Abstract
Although the exact etiology of Alzheimer’s disease (AD) is poorly understood, experimental and clinical evidences suggest the contribution of neuroinflammation in the pathogenesis of AD. Pathologically, AD brain is characterized by an imbalance in redox status, elevated endoplasmic reticulum (ER) stress, synaptic dysfunction, inflammation, and progressive neurodegeneration. It has been noted that continuous accumulation of amyloidbeta (Aβ) and intracellular neurofibrillary tangles (NFTs) in AD brain trigger ER stress, which contributes to neurodegeneration. Similarly, experimental evidences supports the hypothesis that thioredoxin-interacting protein (TXNIP), an endogenous regulator of redox regulator thioredoxin (TRX), is activated by ER stress and contributes to activation of NLRP3 (NOD-like receptor protein 3) inflammatory cascade in hippocampus of the AD brain. Hippocampus of postmortem human AD and aged matched non-AD controls were analyzed for the expression ER stress markers and TXNIP-NLRP3 inflammasome at cellular and molecular levels. We found higher expression of TXNIP at protein and transcript levels in close association with pathological markers of AD such as Aβ and NFTs in AD hippocampus. In addition, our results demonstrated that TXNIP was co-localized in neurons and microglia. Moreover, expression of binding immunoglobulin protein (BiP), activated eukaryotic initiation factor-2α (eIf2α) and C/EBP homology protein (CHOP), proteins involved the development of ER stress, were elevated in AD hippocampus. Further, elevated expression of effector molecules of NLRP3 inflammasome activation such as apoptosis associated speck-like protein (ASC), cleaved caspase-1 and cleaved interleukin-1β were observed in the AD hippocampus. The study suggests that TXNIP could be a link that connect ER stress with neuroinflammation. Thus, TXNIP can be a possible therapeutic target to mitigate the progression of neuroinflammation in the pathogenesis of AD.
Keywords: Alzheimer’s disease, Endoplasmic reticulum stress, Thioredoxin-interacting protein, NLRP3 inflammasome
1. Introduction
Alzheimer’s disease (AD) is the most common age-related neurodegenerative disease in the elderly, characterized by progressive decline in cognitive function and is the fifth leading cause of death in aged above 65 (Hebert et al., 2013). According to the latest Alzheimer’s association report, one in 10 people of age 65 and older has AD and its prevalence increases with age (“2020 Alzheimer’s disease facts and figures,” 2020). AD involves irreversible neuronal degeneration which gives rise to a multiple clinically detectable neurological impairments that dramatically affect the quality of life (Hersi et al., 2017). Multiple pathogenic hypothesis have been put forward about the pathogenies of AD including extracellular beta-amyloid (Aβ) peptide deposition, intracellular accumulation of hyperphosphorylated tau, cholinergic dysfunction and oxidative stress (Busche et al., 2016; Cacabelos et al., 2014; Latta et al., 2015; Liu et al., 2015; Lloret et al., 2011). Sustained inflammatory/immune response is considered as the progressive factor that has exacerbated AD pathology for last decade (Kinney et al., 2018). Accumulating evidences have shown that endoplasmic reticulum (ER) stress and neuroinflammation are potential mediator AD pathology and progressive cognitive impairment (Gomez-Nicola and Boche, 2015; Kumar, 2018; Salminen et al., 2009). Continuous accumulation of Aβ and intracellular neurofibrillary tangles (NFTs) in AD brain trigger ER stress, which contributes to neurodegeneration. Aberrant neuroinflammatory pathways activation is characterized by glial activation, increased secretion of proinflammatory cytokines and decreased anti-inflammatory responses; all of which makes the CNS more susceptible to neuronal degeneration and progressive cognitive impairment (Kinney et al., 2018). Hence, there is an urgent need to better understand the pathways/targets that are involved in the progression of neuroinflammation in the pathogenesis of AD, in order to develop effective therapeutic strategies for better management of AD.
Thioredoxin-interacting protein (TXNIP) is an endogenous negative regulator of redox regulator, thioredoxin (TRX) and belongs to the α-arrestin superfamily. TXNIP is known to be associated with redox imbalance, neuroinflammation and neurodegeneration (Devi et al., 2012; Nasoohi et al., 2018; Y. Wang et al., 2019). In addition, TXNIP is induced by protein kinase RNA-like endoplasmic reticulum (ER) kinase (PERK), which is activated in response to ER stress (Oslowski et al., 2012). TXNIP is considered as critical link between neuroinflammation and ER stress in β cell death (Oslowski et al., 2012). TXNIP mediates inflammatory responses by modulating inflammatory cascades in AD brain through direct interaction with NLRP3 (NOD-like receptor protein 3) inflammasome. Activated NLRP3 initiates the oligomerization of apoptotic-associated speck-like protein (ASC) and induces the cleavage of caspase-1 to cleaved caspase-1. Further, cleaved caspase induces the maturation of inflammatory mediator interleukin 1 beta (IL-1β), initiating inflammatory response. Elevated expression of NLRP3 inflammasome components were observed in APP/PS1 mice and postmortem AD subjects (Blum-Degen et al., 1995; Heneka et al., 2013).
The hippocampus is the brain area responsible for learning as well as memory and is susceptible to damage at the early stages of AD, leading to memory deficit (Mu and Gage, 2011). Consistently, hippocampal inflammation has been described to contribute to cognitive impairment and progression of AD (Feng et al., 2017; Zarifkar et al., 2010). In the present study we analyzed the potential contribution ER stress induced-TXNIP upregulation, which activates NLRP3 associated neuroinflammation, and its cell specific expression in the postmortem hippocampus samples from AD and non-AD brain. We found the activation of ER stress associated TXNIP-NLRP3 inflammasome activity in the hippocampus of AD brain.
2. Materials and methods
2.1. Ethical approval
The study was approved by the UTHSC Institutional Review Board (IRB #17-05716-NHSR; Exempt Application 665,258), Memphis, TN, USA, and was performed in accordance with standard ethical guidelines. All the appropriate personal protection safety procedures were followed to handle the human samples.
