FKSI-19 DRS-v1 and EQ-5D-3L VAS) over time. In contrast, patients receiving sunitinib experienced a decline from baseline in scores (ie, a deterioration in health status) across all instruments. None of the changes from baseline exceeded the predefined clinically meaningful threshold in any arm except deterioration of EQ-5D-3L UK utility index values in the sunitinib arm, which was clinically meaningful at several timepoints after week 55. A similar trend was observed for the mean observed scores (appendix page 8–9).
Figure 2: Change from baseline in (A) FKSI-19 total score, (B) FKSI-19 DRS-v1 score, (C) EQ-5D-3L VAS score, and (D) EQ-5D-3L UK utility index value – MMRM results.
CI=confidence interval. DRSv1=disease-related symptoms version 1. EQ-5D-3L=three-level version of the EQ-5D. FKSI-19=Functional Assessment of Cancer Therapy-Kidney Symptom Index. MMRM=mixed-model repeated measures. SD=standard deviation. SE=standard error.
Mean change from baseline in FKSI-19 and EQ-5D-3L scores consistently favoured nivolumab plus cabozantinib versus sunitinib, with nominally statistically significant differences between treatment arms at most timepoints through week 115. The overall difference in mean score change from baseline through week 115 was nominally statistically significant in favour of nivolumab plus cabozantinib versus sunitinib for FKSI-19 total score (difference, 2·38 points; 95% CI 1·20–3·56; nominal p<0·0001, ES 0·33), FKSI-19 DRS-v1 (difference, 1·33 points; 95% CI 0·84–1·83; nominal p<0·0001, ES 0·45), EQ-5D-3L VAS (difference, 3·48 points; 95% CI 1·58–5·39]; nominal p=0·0004, ES 0·30), and EQ-5D-3L UK utility index (difference, 0·04 points; 95% CI 0·01–0·07; nominal p=0·0036, ES 0·25). PMM sensitivity analysis results generally aligned with the MMRM results (appendix page 10).
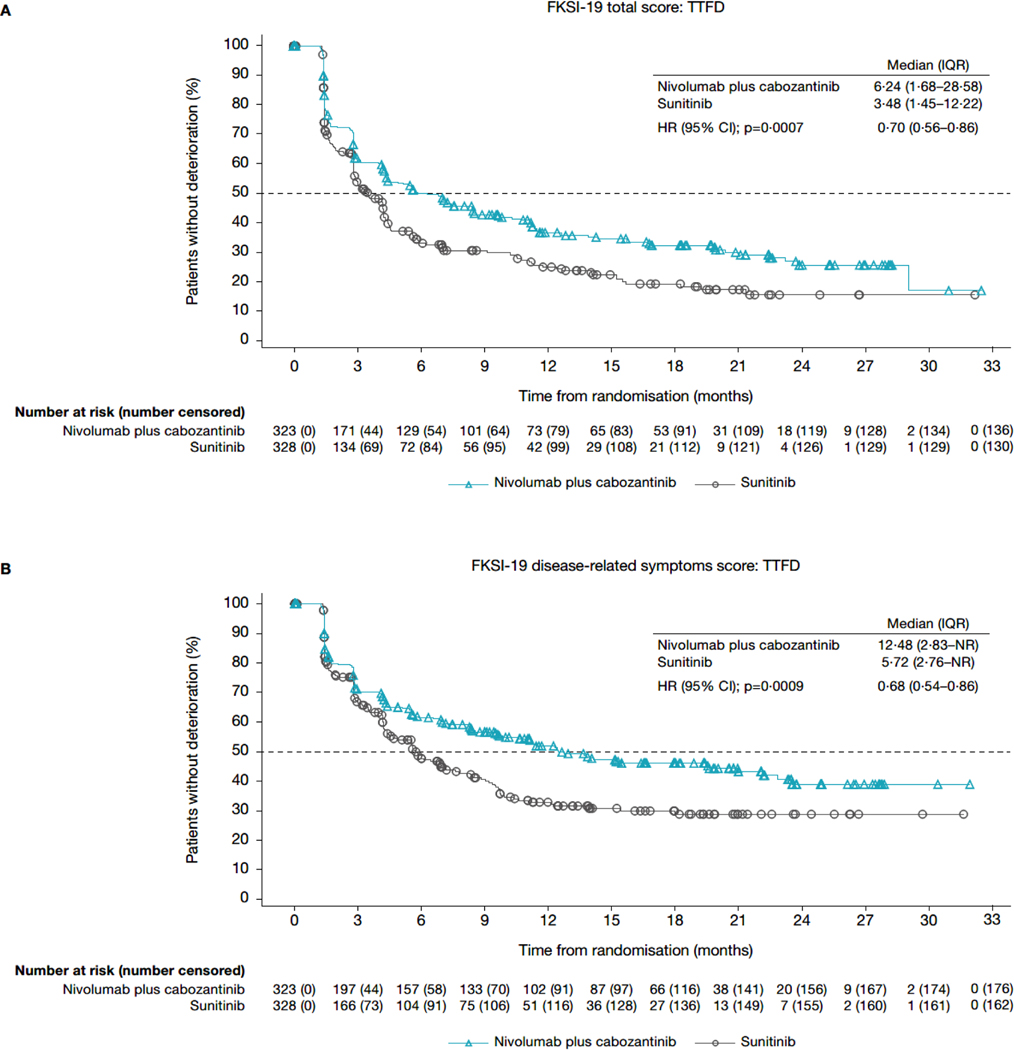
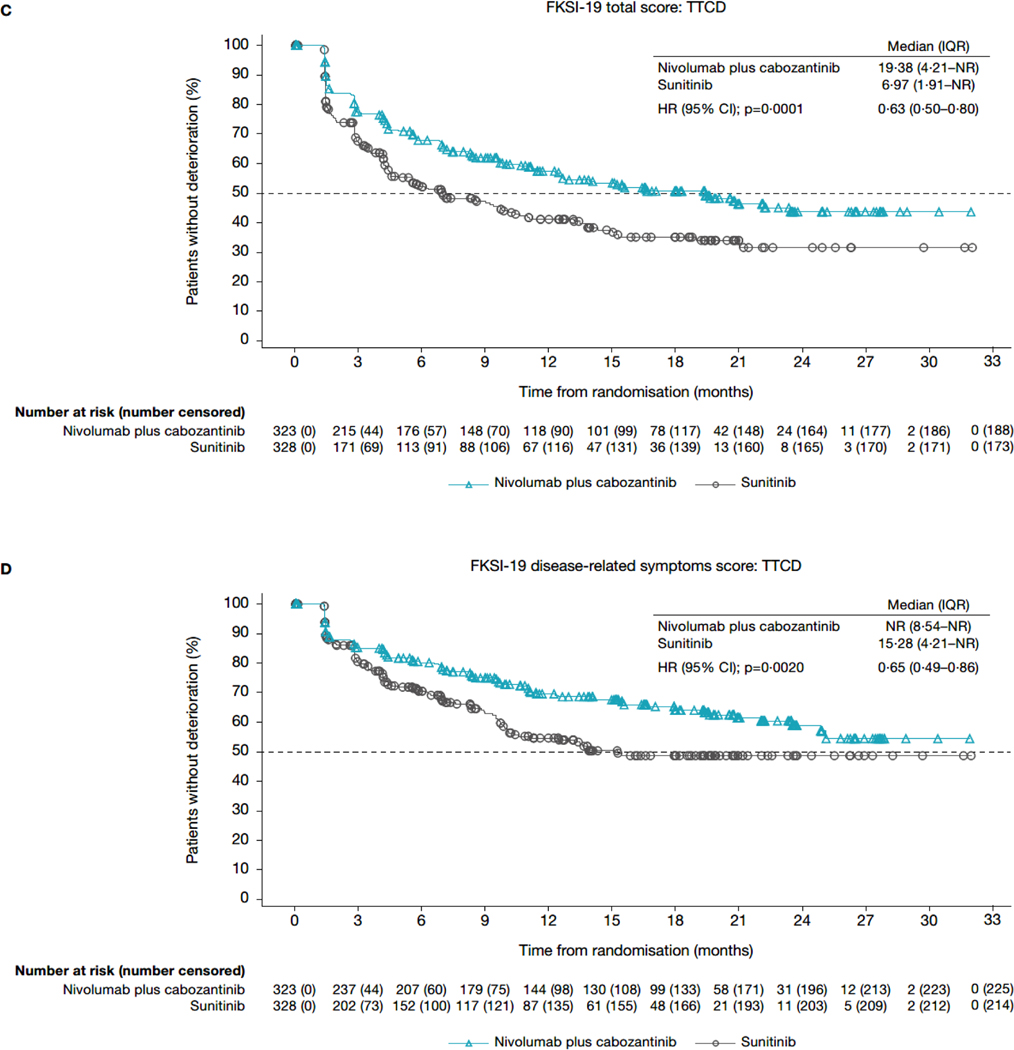
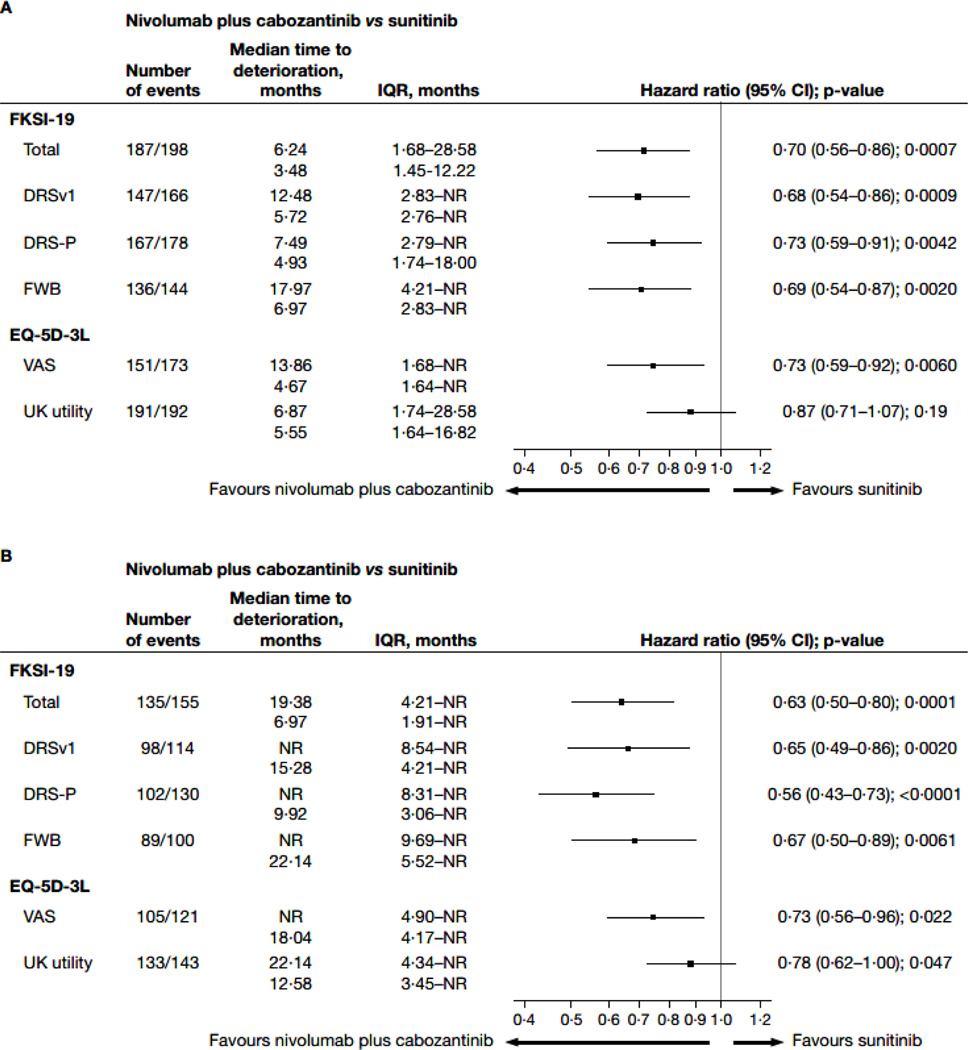
In the TTD analyses for FKSI-19 total score, patients in the nivolumab plus cabozantinib arm had a longer median TTFD in FKSI-19 total score (6·24 months; IQR 1·68–28·58) than did patients in the sunitinib arm (3·48 months; 1·45–12·22; HR 0·70; 95% CI 0·56–0·86; nominal p=0·0007; figures 3A, 4A). Median TTCD (IQR) was 19·38 months (4·21-not estimable) in the nivolumab plus cabozantinib arm and 6·97 months (1·91-not estimable) in the sunitinib arm (HR 0·63; 95% CI 0·50–0·80]; nominal p=0·0001; figures 3C, 4B). Additionally, nivolumab plus cabozantinib significantly reduced the risk of first and confirmed deterioration in all other FKSI-19 scores (HR range 0·56–0·73; nominal p<0·05 for all scores; figures 3, 4) relative to sunitinib. Similar results were observed when sensitivity thresholds were applied (appendix pages 4 and 11).
Figure 3: Kaplan-Meier plot of TTFD and TTCD in FKSI-19 scores. (A) FKSI-19 total score: TTFD. (B) FKSI-19 disease-related symptoms score: TTFD. (C) FKSI-19 total score: TTCD. (D) FKSI-19 disease-related symptoms score: TTCD.
CI=confidence interval. DRSv1=disease-related symptoms version 1. FKSI-19 = Functional Assessment of Cancer Therapy-Kidney Symptom Index. HR=hazard ratio. IQR=interquartile range. NR=not reached. TTCD=time to confirmed deterioration. TTFD=time to first deterioration.
Figure 4: Forest plots for time to first deterioration (A) and time to confirmed deterioration (B) in FKSI-19 scores.
This analysis includes all patients in the intention-to-treat population (nivolumab plus cabozantinib, n=323; sunitinib, n=328).
