Abstract
The staphylococcal virulon is activated by the density-sensing agr system, which is autoinduced by a short peptide (autoinducing peptide [AIP]) processed from a propeptide encoded by agrD. A central segment of the agr locus, consisting of the C-terminal two-thirds of AgrB (the putative processing enzyme), AgrD, and the N-terminal half of AgrC (the receptor), shows striking interstrain variation. This finding has led to the division of Staphylococcus aureus isolates into three different agr specificity groups and to the division of non-aureus staphylococci into a number of others. The AIPs cross-inhibit the agr responses between groups. We have previously shown that most menstrual toxic shock strains belong to agr specificity group III but that no strong clinical identity has been associated with strains of the other two groups. In the present report, we demonstrate a fourth agr specificity group among S. aureus strains and show that most exfoliatin-producing strains belong to this group. A striking common feature of group IV strains is activation of the agr response early in exponential phase, at least 2 h earlier than in strains of the other groups. This finding raises the question of the biological significance of the agr autoinduction threshold.
The agr locus (Fig. 1) regulates the production of most staphylococcal exoproteins, including exoenzymes, toxins, surface proteins, and other virulence factors, by means of a density-dependent autoinducible signal transduction system driven by a short posttranslationally processed peptide (autoinducing peptide [AIP]) (4). Autoinduction activates synthesis of a 0.5-kb highly conserved agr transcript, RNAIII, that is the actual effector of exoprotein gene regulation (10). Although the agr locus is conserved throughout staphylococci, the autoinduction circuit, consisting of the signal receptor AgrC, the AIP, processed from AgrD, and the putative AIP processing-secretion protein AgrB, shows striking sequence variation (see the hypervariable region in Fig. 1), which serves to define distinct specificity groups (5). Within any one group, these sequences are highly conserved and mutual cross-activation is observed. Between groups, however, there is mutual cross-inhibition of the agr signaling pathway (5), giving rise to a novel type of bacterial interference that may have a role in colonization and/or infection. In an earlier study (5), three distinct agr specificity groups of Staphylococcus aureus strains were defined on the basis of mutual cross-inhibition of agr function, and we and others have observed that coagulase-negative staphylococcal species produce an additional series of variant peptides (12, 14; unpublished data). Those that have been examined inhibit agr expression in all three S. aureus groups (12; unpublished data).
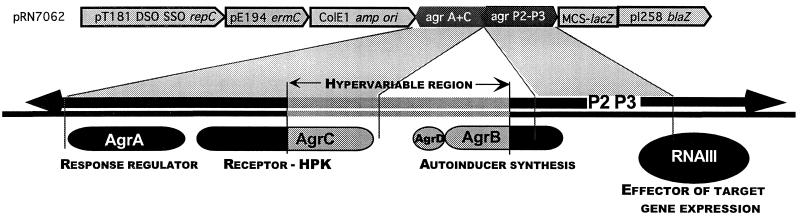
FIG. 1.
S. aureus agr locus. At the top is a schematic diagram of a shuttle vector, pRN7062, containing two segments of the agr locus, agrA plus agrC and the P2-P3 region, and below is a diagram of the agr locus, as described in the text, showing the approximate locations of the segments used in the vector. DSO, double-strand origin; SSO, single-strand origin; MCS, multiple cloning site; HPK, histidine protein kinase.
Surveys of naturally occurring S. aureus isolates, by dot blot hybridization using agr group-specific DNA probes based on divergent regions of agrC (5), have revealed several strains that do not belong to any of the three known groups. The chromosomal DNAs of these strains did not hybridize with any of the three group-specific probes. Many of these strains produce exfoliatin (ET), a toxin that is responsible for staphylococcal scalded-skin syndrome (6, 8). In earlier studies, we have not detected ET production among strains of the three known groups. In this report, we present data demonstrating a fourth agr specificity group in S. aureus, associated with ET production, and show that occasional strains belonging to the previously defined agr specificity groups I and II also produce ET.