2.2. Human brain specimens
Table 1 demonstrates the characteristics of AD patients and the brain specimens that were used in western blotting and immunohistochemistry analysis. For western blot analysis, non-AD and AD brain specimens were collected from the Human Brain and Spinal Fluid Resource Center, which is sponsored by NIHDS/NIMH, National Multiple Sclerosis Society, and the Department of Veterans Affairs. Samples were collected from short-postmortem interval (PMI) autopsies from the University of Kentucky AD Center (UK-ADC) cohort. As addressed in our earlier report (Kim et al., 2017), the neuropathological evaluations were performed using standard neuropathological assessments as described elsewhere (Nelson et al, 2009, 2010). For western blotting analysis, hippocampal sections were snap-frozen following the autopsy and stored at — 80 °C till the time of immunoblotting. The inclusion criteria included: PMI <4 h; no clinical evidence of frontotemporal dementia or pathologic evidence of frontotemporal lobar degeneration; no large infarctions in the whole brain, or micro-infarcts found within 3 cm of the brain tissue samples and no cancer in the brain parenchyma.
Table 1.
Demographic parameters of human specimens.
| Table 1: Brain tissue samples | |||
|---|---|---|---|
| For Western blotting | |||
| Age | Sex | PMI | |
| Non AD (n = 5–7) | 68 ± 8.42 | 4 male, 2 female | <4 h |
| AD (n = 6–8) | 79 ± 3.6 | 5 male, 3 female | <4 h |
| For Real-time PCR | |||
| Non AD (n = 4) | 76 ± 13.78 | 2 male, 2 female | <4 h |
| AD (n = 8) | 79 ± 10.23 | 4 male, 4 female | <4 h |
| For immunohistochemistry | |||
| Non AD (n = 4) | 88 ± 2.98 | 2 male, 2 female | 3–8 h |
| AD (n = 4) | 83 ± 2.06 | 2 male, 2 female | 3–8 h |
For Immunofluorescence staining, postmortem AD human brain samples with a remarkable history of dementia (5–12 years) and neuropathological criteria for definite AD were obtained from the University of Iowa Deeded Body Program, Iowa City. IA, USA. The age-matched control brain (non-AD) samples were obtained at routine autopsy, from patients dying without any history of neurological or psychiatric ailments. The blocks were dissected and immersion-fixed in 4% paraformaldehyde solution and were cryoprotected with 30% sucrose until sunk. The frozen sections were cut at 40 μm on a sliding microtome and were collected in phosphate buffer saline (PBS) and stored in cryostorage solution (glycerol 30 ml, ethylene glycol 30 ml, 40 ml 0.1 M PBS) until the time of immunofluorescence staining.
2.3. Quantitative real-time PCR
In order to quantify the Txnip mRNA level in AD hippocampus, real-time PCR was performed as previously reported (Li et al., 2019). Briefly, total RNA was isolated from AD and non-AD brain tissues using RNeasy® Mini Kit (Cat. #74104, Qiagen, Germany). Genomic DNA contamination was removed with DNase treatment and single-strand complementary DNA (cDNA) was prepared using QuantiNova™ Reverse Transcription Kit (Cat.#205413, Qiagen, Germany). The primer pair 5′-CAGCAGTGCAAACAGACTTCGG-3’ (forward) and 5′-CTGAGG AAGCTCAAAGCCGAAC-3′ (reverse) specific for human Txnip were carefully chosen for qPCR amplification (Integrated DNA Technologies, IA). Real-time quantitative PCR was performed using PowerUp™ SYBR™ Green Master Mix (Applied Biosystems) and Bio-Rad CFX connect™ Real-Time system (Serial#BR003568, Bio-Rad Laboratories, CA). The thermal profiles were as follows: UDG activation at 50 °C for 2 min, and Dual-Lock™ DNA polymerase activation at 95 °C for 2 min, followed by amplification of cDNA for 40 cycles with denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. The Ct values were normalized using human β-actin mRNA level as an endogenous internal standard (specific oligonucleotide primer forward 5′-CACCATT GGCAATGAGCGGTTC-3′, reverse 5′-AGGTCTTTGCGGATGTCCACGT-3′, Integrated DNA Technologies, IA).
2.4. Western blotting
Brain lysates were prepared from human brain hippocampal specimens with radioimmunoprecipitation assay (RIPA) buffer. 30-μg protein were electrophoresed and electro blotted to PVDF membranes. The membranes were washed and nonspecific binding is blocked with 5% non-fat skimmed milk. Then the membranes were incubated with the specific primary antibodies, specified in Table 2 at 4 °C overnight. The membranes were washed with tris-buffer saline-tween 20 (TBS-T) and then incubated with a horseradish peroxidase (HRP)–conjugated secondary antibody (1: 10,000, Sigma). The specific bands were then detected by means of an enhanced chemiluminescent substrate system (Thermo Fisher Scientific) and evaluated by Image J software, normalized with β-actin as loading controls, and represented as fold change.
Table 2.
Primary antibodies probed in western blotting and immunostaining.
