Abstract
Background:
The crux in high-grade glioma surgery remains maximizing resection without affecting eloquent brain areas. Toward this, a myriad of adjunct tools and techniques has been employed to enhance surgical safety and efficacy. Despite intraoperative MRI and advanced neuronavigational techniques, as well as augmented reality, to date, the only true real-time visualization tool remains the ultrasound (US). Neuroultrasonography is a cost-efficient imaging modality that offers instant, real-time information about the changing anatomical landscape intraoperatively. Recent advances in technology now allow for the integration of intraoperative US with neuronavigation.
Case Description:
In this report, we present the resection technique for three cases of high-grade gliomas (two glioblastomas and one anaplastic astrocytoma). The patient presented with a variable clinical spectrum. All three cases have been performed using the Brainlab® neuronavigation system (BrainLAB, Munich, Germany) and the bk5000 US Machine® (BK Medical, Analogic Corporation, Peabody, Massachusetts, USA).
Conclusion:
Gross total resection was achieved in all three cases. The use of 3D navigated US was a reliable adjunct surgical tool in achieving favorable resection outcomes in these patients.
Keywords: 3D, High-grade, Navigated, Report, Ultrasound

INTRODUCTION
Gliomas are among the most devastating diseases known to mankind and currently, safe maximal surgical resection followed by adjuvant chemoradiation therapy is the standard of care.[1,41] Numerous studies demonstrated the benefit of gross total resection in improving patient survival times as an independent prognostic factor.[2,4,8,33,36]
Preoperative imaging and neuronavigational systems are essential tools for routine surgical planning and resection of brain tumors.[7,18,37,43] Conventional image-guided surgery, or neuronavigation, relies mainly on preoperative computed tomographic (CT) and magnetic resonance (MR) images.[43] Yet, intraoperative neuronavigation is at times rendered inaccurate as brain shift occurs.[13,17,43] To tackle this, intraoperative MRI (iMRI) has been introduced to provide near real-time imaging to the surgeon.[32,43] However, iMRI is less useful in more urgent surgical settings such as suspected hemorrhage or to repeatedly account for brain shift; it is also a higher cost and logistic endeavor only applicable to select neurosurgical practices.[43]
Intraoperative ultrasonography (IoUS) is a versatile alternative that has been used broadly across surgical disciplines, such as in breast, liver, and pancreatic operations.[3,25,35] IoUS has been a staple in neurosurgical oncology since its inception in the late 1970s by Reid when it was used to visualize cervical intramedullary diffuse glioma.[28] The fact that IoUS can provide instant, real-time updates of the operative field without hindering the surgical workflow explains its widespread use among oncologic neurosurgeons.[23,30,39,40] The development of three-dimensional (3D) ultrasound (US) technology has opened an avenue for navigational integration and helped overcome issues posed by standard two-dimensional US, namely, interpreting atypical planar images and orienting using a restricted field of view.[30] Further advancements in ultrasonography image resolution and postimage processing have improved the quality of ultrasonography, created smaller probes better suited for neurosurgery, and integrated US with existing neuronavigation systems.[30,33] This integration allows surgeons to visualize the surgical field in real-time while tracking the probe’s location on the preoperative MRI.[33]
The current literature lacks sufficient evidence of the efficacy and the applicability of navigated 3D US in neurosurgical oncology. In this article, we aim to demonstrate and assess the value of navigated 3D US in resecting HGGs, with an emphasis on its supporting role in tumor mapping, preservation of key structures, locating residual tumor, and identifying possible hematoma accumulation during closure. Here, we report three consecutive HGG cases where integration of navigated 3D US in the surgical workflow aided in achieving maximal safe resection of tumors. We also discuss the literature examining the benefits and limitations of navigated 3D US compared to other neurosurgical imaging modalities, as well as examine its potential role in the future of neurosurgical oncology.
CASE REPORTS AND CLINICAL MATERIALS
Patients and 3D navigated US
Retrospective data collection was performed on three patients; (three females) with a mean age of 56 years (Range 32–68). All patients presented with diagnosed high-grade gliomas (two glioblastomas [GBMs] and one anaplastic astrocytoma). All three cases have been performed using the Brainlab® neuronavigation system (BrainLAB, Munich, Germany) and the bk5000 US Machine® (BK Medical, Analogic Corporation, Peabody, Massachusetts, USA). The operating room setup during US-guided resection of the three cases is shown in Figure 1. Technique in brief, once the skull flap is removed, the navigated US probe is used to acquire datasets that are volumetrically merged with each other and also can be fused to the preoperative navigational CT and MRI sequences. US echogenicity can then be used as a valuable additional imaging modality toward navigation. The process of acquiring 3D neuroultrasonographic datasets can be repeated as needed to generate a real-time high-quality navigational sequence (e.g., to account for brain shift further into the surgery).
