Metabolic syndrome – is it right to focus on it separately?
Metabolic syndrome (MetS), which is a cluster of comorbid conditions including obesity, hypertension, and disordered carbohydrate and lipid metabolism, constitutes a significant health and social problem in Poland. The purpose of this paper is not to create a separate condition, but rather to emphasize that what we know as ‘metabolic syndrome’ involves a combination of significant and modifiable cardiovascular (CV) risk factors.
Poland is classed as a high CV risk country [1]. Optimum management to reduce the CV risk should not focus narrowly on altering individual risk factors but instead take a broader look and address the comorbid risk factors simultaneously, as there is often a cause-and-effect relationship between them, to achieve a significant CV risk reduction. Obesity does not only coexist with hypertension as well as disordered glucose and lipid metabolism; if properly treated, it is also a reversible cause of them. The development of obesity, progression of hypertension and increasing severity of metabolic disorder lead to the development of further conditions, which additionally increase the cardiovascular risk [2]. From that perspective, the concept of the “metabolic syndrome” seems accurate, as it enables a holistic approach to a person with obesity indicating the need to identify and modify the concomitant CV risk factors and to introduce measures for their early prevention.
The 2022 metabolic syndrome definition
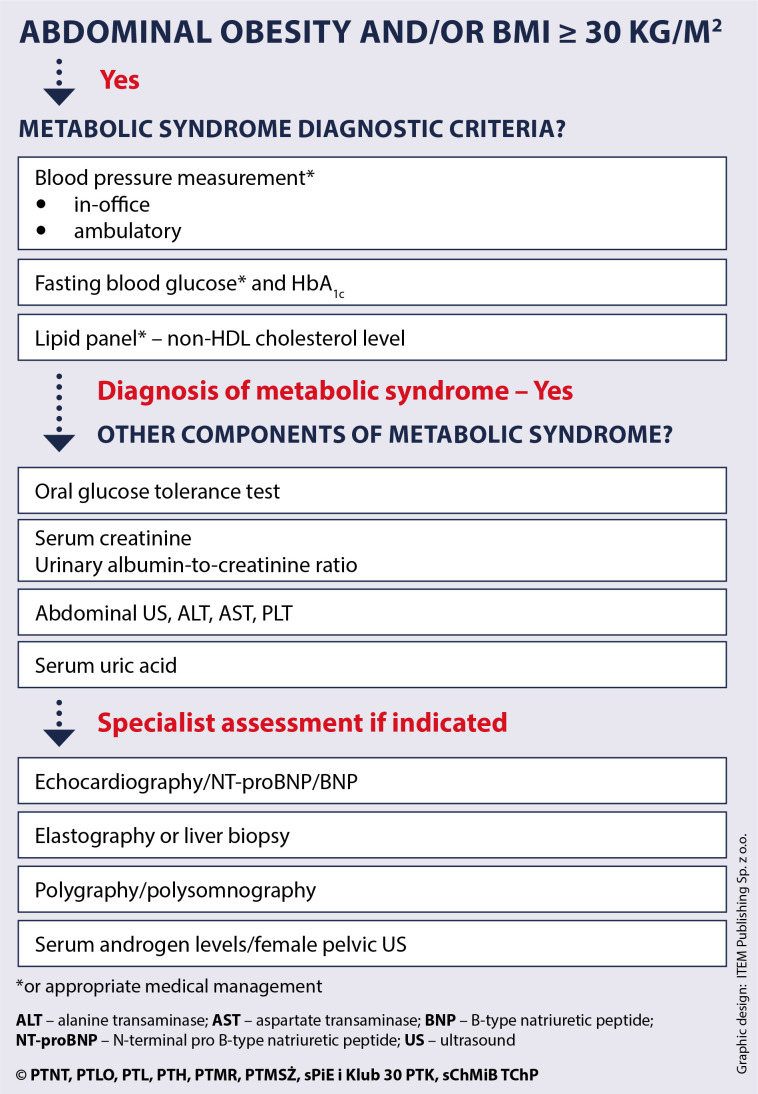
Considering the progress in understanding individual components of MetS and the most current guidance on management of each individual condition, the authors propose that the definition of MetS encompass the presence of obesity and two of the three following criteria: high blood pressure, impaired glucose metabolism, elevated non-high-density lipoprotein (non-HDL) cholesterol level (atherogenic dyslipidaemia). The MetS diagnostic criteria are presented in Figures 1 and 2 and explained in the subsequent sections.
Figure 1.
Main conditions of the metabolic syndrome
Figure 2.
Metabolic syndrome diagnostic criteria
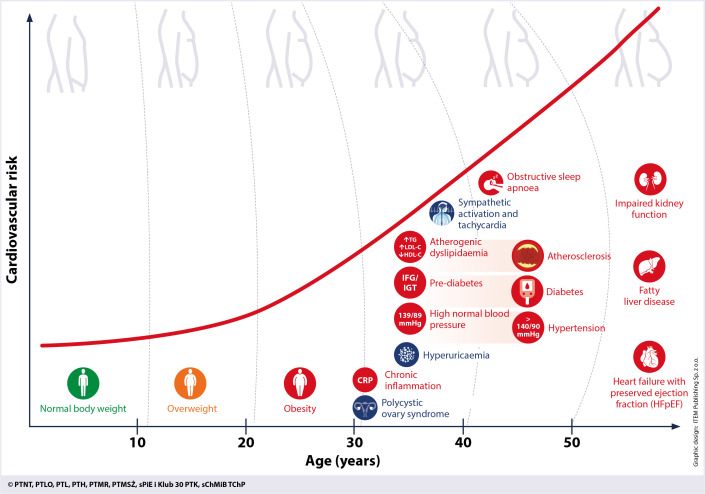
Furthermore, as shown in Figure 3, apart from the main components, MetS also encompasses such additional conditions as impaired kidney function, hepatic steatosis, obstructive sleep apnoea, heart failure with preserved ejection fraction, polycystic ovary syndrome, chronic inflammation, sympathetic activation and hyperuricaemia.
Figure 3.
Main and additional conditions of the metabolic syndrome as the consequences of obesity
Any patient diagnosed with metabolic syndrome should at least be seen as a high cardiovascular risk patient. A comprehensive assessment of the main and additional conditions of the metabolic syndrome is advised, as well as implementing lifestyle modifications alongside appropriate medical treatment. Early intervention can prevent the development or slow the progression of individual components of the metabolic syndrome.
Metabolic syndrome epidemiology
The prevalence of MetS has been assessed in Poland in at least four studies: NATPOL 2002 and 2011, WOBASZ (2003–2005) and WOBASZ II (2013–2014). These studies indicate that the prevalence of MetS has been rising in the 21st century in Poland. The process is best described in the last publication comparing the WOBASZ study findings in the population aged 20–74. In 2014, the prevalence of MetS was 33% and 39% in women and men, respectively. That indicates an increase since 2003 by almost 3% and 9% in women and men, respectively. A particularly significant increase (from 43% to 57%) was seen in males aged 60–74, with increased prevalence of disordered carbohydrate metabolism, abdominal obesity and dyslipidaemia cited as the fundamental causes. Hypertension was the only MetS criterion to become slightly less prevalent over the study period [3].
In 2014, the most common feature of MetS in women (65%) was abdominal obesity, whereas it was hypertension in men (62%). While the prevalence of MetS clearly increases with age, there are also sex-based differences. A significant sex-based difference in MetS prevalence was seen in two age groups: 20–39 years and 40–59 years (22% vs. 9% and 50% vs. 36% in men and women, respectively), which indicates earlier CV risk factor accumulation in men, potentially translating into their reduced life expectancy. These differences were not significant in the age group of 60–69 years (59.7% vs. 56.2% in women and men, respectively) [3].