| Application | Target | Company | Catalogue No |
Dilution |
|---|---|---|---|---|
| Western Blotting | TXNIP | Novus Biologicals | NBP1-5 4578 | 1:1000 |
| TRX | Cell Signaling Technology | C63C6 | 1:1000 | |
| NLRP3 | Adipogen Life Sciences | AG-20B-0014 | 1:1000 | |
| ASC | Adipogen Life Sciences | AG-25B-0006 | 1:1000 | |
| cleaved caspase-1 | Adipogen Life Sciences | AG-20B-0042 | 1:1000 | |
| cleaved IL-1β | Abcam | ab9722 | 1:1000 | |
| BiP | Cell Signaling Technology | 3183 | 1:1000 | |
| p-eIF2α | Cell Signaling Technology | 3398 | 1:1000 | |
| eIF2α | Santa Cruz Biotechnology | SC133132 | 1:1000 | |
| CHOP | Cell Signaling Technology | 2895 | 1:1000 |
2.5. Immunofluorescence staining
Brain sections were subjected to antigen retrieval with sodium citrate buffer (pH 6) and were blocked with 5% Bovine Serum free protein block (X0909, Dako) at room temperature for 1 h. Further, the sections were incubated with specific primary antibodies at 4 °C overnight (Table 2). The sections were then washed three times with PBS followed by incubation with the respective Dyligt- 488 (042-03-18-06, Sera Care) and/or Alexa Fluor −594 (A-11012, Invitrogen) tagged secondary antibodies (1:200) for 1h at room temperature. For double staining, the process was repeated with primary antibodies against Neu-N (1:250; ABN78, Millipore), Ib-a1 (1:250; WDF6884; WAKO) or GFAP (1:500; 20,334, Dako). After single or double-staining, sections were mounted with ProLong™ Diamond Antifade Mountant with DAPI (Invitrogen), and analyzed using a Zeiss 710 confocal laser-scanning microscope. Negative controls were prepared by excluding the primary antibodies.
2.6. Statistical analysis
Quantitative data in all graphs were presented using means ± SEM (standard error of mean). One-way ANOVA was carried out with GraphPad Prism Instat 7.0 software for statistical analyses. Non-parametric Mann Whitney U tests were used to analyze statistical significance between AD and non-AD brains.
3. Results
3.1. Elevated expression of TXNIP in the hippocampus of AD brain
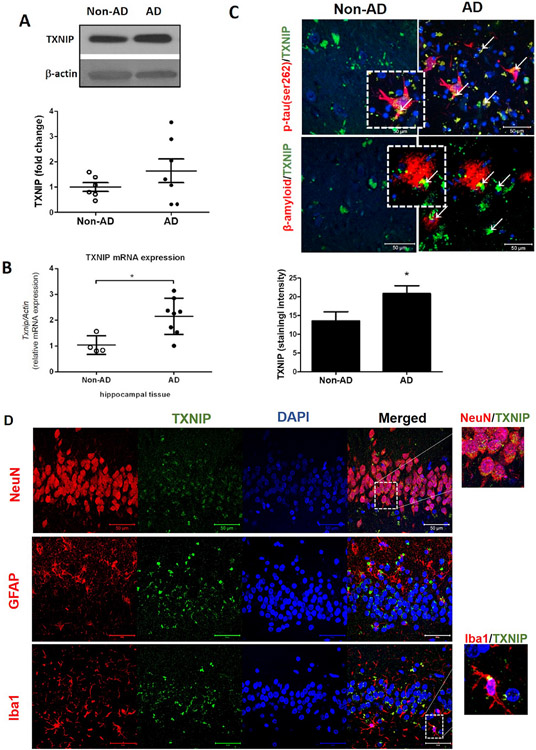
Western blot analysis of control and AD brain showed elevated expression of TXNIP in the hippocampus tissue (1A). Consistent with observed protein expression, real time PCR analysis revealed that AD brain had increased Txnip mRNA expression (p < 0.05, Fig. 1B) in the hippocampus compared to aged matched non-AD control subjects. Additionally, immunostaining analysis of AD brain confirmed the significant overexpression of TXNIP in the hippocampus (Fig. 1C). Further, double immunostaining analysis showed that TXNIP was overexpressed in the hippocampus of the AD brain in close association with key markers of AD such as phosphorylated tau and β-amyloid plaque. This further confirm the pathological contribution of TXNIP in AD (Fig. 1C). In order to find the cell specific expression of TXNIP, we used colocalization of TXNIP with cell specific markers such as NeuN, GFAP and Iba-1. We found that TXNIP was predominantly colocalized with NeuN and with Iba-1 for less extent confirming expression of TXNIP in neurons and microglia (Fig. 3A).
Fig. 1. TXNIP/NLRP3-inflammasome expression and localization in postmortem human AD and non-AD hippocampus.
Hippocampal samples were collected from AD and age-matched non-AD brains and were subjected to immunoblot analysis. As obvious in representative blots, (A) higher levels of TXNIP proteins and transcripts (B) in AD brains compared to age-matched non-ADs. Regional TXNIP overexpression was confirmed by signal intensity analysis in hippocampal specimens (C), indicating a remarkable TXNIP (green, represented by white arrows) over expression in the vicinity of β-amyloid (red) and p-Tau (red) accumulation. Our double-staining experiments further indicated (D) TXNIP (green) was mainly co-localized in neural (NeuN positive; red) and microglial (Iba1 positive; red) cells rather than astrocytes (GFAP; red). Magnification 40 × . Scale bar = 50 μm. Values are expressed as mean ± SEM, *p < 0.05 vs non-AD samples, n = 4–8/group GFAP, glial fibrillary acidic protein; NeuN, neuronal nuclei; GFAP, glial fibrillary acidic protein; DAPI, diamidino phenylindole; TXNIP, TRX interacting protein.
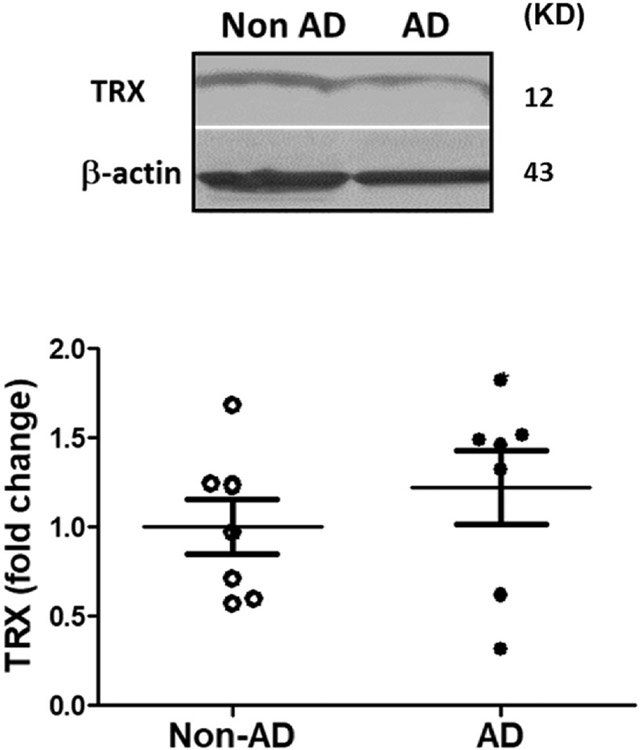
Fig. 3. TRX expression in postmortem human AD and non-AD hippocampus.