CI=confidence interval. DRS-P=disease-related symptoms-physical. DRSv1=disease-related symptoms version 1. EQ-5D-3L=three-level version of the EQ-5D. FKSI-19=Functional Assessment of Cancer Therapy-Kidney Symptom Index. FWB=functional wellbeing. IQR=interquartile range. NR=not reached. VAS=visual analogue scale.
For EQ-5D-3L, patients receiving nivolumab plus cabozantinib had a significantly longer median TTFD in VAS score (13·86 months; IQR 1·68-not estimable) than did patients in the sunitinib arm (4·67 months; 1·64-not estimable; HR 0·73; 95% CI 0·59–0·92; nominal p=0·0060; figure 4A). Median TTFD in EQ-5D-3L UK utility index was also qualitatively longer for nivolumab plus cabozantinib relative to sunitinib arms (6·87 months; 1·74–28·58 vs 5·55 months; 1·64–16·82), but not nominally statistically significant (HR 0·87; 95% CI 0·71–1·07; p=0·19; figure 4A). Risk of confirmed deterioration in both EQ-5D-3L VAS and UK utility index was significantly reduced for the nivolumab plus cabozantinib arm relative to sunitinib (nominal p<0·05, figure 4B).
Results of the post-hoc analysis suggest which symptoms may drive the observed time-to-event differences. For the DRS-P scale, items for fatigue, pain, weight loss, appetite, and sleep demonstrated the highest contribution to the observed deterioration for the scale. Additionally, a modest contribution was observed for bone pain, cough, and dyspnoea, while fever and haematuria did not seem to contribute appreciably to the observed deterioration (appendix page 12).
At baseline, consistent with patients with no previous treatment for aRCC, nearly 80% (nivolumab plus cabozantinib: 223/279; sunitinib: 235/300) of patients reported “not at all” in response to the FKSI-19 GP5 item, “I am bothered by side effects of treatment” (appendix page 13). Distributions of item responses were comparable between arms. Through the first year of the study (up to week 55), when toxicity is most evident, the proportion of patients who felt “quite a bit” or “very much” bothered by side effects of treatment was low (peaking at 5·0% [13/261 at week 13] “quite a bit” and 2·9% [8/274 at week 7] “very much”) in the nivolumab plus cabozantinib arm, suggesting that treatment was generally well tolerated. In contrast, 11·4% [19/166 at week 31] and 4·1% [11/270 at week 7] of the patients in the sunitinib arm reported being “quite a bit” or “very much” bothered, respectively). Overall, at all timepoints the proportion of patients who reported little to no bother was greater with nivolumab plus cabozantinib than with sunitinib (appendix pages 13 and 14).
Discussion
This study suggests a treatment benefit of the combination of nivolumab plus cabozantinib over sunitinib monotherapy in HRQoL as measured by FKSI-19 and EQ-5D-3L for previously untreated patients with aRCC during the first 115 weeks from treatment initiation, corroborating findings from the previous data cut.13 By making use of established generic and disease-specific PRO measures, this research helps to clarify the patient experience of therapies for aRCC. After an initial worsening in both treatment arms, likely due to early toxicity, nivolumab plus cabozantinib showed a HRQoL benefit over sunitinib, with nominally statistically significant differences at multiple timepoints between treatment. Throughout the trial, PRO scores were maintained or improved with nivolumab plus cabozantinib versus sunitinib; sunitinib was associated with deteriorating scores and a greater proportion of patients self-reporting being bothered by side effects over time. These changes appear to be driven by symptom deterioration relating mostly to fatigue, pain, weight loss, appetite, and sleep.
Furthermore, findings from the disease-specific FKSI-19 and general EQ-5D-3L were comparable. Treatment with nivolumab plus cabozantinib significantly delayed TTD (with or without confirmation) as well as the risk of deterioration in all scores of the FKSI-19, including disease-related symptoms, versus sunitinib. PROs were previously reported from the CheckMate 214 trial of nivolumab plus ipilimumab versus sunitinib in patients with previously untreated advanced renal cell carcinoma. In this analysis, PRO scores were maintained or improved from baseline with nivolumab and were consistently better than with sunitinib in patients with intermediate or poor IMDC risk, consistent with our PRO results from CheckMate 9ER.25
Because there are no formally validated thresholds for defining change in FKSI-19 scores as “clinically meaningful” at the group level, our study relied on within-subject thresholds from the literature and statistical significance. Within-subject thresholds are known to be larger due to a greater variance than within a group.26,27 Although we have demonstrated that HRQoL was maintained or improved for nivolumab plus cabozantinib from baseline and declined for sunitinib, the changes from baseline in either direction were small, with effect sizes ranging from between 0·2 and 0·5, apart from a sharp deterioration in the sunitinib arm for EQ-5D-3L UK utility index values at a few timepoints. Also, although the between-treatment differences in FKSI-19 and EQ-5D scores were nominally statistically significant, they did not exceed our predefined threshold values for individual change, which typically were set higher than group difference thresholds. At the group level, differences of this magnitude may possibly reflect a clinically meaningful benefit. For example, we required a threshold of 5 units for individual change for FKSI-19 DRS-v1, but a threshold of as low as 1 point has previously been proposed for the DRS-v1 when evaluating between-group differences.28 Using this lower threshold, the group difference in our study would be considered meaningful (difference=1·33; figure 3). While it can be argued that the findings of the longitudinal change from baseline analyses may reflect a small group difference with questionable clinical relevance, nivolumab plus cabozantinib significantly reduced the risk of clinically meaningful deterioration (with or without confirmation) in all scores of the FKSI-19, including disease-related symptoms, versus sunitinib. Future studies may help determine the minimum clinically meaningful thresholds for group-level differences and change.
