Abstract
An Asp or Asn substitution for Gly247 in transmembrane helix 8 (TM-8) of Tet(B), the tetracycline efflux protein, eliminated tetracycline resistance. Second site suppressor mutations which partially restored resistance were located in TM-5, -8, -10, or -11 or in cytoplasmic loop 8-9 or loop 10-11. These results indicate physical proximity or functional relationships between TM-8 and these other regions of Tet(B).
Tet proteins mediate tetracycline (Tc) resistance through the energy-dependent efflux of the drug out of the cell. To date, eight different classes of Tet proteins have been identified among gram-negative bacteria. Predicted to consist of 12 transmembrane (TM) regions (alpha helices), the N- and C-terminal (alpha and beta) halves of the protein, each with 6 TM helices, are joined by a large interdomain cytoplasmic loop (1, 3, 10, 12). Both domains are required for Tet function.
Tet is a member of the major facilitator superfamily (MFS) (15), which includes uniporters, symporters, and antiporters of a wide range of substrates. While little is known about the arrangement of the TM helices of Tet proteins, genetic evidence suggests that the alpha and beta domains of Tet(B) interact between TM helix 5 (TM-5) and TM-8 (18) and between TM-2 and TM-11 (11).
An inactive hybrid Tet protein consisting of a class C alpha domain and class B beta domain [Tet(C/B)] could be rendered functional by an Asp replacement of Gly247 (18), a residue near the periplasmic side of TM-8 in Tet(B). However, the introduction of the Gly247Asp mutation into Tet(B) caused a 200-fold loss of Tc resistance, with 40 to 80% reduction in the amount of the mutant protein (18) (Table 1). The change of Gly247 to Asn also led to loss of Tc resistance but little loss of the protein (Table 1). These results suggest that changes at residue 247 can affect both Tet stability and function. In the present work, suppressor mutations which restored resistance to the Gly247Asp or Gly247Asn mutants were found and characterized.
TABLE 1.
Secondary amino acid substitutions which restore Tc resistance to Tet(B) Asp247 or Asn247 mutants
| Original mutation | Second-site mutation | Location of second mutation | No. isolated | MIC (μg/ml) | Protein detected (% of wild type) | Relative sp acta |
|---|---|---|---|---|---|---|
| Gly247Asp | None | 1 | 20 | <0.019 | ||
| Gly132Asp | TM-5 | 3 | 12 | 96 | 0.05 | |
| Ala136Val | TM-5 | 4 | 12 | 63 | 0.07 | |
| Gly254Ser | TM-8 | 1 | 24 | 12 | 0.8 | |
| Ala262Thr | TM-8 | 4 | 24 | 139 | 0.07 | |
| Glu274Lys | Cytoplasmic loop 8-9 | 3 | 16 | 7 | 0.9 | |
| Gly320Lys | TM-10 | 1 | 24 | 10 | 0.9 | |
| Gly320Glu | TM-10 | 1 | 12 | 7 | 0.7 | |
| Gln335Lys | Cytoplasmic loop 10-11 | 1 | 12 | 7 | 0.7 | |
| Ser340Asn | TM-11 | 2 | 12 | 7 | 0.7 | |
| Gly247Asn | None | 1 | 80 | <0.005 | ||
| Gly135Arg | TM-5 | 4 | 24 | 130 | 0.07 | |
| Ala136Val | TM-5 | 1 | 12 | 120 | 0.04 | |
| Pro146Leu | TM-5 | 1 | 24 | 125 | 0.07 | |
| Gly254Ser | TM-8 | 11 | 24 | 57 | 0.17 | |
| Ala262Thr | TM-8 | 1 | 24 | 69 | 0.14 | |
| Gly346Asp | TM-11 | 2 | 24 | 90 | 0.1 |
That is, the MIC divided by the percent protein detected, normalized to the ratio for wild-type protein (whose ratio is 2.6). Proteins were quantitated by Western blot as described previously (18).