Hippocampal samples were collected from AD and age-matched non-AD brains and were subjected to immunoblot analysis. As obvious in representative blots, AD and non-AD samples did not show remarkable difference in TRX. Values are expressed as mean ± SEM, n = 6–8/group. TRX; thioredoxin.
3.2. Activation of NLRP3 inflammasome in the hippocampus of AD brain
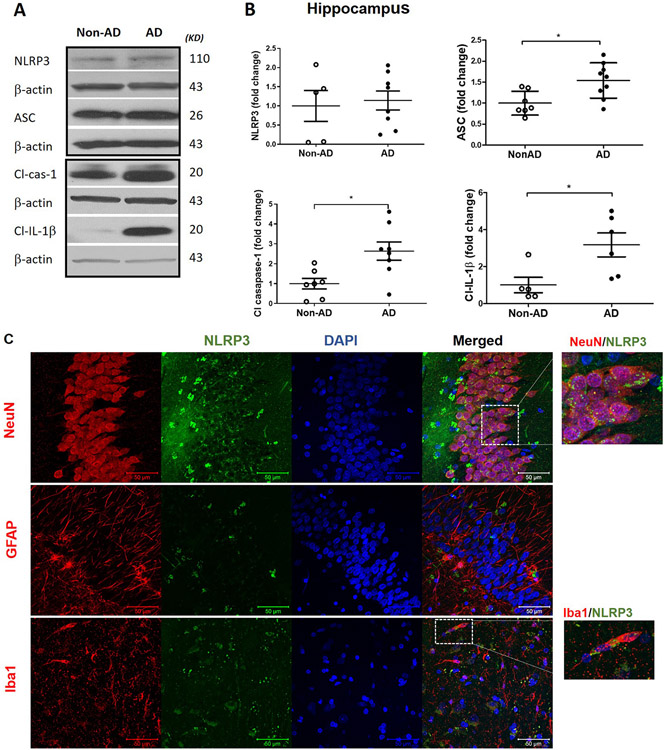
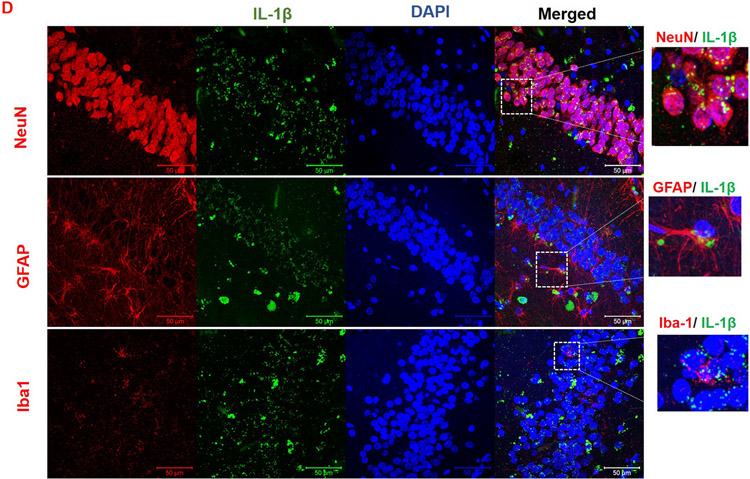
To evaluate the association between TXNIP and NLRP3 inflammasome activation in AD brain, hippocampal tissue was subjected immunoblotting. We found elevated expression of NLRP3 inflammasome components in AD brain (Fig. 2A and B). The expression of inflammasome components such apoptotic speck like protein (ASC), Cleaved Caspase-1 and mature IL-1β were significantly elevated in the AD brain (p < 0.05) despite any change in NLRP3 expression confirming the activation of NLRP3 inflammasome. To identify the cell specific expression of NLRP3 and IL-1β, we co-localized these mediators with cell specific markers such as NeuN, GFAP and Iba-1. NLRP3 is predominantly co-localized with NeuN and Iba-1 in the hippocampus of AD brain (Fig. 2C). However, the expression of IL-1β is co-localized with variety of cell specific markers such as NeuN, GFAP and Iba-1 (Fig. 2D), confirming activation of inflammatory pathway in variety of cell types in the brain.
Fig. 2. Immunoblotting analyses of principal components of NLRP3 inflammasome activation in postmortem human AD and non-AD hippocampus.
(A) Representative and (B) quantitative analysis of NLRP3, ASC, cleaved caspase-1 and cleaved IL-1β protein expression demonstrate the corresponding differences between hippocampus in AD and non-AD brain. AD and non-AD samples did not show a remarkable difference in the NLRP3 but revealed a significant increase in ASC as well as cl-caspase-1 and IL-1β levels in old AD brain specimens indicating higher cleavage activity of NLRP3-inflammasome. Co-localization of TXNIP with cells specific markers showed that (C) NLRP3 (green) is localized in neurons (red) and microglia (red) whereas its product (D) IL-1β (green) is mainly co-localized in neural (NeuN positive; red), astrocytes (GFAP positive; red) and microglial (Iba1 positive; red) cells suggesting NLRP3 inflammasome/IL-1β links with AD pathology. Values are expressed as mean ± SEM. *p < 0.05 vs non-AD brains, n = 5–8/group. NLRP3, nucleotide oligomerization domain (NOD)-like receptor protein-3; ASC, apoptosis associated speck-like; Cl. Caspase-1, cleaved caspase-1; IL-1β, cleaved interleukin-1 beta.