Figure 1:
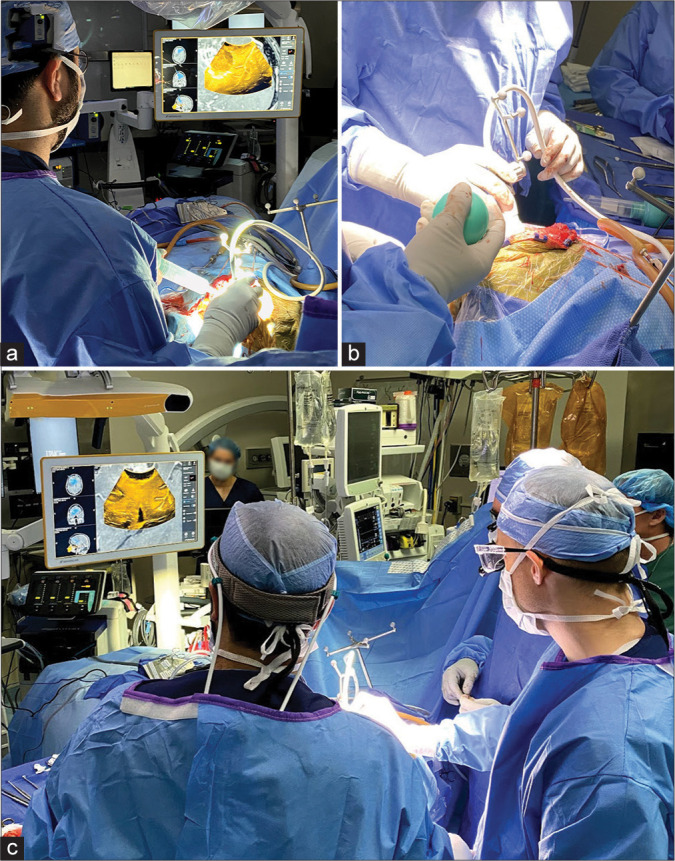
A figure representation of the operating room setup during ultrasound-guided resection of high-grade gliomas. Note the digital integration of the bk5000 ultrasound device images with the conventional Brainlab navigation console. The Brainlab ultrasound navigation integration provides updated and accurate real-time overlay of ultrasound imaging on preoperative MRI/CT providing immediate and accurate information that accounts for brain shift during resection.
Patient 1
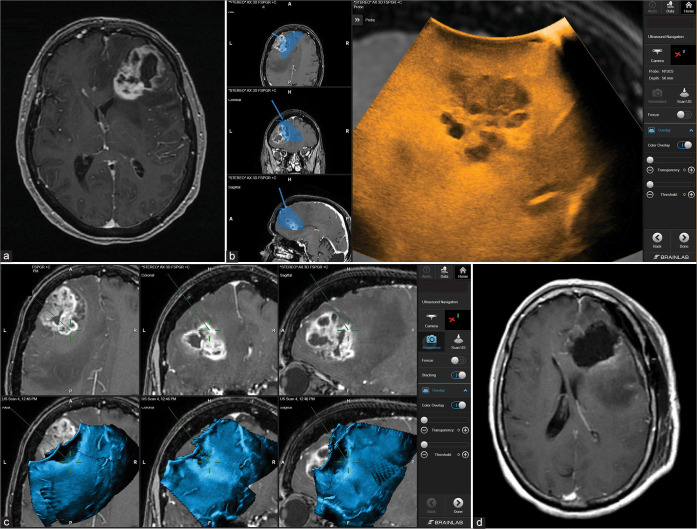
The patient is a 68-year-old Caucasian female who presented with worsening gait instability and recurrent falls. The patient was diagnosed with GBM WHO Grade IV and was initially treated at an outside facility with subtotal resection, concomitant radiation, and three cycles of temozolomide. Six months later, the patient presented to our facility with personality changes and mild expressive aphasia, increased nodular contrast enhancement inside, and extending from the prior resection cavity, as well as increasing enhancement tracking through the genu of the corpus callosum with marked surrounding vasogenic edema extending through the left frontal lobe [Figures 2a-d]. The patient was appropriate for awake craniotomy for resection of this recurrent tumor. At surgery, following dural opening, 3D navigated US was used to map out the tumor and adjacent brain. Two US 3D datasets were acquired that were merged, overlaid with the navigational MRI, and then used to navigate off using the microscope and navigation wand. 5-ala was used for the tumor resection and once maximum safe resection had been performed; a 3D dataset was acquired and resection margins were verified using the navigation wand and the newly acquired brain shift independent US 3D dataset. It was then noticed that a small residual tumor nodule was still present deep abutting the ventricle which was then resected using the cavitron ultrasonic surgical aspirator. Once gross total resection was achieved according to 5-ala, US, and navigation, meticulous final hemostasis was obtained. The US was again used after dural closure to demonstrate the absence of any untoward findings in the resection cavity or ventricles. The pathology report demonstrated that the tumor was a WHO Grade IV GBM, IDH1 wildtype, MGMT promoter methylated, TERT mutation, PTEN mutation, EGFR mutation, and GOPC-ROS1 fusion. Postoperatively, the patient’s aphasia improved without additional focal neurological deficits. The patient received postoperative radiotherapy and three cycles of temozolomide and remained in remission for a total of a 9 months. The patient developed recurrence after 9 months and was deceased.
