Abstract
A mutant of Synechocystis sp. strain PCC 6803 disrupted for sll1878 exhibited greatly reduced Fe3+ transport activity. The Km value of sll1878-dependent Fe3+ transport in cells grown in iron-replete medium was 0.5 μM. Both the maximal rate and Km value were increased in iron-starved cells.
While the iron concentration in terrestrial environments is high, the biological availability of this element can be very low, since under aqueous, oxygenic conditions, iron is present as Fe3+ (ferric iron), which forms insoluble hydroxides. To promote the acquisition of this element, many bacteria produce extracellular, iron-specific chelators known as siderophores (2, 3, 5, 8). Iron chelation and uptake by cyanobacteria have been reviewed by Boyer et al. (2). The Synechocystis sp. strain PCC 6803 genome contains 32 genes that potentially code for nucleotide-binding components of ATP-binding cassette transporters that have no other strong similarity to functionally identified transport polypeptides (6). To determine which of these genes is involved in iron transport, we have analyzed the growth and iron uptake of Synechocystis strains in which these putative transport genes have been disrupted. The results suggest that the protein encoded by sll1878 is a novel iron transporter.
Cells were grown in BG-11 medium (7) buffered by 20 mM N-Tris(hydroxymethyl) methyl-2-aminoethanesulfonic acid (TES)–KOH at pH 8.0 under 3% CO2 in air (vol/vol). To make iron-free BG-11, MgSO4 was replaced by K2SO4 and the citric acid, ferric ammonium dicitrate, CaCl2, and trace elements were not initially added to the medium. The medium was treated with Chelex 100 resin (Bio-Rad, Hercules, Calif.) and then supplemented with trace elements and ultrapure MgCl2 and CaCl2 (Ultrapure Chemicals Co., Saitama, Japan). To starve Synechocystis for iron, cells were grown in normal BG-11 medium, washed by 20 mM TES-KOH (pH 8.0), and then grown in fresh iron-free BG-11 overnight under continuous illumination with fluorescent lamps at 60 μE m−2s−1.
The mutant lacking sll1878 (designated M-1) constructed in this study has been deposited in the web site “CyanoMutants” (http://www.kazusa.or.jp/ cyano/mutants/), where the site of insertion of the kanamycin resistance cassette is shown. The wild-type and mutant cells before and after iron starvation were washed with 20 mM TES–KOH buffer and resuspended in fresh iron-free BG-11 at 2 × 109 cells/ml. 59FeCl3 solution was added to iron-free BG-11 medium supplemented with various concentrations of cold FeCl3. An aliquot (250 μl) of this solution was mixed with an equal volume of cell suspension in the presence of 1 mM ferrozine (Sigma Chemical Co., St. Louis, Mo.) and incubated at 30°C, either in the dark or light (at 700 μE m−2 s−1). Uptake was terminated by centrifugation, and the pellet was washed twice with 20 mM Tes–KOH containing 10 mM EDTA before being analyzed for the incorporation of 59FeCl3.
Out of 32 Synechocystis genes encoding nucleotide binding components of ATP-binding cassette transporters that have not been ascribed any function, we were able to construct 24 separate mutants by inactivating the transporter genes but were unable to attain complete disruption of the remaining 8 genes (sll0759, sll0912, sll1276, sll1623, slr0075, slr0251, slr0354, and slr1735). All of the mutants except for the one lacking sll1878 (M-1) grew as well as the wild type on solid, iron-free BG-11 medium, probably utilizing iron that contaminates the iron-free medium or that is carried over from the cell cultures used for the initial inoculum. Wild-type cells grew at a maximal rate at 1 μM Fe3+, while the M-1 mutant grew more slowly at this Fe3+ concentration.