3.3. Expression of TRX in the hippocampus of AD brain
TXNIP is negative regulator of antioxidant thioredoxin(TRX). In order to evaluate the consequence of elevated TXNIP expression on TRX. Densitometric analysis of the western blot showed that the expression of TRX did not change between the groups (Fig. 3).
3.4. Elevated expression of ER stress markers in the hippocampus of AD brain
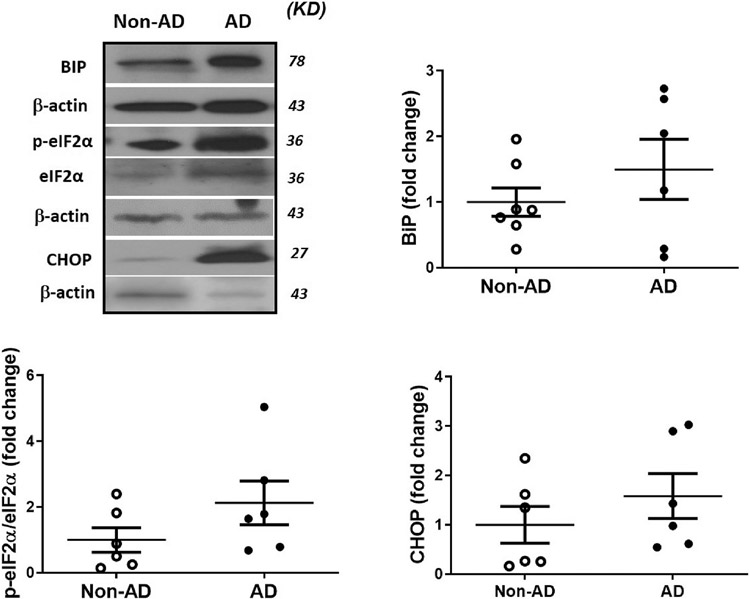
Earlier findings revealed that TXNIP expression is also induced by the ER stress; hence, we evaluated the expression ER stress mediatotrs such as binding immunoglobulin protein (BiP), phospho-eukaryotic initiation factor-2α (eIf2α) and CHOP in AD hippocampus. BiP is an ER specific chaperone involved in UPR, also known as GRP78. Activated eIF2α is an ER stress sensor which inhibit the translational assembly of the 80S ribosome to facilitate UPR (Hoozemans et al., 2005). CHOP is a transcription factor; increased expression is an indication of elevated ER stress associated apoptosis. We found a marginal increase in BiP, ratio of phospho-eIf2α to total eIF2α and CHOP expression in AD hippocampus in comparison with age matched control subjects (Fig. 4) (see Fig. 5).
Fig. 4. Expression ER stress mediators in the postmortem human AD and non-AD hippocampus.
Expression of (A) BIP, (B) p-eIF2α/eIF2α and (C) CHOP in AD hippocampal samples were analyzed by western blotting as an indicator of ER stress, as obvious in representative blots, AD hippocampus showed a marginal increase in the BiP, p-eIF2α/eIF2α and CHOP expression in comparison with age matched control. Values are expressed as mean ± SEM, n = 6–8/group. BIP; Binding immunoglobulin protein, eIF2α; eukaryotic elongation factor 2α, CHOP; C/EBP homology protein.
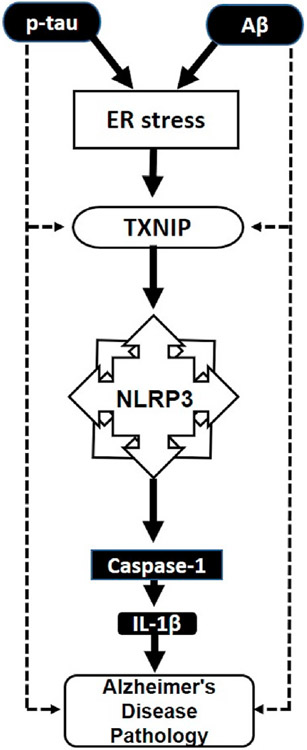
Fig. 5. Schematic representation of possible link between ER stress and TXNIP with AD pathology.
Overexpression of TXNIP along with phospho-tau and Aβ in AD brain demonstrated the contribution of TXNIP in AD pathology. In the AD brain, phospho-tau and Aβ may induce ER stress, as denoted by dotted line, which in turn stimulates the activation of TXNIP. Activated TXNIP is known to directly interact with NLRP3 inflammasome and induces the activation of caspase and IL-1β. TXNIP can also be directly activated by phosphor tau and Aβ in the AD brain to propagate AD pathology. Hence TXNIP may be a link that connect ER stress with AD pathology. Aβ, amyloid-β protein; p-tau, phosphorylated tau protein; ER stress, Endoplasmic reticulum stress; TXNIP, thioredoxin interacting protein; IL-1β, interleukine-1β.
4. Discussion
In the present study, we found that TXNIP expression is elevated in the hippocampus of the AD brain at protein and mRNA level when compared to age-matched control. This is consistent with our previous observation that increased TXNIP expression is found in the prefrontal cortex of AD brains (Li et al., 2019). However, we could not find any notable difference in expression of TRX, an intrinsic negative regulator of TXNIP, in AD hippocampus. This is consistent with in vivo observation that showed increased TXNIP expression in a mouse model of AD without change in TRX expression (Y. Wang et al., 2019). Elevated TXNIP expression is reported in the hippocampus of transgenic 5xFAD (expresses neuronal human APP carrying three AD familiar mutation and two presenilin 1 mutations) mouse model of AD association with neuroinflammation and cognitive decline (Perrone et al., 2012). Wang et al. also demonstrated that increased expression of TXNIP in the frontal cortex and hippocampus of APP/PS1 mice (Y. Wang et al., 2019). Additionally, experimental studies in fruit flies showed that TXNIP is a candidate gene for life expectancy (Oberacker et al., 2018). Several pharmacological agents as are known to modulate TXNIP expression and ameliorated inflammation and AD pathology in-vivo and in-vitro (Feng and Zhang, 2019; Friedemann et al., 2014; Gao et al., 2015; Melone et al., 2018; C.-Y. Wang et al., 2019). Altogether, these studies emphasize that TXNIP contributes to the pathogenesis of AD.