Figure 2:
Patient 1. Preoperative axial MRI with contrast showing the extent of tumor involvement in the left frontal lobe (a). Intraoperative Images showing the integration of real-time ultrasound images with the Brainlab navigation system (b). Using real-time ultrasound to safely and efficiently determine the extent of resection of the tumor bed and effectively accounting for possible brain shift towards the end of surgery (c). Postoperative axial MRI with contrast showing complete tumor resection (d).
Patient 2
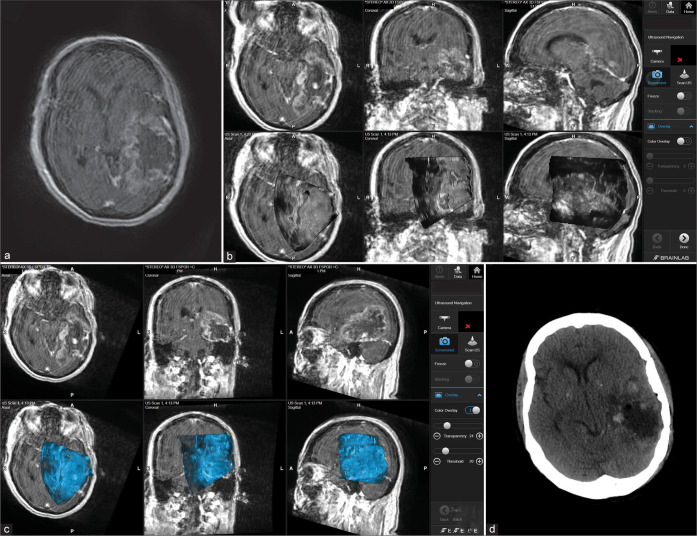
The patient is a 68-year-old female with a medical history of sarcoidosis presented with a 1-month history of mental status change, right-sided headaches, and difficulty finding words that have significantly worsened over the past 2 weeks before admission. MRI demonstrated an ill-defined heterogeneously enhancing 8.7 cm mass in the left parietal and temporal lobes with mass effect [Figures 3a-d]. At surgery, dura was opened and the corticectomy was started from the inferior and middle temporal gyrus from just behind the temporal tip. The tumor was extremely swollen, hemorrhagic, and fibrous in nature which resulted in the tumor being adherent to neighboring blood vessels. Navigated 3D US was used to navigate through the swollen temporal lobe and the vein of Labbe was preserved. The tumor was removed to achieve a maximum safe resection. However, due to the fibrous nature of the tumor and its adherence to a major blood vessel, subtotal resection was achieved. Following final hemostasis, navigated US was again used after dural closure to demonstrate if there was any hematoma in the resection cavity. Pathology demonstrated GBM, IDH wild type, TERT, and P53 mutated, and MGMT promoter unmethylated. The patient received adjuvant treatment in an outside facility and remained in remission currently at the time of writing this report for a total follow-up period of 23 months.
Figure 3:
Patient 2. Preoperative axial MRI with contrast showing massive temporoparietal involvement with high-grade glioma (a). Intraoperative images showing the integration of real-time ultrasound images with the Brainlab navigation system during the course of surgery (b and c). Postoperative axial CT scan showing complete tumor resection (d).