Analysis of the GP5 item through week 55 also favoured nivolumab plus cabozantinib compared with sunitinib, with a smaller proportion of patients receiving nivolumab plus cabozantinib reporting that they were bothered by treatment side effects than those receiving sunitinib. These findings are particularly relevant because the PRO analysis may have introduced bias in favour of sunitinib. All PRO data assessments included in these analyses were collected at the beginning of each treatment cycle, when patients in the sunitinib arm had been off treatment for 2 weeks, versus 1 week for patients receiving nivolumab (and no break for cabozantinib treatment). Thus, patients in the sunitinib arm had more time to recover from any treatment-related side effects than patients receiving nivolumab, while there was no planned treatment interruption of cabozantinib.
With the advent of multiple effective options, selecting the optimal first-line therapy can be challenging. These results suggest that the superior clinical efficacy of nivolumab plus cabozantinib over sunitinib is accompanied by the additional small but significant benefit of improved HRQoL, making this combination a strong candidate for treatment selection. Furthermore, while combination therapies may raise concerns about overlapping toxicity, CheckMate 9ER data indicate that treatment with nivolumab plus cabozantinib is associated with better disease symptom control than sunitinib, and treatment-related adverse events can be managed with dose holding or dose reductions.9 This symptom control may be related to the advantage of this combination therapy. Future studies would benefit from an increased understanding of the relationship between clinical efficacy and the patient’s treatment experience, as in this trial where both favoured nivolumab plus cabozantinib.
Our HRQoL analysis has several limitations. The open-label nature of the CheckMate 9ER trial may be considered a potential cause of bias for PRO data capture. Recent research has suggested that the magnitude of this type of bias may be less prominent than initially considered.21,29,30 Another limitation is the sample size at later timepoints (eg, beyond week 55) with lower numbers in the sunitinib arm compared with nivolumab plus cabozantinib. Per study protocol, patients who discontinued treatment had only two follow-up visits, and thereafter all PRO assessments were discontinued except for EQ-5D-3L, which caused a reduction in available PRO data as the trial progressed. Most of this discrepancy was related to disease progression occurring earlier in patients receiving sunitinib. While the longitudinal change from baseline analysis included only assessments during treatment period, the time-to-deterioration analysis included all available assessments, including the two follow-up visits that took place after discontinuation of the study drug and therefore reflected the PRO scores beyond treatment discontinuation. A third limitation is that our longitudinal change from baseline analysis relies on the modelling assumptions. Because missing data assumptions are not testable, no single method can be counted on to give a comprehensive treatment of missing data. In order to stress-test the missing-at-random assumptions made by the MMRM, we also implemented the PMM analysis, which assumed four clinically feasible missing patterns where nivolumab plus cabozantinib and sunitinib arms were treated the same way for patterns 1 (death), 3 (progression), and 4 (other reasons), and pattern 2 (due to AE) penalises the nivolumab plus cabozantinib arm more. Because the PMM results align with the MMRM results reported here, we have added confidence in the fact that the longitudinal findings were not strongly dependent on the nature of the missing data.
Additional limitations include the distinct administration routes of the respective treatments and the differing schedules for nivolumab plus cabozantinib and sunitinib; these factors are common to first-line RCC trials. There were also differing schedules for collection of PRO data for patients in the nivolumab plus cabozantinib arm (every 6 weeks) and those in the sunitinib arm (every 2 weeks), which could contribute to possible bias in our analysis. Another consideration is that all analyses were exploratory and did not account for multiplicity. In addition, because PRO analyses were confined only to timepoints common between trials, it is possible that deterioration events were missed. We also noted that, for the EQ-5D-3L results, there was some inconsistency between the VAS and utility index (ie, stable vs improved); this may be because utility scores are designed for economic rather than patient outcome evaluations, and focus only on the five dimensions, whereas the VAS is a more holistic measure.