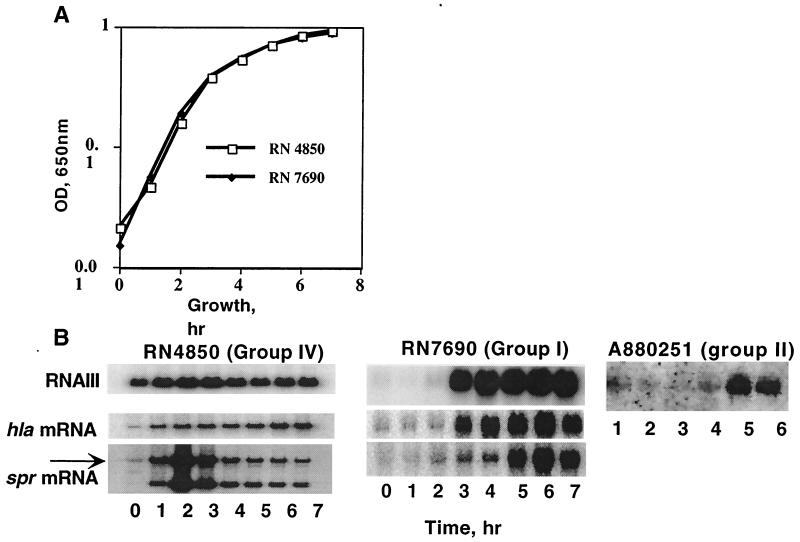
To begin the characterization of ET-producing strains that did not belong to agr groups I to III, we tested several for the production of agr AIPs. In this test a post-exponential-phase culture supernatant is added to an early-exponential-phase culture of the same strain and induction of RNAIII synthesis is evaluated by Northern hybridization, now a standard test for agr autoinduction (4). In Fig. 2A we show a Northern hybridization pattern demonstrating a positive result in this test, using strain RN4850, a typical ET producer. Note that although the bacteria used for this test were at a very early stage in exponential-phase growth, at a cell density of ∼5 × 107/ml, there was already a substantial RNAIII signal. In strains of the three existing groups, there is generally no detectable RNAIII signal until 2 to 3 h later, in mid-exponential phase (A. M. S. Figueiredo, H. F. Ross, Y. Fang, and R. P. Novick, submitted for publication). The early RNAIII signal suggested that RNAIII timing might differ among these strains, and a time course of RNAIII expression revealed that this is indeed the case, as shown in Fig. 3A. Here, the results for the ET strain RN4850 are compared with the time course for a typical group I strain, RN7690 (Figueiredo et al., submitted). As can be seen, the onset of RNAIII synthesis in the former was at a cell density between 1.5 × 107 and 3 × 107/ml, occurring nearly 3 h earlier than in the group I strain. This difference was paralleled by differences in the times of onset of transcription of two agr-regulated genes, hla (α-hemolysin) and spr (V8 serine protease), and the blot also revealed another difference, namely, the presence of a second spr transcript, much smaller than the full-sized transcript containing the gene. This second spr transcript was seen in other group IV strains but not in strains of the other groups (Figueiredo et al., submitted). It has not been characterized further.
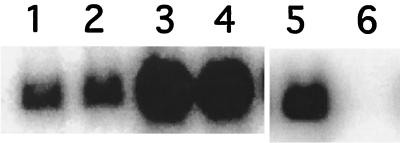
FIG. 2.
Activation and inhibition of RNAIII transcription in agr group IV strains by culture supernatants. Early-exponential-phase bacteria (5 × 107 cells/ml, strain RN4850, lanes 1, 3, 5, and 6, or RN4846, lanes 2 and 4) were treated with Casamino Acids-yeast extract broth (lanes 1, 2, and 5), with supernatant from post-exponential-phase cells of the same strain (lanes 3 and 4), or with supernatant from a group II strain (RN6607) (lane 6). Whole-cell RNA samples were prepared after 1 h (lanes 1 to 4) or after 80 min (lanes 5 and 6) (5) and analyzed by Northern hybridization with an RNAIII-specific probe.
FIG. 3.
Timing of RNAIII, α-hemolysin (hla) and serine protease (spr) gene transcription. Cultures were started in early exponential phase (5 × 107 cells/ml), and samples were removed at hourly intervals for determination of cell density and for preparation of whole-cell RNA for Northern hybridization analysis. The amount of RNA in each sample was normalized to that of the rRNA, which was quantitated by scanning an ethidium bromide-stained agarose gel with an Alpha-Inotech video-imager. (A) Growth curves of RN4850 and RN7690. Cell densities (optical densities [OD]) were measured with a Molecular Devices microplate reader at 650 nm. (B and C) Northern hybridization analysis using a probe specific for RNAIII, hla, or spr. The arrow indicates the full-sized transcript containing the gene.