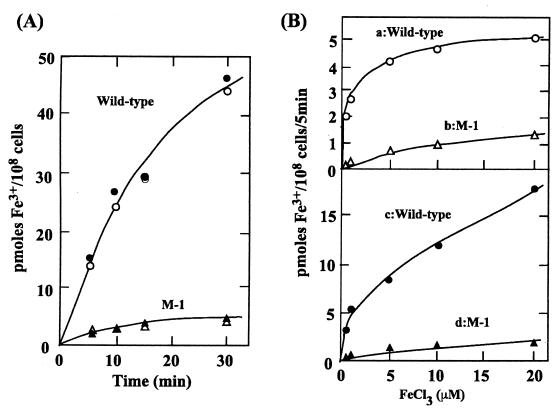
The slow growth of the M-1 mutant in iron-free medium was ascribed to a defect in iron acquisition. We assayed the wild-type and mutant strains for the rate of Fe3+ transport using 59FeCl3 in the presence of ferrozine (inhibits Fe2+ transport) (4). Figure 1A shows time courses of 59Fe3+ accumulation by iron-deprived wild-type and M-1 mutant cells incubated with 10 μM 59FeCl3 in the light or dark. The Fe3+ uptake proceeded in the dark; light did not have a stimulatory effect on the accumulation of iron over at least a 30-min period. Hence, respiration and other dark metabolic reactions must generate a sufficient supply of ATP to energize Fe3+ transport. This is in contrast to the transport of other ions such as Mn2+ that is light dependent (1). Since the amount of Mn2+ taken up by Synechocystis strain PCC 6803 cells is not more than that of Fe3+, it might be expected that ATP produced in the dark would be sufficient to drive Mn2+ uptake. However, Mn2+ uptake may be linked to immediate incorporation of the cation into protein(s), a process that might be light dependent.
FIG. 1.
(A) Time course of 59Fe3+ uptake by iron-deprived wild-type and M-1 cells, either in the dark (filled symbols) or in the light (open symbols). The concentration of 59FeCl3 was 10 μM. (B) Concentration-dependent uptake of 59Fe3+ by wild-type and M-1 cells grown in complete medium (curves a and b in upper panel) or by iron-deprived cells (curves c and d in lower panel) during a 5-min incubation in the dark.
Figure 1B shows uptake of Fe3+ by wild-type and M-1 mutant cells grown in nutrient-replete medium (upper panel) or by iron-deprived cells (lower panel). The cells were incubated for 5 min in the dark with various concentrations of FeCl3 in the presence of 1 mM ferrozine. Fe3+ uptake by the M-1 strain was about one-fifth that of wild-type cells. Fe3+ transport activity increased more than fivefold in wild-type cells and two to three times in the M-1 mutant following iron deprivation. The low-level Fe3+ transport activity retained in the M-1 mutant suggests the presence of additional Fe3+ transporter(s). The difference between the two curves a-b and c-d approximates the activity of the sll1878-dependent Fe3+ transport. The Km and Vmax values for sll1878-dependent Fe3+ transport, determined by plotting the reciprocals of curve a-b and curve c-d against the reciprocals of the substrate concentration were 0.5 μM and 3.9 pmol/108 cells/5 min, respectively, in the cells grown in nutrient-replete medium and 2.5 μM and 25 pmol/108 cells/5 min, respectively, in the iron-deprived cells. Thus, the activity of the sll1878-dependent Fe3+ transport increased about sixfold after iron deprivation treatment. The affinity of the transporter for the substrate decreased fivefold in the iron-deprived cells.
The product of sll1878 appears to be a peripheral membrane protein. No citrate is required for sll1878-dependent Fe3+ uptake, demonstrating that the citrate-iron chelate is not the substrate for this transporter. However, the substrate may be a complex between ferric iron and siderophores produced by Synechocystis in response to iron deprivation.
Acknowledgments
This work was supported by a grant, JPSP-RFTF96L00105, from the Japan Society for the Promotion of Science and a grant from the Human Frontier Science Program to T.O. and by National Science Foundation grant MCB 9727836 to A.R.G.
REFERENCES
- 1.Bartsevich V V, Pakrasi H B. Manganese transport in the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem. 1996;271:26057–26061. doi: 10.1074/jbc.271.42.26057. [DOI] [PubMed] [Google Scholar]
- 2.Boyer G L, Gillam A H, Trick C. Iron chelation and uptake. In: Fay P, Van Baalen C, editors. The cyanobacteria. Amsterdam, The Netherlands: Elsevier; 1987. pp. 415–436. [Google Scholar]
- 3.Braun V, Hantke K, Koester W. Bacterial iron transport: mechanisms, genetics, and regulation. Metal Ions Biol Sys. 1998;35:67–145. [PubMed] [Google Scholar]
- 4.Ecker D J, Emery T. Iron uptake from ferrichrome A and iron citrate in Ustilago sphaerogena. J Bacteriol. 1983;155:616–622. doi: 10.1128/jb.155.2.616-622.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerinot M L. Microbial iron transport. Annu Rev Microbiol. 1994;48:743–772. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 7.Stanier R Y, Kunisawa R, Mandel M, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococales) Bacteriol Rev. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straus N A. Iron deprivation: physiology and gene regulation. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer; 1994. pp. 731–750. [Google Scholar]