Plasmid DNA (derived from pLR1068 [18]) which encoded the Gly247Asp or Gly247Asn mutation in Tet(B) was mutagenized as previously described (18) and transformed into Escherichia coli DH5α cells. Since pLR1068 also encoded the tet repressor TetR(B), the gratuitous inducer 5a,6-anhydrotetracycline (kindly prepared by Mark Nelson, Paratek Pharmaceuticals, Boston, Mass.) was added during the determination of Tc susceptibility. Colonies resistant to 5 μg of Tc per ml were selected, and the plasmid providing resistance was isolated and retested. The entire tet(B) efflux gene was sequenced as described earlier (18) to verify the presence of the original mutation as well as to determine the site of a second mutation. Analysis of 20 suppressor mutants of each of the two original Gly247 mutations identified secondary mutations at 11 sites (Table 1); no reversion to the wild-type codon was seen.
In many cases, the same secondary substitution suppressed both the Asp247 and the Asn247 primary mutation. All of the secondary mutations which suppressed the substitutions at position 247 in TM-8 were found in TM-5, -8, -10, and -11 or in putative cytoplasmic loop 8-9 or loop 10-11 (Fig. 1 and Table 1). Of the 11 different residues replaced in the suppressor mutants, 10 were conserved in at least six, if not all eight, classes of gram-negative Tet proteins. Most of the suppressor mutations replaced a Gly or Ala residue with a larger, sometimes charged, residue.
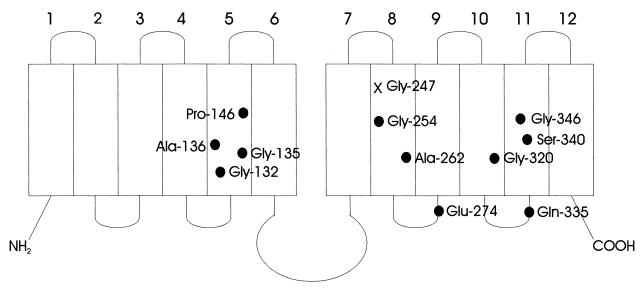
FIG. 1.
Topological model of Tet(B). The location of Gly247 is designated with an X, and second-site mutations which suppress mutations at position 247 are indicated with black circles.
Three of the Gly247Asp suppressors, Gly132Asp and Ala136Val (both in TM-5) and Ala262Thr (in TM-8), appeared to increase protein amount and specific activity at least severalfold, while the rest of the suppressors reduced protein amounts severalfold, but enhanced specific activity almost to wild-type levels (Table 1). The Gly247Asn suppressors activated the protein 4 to 7% of the wild-type specific activity in TM-5 and 10 to 17% of the wild-type level in TM-8 and TM-11; lesser effects on protein stability were also seen (Table 1).
Remarkably, of the 40 suppressing mutations examined, 17 were at one of two different sites, Gly254Ser and Ala262Thr, in the same transmembrane helix (TM-8) as the original mutation at Gly247 (Fig. 1). Gly254 is predicted to be on the same face of TM-8 as Gly247 but displaced two alpha-helical turns toward the C terminus. Ala262 is close to this face, displaced by four helical turns. Functionally relevant hydrophilic residues such as His and Asn located on this face may line a substrate translocation channel (21, 22).
Three of the Gly247Asp suppressor mutants were in putative cytoplasmic loop 8-9 and contained a Glu274Lys replacement (Table 1). Glu274 is part of a conserved sequence motif. While we found that the Glu274Lys mutation alone eliminated Tc resistance (Table 2), a Cys or an Ala can be tolerated at this position (23).
TABLE 2.