Specific to this trial, PRO data were collected for the sunitinib arm after the 2-week treatment-free period in each sunitinib cycle, whereas for the nivolumab plus cabozantinib arm, PRO assessment occurred after a 1-week treatment-free period for nivolumab with no treatment break for cabozantinib; it is possible that this may have alleviated treatment-related toxicity and impacted comparisons between the treatment arms in favour of sunitinib. Although almost 80% of patients reported not being bothered by treatment side effects at baseline, some patients reported treatment side effects before the initiation of the trial; this may have been associated with factors such as concomitant comorbidities or side effects of previous nephrectomy or local radiotherapy.
This study has the advantage of a large patient sample (>650 patients) and high PRO completion rates (>75% across most timepoints). We employed both a widely used generic instrument (EQ-5D-3L) as well as a cancer-specific instrument (FKSI-19) to gain insights into patient-reported HRQoL. Additionally, we determined prespecified thresholds to define deterioration.
In conclusion, CheckMate 9ER demonstrates that the clinical benefit of nivolumab plus cabozantinib is accompanied by maintenance or improvement in patient HRQoL, functioning, and disease-related symptoms compared with treatment with sunitinib. PROs of nivolumab plus cabozantinib show that the treatment is well tolerated, with smaller proportions of patients reporting being bothered by treatment side effects in contrast to sunitinib. These analyses of CheckMate 9ER PROs demonstrate the positive impact that nivolumab plus cabozantinib can have on HRQoL in patients with aRCC.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for “renal cell carcinoma,” “RCC,” “kidney cancer,” “health related quality of life,” “HRQoL,” “patient reported outcomes [PRO],” “FKSI-19”, and “EQ-5D” on April 23, 2021, and found a single result published in 2019. This study reported on the PRO results of CheckMate 214, an open-label, randomised, controlled, phase 3 clinical trial in patients with advanced RCC (aRCC) treated with nivolumab plus ipilimumab or with sunitinib. Using data from the Functional Assessment of Cancer Therapy Kidney Symptom Index-19 (FKSI-19), Functional Assessment of Cancer Therapy-General (FACT-G), and EuroQol five dimensional three level (EQ-5D-3L), this study demonstrated that combination therapy with nivolumab plus ipilimumab is associated with a benefit to health-related quality of life (HRQoL) in patients with aRCC when compared with sunitinib. In addition, we identified via targeted literature search a review published in 2020 that summarises available first-line treatment options for patients with aRCC, including combination therapies nivolumab plus ipilimumab, as well as pembrolizumab or avelumab plus axitinib. Both pembrolizumab and avelumab plus axitinib—despite offering a survival advantage over sunitinib—do not appear to offer any improvement to HRQoL.
Added value of this study
The current study provides a summary of patient-reported outcome (PRO) measure analyses for the CheckMate 9ER phase 3 clinical trial, wherein patients were treated with nivolumab plus cabozantinib or sunitinib. Efficacy results from CheckMate 9ER indicate that patients with renal cell carcinoma treated with nivolumab plus cabozantinib experience survival benefits and increased probability of response compared with those treated with sunitinib. In the current study, PROs assessed via the FKSI-19 and the EQ-5D-3L as exploratory endpoints further support the use of nivolumab plus cabozantinib over sunitinib, with PRO-related findings consistently favouring nivolumab plus cabozantinib throughout the trial. Findings suggest that patients receiving combination nivolumab plus cabozantinib treatment experienced maintenance or improvement in PRO scores, while those receiving sunitinib experienced a decline. In addition, time to deterioration also tended to be longer for the nivolumab plus cabozantinib arm compared with sunitinib. Moreover, a lower proportion of patients receiving nivolumab plus cabozantinib than patients receiving sunitinib reported that they were bothered by treatment side effects, suggesting a reduction in toxicity without a corresponding reduction in efficacy for this combination therapy. Taken together, these results describe a positive overall risk-benefit profile for nivolumab plus cabozantinib.
Implications of all the available evidence
Our results suggest that, in contrast to treatment with sunitinib for patients with aRCC, nivolumab plus cabozantinib may be associated with maintenance or improvement of HRQoL in addition to the previously established survival benefit. Compared with sunitinib, nivolumab plus cabozantinib was well tolerated, with few patients reporting being bothered by the side effects of treatment.
Acknowledgments
Bristol Myers Squibb (Princeton, NJ) and Ono Pharmaceutical Company Ltd. (Osaka, Japan). This study was supported by Bristol Myers Squibb. Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant (Core Grant, number P30 CA008748). All authors contributed to and approved the presentation; writing assistance was provided by Emily Ruzich, PhD, of IQVIA, funded by Bristol Myers Squibb. Editorial support was provided by Parexel, funded by Bristol Myers Squibb.