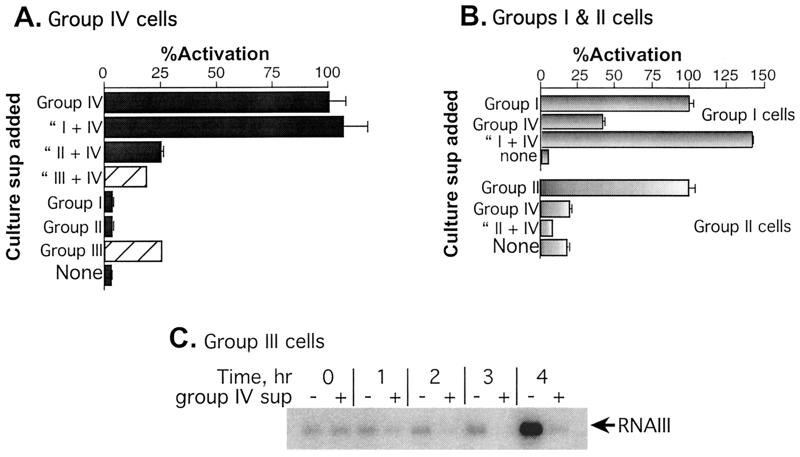
Tests for autoinduction specificity involved determinations of agr activation and inhibition activity by each of the existing groups toward the ET strains. For these tests, we used different plasmid constructs, each containing agrAC from one of the four groups, and introduced each of these into an agr-null derivative of the standard strain from which the agrAC region was cloned (Figueiredo et al., submitted), along with a second plasmid containing an agr-P3::blaZ fusion (Lyon et al., submitted for publication). A diagram of one of these constructs, pRN7062, is shown in Fig. 1 (top), and a list of the strains and plasmids used is presented in Table 1. With these strains, β-lactamase is produced only when agrAC signaling is induced by the autologous AIP, added externally, and inhibition of this response can be demonstrated by the simultaneous addition of autologous and heterologous AIPs. Thus, AIP activities could be tested without interference from the endogenous peptides (4). This strategy, however, could not be used for agr group III, as all of our group III strains possess a chromosomal β-lactamase gene (unpublished data). As shown in Fig. 4A, there was no detectable RNAIII activation in cultures of RN9371 (the agr-null derivative of RN4850 containing cloned RN4850 agrAC) treated with supernatants from strains of each of the three known groups, whereas there was a strong signal in the RN9371 culture treated with the autogenous (RN4850) supernatant. The group II and III supernatants inhibited activation by the RN4850 supernatant, as is usually the case with heterologous AIPs (5). The group I supernatant, however, did not inhibit agr activation by RN4850 (group IV) supernatant. Conversely, as shown in Fig. 4B and C, the RN4850 supernatant inhibited RNAIII production by the standard strains of groups II and III; however, it did activate the group I strain to approximately 30% of the level seen with the group I supernatant. These results were confirmed for groups I and II with synthetic AIPs (7). Because of its endogenous β-lactamase, the group III strain was tested by Northern hybridization (Fig. 4C) and showed strong inhibition of agr activation by the RN4850 supernatant.
TABLE 1.
Strains and plasmids
| Strain | Plasmid | Description | Reference |
|---|---|---|---|
| RN6734 | Group I prototype, derived from NTCT8325 | 2 | |
| RN7690 | Group I prototype (V8 serine protease strain) | 1 | |
| RN6607 | Group II prototype (strain 502A) | 5 | |
| RN8465 | Group III prototype (menstrual toxic shock syndrome strain)a | ||
| RN4846 | Group IV prototype (ET producer) University of Minnesota strain TAa | ||
| RN4850 | Group IV prototype (ET producer) University of Minnesota strain KGa | Figueiredo et al., submitted | |
| RN5881 | Group IV prototype | This work | |
| RN7206 | Group I prototype, RN6734, with tetM replacing agr (agr null) | 10 | |
| RN9365 | pRN7062 | RN7206 with shuttle vector containing group I agrAC and the agr-P3::blaZ fusion | Lyon et al., submitted |
| RN9120 | Group II prototype, RN6607, with tetM replacing agr (agr null) | Figueiredo et al., submitted | |
| RN9372 | pRN7105 | RN9120 with the shuttle vector containing group II agrAC and the agr-P3::blaZ fusion | Lyon et al., submitted |
| RN9121 | Group IV prototype, RN4850, with tetM replacing agr (agr null) | Figueiredo et al., submitted | |
| RN9371 | pRN7107 | RN9121 with the shuttle vector containing group IV agrAC and the agr-P3::blaZ fusion | Lyon et al., submitted |
Kindly provided by Patrick Schlievert.