Patient 3
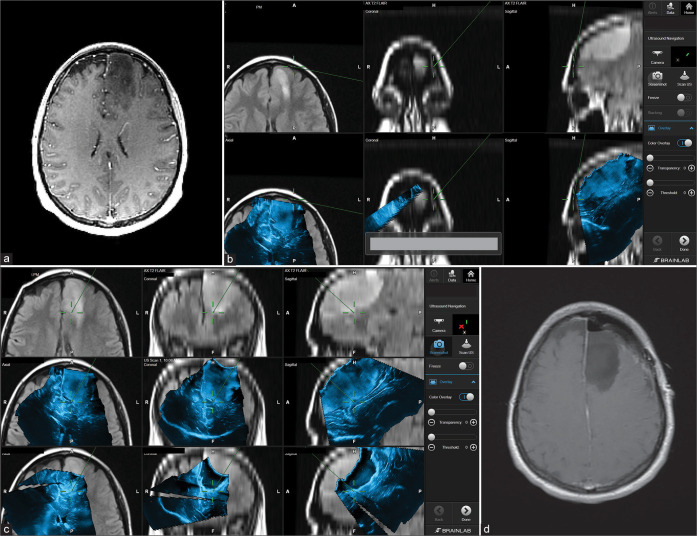
The patient is a 32-year-old female with no significant medical history who presented to our institution from an outside hospital after an incidental brain mass was found 1 month before presentation. On examination, the neurological examination was normal. MRI demonstrated an anterior frontal lobe mass which was predominantly extra-axial with T2 sequence hyperintensity and CSF cleft sign on sagittal T1-weighted images. The mass showed vague contrast-enhancement and minimal vasogenic edema. On FLAIR images, the mass was hyperintensity and measured 5.7 × 4.4 cm [Figures 4a-d]. At surgery, the tumor was then mapped out and the incision was marked in a hair-sparing fashion. The dura was opened and then an US was used to ensure that the craniotomy was adequate. Navigated US was used along with conventional neuronavigation to map out the tumor. We then acquired a 3D data set for navigated US guidance. The corticectomy was performed in a microsurgical fashion using loupe magnification and microsurgical techniques. The tumor was anatomically resected. We then used navigation to delineate the tumor borders deep and medial, then the tumor was removed in an en bloc fashion. The role of navigated US became invaluable when we assessed for residual tumor. We were able to locate additional residual tumor bits, mostly posteriorly and far anteriorly where the residual tumor was suspected. Following final hemostasis, navigated US was again used after dural closure to demonstrate if there was any hematoma in the resection cavity. Pathology demonstrated an anaplastic astrocytoma IDH-mutant with ATRX mutations identified with negative MGMT promoter methylation. The patient received adjuvant six cycles of temozolomide in an outside facility and remains in remission currently at the time of writing this report for a total follow-up period of 21 months.
Figure 4:
Patient 3. Preoperative axial MRI with contrast showing left frontal lobe involvement with high-grade glioma (Anaplastic astrocytoma) (a). Intraoperative images showing the integration of real-time ultrasound images with the Brainlab navigation system during the course of surgery (b and c). In this case, it is apparent how navigated ultrasound can also help in planning the surgical course at the beginning of the surgery (b) and the role of assuring tumor bed safe margins toward the end of the resection (c). Postoperative axial MRI scan showing complete tumor resection (d).
