Abstract
Background:
Novice neurosurgeons require neurosurgical technique training, but the current method is demanding and time consuming. Therefore, it is crucial to perform training using an appropriate and informative method. In this report, we describe our attempts to provide training in neurosurgical techniques using goat in vivo brain model and to demonstrate the effectiveness of this model.
Methods:
Under general anesthesia, the surgery was performed on a male goat in the prone position. A midline liner skin incision was made in the scalp, six burr holes were drilled, a craniectomy was performed, and the dura was incised in an arcuate fashion. We attempted the interhemispheric approach and a retrosigmoid approach.
Results:
It was confirmed that common neurosurgical approaches are achievable in this model. Furthermore, anatomical structures such as nerves and blood vessels were similar to those of humans. Moreover, the goat brain was similar in color and texture to that of humans.
Conclusion:
Unlike a cadaver brain, in vivo brain requires hemostasis and careful dissection, which provides the surgeons a realistic experience of actual neurosurgery.
Keywords: Brain model, Cadaver dissection, Goats, Hemostasis, Neurosurgery

INTRODUCTION
At present, there are two ways to learn for novice and inexperienced neurosurgeon: By viewing operative videos and observing the surgical techniques of exceptional neurosurgeons, or by joining a cadaver dissection course and learning through hands-on practice.[2] It would be beneficial to learn through hands-on experience. At present, there are no reports of neurosurgical procedure training using the goat brain. The previously studied porcine brain model is relatively fragile than the goat brain.[9] Bovine and ovine models are more expensive than goat brain. Thus, the goat brain model may be more suitable for neurosurgical training. In this study, we report an attempt to provide neurosurgical training using an in vivo goat brain model.
MATERIALS AND METHODS
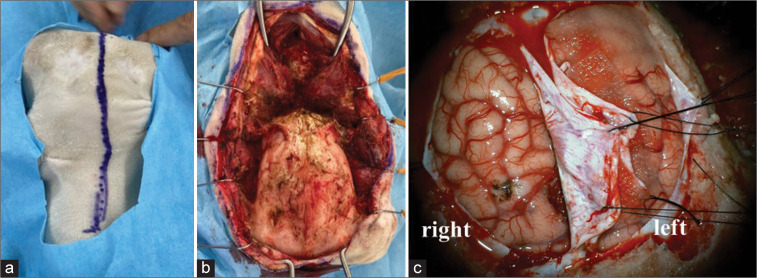
In this study, we used two female goats. They weighed 27.5 and 28.0 kg, respectively. Under general anesthesia, xylazine 0.4 mg/0.02/kg and atropine 0.5 mg were injected intramuscularly, and isoflurane 5% and O2 100% 1.5–2.0 ml/min were administered for anesthesia induction. The goat was intubated and ventilated to control breathing. Anesthesia was maintained with 2% isoflurane and 100% O2 at 1.5–2.0 ml/min. The goat was placed in the prone position and the head was patently fixed. A midline skin incision was made in the scalp [Figure 1a]. The skull was exposed [Figure 1b], and six burr holes were drilled using an air tome for craniotomy. The craniotomy was performed to the size of 6 cm × 6 cm. Hemorrhage from the superior sagittal sinus was hemostatically treated with DuraGen®, and a hemicircular incision was made in the dura mater [Figure 1c]. Under a surgical microscope, the right interhemispheric approach was performed. The interhemispheric fissure was further opened posteriorly to reach the cerebellar tentorium. Next, the skin incision was extended to the spinal level of the second cervical vertebra, exposing the suboccipital bone, the first and second cervical vertebrae. Several burr holes were made in the suboccipital bone. The craniectomy was performed until fully exposing the transverse and sigmoid sinus. The dura mater of the posterior cranial fossa was incised in a V-shape, and a spatula was inserted and retracted in the lateral side of the cerebellum to observe the posterior fossa base. In addition, after the wound was sutured, the goat was placed in the supine position, and the femoral artery was exposed for end-to-end anastomosis training (data not shown). Both the goats underwent the surgery with minimum suffering under the supervision of veterinarians. We assure that the study was conducted under the conformity of the international, national, and institutional rules considering animal experiments, clinical studies, and biodiversity rights.