Lifestyle as the leading cause of metabolic disorder
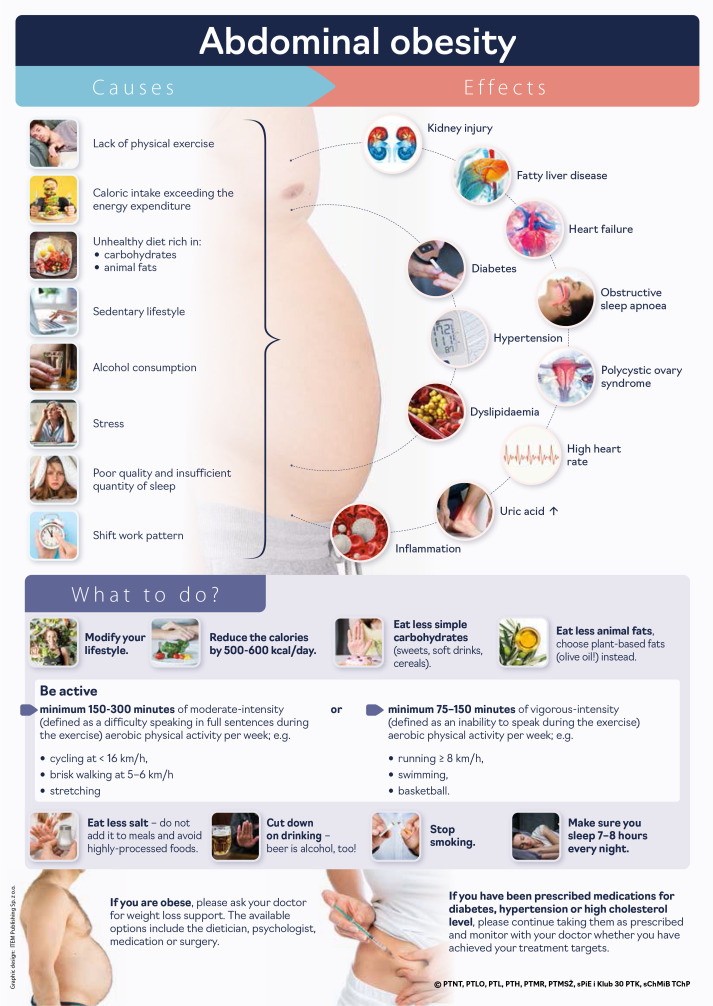
All components of MetS can be seen as the aftermath of an ‘unhealthy’ lifestyle (Patient infographic #1). Effective lifestyle interventions are key for prevention and treatment of MetS and associated conditions. Interventions aimed at preventing substance misuse and promoting healthy eating, physical activity and sleep hygiene are particularly vital in the context of metabolic syndrome [4].
Diet
Weight loss is one of the primary interventions to positively affect all MetS conditions [4, 5]. The imbalance between energy intake and expenditure is the key cause of overweight and obesity, which are a part of the metabolic syndrome [6]. The problem affects almost 60% of the Polish population, and unhealthy diet is responsible for 20% of deaths in Poland [7]. The management of MetS should include the following dietary modifications [8]:
Reducing the intake of the trans-unsaturated fatty acids (present in highly processed foods, including commercial bakery products and some hydrogenated fats) and saturated fatty acids present in meat, dairy, coconut and palm oil [9] (the benefits include reducing triglyceride levels and increasing the HDL-C level);
Increasing the amount of dietary fibre, by eating e.g. pulses, vegetables, fruit and whole-grain products (the benefits include reducing triglyceride levels, increasing the HDL-C level, improved control of blood pressure, body weight and glycaemia) [10]. Vegetables are also a good source of potassium, which positively affects blood pressure regulation;
Increasing the intake of the omega-3 fatty acids, by eating e.g. fish (the benefits include reducing triglyceride levels);
Reducing the proportion of dietary carbohydrates (especially simple) to below 50% of all caloric intake, in particular by reducing the intake of sugary drinks (the benefits include reducing triglyceride levels);
Reducing salt intake (the benefits include decreasing blood pressure).
Lack of physical exercise
Although health benefits of physical activity are both clinically proven and commonly known, almost 70% of Polish men and over 60% of Polish women do not exercise regularly [7]. From the MetS perspective, the crucial effects of physical activity include increase of HDL-C levels, reduction of triglyceride levels, improvement of glycaemic control due to increased tissue sensitivity to insulin, and blood pressure reduction. The latest guidance by the European Society of Cardiology [10] recommends that to reduce all-cause mortality, CV mortality, and morbidity, the physical activity of an adult per week should be at least (Patient infographic # 1):
150–300 min of moderate-intensity aerobic physical activity (defined as difficulty speaking in full sentences during the exercise);
75–150 min of vigorous-intensity aerobic physical activity (defined as inability to speak during the exercise);
or an equivalent combination of moderate- and vigorous-intensity activity. The guidelines indicate additional benefits of resistance exercise, recommending it in addition to aerobic activity on 2 or more days per week.
There is not a single recommended exercise type – the activities should be tailored to match a patient’s health status, skills and interests. Apart from organised activities, the patients should be encouraged to increase their daily physical activity outside workouts, such as walks, using stairs rather than lifts, doing daily chores, etc.
The physical activity recommendations can be formulated as an “exercise prescription”, using the FITT principles, which helps to communicate the aspects of recommended activity in a simple way [11]:
F = frequency, the number of days per week that exercise is performed,
I = intensity, the difficulty level of exercise performed (measured as maximal oxygen consumption (VO2 max) percentage, maximum heart rate, heart rate reserve, metabolic equivalent of task (MET) or Borg rating of perceived exertion (RPE)),
T = time, duration of a single workout/activity,
T = type, type of activity, e.g. a brisk walk, cycling.
Alcohol consumption
The mean alcohol consumption in Poland is approximately 10.6 l/person/year. Large-scale populational studies show that “the safest level of drinking is none” [12], and that alcohol consumption increases the risk of not only cancer [13], depression and suicide [14], but also overweight, obesity and CV disease. The adverse effect of alcohol on body weight is due to its high caloric content with no nutrients. In the context of MetS, alcohol also increases blood levels of triglycerides and uric acid as well as blood pressure [10].
Alcohol intake should be ascertained as a part of the medical history. The Alcohol Use Disorders Identification Test (AUDIT) should be administered [15] if a patient reports using alcohol. They should also be encouraged to reduce their alcohol consumption or give it up completely. If alcohol addiction is identified, a patient should be referred to specialist services. It is also recommended to administer depression and anxiety screening to patients who use alcohol [16].
Sleep and circadian rhythm
Sufficient quantity and quality of sleep are crucial for maintaining optimum body weight. Impaired quantity and/or quality of sleep is associated with a risk of weight gain and numerous complications of MetS [17].
It is recommended to include questions about the quality of sleep as a part of the medical history. Apart from asking about the duration of sleep, its quality should also be ascertained. (e.g. Do you wake up refreshed?) Below, we present the main principles of sleep hygiene:
one should have approximately 6 to 8 h of sleep per night (1/3 to 1/4 of a day);
with regular bedtime and wake-up times;
the bedroom should be as dark as possible, with the bed used only for sex and sleep;
exposure to blue light from light-emitting electronic devices (e.g. smartphones, tablets) should be minimised at least 1 h before the planned bedtime;
vigorous physical exercise should be avoided within at least 3 h and meals within at least 4 h before the planned bedtime;
alcohol should be avoided in the evening, to ensure optimum quality of sleep.
Obesity as a basic element of metabolic syndrome
Almost 60% of Polish adults are overweight; this includes 85% of those in the highest CV risk group [18]. One in five adults in Poland is obese. Obesity is a disorder of energy homeostasis which manifests as excessive adipose tissue accumulation. As there are no biological markers of obesity, it is diagnosed based on the body mass index (BMI) assuming that values above 30 kg/m2 confirm the diagnosis. The BMI, however, does not provide information on adipose tissue distribution (visceral or femoral-gluteal), so waist circumference measurements are used (the midpoint between the iliac crest and the lowest rib along the midaxillary line defines the measurement level). According to the criteria of the International Diabetes Federation (IDF), central obesity is diagnosed in European adults based on the waist circumference of ≥ 80 cm and ≥ 94 cm in women and men, respectively. A significantly increased risk of metabolic complications is found in women with a waist circumference of ≥ 88 cm and men with a waist circumference of ≥ 102 cm.
Diagnosing obesity, or even overweight, must entail treatment commencement, as further progression increases the risk of premature death, as well as social exclusion and often also disability. Excessive accumulation of adipose tissue, in particular abdominal obesity, is a cause of over 200 complications, including type 2 diabetes mellitus, hypertension and dyslipidaemia – key components of MetS – as the most common ones [19].
The goal of obesity treatment is to stop its progression, that is, further body weight increase, and subsequently to lose weight. Even a modest weight loss of 5% to 10% of total body weight is likely to produce health benefits [19, 20].
Non-medical management of obesity
Non-medical management of obesity encompasses nutritional therapy, change of eating habits and increased volitional physical activity. Dietary guidance should be personalised to match individual energy demand, preferences and treatment goals of a patient. It should also ensure sufficient nutrition and be sustainable long-term. To lose approximately 0.5 kg a week, a well-balanced diet is recommended, which reduces the daily caloric intake by 500–600 kcal (Patient infographic #2). In patients with obesity and pre-diabetes, the treatment goal should be a weight loss of at least 5–7%. In patients with obesity and diabetes, the treatment goal should be a weight loss of at least 7–15%. Alongside the dietary intervention, it is recommended to increase the physical activity. The prescribed physical activity should be described by type, intensity, frequency and duration. At least 150 min of moderate-intensity aerobic physical activity, such as brisk walking, swimming, cycling or water aerobics, are recommended. Cognitive-behavioural therapy including modification of eating behaviours and/or an eating disorder should be delivered by a qualified psychologist/psychological therapist [20–22].