REVIEW OF THE LITERATURE AND DISCUSSION
The application of US in neurosurgery dates back to the 1950s when French et al. used ultrasonics to locate subcortical neoplasms in autopsied brains.[9] Initially, the anatomical constraints, including the inability of high-frequency waves to pass through the skull, slowed the assimilation of the use of USs into the diagnostic repertoire of neurosurgery.[5,11] However, the interest in the intraoperative ultrasound (IoUS) was revived when surgeons realized that they could use IoUS as a real-time guidance system during the placement of ventriculoperitoneal shunts and identification and resection of solid and metastatic CNS tumors.[5,11,27,28,30] This paved the way for the advancement and evolution of IoUS imaging modalities we see today (intraoperative contrast-enhanced US [iCEUS], Doppler US, navigated 3D US, etc.) that have become an essential part of current practice by aiming to assess the changing anatomical landscape during neurosurgical procedures.[10,11,24,28,43]
Over the past 20 years, neuronavigation has become an incredibly useful tool in providing guidance during neurosurgical procedures. The brain is a geometric volume; therefore, it can be divided into Cartesian planes, and any space within those planes can be defined by a set of coordinates. Neuronavigational systems take advantage of this by utilizing the tip of a probe to define a space within the brain in terms of these coordinates and then matching those coordinates to a parallel coordinate system in a 3D image. This allows surgeons to recognize the specific location of the brain; they are operating on, in the context of the preoperative scans, and can guide resection plans with great spatial accuracy. It is especially helpful in procedures that involve deep structures of the brain with highly important neurovascular structures.[12,42] Originally, neuronavigational systems consisted of a dual infrared light source that was fixed to a surgical instrument (digitizer), and the data from this digitizer were correlated to a reference preoperative image. However, this system could not track the shift of brain structures during surgery, and thus, the need for a modality that could provide real-time data was recognized.[20,42] This led to the introduction of navigated 3D U/S technology, namely, the Sonowand, in which a neuronavigational system is built into a high-end US scanner. The US scanner emits high-frequency sound pulses that are partially reflected off tissue. Reflected waves are then repicked up by the scanner, and the scanner can use the new frequencies to create an image that shows the distance objects are from it. With the SonoWand, neurosurgeons can acquire serial temporal images of the brain and reconstruct a 3D volumetric scan that identifies and quantifies brain shift, as well as precisely shows the location of residual tumor remnants in real-time.[14,15,24,43] This technology allows neurosurgeons to constantly refine their surgical plan based on the changing surgical landscape and has led to an excellent extent of a resection assessment tool for brain tumors that complements neuronavigational systems.[22,34] Given that the extent of surgical resection of most brain tumors is correlated to overall survival, the correct implementation of navigated 3D U/S has the potential to significantly improve neurosurgical outcomes.
The low cost, absence of radiation, real-time feedback, and minimal anatomical disruption make the use of IoUS in neurosurgical practice extremely appealing, especially with the minimal efficacy sacrifice that it has to offer.[10,24] Studies have shown that the ability of 3D U/S to delineate tumor borders is equivalent to that of T2-weighted MRI and 3D MRI and is better than T1-weighted MRI.[24,30,38] Unsgaard et al. found that the correlation between U/S images and histopathology was about the same as the correlation between T2-weighted MRI images and histopathology for the detection of astrocytomas (74–59%) and GBMs (79–69%). Furthermore, they showed that the correlation between U/S images and histopathology was higher than the correlation between T1-weighted MRI images and histopathology for the detection of astrocytomas (74–35%).[38] Since efficacy is maintained while using IoUS in comparison to MRI, it is expected that the intraoperative MRI could be completely replaced by IoUS images in the future.[24] The efficacy of IoUS is only achieved, however, if the operator of the US is properly trained. One of the largest limitations of U/S use is its technical complexities and artifacts.[30,31] In addition, the plane of insolation does not always match with the axial plane surgeons who are familiar with, and the accuracy of the image is typically diminished with each stage of the surgical procedure in which the anatomy may be distorted.[10,24,30] Thus, it remains clear that ultrasonography training is required for neurosurgeons to ensure that the efficacy of this tool is maintained during surgical use.[3]
As navigated 3D US techniques continue to evolve, an increase in image definition is expected. While image fusion between preoperative MRIs and navigated 3D U/S has eliminated the issue surrounding the plane of insolation and interpretation of U/S images in comparison to other modalities, the issue of artifacts still remains.[24] Fluids like saline often used to wash the resection cavity during neurosurgical procedures and conduct speed slower than homogenous brain tissue does. This can result in brightness artifacts on the U/S image that may obscure the surgical view.[30] Furthermore, the use of nanoparticles (NPs) is being investigated as a possible contrast agent that could enhance the visibility of brain structures during iCEUS – a relatively new neurosurgical imaging technique that allows for vascular mapping of the brain.[10,19,21,26] NPs are able to avoid serum protein binding, and thus, can concentrate in an organ of interest and produce high signal-to-background ratios that enhance image definition.[6] Given that some NPs can cross the blood-brain barrier, iCEUS could be used to map out the vascular architecture of tumors during neurosurgical procedures; this could help neurosurgeons avoid inadvertently injuring vascular structures during tumor resection.[6,19]
CONCLUSION
Surgical eradication of high-grade gliomas remains a challenge for neurosurgeons due to the complex anatomy and the invasive nature of such tumors. The introduction of novel neuronavigation modalities combined with advanced imaging techniques could facilitate achieving a maximal resection which, in turn, correlates with better survival rates for patients with high-grade gliomas.[16] Navigated 3D US is a relatively novel adjunct surgical tool that greatly enhances conventional neuronavigation. The current literature discussing 3D navigated US’s role in neurosurgery despite being scarce does point toward a favorable impact of utilizing this modality as a cost-efficient, relatively accurate, and real-time source of information within the ever-shifting brain anatomy during tumor resection.[24,29,43] The ability to obtain real-time feedback regarding brain shift during surgery, especially with larger and more superficial tumors, is beneficial to achieving favorable resection outcomes. Nevertheless, achieving such outcomes should pivot on a firm familiarization with the technical aspect of this modality as well as the ability to identify possible image artifacts intraoperatively. Finally, 3D navigated US if optimally used could play a role in attaining better survival outcomes for glioma patients.