Upregulation of TXNIP is associated with NLRP3 inflammasome activation in AD brain (Feng and Zhang, 2019; Li et al., 2019; C.-Y. Wang et al., 2019). We found that elevated expression of NLRP3 inflammasome components: expression of ASC, cleaved caspase-1 and IL-1β; were significantly elevated in the hippocampus of the AD brain, despite no change in NLRP3 expression. Accordingly, we may conclude that while NLRP3 expression did not change, its assembly and cleavage activity has raised enough to cause a remarkable change in IL-1β and caspase-1 cleavage. Previously, we have demonstrated that TXNIP associated NLRP3 inflammasome activation in the cortex of the AD brain (Li et al., 2019). Consistently, activation of TXNIP has been associated with activation of NLRP3 inflammasome, microglial activation and subsequent development of AD (Nasoohi et al., 2018). Aβ induces TXNIP upregulation in BV2 cells and consequent microglial activation and inhibition of TXNIP is associated with reversal of these changes (Feng and Zhang, 2019). Similarly, Aβ can activate NLRP3 inflammasome, which in turn mediates synaptic dysfunction, neuronal cell death, and eventually leads to cognitive impairment. Moreover, clinical and experimental studies demonstrated the contribution of NLRP3 in AD pathology (Heneka et al., 2013), genetic deletion of NLRP3 and caspase-1 protected the APP/PS1 mice from memory deficit and AD pathology.
AD patients commonly have increased expression of inflammatory cytokines, such as IL-1β in the brain, suggesting that inflammation is closely linked to the pathology of AD. IL-1β is a critical regulator of brain inflammatory cascade, which regulate the expression of other inflammatory molecules including tumor necrosis factor-α (TNFα) and IL-6 (Singh-Manoux et al., 2014). IL-1β is found to be elevated early stages of AD, remain elevated during the progression of AD and considered as essential for Aβ deposition (Akiyama et al., 2000). IL-1β increases Aβ deposition by regulating APP processing by activation of protein kinase C and γ-secretase (Buxbaum et al., 1992; Sadigh-Eteghad et al., 2015). IL-1β is proinflammatory in nature and detected in cortex and hippocampus of AD brain (Buxbaum et al., 1992; Li et al., 2019). Consistently, increased expression of IL-1receptor is found in the dentate gyrus and pyramidal cells in the hippocampus, which is considered as the site for early development of AD pathology (Farrar et al., 1987).
ER is the primary site for endomembrane trafficking, actively involved posttranslational processing, and folding of naive proteins (Roussel et al., 2013). Various pathological signals in the brain are known to activate unfolded protein responses in the ER and initiates the development of ER stress associated signaling. BiP is most abundant ER specific molecular chaperon involved in protein folding, also known as GPR78 (Santos and Ferreira, 2018).We found an increased expression of BiP in the hippocampus of the AD brain. Consistently, elevated expression of BiP was previously reported in the temporal cortex and hippocampal neurons of AD brain (Hoozemans et al., 2005). PKR-like ER kinase (PERK) is an ER transmembrane UPR sensor protein regulated by BiP (Jansen et al., 2012). Activated PERK further inhibits translational ribosomal assembly through the phosphorylation of eIF2α (Santos and Ferreira, 2018). We found an increased expression of phosphorylated eIF2α in the AD hippocampus (Fig. 4b), confirming the activation of ER stress in the hippocampus. Elevated ER stress culminates in the activation of CHOP mediated cellular apoptotic pathway (Li et al., 2015; Szegezdi et al., 2006). CHOP mediates the activation of GADD34, which increases activation of proapoptotic genes and oxidative stress culminates in programmed cell death. We found an increased expression of CHOP in AD hippocampus, which is consistent with previous finding. Yoon et al. demonstrated an elevated expression of CHOP in the cortex of the AD brain along with other ER stress sensors such as eIF2α, PERK and ATF6 (Yoon et al., 2012, p. 3). Multiple evidences support the existence of ER stress in AD brain (Gerakis and Hetz, 2018). Aβ plaques and NFTs in the AD brain induces imbalances in calcium homeostasis and unfolded protein response in the ER (Alberdi et al., 2013, p. 2; Viana et al., 2012).
It was reported that TXNIP is activated by ER stress sensors such as inositol-requiring enzyme 1α (IRE1α) and PERK-eIF2α signaling (Oslowski et al., 2012). Pharmacological inhibition of ER stress sensor PERK inhibited the expression of TXNIP and its transcriptional modulator ChREBP (Zhao et al., 2017), confirming the contributory role of ER stress in the activation of TXNIP. Consistently, TXNIP level is also modulated by IRE1α post-transcriptionally by destabilizing miRNA and miR-17, resulting in the elevated TXNIP expression (Zhou et al., 2010).
In summary, we found a clinical evidence for the association between ER stress and TXNIP/NLRP3 inflammasome activation in the hippocampus of AD brain. TXNIP is the connecting link between increased ER stress and activated neuroinflammation in AD brain. TXNIP can be considered as the key molecular target for the development of novel therapies for the clinical management of neuroinflammation associated AD pathogenesis. To offset the physiological anti-tumorigenic effects of TXMIP, further examinations are essential to selectively target its deteriorating pro-oxidant activity, to approach promising translational implementation. In conclusion, our results suggest an important role played by TXNIP-NLRP3 activation in AD brains. Further investigations are needed to determine the specific contribution and the mechanism of TXNIP associated NLRP3 activation in AD pathogenesis.