Medical management of obesity
Medical treatment is a part of a complex management strategy and is used when non-medical interventions prove ineffective. Failure to attempt to use the effective treatment approaches, including medical therapy, should be considered as an act of omission, as is the case with the failure to use appropriate treatments in patients with hypertension, diabetes, hypercholesterolaemia and other chronic diseases [19].
Medical management should also be considered in all patients with BMI ≥ 30 kg/m2 as well as those with BMI ≥ 27 kg/m2 and at least one overweight-related disease. Thus, all patients with MetS should be considered a priori as potential clients for medical treatment. The medical management of obesity should be continued for at least 12 months, for as long as it is effective and well tolerated, as obesity is a chronic condition which does not tend to resolve spontaneously [22].
Currently, the following drugs are approved and available for the treatment of obesity in Poland:
orlistat, a lipase inhibitor;
bupropion hydrochloride and naltrexone hydrochloride in a fixed-dose combination medication;
glucagon like peptide-1 receptor agonists (GLP-1RA) – liraglutide (target dose of 3 mg), semaglutide (target dose of 2.4 mg).
GLP-1RA should be used as a drug of choice in patients with overweight and obesity with comorbid MetS, pre-diabetes, type 2 diabetes mellitus, hypertension, atherosclerosis and its clinical sequelae such as OSA, fatty liver disease and polycystic ovary syndrome. Liraglutide is the only drug whose efficacy and safety have been proven in patients with obesity before or after bariatric surgery.
Naltrexone/bupropion in a fixed-dose combination medication should be primarily considered in patients with obesity comorbid with depression, those who decided to stop smoking and those whose body weight increased following smoking cessation. It should also be considered in patients whose obesity is due to snacking. Orlistat is recommended as the second or third choice therapy [19, 22].
Multidisciplinary approach to the management of obesity
Obesity management encompasses the cooperation between the patient and the multidisciplinary team of different healthcare professionals, including physicians representing various medical specialties, dieticians, psychologists and physiotherapists. This approach, which emphasizes the role of representatives of different healthcare professions in order to achieve the best possible treatment outcomes, is justified by the complexity of obesity and plethora of research supporting the need for the involvement of: a physician (diagnosis, planning the treatment strategy and its oversight), dietician (nutritional education), psychologist (improving the patient’s emotional state and supporting behavioural modification to aid treatment compliance) or physiotherapist (improving fitness and stamina) [19].
Surgical management of obesity
Bariatric surgery can lead to a complete, permanent weight loss and remission of obesity-related diseases, such as type 2 diabetes mellitus, hypertension, and dyslipidaemia – components of MetS.
Based on their BMI levels, the following patients are eligible for bariatric surgery:
those with BMI > 40 kg/m2;
those with BMI of 35–39.9 kg/m2 and ≥ 1 obesity-related disease (e.g. type 2 diabetes mellitus, hypertension, severe osteoarthritis, dyslipidaemia, severe OSA);
those with BMI of 30–35 kg/m2 and type 2 diabetes mellitus which remains uncontrolled despite appropriate medical treatment [23].
Pre-operative weight loss (5–10%) is indicated. However, even if a patient’s BMI after the pre-operative weight loss falls below the eligibility criteria, the surgery may still be performed provided that this criterion has been met and documented in the past.
The history of bariatric surgery dates back to the mid-twentieth century. All several dozen procedures available nowadays share one common element, that is, laparoscopic technique, considered the ‘gold standard’ in bariatric surgery. The procedure should be decided upon on a case-to-case basis, considering the patient’s preferences, age or comorbidities. The available research confirms a sustainable weight reduction effect and resolution of obesity complications as well as reducing the risk of death in long-term follow-up as compared to non-surgical treatment [24, 25].
Impaired glucose metabolism
The risk of developing type 2 diabetes mellitus is 3–5-fold higher in patients with MetS compared to the general population and it is proportionate to the number of components of the metabolic syndrome [26]. Type 2 diabetes mellitus and MetS share common underlying mechanisms including insulin resistance and metabolic abnormalities linked to the excess adipose tissue and its dysfunction [27]. Hyperglycaemia and lipid abnormalities (mainly hypertriglyceridemia) develop as a result of impaired tissue sensitivity to insulin. Increased insulin release from islet β-cells gradually leads to their exhaustion, followed by pre-diabetes and diabetes. Hyperglycaemia, dyslipidaemia and hyperinsulinaemia cause vascular endothelial dysfunction and vascular wall remodelling, thus accelerating the development of atherosclerosis and the onset of its clinical consequences [28].
Figure 4 shows the principles to guide the diagnostic assessment for diabetes and pre-diabetes. Table I lists symptoms of hyperglycaemia and high high-risk groups for diabetes.
Figure 4.
Principles to guide the diagnostic assessment of impaired glucose tolerance in the general population
Pre-diabetes: IFG – impaired fasting glucose and IGT – impaired glucose tolerance, HbA1c – glycated haemoglobin, OGTT – oral glucose tolerance test.
Table I.
Symptoms suggestive of hyperglycaemia and high risk of diabetes
| Symptoms suggestive of hyperglycaemia | High risk of diabetes |
|---|---|
|
Both sexes:
|
Women:
|
Management of pre-diabetes
The management of pre-diabetes primarily involves reducing insulin resistance, mainly through weight loss. Weight loss of 5% improves glycaemic control, blood pressure and lipid profile, thus significantly reducing the risk of type 2 diabetes mellitus and its CV complications. The greater the weight loss, the more significant is the expected improvement of carbohydrate and lipid metabolism as well as blood pressure [29, 30]. All patients with pre-diabetes, regardless of their BMI, should be encouraged to implement lifestyle modifications (dietary changes and increased physical activity) [31]. Metformin should be considered to prevent the development of type 2 diabetes mellitus in the following groups of patients with pre-diabetes: those aged < 60 with BMI ≥ 35 kg/m2, and women with a history of gestational diabetes [32, 33]. Furthermore, glucagon-like peptide-1 receptor agonists (GLP-1RA) can be considered in patients with BMI ≥ 27 kg/m2 to reduce the risk of progression of pre-diabetes to type 2 diabetes mellitus (liraglutide – target dose of 3 mg; semaglutide – target dose of 2.4 mg) [34]. The risk of MetS in patients with pre-diabetes (especially where abnormal fasting glycaemia is concomitant with impaired glucose tolerance) is comparable to that of patients newly diagnosed with type 2 diabetes mellitus [35, 36].
Management of diabetes
The treatment goal in diabetes (regardless of its aetiology) is to reduce the risk of long-term complications, including CV risk. This can be done by attempting to achieve target glycaemia, blood pressure, low-density lipoprotein (LDL) and non-HDL cholesterol as well as body weight – basic components of MetS – and by using drugs with proven advantageous effects on cardiovascular risk and body weight. A multi-modal approach is needed, which encompasses the above-mentioned behavioural interventions, alongside medications, patient education and bariatric surgery in some cases. According to the guidelines of the Polish Society of Diabetology, metformin is primarily used for medical management of type 2 diabetes mellitus. In cases with concomitant overweight/obesity, atherosclerotic cardiovascular disease, chronic kidney disease or very high cardiovascular risk, dual therapy involving metformin and another agent with a proven effect on cardiovascular risk, GLP-1RA or sodium glucose co-transporter 2 inhibitor (SGLT2i) is recommended. According to the American and European guidelines, GLP-1RA or SGLT2i monotherapy is possible in patients with type 2 diabetes mellitus. Eligibility assessment for metabolic surgery is recommended in patients with type 2 diabetes mellitus and BMI ≥ 35 kg/m2, as well as those with BMI ≥ 30 kg/m2, in whom medical management fails to achieve optimum glycaemic control [33].
Dyslipidaemia
Atherogenic dyslipidaemia
Patients with MetS often have atherogenic dyslipidaemia, which includes elevated triglyceride levels, low HDL levels and normal to elevated LDL levels, with predominance of small, dense low-density lipoprotein (sdLDL) which additionally increases the CV risk [37–39]. Most recent studies unequivocally indicate that the HDL cholesterol determination has no predictive role, supporting the replacement of triglyceride level determination with that of non-HDL cholesterol and/or apolipoprotein B [10]. Due to the limited availability of the apolipoprotein B assay, the current document uses the non-HDL cholesterol level ≥ 130 mg/dl as a diagnostic criterion of metabolic syndrome. The most recent (2021) Polish guidelines on the management of dyslipidaemia recommend the determination of both LDL and non-HDL cholesterol in all patients [40] (Figure 5).
Figure 5.
Changes in lipid levels in patients with atherogenic dyslipidaemia
The non-HDL cholesterol assay reflects the plasma concentration of all lipoproteins containing apolipoprotein B: LDL, very-low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), chylomicrons, chylomicron remnants, VLDL remnants and lipoprotein(a), involved in atherogenesis and plaque destabilisation [41] (Figure 6). Non-HDL cholesterol level, as a marker of atherogenic lipoprotein concentration, is therefore crucial for CV risk assessment and should be a regular element in the lipid panel. It is of particular diagnostic value when LDL cholesterol level calculation offers limited accuracy. The research shows that the non-HDL cholesterol level is a better predictor of CV risk than the LDL cholesterol level [40, 41].