Footnotes
How to cite this article: Habib A, Jovanovich N, Hoppe M, Hameed NU, Edwards L, Zinn P. Navigated 3D ultrasound-guided resection of high-grade gliomas: A case series and review. Surg Neurol Int 2022;13:356.
Contributor Information
Ahmed Habib, Email: habiba@upmc.edu.
Nicolina Jovanovich, Email: jovanovich.nicolina@medstudent.pitt.edu.
Meagan Hoppe, Email: meagan.hoppe@pitt.edu.
N.U. Farrukh Hameed, Email: hameedf@upmc.edu.
Lincoln Edwards, Email: edwardsl7@upmc.edu.
Pascal Zinn, Email: zinnpo@upmc.edu.
Declaration of patient consent
Patients’ consent not required as patients’ identities were not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K, et al. Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): A randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17:1521–32. doi: 10.1016/S1470-2045(16)30313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16:81–91. doi: 10.1093/neuonc/not159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozinov O, Burkhardt JK, Fischer CM, Kockro RA, Bernays RL, Bertalanffy H. Advantages and limitations of intraoperative 3D ultrasound in neurosurgery. Technical note. Acta Neurochir Suppl. 2011;109:191–6. doi: 10.1007/978-3-211-99651-5_30. [DOI] [PubMed] [Google Scholar]
- 4.Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016;2:1460–9. doi: 10.1001/jamaoncol.2016.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandler WF, Knake JE, McGillicuddy JE, Lillehei KO, Silver TM. Intraoperative use of real-time ultrasonography in neurosurgery. J Neurosurg. 1982;57:157–63. doi: 10.3171/jns.1982.57.2.0157. [DOI] [PubMed] [Google Scholar]
- 6.Choi HS, Frangioni JV. Nanoparticles for biomedical imaging: Fundamentals of clinical translation. Mol Imaging. 2010;9:291–310. [PMC free article] [PubMed] [Google Scholar]
- 7.Coenen VA, Krings T, Mayfrank L, Polin RS, Reinges MH, Thron A, et al. Three-dimensional visualization of the pyramidal tract in a neuronavigation system during brain tumor surgery: First experiences and technical note. Neurosurgery. 2001;49:86–92. doi: 10.1097/00006123-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Doyle J, Khalafallah AM, Yang W, Sun Y, Bettegowda C, Mukherjee D. Association between extent of resection on survival in adult brainstem high-grade glioma patients. J Neurooncol. 2019;145:479–86. doi: 10.1007/s11060-019-03313-w. [DOI] [PubMed] [Google Scholar]
- 9.French LA, Wild JJ, Neal D. The experimental application of ultrasonics to the localization of brain tumors; preliminary report. J Neurosurg. 1951;8:198–203. doi: 10.3171/jns.1951.8.2.0198. [DOI] [PubMed] [Google Scholar]
- 10.Ganau M, Ligarotti GK, Apostolopoulos V. Real-time intraoperative ultrasound in brain surgery: Neuronavigation and use of contrast-enhanced image fusion. Quant Imaging Med Surg. 2019;9:350–8. doi: 10.21037/qims.2019.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganau M, Syrmos N, Martin AR, Jiang F, Fehlings MG. Intraoperative ultrasound in spine surgery: History, current applications, future developments. Quant Imaging Med Surg. 2018;8:261–7. doi: 10.21037/qims.2018.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganslandt O, Behari S, Gralla J, Fahlbusch R, Nimsky C. Neuronavigation: Concept, techniques and applications. Neurol India. 2002;50:244–55. [PubMed] [Google Scholar]
- 13.Gerard IJ, Kersten-Oertel M, Petrecca K, Sirhan D, Hall JA, Collins DL. Brain shift in neuronavigation of brain tumors: A review. Med Image Anal. 2017;35:403–20. doi: 10.1016/j.media.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Gronningsaeter A, Kleven A, Ommedal S, Aarseth TE, Lie T, Lindseth F, et al. SonoWand, an ultrasound-based neuronavigation system. Neurosurgery. 2000;47:1373–9. [PubMed] [Google Scholar]