Figure 1:
From scalp incision to dural incision (a) midline scalp skin incision, (b) a craniotomy performed using an air tome, and (c) arc-shaped dural incision was made. Color and texture of the brain surface were similar to those of the human brain.
RESULTS
The dura mater was as thin as a sub-adult. The interhemispheric fissure showed the same morphology as that of a human. The bridging vein was fragile and it was then coagulated and cut. Pia matter, the texture of the brain, was similar to that of the human brain. The interhemispheric approach allowed us to reach the pericallosal artery [Figure 2a] and the corpus callosum by dissection to a depth of about 2 cm. When the interhemispheric fissure was opened posteriorly, the Galenic vein [Figure 2b] and cerebellar tentorium were identified immediately. The falx and cerebellar tentorium were very thick. In the right retrosigmoid approach, it was observed that the lower cranial nerves were entering the jugular foramen [Figure 3a] and the 7th–8th cranial nerve complex [Figure 3b] was entering the internal auditory canal. The lower cranial nerves, that is, the 9th, 10th, and 11th cranial nerves, could be identified. Their morphology was identical to that of humans. Furthermore, it was confirmed that the running facial and vestibular auditory nerve could be recognized in detail, and that it showed the same form as that of humans.
Figure 2:
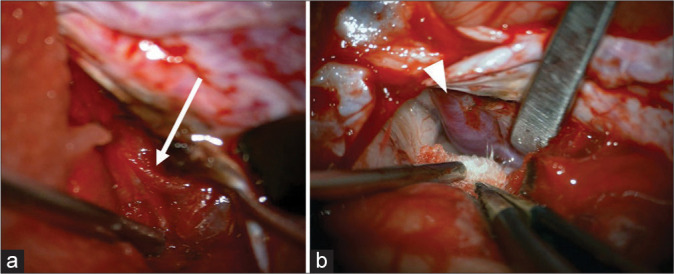
Interhemispheric approach (a) pericallosal artery (arrow) was identified and dissected from the surrounding tissue and (b) posteriorly proceeding to interhemispheric dissection, galenic vein (arrow head) was identified.
Figure 3:
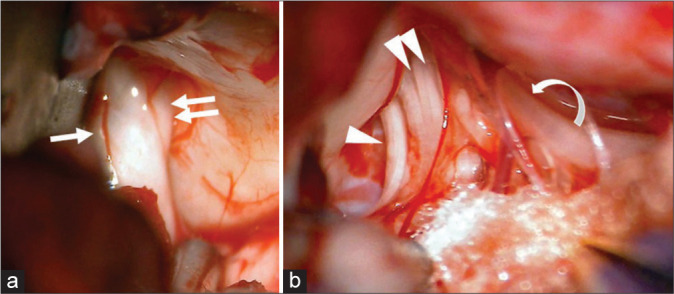
Retrosigmoid approach (a) when the lateral cerebellum was compressed with a spatula, the facial nerve (arrow) and vestibulocochlear nerve (double arrows) entering the internal auditory canal were identified, (b) lower cranial nerve such as the glossopharyngeal nerve (arrow head), vagus nerves (double arrow heads), and accessory nerve (curbed arrow) were identified to be entering the jugular foramen.