Figure 6.
Triglyceride content in individual components of non-HDL cholesterol
Management of dyslipidaemia
Patients with MetS are considered to have at least high CV risk (Table II). The non-HDL cholesterol level is a primary parameter in the lipid panel which determines the cardiovascular risk and targets of lipid-lowering therapy. The treatment goal in dyslipidaemia is to achieve the target LDL cholesterol levels and, secondarily, also target non-HDL cholesterol levels [1, 40]. Broad lifestyle modification is the key to non-medical management of MetS and dyslipidaemia. Diet and physical exercise (especially aiming at weight loss) may significantly (even by 20–25%) reduce the LDL cholesterol levels. Reducing alcohol consumption can also contribute to decreasing the triglyceride levels.
Table II.
Target levels of LDL cholesterol and non-HDL cholesterol in patients with metabolic syndrome
| High CV risk | Very high CV risk | |
|---|---|---|
| Factors associated with a given CV risk category | High risk as per SCORE2 or SCORE2-OP Chronic kidney disease (eGFR 30–60 ml/min/1.73 m2) |
Very high risk as per SCORE2 or SCORE2-OP Atherosclerotic cardiovascular disease Diabetes mellitus Chronic kidney disease (eGFR < 30 ml/min/1.73 m2) |
| Target LDL cholesterol level | < 70 mg/dl (1.8 mmol/l) and reduction by ≥ 50% compared to baseline |
< 55 mg/dl (1.4 mmol/l) and reduction by ≥ 50% compared to baseline |
| Target non-HDL cholesterol level | < 100 mg/dl (2.6 mmol/l) | < 85 mg/dl (2.2 mmol/l) |
CV – cardiovascular, eGFR – estimated glomerular filtration rate, HDL – high-density lipoprotein, LDL – low-density lipoprotein.
Medical management of dyslipidaemia in patients with MetS includes [1, 40]:
high-intensity statin therapy up to the maximum tolerated dose to achieve the target LDL cholesterol level for a given risk group;
should that not be achieved with the maximum tolerated dose, ezetimibe can be prescribed as an add-on;
for primary prevention, a PCSK9 inhibitor can be considered in combination with the maximum tolerated dose of a statin and ezetimibe, should the two latter alone fail to reduce the LDL cholesterol level to the target values;
for secondary prevention, a PCSK9 inhibitor is recommended in combination with the maximum tolerated dose of a statin and ezetimibe, should the two latter alone fail to reduce the LDL cholesterol level to the target values;
after the LDL cholesterol level has been reduced to the target values, an attempt to reduce the non-HDL cholesterol to the target values can be considered (Table III);
combination therapy using omega-3 fatty acids (PUFA, at 2–4 g/day) and statin can be considered in patients with triglyceride concentrations above 2.3 mmol/l (200 mg/dl) despite statin treatment;
combination therapy using choline fenofibrate and statin can be considered as a part of primary prevention in patients with triglyceride concentrations above 2.3 mmol/l (200 mg/dl) whose LDL cholesterol levels have been reduced to the target values, especially where the HDL cholesterol levels are low;
combination therapy using choline fenofibrate and statin should be considered in high-risk patients with triglyceride concentrations above 2.3 mmol/l (200 mg/dl) whose LDL cholesterol levels have been reduced to the target values, especially where the HDL cholesterol levels are low.
Table III.
Kidney function assessment in patients with metabolic syndrome according to KDIGO 2012
| Kidney function assessment | Method | Diagnostic criteria |
|---|---|---|
| eGFR | Serum creatinine level and eGFR calculation (ml/min/1.73 m2) | G2 60–89 mild impairment G3a 45–59 mild to moderate impairment G3b 30–44 moderate to severe impairment G4 15–29 severe impairment G5 < 15 kidney failure |
| Urinary albumin | Assessment of albumin-to-creatinine ratio in a urine sample (mg/g) | A1: < 10 normal or 10–30 mildly elevated A2: 30–300 moderately elevated A3: > 300 albuminuria |
eGFR – estimated glomerular filtration rate.
Hypertension
Overweight and obesity are the reversible – if properly treated – causes of increased blood pressure and hypertension. There is a positive, linear correlation between BMI and the risk of hypertension, observable as early as the first decades of life [42].
Blood pressure (BP) monitoring should be primarily based on ‘out-of-office’ or ambulatory blood pressure measurements. Patients with high normal blood pressure values in ‘in-office’ measurements should be requested to perform ambulatory blood pressure measurements. The diagnostic criteria of metabolic syndrome include systolic blood pressure (SBP) ≥ 130 mm Hg and/or diastolic blood pressure (DBP) ≥ 80 mm Hg obtained in ambulatory BP measurements (or a mean value of 24-hour ambulatory blood pressure monitoring). This is approximately in keeping with the upper limit of the high normal blood pressure (SBP ≥ 130 mm Hg and/or DBP ≥ 80 mm Hg) obtained in an in-office measurement, which is also the diagnostic criterion of metabolic syndrome [43–45].
To ensure reliability, ambulatory blood pressure measurements need to meet several criteria [43–45]: for the diagnosis of hypertension or ongoing monitoring, to assess treatment efficacy in patients on long-term treatment prior to a medical appointment, the measurements should be carried out over 7 consecutive days, two consecutive measurements at a time, both in the morning and evening (before a meal and medications); several additional measurements at random times should be carried out over the week/month, outside the above schedule – always two consecutive measurements at a time; ensuring the right size cuff is used – the large-sized (L) cuff (32–42 cm) or a combined medium-to-large-sized (M/L) cuff (22–42 cm) may be needed in patients with obesity.
According to current guidelines, anti-hypertensive treatment should be started in individuals with blood pressure ≥ 135/85 mm Hg assessed through ambulatory BP measurement (or the mean of the 24-hour ambulatory blood pressure monitoring) or ≥ 140/90 mm Hg assessed through in-office measurements. The target blood pressure values are < 130/80 mm Hg in in-office and ambulatory measurements (or the mean of the 24-hour ambulatory blood pressure monitoring) in individuals under 70 years of age, and < 140/90 mm Hg in in-office measurements and < 135/85 mm Hg in ambulatory measurements (or the mean of the 24-hour ambulatory blood pressure monitoring) in individuals over 70 years of age [43–45].
All patients with hypertension concomitant with metabolic syndrome should be offered non-medical management aiming at significant lifestyle modifications, to include in particular:
weight loss;
reduced salt intake;
increased physical activity.
Where weight loss cannot be achieved through non-medical management, medical treatments (preferably with GLP1-RA due to its superior efficacy in inducing weight loss and therefore greater blood pressure reduction) or bariatric surgery should be considered [46].
Choosing anti-hypertensive drugs [43, 45]
The first-line treatment should involve a combination of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin-II receptor blocker (ARB) with a dihydropyridine calcium-channel blocker or a thiazide/thiazide-like diuretic (preferably in a fixed dose combination).
Where the target blood pressure fails to be achieved, the third anti-hypertensive medication should be added after 6–8 weeks, for the treatment to involve a combination of an ACE inhibitor or ARB with a dihydropyridine calcium-channel blocker and a thiazide/thiazide-like diuretic (preferably in a fixed dose combination).
Where the target blood pressure fails to be achieved, the following treatment options (add-ons) should be considered after 6–8 weeks:
a mineralocorticoid receptor antagonist (MCRA), which plays a role in limiting the adverse effect of obesity and hypertension on kidney damage [42], or
a β-blocker (to inhibit sympathetic overactivity in patients with obesity, nebivolol or bisoprolol are preferred due to the metabolic profile) [47].
Subsequently, a centrally acting agent (clonidine) can be considered, especially in patients with adrenergic overactivity and/or mood disorders [48].
While obesity is one of the key predictors of refractory hypertension, multiple studies have demonstrated that antihypertensive agents decreased blood pressure to a comparable degree in obese vs. non-obese patients. The difficulties in managing hypertension may be due to its complex pathogenesis and the need to address its multiple mechanisms – hence the need for treatment regimens including four, five or more antihypertensive agents in some cases.
Whenever possible, fixed dose combinations (including two or three antihypertensive medications) should be used in the management of hypertension, to improve compliance. In patients with concomitant hypertension and hypercholesterolaemia, using a fixed dose combination of two antihypertensive agents with a statin may improve compliance in the management of both hypertension and hypercholesterolaemia.