Effects of subcloning second site mutations into wild-type Tet(B)
| Mutation | MIC (μg/ml) | Protein detected (% wild type) | Relative sp acta |
|---|---|---|---|
| None | 260 | 100 | 1.0 |
| Gly132Asp | 1 | 10 | 0.04 |
| Ala136Val | 1 | 5 | 0.08 |
| Gly135Arg | 3 | 120 | 0.01 |
| Pro146Leu | 64 | 57 | 0.43 |
| Glu274Lys | 3 | 80 | 0.014 |
| Gly320Lys | 4 | 9 | 0.17 |
| Gly320Glu | 1 | 3 | |
| Gln335Lys | 1 | 1 | |
| Ser340Asn | 24 | 17 | 0.54 |
| Gly346Asp | 3 | 54 | 0.02 |
That is, the MIC divided by the percent protein detected, normalized to wild type (first row). Mutations in TM-5 of Tet protein were introduced into the tetA gene on an EcoRI fragment. With the exception of Gly245Ser and Ala262Thr, which could not be subcloned by existing restriction endonuclease sites, mutations in TM-8, TM-10, TM-11, or loop 8-9 or loop 10-11 were introduced into tetA on a BglI/PvuI fragment.
Of the suppressor mutants, 13 had mutations in TM-5 (Table 1), a finding supporting our previous work (18) which indicated a TM-5 and TM-8 interaction. For both the 247Asp and the 247Asn mutations, the second mutation in TM-5 increased the quantity of protein and raised the specific activity to modest levels (Table 1). These substitutions of larger side chain volume and/or charged residues into key positions in TM-5 could restore or stabilize functional interactions between TM-5 and TM-8. Gly residues 132 and 135 and Ala136 are predicted to be on the same face of TM-5 which may interface with TM-8, at or adjacent to Gly247. In particular, Gly150 is also on this side of TM-5; Gly150 of Tet(B) corresponds to Gly152 of Tet(C), which appears to be a point of interaction with TM-8 in active mutant Tet(C/B) hybrids (18). The current functional model for the lac permease also holds that TM-5 and TM-8 are adjacent to one another (19) and participate in lactose binding (4, 17).
From molecular modeling of the class C Tet protein, Varela et al. (19) proposed that an amino acid motif conserved in TM-5 among antiporters of the MFS could form a Tc binding pocket devoid of side chains due to the many Gly residues in the helix. Of note, the presence of the Pro146Leu mutation alone in Tet(B) did not greatly alter the specific activity (Table 2), while this and other substitutions at the corresponding residue (P156) of TetA(K), the class K efflux protein, greatly lowered the resistance (5). Introduction of the other TM-5 mutations into Tet(B), however, greatly diminished or eliminated Tc resistance (Table 2, residues 132, 135, and 136).
Two of the suppressors of Gly247Asp involved amino acid substitutions within TM-10 at position 320 (Table 1). To our knowledge this is the first evidence that TM-8 and TM-10 may interact in the tertiary arrangement of helices in Tet(B). In a study of the lactose permease, He and Kaback (7) presented evidence that specific amino acid residues within TM-8 and TM-10 interact and serve to stabilize an interface between TM-5 and TM-8 to promote substrate binding. The location of mutations in either TM-5 or TM-10 which suppress defects in TM-8 suggests a close proximity and/or a functional interaction between these three regions of Tet(B).
Two additional second site mutations were located within TM-11 (Table 1). Also, at the junction of cytoplasmic loop 10-11 and TM-11 (13) is Gln335Lys. The isolation of these mutants could indicate that TM-8 and TM-11 are adjacent to one another in the membrane. To date, however, there is no other genetic evidence which supports this interpretation, although these two helices face each other across the putative transport channel in the symmetrical arrangement of transmembrane segments in a model for MFS members (6).