Acknowledgments
This work was supported by startup funds from the UTHSC Department of Anatomy and Neurobiology (TI) and by a grants R01-NS097800 (TI), 1R01-AG058467-03 (FL), 1R01 NS120327-01 (FL) and R21-NS101703 (KS) from the National Institutes of Health. We thank Dr. Peter T. Nelson (University of Kentucky Sanders-Brown Center on Aging) and Human Brain and Spinal Fluid Resource center, Los Angeles, CA for providing human brain specimens from the University of Kentucky AD Center (UK-ADC) cohort. Authors are also grateful for Dr. Zaheer, Department of Neurology, University of Missouri, Columbia, MO, USA, for providing human brain sections for immunostaining.
Footnotes
Credit author statement
SI conducted all the experiments, prepared the draft of the MS, WW helped in tissue processing and Western blot analysis; KS, FFL, MM, and TI contributed in reviewing the manuscript; TI supervised the project, including experimental design, data analysis and arranging the overall manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
This study was approved by the UTHSC Institutional Review Board (IRB #17-05716-NHSR; Exempt Application 665,258), Memphis, TN, USA, and was performed in accordance with standard ethical procedures. All the appropriate personal protection safety procedures were followed to handle the human samples.
Availability of data and material
The data generated and analyzed in this study will be made available from the corresponding author on reasonable request.
References
- Alzheimer’s disease facts and figures, 2020. Alzheimers Dement 16, 391–460. 10.1002/alz.12068. [DOI] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WST, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata–Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss–Coray T, 2000. Inflammation and Alzheimer’s disease. Neurobiol. Aging 21, 383–421. 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberdi E, Wyssenbach A, Alberdi M, Sánchez-Gómez MV, Cavaliere F, Rodríguez JJ, Verkhratsky A, Matute C, 2013. Ca(2+) -dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid β-treated astrocytes and in a model of Alzheimer’s disease. Aging Cell 12, 292–302. 10.1111/acel.12054. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P, 1995. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci. Lett 202, 17–20. 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Busche MA, Staufenbiel M, Willem M, Haass C, Förstl H, 2016. [Mechanisms of Alzheimer’s disease : neuronal hyperactivity and hypoactivity as new therapeutic targets]. Nervenarzt 87, 1163–1174. 10.1007/s00115-015-0041-5. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P, 1992. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc. Natl. Acad. Sci. Unit. States Am 89, 10075–10078. 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacabelos R, Cacabelos P, Torrellas C, Tellado I, Carril JC, 2014. Pharmacogenomics of Alzheimer’s disease: novel therapeutic strategies for drug development. Methods Mol. Biol. Clifton NJ 1175, 323–556. 10.1007/978-1-4939-0956-8_13. [DOI] [PubMed] [Google Scholar]
- Devi TS, Lee I, Hüttemann M, Kumar A, Nantwi KD, Singh LP, 2012. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: implications for diabetic retinopathy. Exp. Diabetes Res, 438238 10.1155/2012/438238, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB, 1987. Visualization and characterization of interleukin 1 receptors in brain. J. Immunol. Baltim 139, 459–463. Md 1950. [PubMed] [Google Scholar]
- Feng L, Zhang L, 2019. Resveratrol suppresses aβ-induced microglial activation through the TXNIP/TRX/NLRP3 signaling pathway. DNA Cell Biol. 38, 874–879. 10.1089/dna.2018.4308. [DOI] [PubMed] [Google Scholar]
- Feng X, Valdearcos M, Uchida Y, Lutrin D, Maze M, Koliwad SK, 2017. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight 2, e91229. 10.1172/jci.insight.91229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedemann T, Otto B, Klätschke K, Schumacher U, Tao Y, Leung AK-M, Efferth T, Schröder S, 2014. Coptis chinensis Franch. exhibits neuroprotective properties against oxidative stress in human neuroblastoma cells. J. Ethnopharmacol 155, 607–615. 10.1016/j.jep.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Gao J, He H, Jiang W, Chang X, Zhu L, Luo F, Zhou R, Ma C, Yan T, 2015. Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer’s disease. Behav. Brain Res 293, 27–33. 10.1016/j.bbr.2015.06.045. [DOI] [PubMed] [Google Scholar]
- Gerakis Y, Hetz C, 2018. Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J. 285, 995–1011. 10.1111/febs.14332. [DOI] [PubMed] [Google Scholar]
- Gomez-Nicola D, Boche D, 2015. Post-mortem analysis of neuroinflammatory changes in human Alzheimer’s disease. Alzheimer’s Res. Ther 7, 42. 10.1186/s13195-015-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA, 2013. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 80, 1778–1783. 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng T-C, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT, 2013. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678. 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D, 2017. Risk factors associated with the onset and progression of Alzheimer’s disease: a systematic review of the evidence. NeuroToxicology, Determinants of Neurological Disease 61, 143–187. 10.1016/j.neuro.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJM, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P, Scheper W, 2005. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol. 110, 165–172. 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- Jansen G, Määttänen P, Denisov AY, Scarffe L, Schade B, Balghi H, Dejgaard K, Chen LY, Muller WJ, Gehring K, Thomas DY, 2012. An interaction map of endoplasmic reticulum chaperones and foldases*. Mol. Cell. Proteomics 11, 710–723. 10.1074/mcp.M111.016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Sakata K, Liao F-F, 2017. Bidirectional interplay of HSF1 degradation and UPR activation promotes tau hyperphosphorylation. PLoS Genet. 13, e1006849 10.1371/journal.pgen.1006849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT, 2018. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. Transl. Res. Clin. Interv 4, 575–590. 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, 2018. Editorial: neuroinflammation and cognition. Front. Aging Neurosci 10 10.3389/fnagi.2018.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latta CH, Brothers HM, Wilcock DM, 2015. Neuroinflammation in Alzheimer’s disease; A source of heterogeneity and target for personalized therapy. Neuroscience 302, 103–111. 10.1016/j.neuroscience.2014.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-Q, Yu J-T, Jiang T, Tan L, 2015. Endoplasmic reticulum dysfunction in Alzheimer’s disease. Mol. Neurobiol 51, 383–395. 10.1007/s12035-014-8695-8. [DOI] [PubMed] [Google Scholar]