- 15.Hammoud MA, Ligon BL, elSouki R, Shi WM, Schomer DF, Sawaya R. Use of intraoperative ultrasound for localizing tumors and determining the extent of resection: A comparative study with magnetic resonance imaging. J Neurosurg. 1996;84:737–41. doi: 10.3171/jns.1996.84.5.0737. [DOI] [PubMed] [Google Scholar]
- 16.Hervey-Jumper SL, Berger MS. Role of surgical resection in low-and high-grade gliomas. Curr Treat Options Neurol. 2014;16:284. doi: 10.1007/s11940-014-0284-7. [DOI] [PubMed] [Google Scholar]
- 17.Iversen DH, Wein W, Lindseth F, Unsgård G, Reinertsen I. Automatic intraoperative correction of brain shift for accurate neuronavigation. World Neurosurg. 2018;120:e1071–8. doi: 10.1016/j.wneu.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Jung TY, Jung S, Kim IY, Park SJ, Kang SS, Kim SH, et al. Application of neuronavigation system to brain tumor surgery with clinical experience of 420 cases. Minim Invasive Neurosurg. 2006;49:210–5. doi: 10.1055/s-2006-948305. [DOI] [PubMed] [Google Scholar]
- 19.Kearns KN, Sokolowski JD, Chadwell K, Chandler M, Kiernan T, Prada F, et al. The role of contrast-enhanced ultrasound in neurosurgical disease. Neurosurg Focus. 2019;47:E8. doi: 10.3171/2019.9.FOCUS19624. [DOI] [PubMed] [Google Scholar]
- 20.Khoshnevisan A, Allahabadi NS. Neuronavigation: Principles, clinical applications and potential pitfalls. Iran J Psychiatry. 2012;7:97–103. [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JH, Park G, Hong GH, Choi J, Choi HS. Design considerations for targeted optical contrast agents. Quant Imaging Med Surg. 2012;2:266–73. doi: 10.3978/j.issn.2223-4292.2012.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindner D, Trantakis C, Renner C, Arnold S, Schmitgen A, Schneider J, et al. Application of intraoperative 3D ultrasound during navigated tumor resection. Minim Invasive Neurosurg. 2006;49:197–202. doi: 10.1055/s-2006-947997. [DOI] [PubMed] [Google Scholar]
- 23.Moiraghi A, Pallud J. Intraoperative ultrasound techniques for cerebral gliomas resection: Usefulness and pitfalls. Ann Transl Med. 2020;8:523. doi: 10.21037/atm.2020.03.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moiyadi AV, Shetty P. Direct navigated 3D ultrasound for resection of brain tumors: A useful tool for intraoperative image guidance. Neurosurg Focus. 2016;40:E5. doi: 10.3171/2015.12.FOCUS15529. [DOI] [PubMed] [Google Scholar]
- 25.Pan H, Wu N, Ding H, Ding Q, Dai J, Ling L, et al. Intraoperative ultrasound guidance is associated with clear lumpectomy margins for breast cancer: A systematic review and meta-analysis. PLoS One. 2013;8:e74028. doi: 10.1371/journal.pone.0074028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prada F, Mattei L, Del Bene M, Aiani L, Saini M, Casali C, et al. Intraoperative cerebral glioma characterization with contrast enhanced ultrasound. Biomed Res Int. 2014;2014:484261. doi: 10.1155/2014/484261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quencer RM, Montalvo BM. Normal intraoperative spinal sonography. AJR Am J Roentgenol. 1984;143:1301–5. doi: 10.2214/ajr.143.6.1301. [DOI] [PubMed] [Google Scholar]
- 28.Reid MH. Ultrasonic visualization of a cervical cord cystic astrocytoma. AJR Am J Roentgenol. 1978;131:907–8. doi: 10.2214/ajr.131.5.907. [DOI] [PubMed] [Google Scholar]
- 29.Saß B, Bopp M, Nimsky C, Carl B. Navigated 3-dimensional intraoperative ultrasound for spine surgery. World Neurosurg. 2019;131:e155–69. doi: 10.1016/j.wneu.2019.07.188. [DOI] [PubMed] [Google Scholar]