Evidence suggests that the two halves of the lac permease interface between TM-2 and TM-11 (8, 9), as well as between TM-5 and TM-8 (4, 20), and that reversible conformational changes at these interface sites mediate lactose transport. A mutation in TM-11 of the lac permease suppressed a Phe280Leu mutation located at the cytoplasmic end of TM-8 (10). In that study it was proposed that a second-site mutation within TM-11 could adjust the TM-2–TM-11 interface, which, in turn, allowed the lac permease to make conformational changes at the TM-5–TM-8 interface which had been prevented by the original mutation within TM-8. In this case, the effect of the suppressor mutation in TM-11 would be indirect. Kawabe and Yamaguchi (11) recently proposed that loop residues immediately flanking TM-2 and TM-11 in Tet(B) are also sites of interaction between the alpha and beta domains. If the hypotheses that functional interfaces between TM-2 and TM-11 and between TM-5 and TM-8 exist in Tet(B) are true, then substitutions in TM-11 which suppress position 247 mutations in TM-8 could be mediating their effect via an indirect mechanism similar to that proposed for the lac permease.
The second-site mutations which suppress the inactivating mutations Gly247Asp or Gly247Asn in TM-8 of Tet(B) support our previous work which demonstrated an interaction between TM-5 and TM-8. They also suggest intrahelical conformational interactions between residues scattered along one face of TM-8, as well as an influence of TM-8 upon cytoplasmic loop 8-9, TM-10, and cytoplasmic loop 10-11. Finally, they demonstrate either a physical proximity or an indirect conformational relationship between TM-8 and TM-11.
Acknowledgments
This work was supported by National Institutes of Health grant GM55430.
REFERENCES
- 1.Allard J D, Bertrand K P. Membrane topology of the pBR322 tetracycline resistance protein: TetA-PhoA gene fusions and implications for the mechanism of TetA membrane insertion. J Biol Chem. 1992;267:17809–17819. [PubMed] [Google Scholar]
- 2.Curiale M S, McMurry L M, Levy S B. Intracistronic complementation of the tetracycline resistance membrane protein of Tn10. J Bacteriol. 1984;157:211–217. doi: 10.1128/jb.157.1.211-217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckert B, Beck C F. Topology of the transposon Tn10-encoded tetracycline resistance protein within the inner membrane of Escherichia coli. J Biol Chem. 1989;264:11663–11670. [PubMed] [Google Scholar]
- 4.Frillingos S, Kaback H R. The role of helix VIII in the lactose permease of Escherichia coli. II. Site-directed sulfhydryl modification. Prot Sci. 1997;6:438–443. doi: 10.1002/pro.5560060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ginn S A, Brown M H, Skurray R A. The TetA(K) tetracycline/H+ antiporter from Staphylococcus aureus: mutagenesis and functional analysis of motif C. J Bacteriol. 2000;182:1492–1498. doi: 10.1128/jb.182.6.1492-1498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswitz V C, Brooker R J. Structural features of the uniporter/symporter/antiporter superfamily. Prot Sci. 1995;4:534–537. doi: 10.1002/pro.5560040319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He M M, Kaback H R. Interaction between residues Glu269 (helix VIII) and His322 (helix X) of the lactose permease of Escherichia coli is essential for substrate binding. Biochemistry. 1997;36:13688–13692. doi: 10.1021/bi9715324. [DOI] [PubMed] [Google Scholar]
- 8.Jessen-Marshall A E, Brooker R J. Evidence that transmembrane segment 2 of the lactose permease is part of a conformationally sensitive interface between the two halves of the protein. J Biol Chem. 1996;271:1400–1404. doi: 10.1074/jbc.271.3.1400. [DOI] [PubMed] [Google Scholar]