Other components of metabolic syndrome
Impaired kidney function
Increasing body weight initially causes increased sodium reabsorption within the renal tubules. This, in turn, leads to compensatory renal vessel dilatation and an increased estimated glomerular filtration rate (eGFR). Glomerular hyperfiltration, which is a consequence of increased body weight, eventually subsides. Then, eGFR decreases gradually as a result of kidney damage and nephron loss. Increased albuminuria may precede the eGFR reduction even by many years. The obesity-related mechanisms of nephron damage are not fully known. The postulated mechanisms included a combination of haemodynamic, metabolic and inflammatory abnormalities. Sympathetic activation, activation of the renin-angiotensin-aldosterone system (RAAS) and physical compression may contribute to hypertension, which, alongside metabolic disorders (e.g. diabetes), glomerular hyperfiltration and inflammation, may cause kidney injury [42, 49].
Kidney function, including eGFR and urinary albumin and creatinine, should be assessed in each patient with metabolic syndrome (Table III) [50]. Current guidelines recommend the CKD-EPI equation for estimating glomerular filtration rate (GFR) from serum creatinine. While there is a significant correlation between albuminuria (however minor) and cardiovascular risk, the correlation between eGFR and cardiovascular risk is only significant for eGFR values below approximately 60 ml/min/1.73 m2. Weight loss, which translates into reduced albuminuria and improved control of other components of the metabolic syndrome, is the key for nephroprotection. Research has demonstrated a specific nephroprotective effect of angiotensin converting enzyme inhibitors, angiotensin II receptor blockers (ARBs), aldosterone antagonists, SGLT2i and GLP1-AR, which both reduce the risk of kidney injury and, in cases of kidney injury, reduce its severity [43, 51, 52].
Metabolic-associated fatty liver disease
Metabolic-associated fatty liver disease (MAFLD) is an inflammatory liver disease which involves accumulation of lipid molecules in > 5% of hepatocytes, present in 25% of the world population and 15–49% of the European population [53, 54]. Its pathogenesis is moderated by such factors as insulin resistance, lipotoxicity, oxidative stress, genetic factors, adipose tissue endocrine activity and microbiota. MAFLD progression leads to non-alcoholic steatohepatitis (NASH). Fibrosis develops within 8–13 years in 50% of patients, leading to cirrhosis in 5–25% of cases [55]. Fibrosis is the primary prognostic factor affecting both complications and survival in MAFLD. MAFLD, regardless of its stage, carries a higher risk of hepatocellular carcinoma (HCC); it also doubles the risk of type 2 diabetes mellitus, cardiovascular disease, colorectal cancer and breast cancer. The risk of cardiovascular death is 60% higher in individuals with MAFLD [56, 57].
By definition, MAFLD is not equivalent to the diagnosis of non-alcoholic fatty liver disease (NAFLD). Metabolic-associated fatty liver disease is diagnosed based on the finding of hepatic steatosis (seen in diagnostic imaging, elastography or histology), as well as type 2 diabetes mellitus and/or overweight and/or obesity and/or hyperlipidaemia, regardless of alcohol consumption. Thus, any individual with MetS and hepatic steatosis will be diagnosed with MAFLD a priori [53]. Previously, non-alcoholic fatty liver disease could only be diagnosed after excessive alcohol consumption had been ruled out [58].
MAFLD affects 55–68% of patients with type 2 diabetes mellitus, which increases progression of fibrosis and the risk of HCC [59]. Each patient with type 2 diabetes mellitus or MetS needs to be assessed for MAFLD. Similarly, each patient with fatty liver needs to be assessed for type 2 diabetes mellitus and MetS (Table IV). Severity (i.e. the extent of fibrosis) assessment should be carried out in each patient with MAFLD using non-invasive biochemical methods (e.g. FIB-2 calculator, using serum ALT, AST and PLT levels; NAFLD score using the FIB-2 parameters and serum albumin level) or physical methods (elastography, magnetic resonance imaging of the liver), plus liver biopsy in uncertain cases. We recommend using those methods in combination (e.g. FAST score based on AST levels and FibroScan parameters) [60]. Normal ALT does not exclude advanced fibrosis in as many as 40% of cases.
Table IV.
Diagnostic assessment and diagnostic criteria of fatty liver disease
| Diagnostic criteria | Evidence of hepatosteatosis in diagnostic imaging, elastography or histology and MetS |
| Severity criteria | Liver fibrosis (grade F0–F4) |
| Basic/screening investigations* | Abdominal ultrasound Biochemistry panel, e.g. FIB-4/NAFLD-score |
| Specialist investigations | Elastography or liver biopsy |
| Non-medical management | Exercise (≥ 150 min/week), reduced caloric intake (500–600 kcal/day), dietary changes |
| Medical management | Vitamin E, pioglitazone after considering contra-indications Treatment of impaired glucose regulation, dyslipidaemia and hypertension |
MetS – metabolic syndrome, NAFLD – non-alcoholic fatty liver disease.
The treatment of MAFLD primarily involves weight loss of 7–10% (0.5–1 kg per week) through reduced caloric intake (by 500–600 kcal/ day) and ≥ 150 min of aerobic exercise per week [58]. In patients with extreme obesity, bariatric surgery offers the best outcomes. Medical management, including pioglitazone vitamin E supplementation, should only be offered to patients with ≥ grade 2 fibrosis or high risk of progression. It is vital to treat metabolic abnormalities and hypertension. MAFLD is also an indication (not a contraindication) for statin therapy (provided that ALT remains ≤ three times the upper normal limit). Patients with decompensated cirrhosis should be assessed for a liver transplant and be monitored by the oncologist.
Heart failure with preserved ejection fraction (HFpEF)
Heart failure with preserved ejection fraction (HFpEF) is a condition where left ventricular dysfunction and increased left ventricular stiffness jointly increase the pressure in the left atrium and, through retrograde transmission, also in the pulmonary circulation, causing typical symptoms of heart failure, e.g. exertional dyspnoea, fatigue, malaise, palpitations and oedema (Figure 7). HFpEF is associated with an increased risk of death and hospital admission [61].
Figure 7.
Metabolic syndrome in the pathogenesis of heart failure with preserved ejection fraction (HFpEF)
Metabolic syndrome is an important risk factor for HFpEF: hypertension causes concentric left ventricular hypertrophy (and increases left ventricular stiffness), whereas obesity and metabolic disorder further reduce left ventricular function through inflammation and impaired coronary microcirculation [62, 63]. Obesity and insulin resistance predict HFpEF better than HF with reduced EF (HFrEF), and this association is more pronounced in women [64]. This may explain the higher proportion of women in the population of patients with HFpEF compared to those with HFrEF [65]. Older age is another important risk factor for HFpEF, as the impaired left ventricular relaxation progresses with age. The mean age of patients with HFpEF is higher than that of patients with HFrEF [65]. However, HFpEF is a heterogeneous condition and its numerous phenotypes include obesity-related, MetS-related, age-related, arterial stiffness-related, and CKD-related HFpEF, which can overlap [66]. Metabolic syndrome is associated with an increased risk of hospital admission due to HF exacerbation in patients with HFpEF [67].
HFpEF should be suspected in patients with impaired exercise tolerance and a typical clinical profile (older age, hypertension (in particular long-standing and poorly controlled), obesity, MetS, atrial fibrillation) [68]. HFpEF can only be diagnosed in a patient with exertional dyspnoea and EF ≥ 50% when additional investigations demonstrate the left ventricular diastolic dysfunction and/or elevated left ventricular filling pressure (e.g. elevated natriuretic peptide levels, concentric left ventricular hypertrophy and left ventricular diastolic dysfunction, left atrial enlargement, elevated pulmonary artery pressure) (Table V) [69, 70]. Nevertheless, the natriuretic peptide levels can also be normal, particularly in obese patients with HFpEF. On the other hand, elevated natriuretic peptide concentrations can be found in the elderly, patients with atrial fibrillation or chronic kidney disease, without concomitant HF [70].
Table V.
Diagnostic assessment, diagnostic criteria and treatment of heart failure with preserved ejection fraction
| Components of metabolic syndrome | HFpEF |
| Diagnostic criteria | HF symptoms + EF ≥ 50% + objective evidence of structural or functional disorder associated with diastolic dysfunction and/or elevated left ventricular filling pressure (echocardiographic parameters, natriuretic peptides) |
| Severity criteria | NYHA classification |
| Basic/screening investigations* | Natriuretic peptides:
|
| Specialist investigations | Echocardiogram to assess for:
|
| Non-medical management | Assessment of risk factors and management of comorbidities (cardiac and extracardiac) |
| Medical management |
|
AF – atrial fibrillation, ARB – angiotensin II receptor blocker, ARNI – angiotensin receptor-neprilysin inhibitor, EF – ejection fraction, HF – heart failure, HFpEF – HF with preserved EF, BNP – B-type natriuretic peptide, NT-proBNP – N-terminal pro B-type natriuretic peptide, NYHA – New York Heart Association, LAVI – left atrial volume index, LVMI – left ventricular mass index, RWT – relative wall thickness.
Obstructive sleep apnoea
The estimated prevalence of obstructive sleep apnoea (OSA) in Poland is approximately 28% [71]. Obesity is the main environmental risk factor for OSA, and the severity of OSA is linked to the amount of visceral fat [72]. Body weight changes affect the severity of OSA, which is best demonstrated by bariatric surgery leading to remission.