- 30.Sastry R, Bi WL, Pieper S, Frisken S, Kapur T, Wells W, 3rd, et al. Applications of ultrasound in the resection of brain tumors. J Neuroimaging. 2017;27:5–15. doi: 10.1111/jon.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selbekk T, Jakola AS, Solheim O, Johansen TF, Lindseth F, Reinertsen I, et al. Ultrasound imaging in neurosurgery: Approaches to minimize surgically induced image artefacts for improved resection control. Acta Neurochir (Wien) 2013;155:973–80. doi: 10.1007/s00701-013-1647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011;12:997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 33.Shetty P, Yeole U, Singh V, Moiyadi A. Navigated ultrasound-based image guidance during resection of gliomas: Practical utility in intraoperative decision-making and outcomes. Neurosurg Focus. 2021;50:E14. doi: 10.3171/2020.10.FOCUS20550. [DOI] [PubMed] [Google Scholar]
- 34.Šteňo A, Hollý V, Mendel P, Šteňová V, Petričková Ľ, Timárová G, et al. Navigated 3D-ultrasound versus conventional neuronavigation during awake resections of eloquent low-grade gliomas: A comparative study at a single institution. Acta Neurochir (Wien) 2018;160:331–42. doi: 10.1007/s00701-017-3377-8. [DOI] [PubMed] [Google Scholar]
- 35.Sun MR, Brennan DD, Kruskal JB, Kane RA. Intraoperative ultrasonography of the pancreas. Radiographics. 2010;30:1935–53. doi: 10.1148/rg.307105051. [DOI] [PubMed] [Google Scholar]
- 36.Trevisi G, Barbone P, Treglia G, Mattoli MV, Mangiola A. Reliability of intraoperative ultrasound in detecting tumor residual after brain diffuse glioma surgery: A systematic review and meta-analysis. Neurosurg Rev. 2020;43:1221–33. doi: 10.1007/s10143-019-01160-x. [DOI] [PubMed] [Google Scholar]
- 37.Unsgaard G, Ommedal S, Muller T, Gronningsaeter A, Nagelhus Hernes TA. Neuronavigation by intraoperative three-dimensional ultrasound: Initial experience during brain tumor resection. Neurosurgery. 2002;50:804–12. doi: 10.1097/00006123-200204000-00022. [DOI] [PubMed] [Google Scholar]
- 38.Unsgaard G, Selbekk T, Muller TB, Ommedal S, Torp SH, Myhr G, et al. Ability of navigated 3D ultrasound to delineate gliomas and metastases comparison of image interpretations with histopathology. Acta Neurochir (Wien) 2005;147:1259–69. doi: 10.1007/s00701-005-0624-1. discussion 1269. [DOI] [PubMed] [Google Scholar]
- 39.Unsgård G, Lindseth F. 3D ultrasound-guided resection of low-grade gliomas: Principles and clinical examples. Neurosurg Focus. 2019;47:E9. doi: 10.3171/2019.9.FOCUS19605. [DOI] [PubMed] [Google Scholar]
- 40.Villa A, Costantino G, Meli F, Odierna Contino A, Imperato A, Francaviglia N. Ultrasound-based real-time neuronavigated fluorescence-guided surgery for high-grade gliomas: Technical note and preliminary experience. Acta Neurochir (Wien) 2019;161:2595–605. doi: 10.1007/s00701-019-04094-x. [DOI] [PubMed] [Google Scholar]
- 41.Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15:e395–403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 42.Willems PW, van der Sprenkel JW, Tulleken CA, Viergever MA, Taphoorn MJ. Neuronavigation and surgery of intracerebral tumours. J Neurol. 2006;253:1123–36. doi: 10.1007/s00415-006-0158-3. [DOI] [PubMed] [Google Scholar]
- 43.Yeole U, Singh V, Mishra A, Shaikh S, Shetty P, Moiyadi A. Navigated intraoperative ultrasonography for brain tumors: A pictorial essay on the technique, its utility, and its benefits in neuro-oncology. Ultrasonography. 2020;39:394–406. doi: 10.14366/usg.20044. [DOI] [PMC free article] [PubMed] [Google Scholar]