DISCUSSION
There are reports of the use of bovine,[5,10] ovine,[3,6] and porcine,[1,4,8] as large animal models for neurosurgical training. Due to their similar neuroanatomy, the bovine, ovine, and porcine models have proven to be reliable structures in neurosurgical simulations.[7] The bovine brain can reproduce the interhemispheric, transcallosal, and retrosigmoid approaches, along with various approaches to the ring of Willis.[5,10] In ovine models, lumbar discectomy and craniosynostosis surgery have been reported.[3,6] Various cranial procedures have been investigated both in vivo and ex vivo in porcine models.[1,4,8] In ex vivo porcine models, the interhemispheric fissure can be dissected, allowing the inspection of the cingulate gyrus, callosomarginal, and pericallosal arteries.[8] In addition, other reports suggested that an approach to the posterior fossa cranial base was available in ex vivo porcine model.[4] However, porcine brains are relatively fragile,[2] which is better suited for technical training. The goat brain is thought to be more similar to human brain texture and pia, arachnoid, and dura matter structures.[9] The ovine and bovine brain models have higher costs than goat. For these reasons, we employed goat brains in this study.
This is the first report of neurosurgical training using a goat in vivo brain. Previously, only one report of a neurosurgical procedure training using goat ex vivo brain model has been published.[9] The goat brain used in the study was obtained by injecting a silicon dye into the cerebral blood vessels of a goat brain. The dye distinguished arteries and veins are in the same manner as in a human cadaver. The study determined that the color and texture of the goat brain are extremely similar to that of the human brain. In addition, the goat brain is less expensive than a human cadaveric brain and may be suitable for neurosurgical training. In this study, we also confirmed that the texture of the brain is not completely dissimilar from that of the human brain. However, our study uses goat in vivo brain, which is considered more similar to the human brain in texture and color. Here, it was confirmed that the structures of the interhemispheric fissure, cerebellar tentorium, and posterior fossa cranial base were similar to those of the human brain.
It is difficult for young neurosurgeons to fully acquire neurosurgical skills.[4,8] Moreover, we believe that our methods should follow a systematic and meaningful plan to be fully effective in terms of technique training. Thus, various educational surgical approaches should be set up using goat in vivo brain model. Each step should be inspected and evaluated by experts and appropriate guidance must be provided. Goats are relatively easy to handle, and their brain texture is similar to that of humans. Anterior and posterior interhemispheric and retrosigmoid approaches can be adequately performed using goat in vivo brains. Unlike the cadaver dissection, handling a soft brain also enhances the surgeon’s concentration and provides a realistic experience similar to that of a normal neurosurgical procedure. In an actual neurosurgical procedure, hemostasis and dissection of pulsating arteries without damaging them are essential, and these techniques need training using a living brain. The results suggest that the goat in vivo brain model has a high potential to be used for neurosurgical training. Based on the evaluation of possible surgical approaches and anatomical differences from the human brain, therefore, we intend to achieve more effective in vivo neurosurgical methods training using goat brain.
CONCLUSION
Neurosurgical technical training using in vivo brain models would be very useful for young neurosurgeons. Unlike a cadaver brain, in vivo brain requires hemostasis and careful dissection, which provides the surgeons a realistic experience of actual neurosurgery. The in vivo goat brain model has a texture and anatomical structure similar to humans; thus, it has high potential to be used for neurosurgical training.
Ethical approval
All procedures used in this research were approved by the Ethical Committee of International University of Health and Welfare.
Acknowledgment
We would like to express our gratitude to Integra Japan K.K., led by Mr. Ito and Mr. Shibakawa, for their cooperation with goat brain neurosurgery training.
Footnotes
How to cite this article: Onoda K, Fujiwara R, Sashida R, Hirokawa Y, Wakamiya T, Michiwaki Y, et al. In vivo goat brain model for neurosurgical training. Surg Neurol Int 2022;13:344.
Contributor Information
Keisuke Onoda, Email: onoda3883@gmail.com.
Ren Fujiwara, Email: renfujiwara9@gmail.com.
Ryohei Sashida, Email: sashidaryohei@gmail.com, sashidaryohei@gmail.com.
Yu Hirokawa, Email: yuhirokawa13@gmail.com.
Tomihiro Wakamiya, Email: twakamiya@iuhw.ac.jp.