Footnotes
Declaration of interests
DC reports consultant fees from AbbVie, Bristol Myers Squibb (BMS), Exelixis, Merck, Novartis, and Pfizer; licensing fees from FACIT.org; research grants (institutional) from AbbVie, Astellas, Aveo, BMS, GSK, Merck, Novartis, and Pfizer; and is an officer of FACIT.org. RJM reports advisory board fees from AstraZeneca, Aveo Pharmaceuticals, Eisai, EMD Serono, Exelixis, Genentech/Roche, Incyte, Lilly Oncology, Merck, Novartis, and Pfizer; and principal investigator fees (institutional) from BMS, Eisai, Exelixis, Genentech/Roche, Merck, and Pfizer. CS reports advisory board fees from Astellas Pharma, Bayer, BMS, EUSA Pharma, Hoffmann-La Roche, Ipsen, MSD, Novartis, Pfizer, and Sanofi-Aventis; speakers bureau fees from Astellas Pharma, BMS, Hoffmann-La Roche, Ipsen, and Pfizer; and research grants (institutional) from AB Science, Aragon Pharmaceuticals, Astellas Pharma, AstraZeneca AB, Bayer, Blueprint Medicines Corporation, Boehringer Ingelheim España SA, BMS, Clovis Oncology, Exelixis Inc., Genentech, Inc., GlaxoSmithKline SA, Hoffmann-La Roche Ltd, Novartis Farmaceutica SA, Pfizer S.L.U., and Sanofi-Aventis. SIB is an employee of BMS, and has stock ownership in BMS and GlaxoSmithKline. FE is an employee of and has stock ownership in BMS. MH is an employee of and has stock ownership in BMS. JFW is an employee and has stock ownership in Exelixis. BS is an employee of and has stock ownership in BMS. JZ is an employee of and has stock ownership in BMS. CI is an employee of IQVIA. ABA has nothing to disclose. TKC reports advisory board fees from Aptitude Health, AstraZeneca, BMS, Calithera, EMD Serono, Exelixis, Infinity, Merck, Pfizer, and Surface Oncology; speakers fees from Advent Health, ASCO-SITC, ASiM, CancerNet, Ipsen, MD Anderson Cancer Center, MJH Life Sciences, PeerView, Physicians Education Resources, Research To Practice, Springer, and WebMed; consulting fees from The Analysis Group; stock ownership in Pionyr and Tempest; personal grant from Orien; royalties from Up-To-Date online textbook; funding (institutional) from Alliance Cooperative Group, AstraZeneca, BMS, Eisai, EMD Serono, Exelixis, Lilly, Merck, Peloton, Pfizer, Takeda, and Tracon; research grants (institutional) from BMS, Exelixis, and Roche; principal investigator fees (institutional) from GSK, Roche, and Surface Oncology; non-non-financial leadership role interest with Kidney Cancer Research Summit of KidneyCAN (meeting co-chair, 2019, 2020); grant reviewer for the American Association for Cancer Research; track leader, session chair, speaker, and discussant for the American Society of Clinical Oncology; speaker, discussant for the European Society for Medical Oncology; access to genomic database from Foundation Med; access to genomic database (institutional) from Guardant; access to genomic database (institutional) from Invitae; personal non-financial interests for medical writing and editorial assistance from Clinical Thinking, Envision Pharma Group, Fishawack Group of Companies, Health Interactions, Parexel, and Oxford PharmaGenesis; and personal non-financial interests for reviews of papers for various journals.