FIG. 4.
agr activation and inhibition by culture supernatants. (A and B) One-tenth volume of a post-exponential-phase culture supernatant (sup) or CY broth was added to mid-exponential-phase cultures, growth was continued for an additional hour with shaking at 37°C, and samples were then assayed for β-lactamase by the nitrocefin method (11) as adapted for the microplate reader (4). The group I supernatant was from RN6734, the group II supernatant was from RN6607, the group III supernatant was from RN8465, and the group IV supernatant was from RN4850. Note that β-lactamase activity in samples treated with group III supernatant (cross-hatched bars) represents the endogenous β-lactamase activity of the group III strain and does not represent activation of group IV. (C) Northern hybridization analysis of the effects of the RN4850 (group IV) supernatant on agr activation in RN8465 (group III). One-tenth volume of a post-exponential-phase RN4850 supernatant (+) or broth (−) was added to a mid-exponential-phase culture of RN8465, and hourly samples were taken for Northern hybridization analysis using an RNAIII-specific probe.
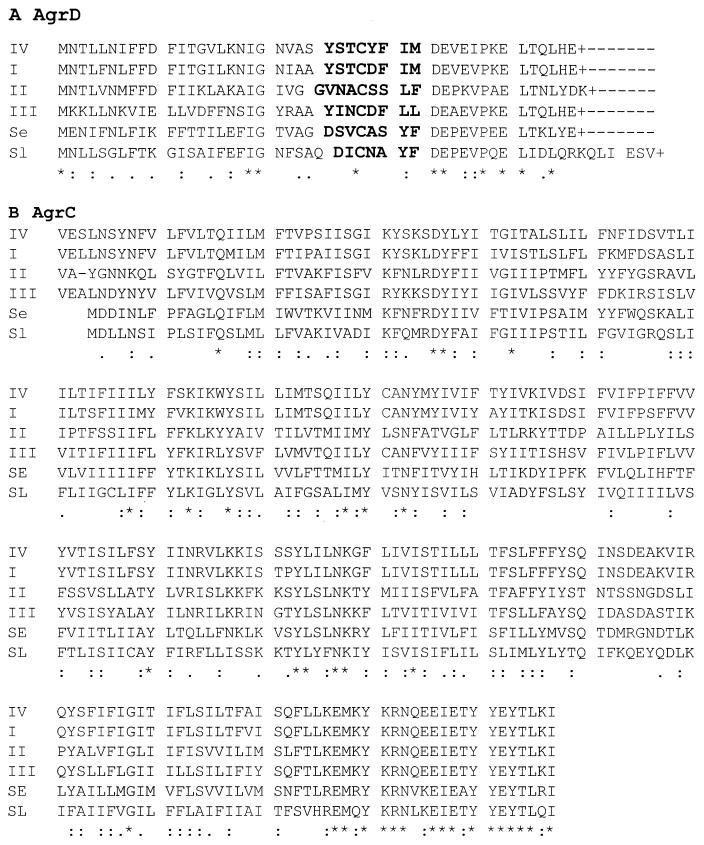
To confirm the identification of a new agr group, we determined the sequences of the agr loci for five different ET-producing strains, including RN4850. Two primers (AGR-1, 5′-ATG CAC ATG GTG CAC ATG CA [forward], and AGR-2, 5′-CAT AAT CAT GAC GGA ACT TGC TGC GCA [reverse]) were designed, according to conserved regions for the three agr groups of S. aureus (GenBank accession numbers M21854, AF001782, and AF001783), to provide specific amplification of an ∼1.2-kb fragment that included the 3′ end of agrB, all of agrD, and the 5′ end of agrC. The five sequences obtained with these different ET-producing strains were essentially identical to one another but differed in typical ways from agr sequences for strains of the other three groups, as shown in Fig. 5. In particular, the predicted AIP sequences were different from those of the other three groups, although, interestingly, there was only a single difference between these and the group I AIP, namely, a replacement of aspartic acid for tyrosine. Additionally, the predicted amino acid sequence of the N-terminal half of group IV AgrC was also close to that of group I agrC (Fig. 5B). It is concluded that the ET-producing strains define a new S. aureus agr group, group IV. The group IV agr locus is most closely related to that of group I, consistent with the observed weak activation of the group I strain by the group IV supernatant. Interestingly, the group IV strains belong to phage group II, which is generally regarded as distant from the other two phage groups.