OSA is diagnosed 2.5-times more often in men than in women. This difference, however, disappears in the population above 50 years of age, where the percentages are comparable for both sexes. The reversible risk factors for OSA are impaired upper airway patency (allergies, nasal polyps, nasal septum deviation) and alcohol consumption. The symptoms are snoring, observable apnoea, nycturia (a very strong association) and daytime sleepiness. The treatment involves weight loss, continuous positive airway pressure (CPAP), ENT surgery and treatment of allergies (Table VI) [73, 74].
Table VI.
Diagnostic assessment, diagnostic criteria and treatment of obstructive sleep apnoea
| Diagnostic criteria | Objective respiratory sleep study, i.e. polysomnography or its limited version, that is, polygraphy (in patients with typical severe symptoms of OSA). Number of apnoea and hypopnoea episodes per hour ≥ 5 (AHI ≥ 5 or REI ≥ 5) |
| Severity criteria |
|
| Screening tests | Questionnaires (e.g. NoSAS, STOP-Bang) Diagnosis of metabolic syndrome is a very sensitive marker of obstructive sleep apnoea |
| Specialist investigations | Objective respiratory sleep study, i.e. polysomnography or polygraphy (in patients with a typical presentation without significant comorbidities) |
| Treatment | Causal treatment:
|
AHI – apnoea/hypopnoea index, CPAP – continuous positive airway pressure, OSA – obstructive sleep apnoea, REI – respiratory event index.
OSA is the cause of secondary hypertension and organ damage (myocardial infarction and stroke). Effective treatment (by using CPAP every night) reduces the CV risk in patients with OSA through e.g. improved control of blood pressure and glycaemia, facilitating weight loss and other, complex mechanisms [43, 75, 76].
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS, Table VII for diagnostic criteria) is one of the most common endocrine abnormalities, diagnosed in 6–10% of women at reproductive age [77]. Women with PCOS have 4-fold higher risk of type 2 diabetes mellitus and 2–3-fold higher risk of MetS, with insulin resistance, found in approximately 70% of women with PCOS regardless of their BMI, as the key underlying mechanism. Obesity exacerbates PCOS symptoms, as increasing insulin resistance promotes hyperinsulinaemia, which stimulates androgen production in ovaries. There is a correlation between hyperandrogenism and CV risk in PCOS, which is further increased by the presence of classic risk factors. That is why women with PCOS should be offered screening for obesity, dyslipidaemia, hypertension and diabetes and, if diagnosed, also appropriate treatment. Prevention of metabolic and CV disorder in all women with PCOS primarily involves lifestyle modification (as in pre-diabetes), and treatment with metformin in those with insulin resistance. In overweight women, treatment with glucagon like peptide-1 receptor agonists (GLP-1RA) – liraglutide (target dose of 3 mg) or semaglutide (target dose of 2.4 mg) – should be considered. In women with obesity, treatment with GLP-1RA and/or metabolic surgery should be considered (Table VII) [78].
Table VII.
Diagnostic assessment, diagnostic criteria and treatment of polycystic ovary syndrome (PCOS)
| Diagnostic criteria | Rotterdam criteria (2 of 3) 1. Oligoovulation or irregular menstrual cycles – anovulation – < 10 cycles/year – cycle > 35 days 2. Hyperandrogenism (HA) – clinical: hirsutism, acne, androgenic alopecia – biochemical: elevated serum androgen levels 3. Polycystic ovaries (PCO) on ultrasound |
| Severity criteria | PCOS phenotypes: A – irregular ovulations + HA + PCO = Classic B – ovulatory dysfunction + HA C – ovulatory dysfunction + PCO D – HA + PCO Phenotype A - highest cardiometabolic risk Phenotypes B, C and D – risk proportionate to androgen levels and BMI |
| Basic/screening investigations | To be assessed at every appointment: – body weight – waist circumference – blood pressure To be assessed once a year: – lipid panel – fasting blood glucose – OGTT* |
| Specialist investigations | In selected cases: – cardiac assessment – assessing for other possible causes of hyperandrogenism – infertility assessment |
| Non-medical management | Low glycaemic index (GI) diet with limited intake of saturated fats (as in impaired glucose regulation) In overweight/obese patients, the recommended caloric deficit is 500–750 kcal Physical activity –minimum 150 min of aerobic activity per week; 200–300 min in patients with obesity Psychological support Smoking cessation |
| Medical management | Patients with insulin resistance: metformin In patients with BMI ≥27 kg/m2 consider the GLP-1RA approved for the management of overweight and obesity |
In women with BMI > 30 kg/m2 and all women > 40 years of age, history of gestational diabetes and/or family history of T2D. BMI – body mass index, GLP-1RA – glucagon-like peptide-1 receptor agonist, OGTT – oral glucose tolerance test, PCOS – polycystic ovary syndrome.
Hyperuricaemia
Experimental and clinical studies have indicated the role of uric acid in the development of different components of the metabolic syndrome: hypertension, diabetes, fatty liver disease, and chronic kidney disease. Uric acid has been postulated to irreversibly react with nitric oxide, causing endothelial dysfunction and development of hypertension. Nitric oxide deficiency leads to reduced blood flow to insulin-sensitive tissues, which exacerbates insulin resistance. Patients with higher serum uric acid levels have higher cardiovascular morbidity and mortality than those with lower serum uric acid levels. However, there are no large prospective randomised clinical trials (RCTs) that demonstrate CV risk reduction with lowering of serum uric acid levels [79, 80]. The ALL-HEART study, which aims to determine whether allopurinol improves cardiovascular outcomes in patients with ischaemic heart disease, is expected to be completed soon and the report to be published [81].
Hyperuricaemia is defined as an elevated serum uric acid level > 7 mg/dl (420 µmol/l). The Polish Society of Hypertension guidelines, based on epidemiological studies, consider serum uric acid levels > 5–6 mg/dl in patients with high cardiovascular risk to be elevated [43]. Treatment of hyperuricaemia starts with dietary changes – reduced intake of fructose, purines and alcohol – and increased physical activity. Medical management of asymptomatic patients with hyperuricaemia remains controversial – while some experts do not recommend it, others clearly point to potential benefits of reducing the serum uric acid level to < 5 mg/dl in patients with high cardiovascular risk [43, 82].
Sympathetic overdrive and tachycardia
High C-reactive protein (CRP) concentration and elevated white blood cell count, associated with increased inflammatory activity in insulin resistance, have been shown to correlate with tachycardia in patients with MetS [83]. This is explained by the autonomic imbalance, that is, impaired central regulation of sympathetic and parasympathetic activity, where the former is hyperactive [84]. Inflammation, on the other hand, plays the key role in pathogenesis and progression of atherosclerosis. Heart rate assessment in patients with obesity, hypertension, impaired glucose regulation and dyslipidaemia should constitute an obligatory element of every medical appointment. Tachycardia is a cardiovascular risk factor [85] which is also an indication for a medical intervention in those patients. Resting HR > 80 bpm in a patient with MetS suggests a higher CV risk [43–45].
Inflammation
Obesity is a primary contributor to insulin resistance, which in turn leads to production of cytokines exerting a metabolic effect within the adipose tissue. These include e.g. adiponectin, leptin and resistin [86]. Higher levels of leptin correlate with atherogenesis and inflammation in obesity, through its effect on proinflammatory cytokine and fibrinogen release [86, 87]. CRP most likely interferes with the insulin-activated pathway, impairing its metabolic effect. A correlation has been reported between elevated CRP and coronary episodes, high BMI and insulin resistance. However, despite multiple reports, the role of CRP in pathogenesis of those conditions still remains unclear [87, 88]. Elevated CRP levels are also seen in smokers, patients with hypertriglyceridemia and hypertension. CRP is not only a risk marker but also a prognostic marker in cardiovascular incidents [87, 88]. Elevated CRP levels help to identify the highest CV risk group patients, which makes this simple and readily available marker a useful diagnostic tool.
Summary
The key abnormality in MetS is overweight or obesity, particularly abdominal. Metabolic syndrome can be easily diagnosed based on medical history, physical examination, blood pressure measurement and a few simple laboratory tests available at each medical practice. The diagnostic algorithm for metabolic syndrome is shown in Figure 8.
Figure 8.