Yuhei Michiwaki, Email: y.michiwaki@iuhw.ac.jp.
Tatsuya Tanaka, Email: s96047@hotmail.com.
Kazuaki Shimoji, Email: shimoji@iuhw.ac.jp.
Eiichi Suehiro, Email: esuehiro@iuhw.ac.jp.
Fumitaka Yamane, Email: fyamane@iuhw.ac.jp.
Masatou Kawashima, Email: mkawashima@iuhw.ac.jp.
Akira Matsuno, Email: akira.dr.ruby@gmail.com.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Aurich LA, Silva Junior LF, Monteiro FM, Ottoni AN, Jung GS, Ramina R. Microsurgical training model with nonliving swine head. Alternative for neurosurgical education. Acta Cir Bras. 2014;29:405–9. doi: 10.1590/s0102-86502014000600010. [DOI] [PubMed] [Google Scholar]
- 2.Cobb MI, Taekman JM, Zomorodi AR, Gonzalez LF, Turner DA. Simulation in neurosurgery-A brief review and commentary. World Neurosurg. 2016;89:583–6. doi: 10.1016/j.wneu.2015.11.068. [DOI] [PubMed] [Google Scholar]
- 3.Hamamcioglu MK, Hicdonmez T, Tiryaki M, Cobanoglu S. A laboratory training model in fresh cadaveric sheep brain for microneurosurgical dissection of cranial nerves in posterior fossa. Br J Neurosurg. 2008;22:769–71. doi: 10.1080/02688690802477573. [DOI] [PubMed] [Google Scholar]
- 4.Hanrahan J, Sideris M, Pasha T, Tsitsopoulos PP, Theodoulou I, Nicolaides M, et al. Hands train the brain-what is the role of hand tremor and anxiety in undergraduate microsurgical skills? Acta Neurochir (Wien) 2018;160:1673–9. doi: 10.1007/s00701-018-3609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicdonmez T, Hamamcioglu MK, Parsak T, Cukur Z, Cobanoglu S. A laboratory training model for interhemispherictranscallosal approach to the lateral ventricle. Neurosurg Rev. 2006;29:159–62. doi: 10.1007/s10143-005-0014-4. [DOI] [PubMed] [Google Scholar]
- 6.Hicdonmez T, Parsak T, Cobanoglu S. Simulation of surgery for craniosynostosis: A training model in a fresh cadaveric sheep cranium. J Neurosurg. 2006;105:150–2. doi: 10.3171/ped.2006.105.2.150. [DOI] [PubMed] [Google Scholar]
- 7.Morosanu CO, Nicolae L, Moldovan R, Farcasanu AS, Filip GA, Florian IS. Neurosurgical cadaveric and in vivo large animal training models for cranial and spinal approaches and techniques–A systematic review of the current literature. Neurol Neurochir Pol. 2019;53:8–17. doi: 10.5603/PJNNS.a2019.0001. [DOI] [PubMed] [Google Scholar]
- 8.Sauleau P, Lapouble E, Val-Laillet D, Malbert CH. The pig model in brain imaging and neurosurgery. Animal. 2009;3:1138–51. doi: 10.1017/S1751731109004649. [DOI] [PubMed] [Google Scholar]
- 9.Soubam P, Mishra S, Suri A, Dhingra R, Mochan S, Lalwani S, et al. Standardization of the technique of silicon injection of human cadaveric heads for opacification of cerebral vasculature in Indian conditions. Neurol India. 2018;66:439–43. doi: 10.4103/0028-3886.227303. [DOI] [PubMed] [Google Scholar]
- 10.Turan Suslu H, Ceylan D, Tatarlı N, Hıcdonmez T, Seker A, Bayrı Y, et al. Laboratory training in the retrosigmoid approach using cadaveric silicone injected cow brain. Br J Neurosurg. 2013;27:812–4. doi: 10.3109/02688697.2013.772095. [DOI] [PubMed] [Google Scholar]