Data sharing
Bristol Myers Squibb’s policy on data sharing can be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html. De-identified and anonymised datasets of clinical trial information, including patient-level data, will be shared with external researchers for proposals that are complete, for which the scientific request is valid and the data are available, consistent with safeguarding patient privacy and informed consent. Upon execution of an agreement, the de-identified and anonymised data sets can be accessed via a secured portal that provides an environment for statistical programming with R as the programming language. The patient-reported outcomes protocol and statistical analysis plan will also be available. Data will be available for 2 years from the study completion or termination of the program (May 2024).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kotecha RR, Motzer RJ, Voss MH. Towards individualized therapy for metastatic renal cell carcinoma. Nat Rev Clin Oncol 2019; 16: 621–33. [DOI] [PubMed] [Google Scholar]
- 2.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017; 376: 354–66. [DOI] [PubMed] [Google Scholar]
- 3.Gurram S, Al Harthy M, Ball MW. The changing landscape of systemic therapy in metastatic renal cell carcinoma: an update. Discov Med 2020; 29: 191–9. [PubMed] [Google Scholar]
- 4.Grimm MO, Leucht K, Grünwald V, Foller S. New first line treatment options of clear cell renal cell cancer patients with PD-1 or PD-L1 immune-checkpoint inhibitor-based combination therapies. J Clin Med 2020; 9. doi: 10.3390/jcm9020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedke J, Albiges L, Capitanio U, et al. Updated European Association of Urology guidelines on renal cell carcinoma: Nivolumab plus cabozantinib joins immune checkpoint inhibition combination therapies for treatment-naïve metastatic clear-cell renal cell carcinoma. Eur Urol 2021; 79: 339–42. [DOI] [PubMed] [Google Scholar]
- 6.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN trial. J Clin Oncol 2017; 35: 591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013; 369: 722–31. [DOI] [PubMed] [Google Scholar]
- 8.Unverzagt S, Moldenhauer I, Nothacker M, et al. Immunotherapy for metastatic renal cell carcinoma. Cochrane Database Syst Rev 2017; 5: Cd011673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021; 384: 829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motzer RJ, Choueiri TK, Powles T, et al. Nivolumab + cabozantinib (NIVO+CABO) versus sunitinib (SUN) for advanced renal cell carcinoma (aRCC): outcomes by sarcomatoid histology and updated trial results with extended follow-up of CheckMate 9ER. J Clin Oncol 2021; 39: 308.33356420 [Google Scholar]
- 11.Chen RC, Choueiri TK, Feuilly M, et al. Quality-adjusted survival with first-line cabozantinib or sunitinib for advanced renal cell carcinoma in the CABOSUN randomized clinical trial (Alliance). Cancer 2020; 126: 5311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apolo AB, Powles P, Burotto M, et al. Nivolumab plus cabozantinib (N+C) versus sunitinib (S) for advanced renal cell carcinoma (aRCC): Outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. J Clin Oncol 2021; 39(suppl 15): 4553. [Google Scholar]
- 13.Cella D, Choueiri TK, Blum SI, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma (aRCC) treated with first-line nivolumab plus cabozantinib versus sunitinib: The CheckMate 9ER trial. J Clin Oncol 2021; 39(suppl 6): 285.33356422 [Google Scholar]
- 14.Rothrock NE, Jensen SE, Beaumont JL, et al. Development and initial validation of the NCCN/FACT symptom index for advanced kidney cancer. Value Health 2013; 16: 789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.EuroQoL Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 16.Dolan P. Modeling valuations for EuroQol health states. Med Care 1997; 35: 1095–108. [DOI] [PubMed] [Google Scholar]
- 17.Janssen B, Szende A. Population norms for the EQ-5D. In: Szende A, Janssen B, Cabases J, editors. Self-Reported Population Health: An International Perspective based on EQ-5D. Dordrecht: Springer Netherlands; 2014. p. 19–30. [Google Scholar]
- 18.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 2007; 5: 70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cella D, Yount S, Brucker PS, et al. Development and validation of a scale to measure disease-related symptoms of kidney cancer. Value Health 2007; 10: 285–93. [DOI] [PubMed] [Google Scholar]
- 20.O’Kelly M, Ratitch B. Clinical Trials with Missing Data: A Guide for Practitioners. West Sussex, UK: John Wiley & Sons, Ltd; 2014. [Google Scholar]
- 21.Roydhouse JK, King-Kallimanis BL, Roy P, et al. Exploration of baseline patient-reported side effect bother from cancer therapy. Clin Trials 2020; 17: 332–7. [DOI] [PubMed] [Google Scholar]
- 22.Pearman TP, Beaumont JL, Mroczek D, O’Connor M, Cella D. Validity and usefulness of a single-item measure of patient-reported bother from side effects of cancer therapy. Cancer 2018; 124: 991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella D, Escudier B, Rini B, et al. Patient-reported outcomes for axitinib vs sorafenib in metastatic renal cell carcinoma: phase III (AXIS) trial. Br J Cancer 2013; 108: 1571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FACIT Group. FACT-Item GP5. 2021. https://www.facit.org/measures/FACT-Item-GP5 (accessed August 3, 2021).
- 25.Cella D, Grünwald V, Escudier B, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol 2019; 20: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J 2002; 19: 398–404. [DOI] [PubMed] [Google Scholar]
- 27.Fisher AJ, Medaglia JD, Jeronimus BF. Lack of group-to-individual generalizability is a threat to human subjects research. Proc Natl Acad Sci U S A 2018; 115: E6106–E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cella D, Motzer RJ, Rini BI, et al. Important group differences on the Functional Assessment of Cancer Therapy-Kidney Symptom Index Disease-Related Symptoms in patients with metastatic renal cell carcinoma. Value Health 2018; 21: 1413–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atkinson TM, Wagner JS, Basch E. Trustworthiness of patient-reported outcomes in unblinded cancer clinical trials. JAMA Oncol 2017; 3: 738–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouillet G, Efficace F, Thiery-Vuillemin A, et al. Investigating the impact of open label design on patient-reported outcome results in prostate cancer randomized controlled trials. Cancer Med 2020; 9: 7363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.