Metabolic syndrome – diagnostic algorithm
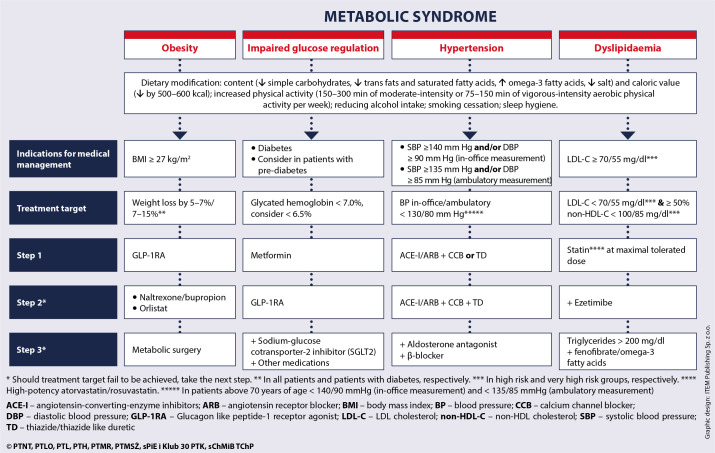
Each patient with MetS should be advised to introduce lifestyle modifications and, in most cases, also offered medication to facilitate weight loss and control the main components of MetS – hypertension, atherogenic dyslipidaemia and impaired glycaemia. The treatment algorithm for metabolic syndrome is shown in Figure 9. Early diagnosis of MetS enables implementation of a complex, multidisciplinary management strategy to control its individual risk factors, which prevents organ and CV complications. It is important to remember that MetS develops over years – initially with the onset of overweight and obesity, followed by individual components of MetS. The earlier the intervention is applied, the earlier and more effective is CV risk reduction (Figure 10). Maximal attainable CV risk reduction in patients with MetS is possible by achieving all treatment targets.
Figure 9.
Metabolic syndrome – treatment algorithm
Figure 10.
Development of obesity and components of metabolic syndrome, and increasing cardiovascular risk
Acknowledgments
Piotr Dobrowolski, Aleksander Prejbisz and Alina Kuryłowicz – co-first authorship. Maciej Banach, Andrzej Januszewicz and Paweł Bogdański shared senior authorship.
Reviewers: Agnieszka Olszanecka, Krzysztof J. Filipiak.
Conflict of interest
The authors declare no conflict of interest.
Patient infographic #1. Abdominal obesity – causes and effects
Patient infographic #1. Sample 500–600 kcal meals
References
- 1.Visseren FLJ, Mach F, Smulders YM, et al. ESC National Cardiac Societies, ESC Scientific Document Group . 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021; 42: 3227-37. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM. Metabolic syndrome: a multiplex cardiovascular risk factor. J Clin Endocrinol Metab 2007; 92: 399-404. [DOI] [PubMed] [Google Scholar]
- 3.Rajca A, Wojciechowska A, Śmigielski W, et al. Increase in the prevalence of metabolic syndrome in Poland: comparison of the results of the WOBASZ (2003-2005) and WOBASZ II (2013-2014) studies. Pol Arch Intern Med 2021; 131: 520-6. [DOI] [PubMed] [Google Scholar]
- 4.Baska A, Grudziąż-Sękowska J, Śliż D. Medycyna stylu życia, zdrowie publiczne i odpowiedzialność za zdrowie. In: Współczesne wyzwania zdrowia publicznego. Pinkas J (ed.). PZWL Wydawnictwo Lekarskie, Warszawa: 2021; 89-108 [In Polish]. [Google Scholar]
- 5.Grundy SM, Cleeman JI, Daniels SR, et al.; American Heart Association, National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735-52. [DOI] [PubMed] [Google Scholar]
- 6.Tomiak E, Koziarska-Rościszewska ME, Mizgała E, et al. Zasady postępowania w nadwadze i otyłości w praktyce lekarza rodzinnego – Wytyczne Kolegium Lekarzy Rodzinnych w Polsce, Polskiego Towarzystwa Medycyny Rodzinnej oraz Polskiego Towarzystwa Badań nad Otyłością. Lekarz Rodzinny 2017; 3: 1-64 [In Polish]. [Google Scholar]
- 7.Warszawa: Narodowy Instytut Zdrowia Publicznego – Państwowy Zakład Higieny; 2020.
- 8.Miller M, Stone NJ, Ballantyne C, et al.; American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, Council on the Kidney in Cardiovascular Disease . Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011; 123: 2292-333. [DOI] [PubMed] [Google Scholar]
- 9.Arnett DK, Blumenthal RS, Albert MA, et al. 2019. ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 140: e596-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visseren FLJ, Mach F, Smulders YM, et al.; ESC Scientific Document Group . 2021. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur J Prev Cardiol 2022; 29: 5-115. [DOI] [PubMed] [Google Scholar]
- 11.Mrozek A, Perl M, Klimkiewicz M, Zabłocka-Leonowicz J, Prochownik M. Aktywność w chorobach przewleklych. Zalecenia, przeciwwskazania, zasady kwalifikacji. Ministerstwo Zdrowia, Warszawa: 2018. [In Polish]. [Google Scholar]
- 12.GBD 2016 Alcohol and Drug Use Collaborators, GBD 2016 Alcohol Collaborators . . . Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018; 392: 1015-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol 2006; 7: 149-56. [DOI] [PubMed] [Google Scholar]
- 14.Dhossche DM. A review of postmortem alcohol detection as a diagnostic test for substance abuse disorders in suicides. Am J Forensic Med Pathol 2000; 21: 330-4. [DOI] [PubMed] [Google Scholar]
- 15.Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption – II. Addiction 1993; 88: 791-804. [DOI] [PubMed] [Google Scholar]
- 16.McHugh RK, Weiss RD. Alcohol use disorder and depressive disorders. Alcohol Res 2019; 40, doi: 10.35946/arcr.v40.1.01, indexed in Pubmed: 31649834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaput JP, Després JP, Bouchard C, et al. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep 2008; 31: 517-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozieł P, Jankowski P, Mirek-Bryniarska E, et al. Obesity in patients with established coronary artery disease over a 20-year period (1997-2017). Pol Arch Intern Med 2021; 131: 26-32. [DOI] [PubMed] [Google Scholar]
- 19.Ostrowska OL, Bogdański P, Mamcarz A. Otyłość i jej powikłania: praktyczne zalecenia diagnostyczne i terapeutyczne. Wydawnictwo Lekarskie PZWL, Warszawa: 2021. [In Polish]. [Google Scholar]
- 20.Obesity and overweight Geneva: World Health Organization; 2020. wwwwhoint/news-room/fact-sheets/detail/obesity-and-overweight (2022 March 13).
- 21.Williams LT, Barnes K, Ball L, et al. Effectiveness of dietetic consultations in primary health care: a systematic review of randomized controlled trials. J Acad Nutr Diet 2017; 117: 1941-62. [DOI] [PubMed] [Google Scholar]
- 22.Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ 2020; 192: E875-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yumuk V, Tsigos C, Fried M, et al. Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes Facts 2015; 8: 402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mingrone G, Panunzi S, De Gaetano A, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2021; 397: 293-304. [DOI] [PubMed] [Google Scholar]
- 25.Kahan S, Williams A, Syn NL, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet 2021; 397: 1830-41. [DOI] [PubMed] [Google Scholar]
- 26.Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 2008; 31: 1898-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest 2017; 127: 1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bornfeldt K, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metabolism 2011; 14: 575-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garvey WT, Mechanick JI, Brett EM, et al. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract 2016; 22 Suppl 3: 1-203. [DOI] [PubMed] [Google Scholar]
- 30.Płaczkiewicz-Jankowska E, Czupryniak L, Gajos G, et al. Management of obesity in the times of climate change and COVID-19: an interdisciplinary expert consensus report. Pol Arch Intern Med 2022; 132 doi: 10.20452/pamw.16216. [DOI] [PubMed] [Google Scholar]
- 31.Diabetes Prevention Program Research Group . Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015; 3: 866-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Diabetes Association Professional Practice Committee . 17. Diabetes Advocacy: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022; 45 (Suppl 1): S254-5. [DOI] [PubMed] [Google Scholar]
- 33.Polskie Towarzystwo Diabetologiczne . [2022 Guidelines on the management of patients with diabetes. A position of Diabetes Poland]. Curr Top Diabetes 2022; 2: 1-130. [Google Scholar]
- 34.le Roux CW, Astrup A, Fujioka K, et al. SCALE Obesity Prediabetes NN8022-1839 Study Group . 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017; 389: 1399-409. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y, Cai X, Mai W, et al. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ 2016; 355: i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurihara O, Takano M, Seino Y, et al. Coronary atherosclerosis is already ongoing in pre-diabetic status: insight from intravascular imaging modalities. World J Diabetes 2015; 6: 184-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banach M, Jankowski P, Jóźwiak J, et al. PoLA/CFPiP/ PCS Guidelines for the Management of Dyslipidaemias for Family Physicians 2016. Arch Med Sci 2017; 13: 1-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oravec S, Dukat A, Gavornik P, et al. Atherogenic versus non-atherogenic lipoprotein profiles in healthy individuals. is there a need to change our approach to diagnosing dyslipidemia? Curr Med Chem 2014; 21: 2892-901. [DOI] [PubMed] [Google Scholar]
- 39.Rizzo M, Barylski M, Rizvi AA, et al. Combined dyslipidemia: should the focus be LDL cholesterol or atherogenic dyslipidemia? Curr Pharm Des 2013; 19: 3858-68. [DOI] [PubMed] [Google Scholar]