- 9.Jessen-Marshall A E, Parker N J, Brooker R J. Suppressor analysis of mutations in the loop 2-3 motif of lactose permease: evidence that glycine-64 is an important residue for conformational changes. J Bacteriol. 1997;179:2616–2622. doi: 10.1128/jb.179.8.2616-2622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jewell J E, Orwick J, Liu J, Miller K W. Functional importance and local environments of the cysteines in the tetracycline resistance protein encoded by plasmid pBR322. J Bacteriol. 1999;181:1689–1693. doi: 10.1128/jb.181.5.1689-1693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawabe T, Yamaguchi A. Transmembrane remote conformational suppression of the Gly-332 mutation of the Tn10-encoded metal-tetracycline/H+ antiporter. FEBS Lett. 1999;457:169–173. doi: 10.1016/s0014-5793(99)01032-7. [DOI] [PubMed] [Google Scholar]
- 12.Kimura T, Ohnuma M, Sawai T, Yamaguchi A. Membrane topology of the transposon 10-encoded metal-tetracycline/H+ antiporter as studied by site-directed chemical labeling. J Biol Chem. 1997;272:580–585. doi: 10.1074/jbc.272.1.580. [DOI] [PubMed] [Google Scholar]
- 13.Kimura-Someya T, Iwaki S, Knnishi S, Tamura N, Kubo Y, Yamaguchi A. Cysteine-scanning mutagenesis around transmembrane segments 1 and 11 and their flanking loop regions of Tn10-encoded metal-tetracycline/H+ antiporter. J Biol Chem. 2000;275:18692–18697. doi: 10.1074/jbc.M000354200. [DOI] [PubMed] [Google Scholar]
- 14.Levy S B, McMurry L M, Barbosa T M, Burdett V, Courvalin P, Hillen W, Roberts M C, Rood J I, Taylor D E. Nomenclature for new tetracycline resistance determinants. Antimicrob Agents Chemother. 1999;43:1523–1524. doi: 10.1128/aac.43.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pao S S, Paulsen I T, Saier M H., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pazdernik N J, Cain S M, Brooker R J. An analysis of suppressor mutations suggests that the two halves of the lactose permease function in a symmetrical manner. J Biol Chem. 1997;272:26110–26116. doi: 10.1074/jbc.272.42.26110. [DOI] [PubMed] [Google Scholar]
- 17.Sahin-Toth M, le Coutre J, Kharabi D, le Maire G, Lee J C, Kaback H R. Characterization of Glu126 and Arg144, two residues that are indispensable for substrate binding in the lactose permease of Escherichia coli. Biochemistry. 1999;38:813–819. doi: 10.1021/bi982200h. [DOI] [PubMed] [Google Scholar]
- 18.Saraceni-Richards C A, Levy S B. Evidence for interactions between helices 5 and 8 and a role for the interdomain loop in tetracycline resistance mediated by hybrid Tet proteins. J Biol Chem. 2000;275:6101–6106. doi: 10.1074/jbc.275.9.6101. [DOI] [PubMed] [Google Scholar]
- 19.Varela M F, Samsom C E, Griffith J K. Mutational analysis and molecular modelling of an amino acid sequence motif conserved in antiporters but not symporters in a transporter superfamily. Mol Membr Biol. 1995;12:313–319. doi: 10.3109/09687689509072433. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Hardy D, Kaback H R. Tertiary contacts of helix V in the lactose permease determined by site-directed chemical cross-linking in situ. Biochemistry. 1999;38:2320–2325. doi: 10.1021/bi982288z. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi A, Adachi K, Akasaka T, Ono N, Sawai T. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by a transposon Tn10: histidine 257 plays an essential role in H+ translocation. J Biol Chem. 1991;266:6045–6051. [PubMed] [Google Scholar]
- 22.Yamaguchi A, Akasaka T, Kimura T, Sakai T, Adachi Y, Sawai T. Role of the conserved quartets of residues located in the N- and C-terminal halves of the transposon Tn10-encoded metal-tetracycline/H+ antiporter of Escherichia coli. Biochemistry. 1993;32:5698–5704. doi: 10.1021/bi00072a027. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi A, Kimura T, Someya Y, Sawai T. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by transposon Tn10: the structural resemblance and functional difference in the role of the duplicated sequence motif between hydrophobic segments 2 and 3 and segments 8 and 9. J Biol Chem. 1993;268:6496–6504. [PubMed] [Google Scholar]