FIG. 5.
Comparisons of the predicted AgrD (A) and partial AgrC (B) products from S. aureus groups I to IV, Staphylococcus epidermidis, and Staphylococcus lugdunensis. The sequences were aligned with the CLUSTAL X software. The sequences are as follows: S. aureus group IV (GeneBank accession number AF288215), group I (GeneBank accession number U85097) (9), group II (GeneBank accession number AF001782), and group III (GeneBank accession number AF001783) and S. epidermidis (Z49220) (Se) (14) and S. lugdunensis (Sl) (GeneBank accession number AF173933). A + indicates a stop codon. All other marks were generated by CLUSTAL X; dashes indicate gaps, asterisks indicate fully conserved residues, and colons and periods, respectively, indicate strongly and weakly similar amino acid groups according to the software. AIP sequences are indicated in boldface.
The possible link between agr group IV and ET production was analyzed with a larger collection of strains by determining the sequence of the agr loci of 15 other ET-producing strains using the method described above. As shown in Table 2, 8 of 15 isolates belong to agr group IV, 2 belong to group I, and 5 belong to group II. It was concluded that while the majority of ET-producing strains were of agr group IV, there was no absolute correlation between ET production and agr group. The kinetics of RNAIII expression in three ET-producing strains belonging to agr groups I (strain A970675), II (strain A970545), and IV (strain A930309) were compared by Northern hybridization. Confirming the results obtained with strain RN4850, the onset of RNAIII synthesis in agr group IV strain A930309 was at a cell density between 106 and 107 CFU/ml (not shown). However, the timing of agr expression in the group I and II ET-producing strains (Fig. 3B) was typical of that in other strains of these two groups (Figueiredo et al., submitted), suggesting that early RNAIII expression is a property of agr group IV strains and not necessarily of ET-producing strains.
TABLE 2.
agr grouping of ET-producing strains
| S. aureus straina | ET toxin gene(s) detectedb | agr groupc |
|---|---|---|
| A 880251 | eta | II |
| A 880663 | eta, etb | IV |
| A 890257 | eta | IV |
| A 890430 | eta | I |
| A 910669 | eta | IV |
| A 910727 | eta | II |
| A 930309 | eta | IV |
| A 940384 | eta, etb | IV |
| A 950202 | etb | IV |
| A 960463 | eta | II |
| A 960645 | etb | IV |
| A 970183 | eta, etb | II |
| A 970529 | eta, etb | IV |
| A 970545 | eta | II |
| A 970675 | eta | I |
| RN4846 | eta | IV |
| RN4850 (MN-KG) | eta | IV |
S. aureus strains were all isolated from different patients with staphylococcal scalded skin syndrome. Isolates were confirmed to be S. aureus by their ability to coagulate citrated rabbit plasma and to produce a clumping factor (6).
eta and etb genes were detected by PCR assay as previously described (6).
agr groups were determined by PCR sequencing analysis.