- Li L, Ismael S, Nasoohi S, Sakata K, Liao F-F, McDonald MP, Ishrat T, 2019. Thioredoxin-interacting protein (TXNIP) associated NLRP3 inflammasome activation in human Alzheimer’s disease brain. J. Alzheimers Dis. JAD 68, 255–265. 10.3233/JAD-180814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li T, Li P, Wei N, Zhao Z, Liang H, Ji X, Chen W, Xue M, Wei J, 2015. The ambiguous relationship of oxidative stress, tau hyperphosphorylation, and autophagy dysfunction in Alzheimer’s disease. Oxid. Med. Cell. Longev, 352723 10.1155/2015/352723, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloret A, Badia M-C, Giraldo E, Ermak G, Alonso M-D, Pallardó FV, Davies KJA, Viña J, 2011. Amyloid-β toxicity and tau hyperphosphorylation are linked via RCAN1 in Alzheimer’s disease. J. Alzheimers Dis. JAD 27, 701–709. 10.3233/JAD-2011-110890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melone MAB, Dato C, Paladino S, Coppola C, Trebini C, Giordana MT, Perrone L, 2018. Verapamil inhibits ser202/thr205 phosphorylation of tau by blocking TXNIP/ROS/p38 MAPK pathway. Pharm. Res. (N. Y.) 35, 44. 10.1007/s11095-017-2276-2. [DOI] [PubMed] [Google Scholar]
- Mu Y, Gage FH, 2011. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener 6, 85. 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasoohi S, Ismael S, Ishrat T, 2018. Thioredoxin-interacting protein (TXNIP) in cerebrovascular and neurodegenerative diseases: regulation and implication. Mol. Neurobiol 55, 7900–7920. 10.1007/s12035-018-0917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Santacruz K, Smith CD, Patel E, Markesbery WR, 2009. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J. Neuropathol. Exp. Neurol 68, 774–784. 10.1097/NEN.0b013e3181aacbe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD, Davis DG, Poduska JW, Patel E, Mendiondo MS, Markesbery WR, 2010. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. Zurich Switz 20, 66–79. 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberacker T, Bajorat J, Ziola S, Schroeder A, Röth D, Kastl L, Edgar BA, Wagner W, Gülow K, Krammer PH, 2018. Enhanced expression of thioredoxin-interacting-protein regulates oxidative DNA damage and aging. FEBS Lett. 592, 2297–2307. 10.1002/1873-3468.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslowski CM, Hara T, O’Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, Greiner D, Kaufman RJ, Bortell R, Urano F, 2012. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metabol. 16, 265–273. 10.1016/j.cmet.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone L, Sbai O, Nawroth PP, Bierhaus A, 2012. The complexity of sporadic Alzheimer’s disease pathogenesis: the role of RAGE as therapeutic target to promote neuroprotection by inhibiting neurovascular dysfunction. Int. J. Alzheimer’s Dis 10.1155/2012/734956, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel BD, Kruppa AJ, Miranda E, Crowther DC, Lomas DA, Marciniak SJ, 2013. Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol. 12, 105–118. 10.1016/S1474-4422(12)70238-7. [DOI] [PubMed] [Google Scholar]
- Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J, 2015. Amyloid-beta: a crucial factor in Alzheimer’s disease. Med. Princ. Pract 24, 1–10. 10.1159/000369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J, 2009. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflammation 6, 41. 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos LE, Ferreira ST, 2018. Crosstalk between endoplasmic reticulum stress and brain inflammation in Alzheimer’s disease. Neuropharmacology, Metabolic Impairment as Risk Factors for Neurodegenerative Disorders 136, 350–360. 10.1016/j.neuropharm.2017.11.016. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A, Kivimaki M, 2014. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology 83, 486–493. 10.1212/WNL.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szegezdi E, Logue SE, Gorman AM, Samali A, 2006. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 7, 880–885. 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana RJS, Nunes AF, Rodrigues CMP, 2012. Endoplasmic reticulum enrollment in Alzheimer’s disease. Mol. Neurobiol. 46, 522–534. 10.1007/s12035-012-8301-x. [DOI] [PubMed] [Google Scholar]
- Wang C-Y, Xu Y, Wang X, Guo C, Wang T, Wang Z-Y, 2019a. Dl-3-n-Butylphthalide inhibits NLRP3 inflammasome and mitigates alzheimer’s-like pathology via nrf2-TXNIP-TrX Axis. Antioxidants Redox Signal. 30, 1411–1431. 10.1089/ars.2017.7440. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Ying, Bharti V, Zhou H, Hoi V, Tan H, Wu Z, Nagakannan P, Eftekharpour E, Wang J-F, 2019b. Upregulation of thioredoxin-interacting protein in brain of amyloid-β protein precursor/presenilin 1 transgenic mice and amyloid-β treated neuronal cells. J. Alzheimers Dis. JAD 72, 139–150. 10.3233/JAD-190223. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Park DJ, Ryu JC, Ozer HG, Tep C, Shin YJ, Lim TH, Pastorino L, Kunwar AJ, Walton JC, Nagahara AH, Lu KP, Nelson RJ, Tuszynski MH, Huang K, 2012. JNK3 perpetuates metabolic stress induced by Abeta peptides. Neuron 75, 824–837. 10.1016/j.neuron.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarifkar A, Choopani S, Ghasemi R, Naghdi N, Maghsoudi AH, Maghsoudi N, Rastegar K, Moosavi M, 2010. Agmatine prevents LPS-induced spatial memory impairment and hippocampal apoptosis. Eur. J. Pharmacol 634, 84–88. 10.1016/j.ejphar.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Che X, Zhang H, Tan G, Liu L, Jiang D, Zhao J, Xiang X, Sun X, He Z, 2017. Thioredoxin-interacting protein mediates apoptosis in early brain injury after subarachnoid haemorrhage. Int. J. Mol. Sci 18 10.3390/ijms18040854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J, 2010. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol 11, 136–140. 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]