- 40.Banach M, Burchardt P, Chlebus K, et al. PoLA/CFPiP/ PCS/PSLD/PSD/PSH guidelines on diagnosis and therapy of lipid disorders in Poland 2021. Arch Med Sci 2021; 17: 1447-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solnica B, Sygitowicz G, Sitkiewicz D, et al. 2020 Guidelines of the Polish Society of Laboratory Diagnostics (PSLD) and the Polish Lipid Association (PoLA) on laboratory diagnostics of lipid metabolism disorders. Arch Med Sci 2020; 16: 237-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall JE, Mouton AJ, da Silva AA, et al. Obesity, kidney dysfunction, and inflammation: interactions in hypertension. Cardiovasc Res 2021; 117: 1859-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tykarski A, Filipiak K, Januszewicz A, et al. 2019. guidelines for the management of hypertension – part 1–7. Arterial Hypertension. 2019; 23: 41-87. [Google Scholar]
- 44.Stergiou GS, Palatini P, Parati G, et al.’ European Society of Hypertension Council and the European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability. 2021 European Society of Hypertension practice guidelines for office and out-of-office blood pressure measurement. J Hypertens 2021; 39: 1293-302.33710173 [Google Scholar]
- 45.Williams B, Mancia G, Spiering W, et al. Authors/Task Force Members. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36: 1953-2041. [DOI] [PubMed] [Google Scholar]
- 46.Hall ME, Cohen JB, Ard JD, et al. American Heart Association Council on Hypertension; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; and Stroke Council. Weight-Loss Strategies for Prevention and Treatment of Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2021; 78: e38-50. [DOI] [PubMed] [Google Scholar]
- 47.Doroszko A, Dobrowolski P, Prejbisz A, et al. Nebiwolol i bisoprolol w terapii nadciśnienia tętniczego i chorób towarzyszących – konsensów ekspertów. Nadciśnienie Tętnicze w Praktyce 2022; 8: 1-16 [In Polish]. [Google Scholar]
- 48.Valensi P. Autonomic nervous system activity changes in patients with hypertension and overweight: role and therapeutic implications. Cardiovasc Diabetol 2021; 20: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Praga M, Morales E. Obesity, proteinuria and progression of renal failure. Curr Opin Nephrol Hypertens 2006; 15: 481-6. [DOI] [PubMed] [Google Scholar]
- 50.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2012; 3: 1-150. [Google Scholar]
- 51.Dobrowolski P, Januszewicz A, Gumprecht J, et al. Why albuminuria should be assessed more frequently in everyday clinical practice? A position statement. Pol Arch Intern Med 2021; 131: 396-406. [DOI] [PubMed] [Google Scholar]
- 52.Khurana N, James S, Coughlan MT, et al. Novel therapies for kidney disease in people with diabetes. J Clin Endocrinol Metab 2022; 107: e1-24. [DOI] [PubMed] [Google Scholar]
- 53.Eslam M, Sanyal AJ, George J, et al.; International Consensus Panel . MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology 2020; 158: 1999-2014.e1. [DOI] [PubMed] [Google Scholar]
- 54.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease. Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64: 73-84. [DOI] [PubMed] [Google Scholar]
- 55.Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc 2015; 90: 1233-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 2016; 65: 589-600. [DOI] [PubMed] [Google Scholar]
- 57.Targher G, Corey KE, Byrne CD, et al. The complex link between NAFLD and type 2 diabetes mellitus – mechanisms and treatments. Nat Rev Gastroenterol Hepatol 2021; 18: 599-612. [DOI] [PubMed] [Google Scholar]
- 58.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO) . EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016; 64: 1388-402. [DOI] [PubMed] [Google Scholar]
- 59.Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019; 71: 793-801. [DOI] [PubMed] [Google Scholar]
- 60.Newsome PN, Sasso M, Deeks JJ, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020; 5: 362-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep 2013; 10: 401-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patel RB, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018; 39: 3439-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Packer M, Lam CSP, Lund LH, et al. Characterization of the inflammatory-metabolic phenotype of heart failure with a preserved ejection fraction: a hypothesis to explain influence of sex on the evolution and potential treatment of the disease. Eur J Heart Fail 2020; 22: 1551-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Savji N, Meijers WC, Bartz TM, et al. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail 2018; 6: 701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kapłon-Cieślicka A, Benson L, Chioncel O, et al.; the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) and the ESC Heart Failure Long-Term Registry Investigators . A comprehensive characterization of acute heart failure with preserved versus mildly reduced versus reduced ejection fraction - insights from the ESC-HFA EORP Heart Failure Long-Term Registry. Eur J Heart Fail 2022; 24: 335-50. [DOI] [PubMed] [Google Scholar]
- 66.Shah SJ, Katz DH, Selvaraj S, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015; 131: 269-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Y, Fu L, Sun J, et al. Association between metabolic syndrome and an increased risk of hospitalization for heart failure in population of HFpEF. Front Cardiovasc Med 2021; 8: 698117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kapłon-Cieślicka A, Kupczyńska K, Dobrowolski P, et al. On the search for the right definition of heart failure with preserved ejection fraction. Cardiol J 2020; 27: 449-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonagh TA, Metra M, Adamo M, et al. ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599-726. [DOI] [PubMed] [Google Scholar]
- 70.Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019; 40: 3297-317. [DOI] [PubMed] [Google Scholar]
- 71.Pływaczewski R, Bednarek M, Jonczak L, et al. Sleep-disordered breathing in a middle-aged and older Polish urban population. J Sleep Res 2008; 17: 73-81. [DOI] [PubMed] [Google Scholar]
- 72.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 2000; 85: 1151-8. [DOI] [PubMed] [Google Scholar]
- 73.Stefan M, Ludger G. Sleep-related breathing disorderes. 3. Clinical picture and diagnosis. European Sleep Research Society; 2016. [Google Scholar]
- 74.Pevernagie D, Sastry M, Van Maanen PD. Sleep-related breathing disorders. 5. Treatment. European Sleep Research Society; 2021. [Google Scholar]
- 75.Zhao X, Zhang W, Xin S, et al. Effect of CPAP on blood glucose fluctuation in patients with type 2 diabetes mellitus and obstructive sleep apnea. Sleep Breath 2022. doi: 10.1007/s11325-021-02556-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chasens ER, Korytkowski M, Burke LE, et al. Effect of treatment of OSA with CPAP on glycemic control in adults with type 2 diabetes: the Diabetes Sleep Treatment Trial (DSTT). Endocr Pract 2022; 28: 364-71. [DOI] [PubMed] [Google Scholar]
- 77.Pasquali R. Metabolic syndrome in polycystic ovary syndrome. Front Horm Res 2018; 49: 114-30. [DOI] [PubMed] [Google Scholar]
- 78.Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab 2010; 95: 2038-49. [DOI] [PubMed] [Google Scholar]
- 79.Soltani Z, Rasheed K, Kapusta DR, et al. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep 2013; 15: 175-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanbay M, Jensen T, Solak Y, et al. Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur J Intern Med 2016; 29: 3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mackenzie IS, Ford I, Walker A, et al. ALL-HEART study group . Multicentre, prospective, randomised, open-label, blinded end point trial of the efficacy of allopurinol therapy in improving cardiovascular outcomes in patients with ischaemic heart disease: protocol of the ALL-HEART study. BMJ Open 2016; 6: e013774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borghi C, Domienik-Karłowicz J, Tykarski A, et al. Expert consensus for the diagnosis and treatment of patient with hyperuricemia and high cardiovascular risk: 2021 update. Cardiol J 2021; 28: 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inoue T, Iseki K, Iseki C, et al. Effect of heart rate on the risk of developing metabolic syndrome. Hypertens Res 2009; 32: 801-6. [DOI] [PubMed] [Google Scholar]
- 84.Vollenweider P, Randin D, Tappy L, et al. Impaired insulin-induced sympathetic neural activation and vasodilation in skeletal muscle in obese humans. J Clin Invest 1994; 93: 2365-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation 2004; 109 (21 Suppl 1): II2-10. [DOI] [PubMed] [Google Scholar]
- 86.Gateva A, Assyov Y, Tsakova A, et al. Classical (adiponectin, leptin, resistin) and new (chemerin, vaspin, omentin) adipocytokines in patients with prediabetes. Horm Mol Biol Clin Investig 2018; 34(1), doi: 10.1515/hmbci-2017-0031. [DOI] [PubMed] [Google Scholar]
- 87.Di Lorenzo C, Dell’agli M, Colombo E, et al. Metabolic syndrome and inflammation: a critical review of in vitro and clinical approaches for benefit assessment of plant food supplements. Evid Based Complement Alternat Med 2013; 2013: 782461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Festa A, D’Agostino R, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000; 102: 42-7. [DOI] [PubMed] [Google Scholar]