To investigate the molecular basis of the early expression of RNAIII by the agr group IV strains, we amplified and sequenced the agr-P2-P3 intergenic region and most of the RNAIII-determining region (511 nucleotides) of agr groups II (strains A970545 and A960463) and IV (strains A930309 and A940384), using primers GL-1 (5′-GCC GCG AGC TTG GGA G [forward]) and GL-2 (5′-GAA GAT ACG TGG CAA ACT GGT C [reverse]). Comparison of the resulting sequences with that of agr group I strain (3) (10) revealed that they were highly similar to each other. Indeed, the RNAIII region, the putative P2 and P3 promoter region, and sar-binding sites showed no sequence variation. However, we observed differences in the −35 consensus sequences of promoter P3 between agr groups I (TTGGAA), II (ATCGAA), and IV (ATGGAA) that may account for differential initiation frequencies (2). At this point we can hypothesize that the −35 region of the P3 promoter, and/or the amino acid sequences of the AIP and of AgrC, may contribute to the early and strong expression of RNAIII in these strains.
The majority of ET producers belong to group IV, and ET production is known to be positively regulated by agr (13). Although there seemed initially to be a correlation between early and strong RNAIII expression in vitro and the production of ET, the finding that ET-producing strains of other agr groups express RNAIII in a manner similar to those of other strains of these groups suggests that the difference in the timings of RNAIII expression among the agr groups cannot represent any pathobiological necessity for early ET expression.
Nucleotide sequence accession number.
The agr sequence for group IV strain RN5881 has been deposited in GenBank (accession no. AF288215).
Acknowledgments
This work was supported by NIH grant R01-AI30138 (to R.P.N.) and by NIH MSTP grant GM07739 (to G.J.L.).
REFERENCES
- 1.Arvidson S, Janzon L, Lofdahl S, Morfeldt E. The exoprotein regulatory region exp of S. aureus. In: Butler L O, Moseley B E B, editors. Genetic transformation and expression. Winborn, Dorset, United Kingdom: Intercept, Ltd.; 1989. pp. 511–518. [Google Scholar]
- 2.Hawley D K, McClure W R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2254. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janzon L, Lofdahl S, Arvidson S. Identification and nucleotide sequence of the delta-lysin gene, hld, adjacent to the accessory gene regulator (agr) of Staphylococcus aureus. Mol Gen Genet. 1989;219:480–485. doi: 10.1007/BF00259623. [DOI] [PubMed] [Google Scholar]
- 4.Ji G, Beavis R, Novick R. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji G, Beavis R, Novick R P. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 6.Lina G, Gillet Y, Vandenesch F, Jones M E, Floret D, Etienne J. Toxin involvement in staphylococcal scalded skin syndrome. Clin Infect Dis. 1997;25:1369–1373. doi: 10.1086/516129. [DOI] [PubMed] [Google Scholar]
- 7.Mayville P, Ji G, Beavis R, Yang H-M, Goger M, Novick R P, Muir T W. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci USA. 1999;96:1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melish M E, Glasgow L A. The staphylococcal scalded skin syndrome: development of an experimental model. N Engl J Med. 1970;282:1114–1119. doi: 10.1056/NEJM197005142822002. [DOI] [PubMed] [Google Scholar]
- 9.Novick R P, Projan S, Kornblum J, Ross H, Kreiswirth B, Moghazeh S. The agr P-2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol Gen Genet. 1995;248:446–458. doi: 10.1007/BF02191645. [DOI] [PubMed] [Google Scholar]
- 10.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Callaghan C H, Morris A, Kirby S M, Shingler A H. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972;1:283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otto M, Süssmuth R, Vuong C, Jung G, Götz F. Inhibition of virulence factor expression in Staphylococcus aureus by the Staphylococcus epidermidis agr pheromone and derivatives. FEBS Lett. 1999;450:257–262. doi: 10.1016/s0014-5793(99)00514-1. [DOI] [PubMed] [Google Scholar]
- 13.Sheehan B J, Foster T J, Dorman C J, Park S, Stewart G S. Osmotic and growth-phase dependent regulation of the eta gene of Staphylococcus aureus: a role for DNA supercoiling. Mol Gen Genet. 1992;232:49–57. doi: 10.1007/BF00299136. [DOI] [PubMed] [Google Scholar]
- 14.Van Wamel W J, van Rossum G, Verhoef J, Vandenbroucke-Grauls C M, Fluit A C. Cloning and characterization of an accessory gene regulator (agr)-like locus from Staphylococcus epidermidis. FEMS Microbiol Lett. 1998;163:1–9. doi: 10.1111/j.1574-6968.1998.tb13018.x. [DOI] [PubMed] [Google Scholar]