Abstract
Postherpetic neuralgia (PHN) is a devastating disease with extraordinarily poor treatment outcomes. Both pulsed radiofrequency (PRF) and ozone have good effects on the treatment of the disease. However, whether PRF and ozone have a synergistic effect on PHN remains unclear. Therefore, this study aimed to assess the therapeutic effects of ozone alone and in combination with PRF in the treatment of PHN. Ninety-one patients with PHN were assigned into two groups: PRF combined with ozone (PRF + ozone group, n = 44) and ozone therapy alone (ozone group, n = 47). In PRF + ozone group, the high-voltage, long-duration PRF was applied to the target dorsal root ganglions. Then ozonated water (11.5 µg/mL) was injected through the inner cannula. In the ozone group, all other processes were the same as those in the PRF + ozone group apart from the electrical stimulation. The therapeutic efficacy was evaluated by visual analog scale and tactile sensation at pre-treatment and post-treatment 3, 6, and 12 months. Compared with pre-treatment data, the visual analog scale score was significantly decreased in both groups after treatment. Compared with the ozone group, the visual analog scale score was significantly decreased in the PRF + ozone group at 3, 6, and 12 months. Similarly, the tactile sensation was also significantly decreased at post-treatment when compared to pre-treatment. However, there were no statistical differences between the two groups. Regression analysis results showed that the history of diabetes mellitus and age had significant negative and positive effects, respectively, on the treatment results. To conclude, the administration of PRF + ozone and ozone therapy alone could both improve pain symptoms. Moreover, treatment effects and total efficacy rates tended to be higher for the combination of PRF and ozone than ozone alone. This conclusion was especially true for long-term therapeutic effects.
Keywords: dorsal root ganglions, Gasserian ganglion, neuromodulatory effects, ozone therapy, ostherpetic neuralgia, pulsed radiofrequency, synergistic effects, total efficacy rate, ultrasound-guided, visual analog scale
INTRODUCTION
Postherpetic neuralgia (PHN) is the most debilitating chronic complication of varicella-zoster virus reactivation, which can persist for months or even years. According to an epidemiological investigation in 2019, the prevalence of PHN in individuals was 2.3% in China.1 In the USA, The overall incidence rate of PHN was 57.5 cases per 100,000 person-years.2 Risk factors include older age (aged ≥ 60 years), immunosuppression, systemic lupus erythematosus and symptoms of personality disorder.3 In a significant percentage of cases, PHN manifests in concert with psychiatric conditions such as anxiety and depression, that severely limit the patients’ quality of life.3
The goals of PHN treatment are to provide effective pain relief, improve sleep quality, and reduce the anxiety and distress associated with chronic pain. PHN treatment includes two broad categories: pharmacological and interventional therapies. There are many drugs available for the treatment of PHN, including calcium channel regulators, lidocaine, antidepressants, aspirin, tramadol, and opioids.4 Despite the fact that such drugs should be trialed when a patient is first diagnosed with PHN,5 no single class of medication can reliably provide ideal efficacy for all patients. Interventional procedures,6 such as paravertebral block, intrathecal drug administration, and selective nerve root injection, are reserved for individuals who are refractory to drug therapy or if medical therapy causes intolerable side effects.
Ozone is a triatomic molecule consisting of three oxygen atoms and is the strongest naturally occurring oxidant in nature.7 Medical ozone (the “ozone” referred to in this report) is actually a mixture of 95–99% oxygen and 1–5% ozone that has been in medical use for over 100 years (German soldiers on the battlefield in World War I used it to disinfect wounds).8 Recent studies have shown that ozone has powerful anti-inflammatory and immune modulating effects9 that can relieve acute and chronic pain such as spinal pain,9 complex regional pain syndrome,10 low back pain,11 and knee osteoarthritis.12 In our previously published clinical studies,13,14,15 we demonstrated that percutaneous ozone injection provides superior efficacy in treating trigeminal neuralgia and PHN while providing a favorable safety profile.
Radiofrequency treatment is a novel therapeutic approach for chronic pain syndromes. There are two types of radiofrequency procedures for clinical application. The first is radiofrequency thermocoagulation. Radiofrequency thermocoagulation is a neuro-destructive procedure that is routinely used to treat trigeminal neuralgia.16 Studies in trigeminal neuralgia have shown that following radiofrequency thermocoagulation, there are several serious complications such as inadvertent mouth penetration (misplaced needle location), facial numbness, and corneal hypoesthesia.17,18 Pulsed radiofrequency (PRF) is an improvement upon radiofrequency thermocoagulation, which is used when treating severe chronic pain such as chronic cervical and lumbosacral pain,19 trigeminal neuralgia,20 or osteoarthritis21; however, the treatment effects are controversial.22 Nevertheless, PRF may be the preferred intervention for PHN treatment due to its characteristic non-neurodestructive effects23 and several studies have demonstrated that PRF achieves satisfactory results.24,25,26,27,28,29,30,31,32,33 In addition, changes in stimulus voltage and duration can alter the effectiveness of PRF. Wan et al.26 indicated that a higher output voltage improved analgesic effects for PHN. Han et al.24 also showed that PFR at 65 V had superior efficacy and safety in PHN treatment compared with PFR at 45 and 55 V.In this study, we utilized high-voltage and long-duration PRF when treating PHN.
Notwithstanding the multiplicity of therapeutic approaches for PHN, no single method has been shown to be completely effective for treating PHN. Therefore, more effective and comprehensive approaches including combination therapy should be considered. The present study was designed to assess the therapeutic effects of ozone alone and in combination with PRF in the treatment of PHN. Further,this study comprehensively compared therapeutic effects and complications resulting from both monotherapy and combination therapy.
SUBJECTS AND METHODS
Subjects
We retrospectively analyzed the clinical data of 91 PHN patients admitted to the Department of Anesthesiology, Pain and Sleep Medicine, Aviation General Hospital of China Medical University from November 2018 to September 2020 (Figure 1). All patients were divided into two cohorts: combination therapy with PRF and ozone (PRF + ozone group, n = 44) and ozone therapy alone (ozone group, n = 47). Sample size was calculated based on preliminary data. The effective rate of ozone therapy for treating PHN was approximately 26%. While the effective rate of PRF was approximately 52%. To achieve a statistical power of 80% and a 2-sided type I error of 5%, 34 patients were needed in each group. With an assumption of an approximate 20% dropout rate, we aimed to enroll 80 patients in total. All patients were supplemented with narcotic analgesics, antiepileptic analgesics, and neurotrophic drugs during inpatient treatment. This retrospective evaluation was approved by the Ethics Committee of Aviation General Hospital of China Medical University (approval No. HK2019-06-12) in 2019. The present evaluation did not include direct contact with patients, and all patient identifiers were removed; hence, no informed consent was required.
Figure 1.
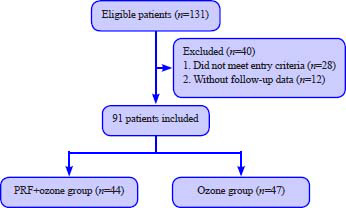
Study flowchart.
Note: PRF: Pulsed radiofrequency.
The inclusion criteria were: (1) a confirmed diagnosis of PHN, that is, pain persisting, or recurring, at the site of shingles at least 1 month after the onset of the acute rash34; (2) age > 18 years old; (3) visual analog scale (VAS) pain scores ≥ 5 points during enrollment35; (4) natural course of disease longer than 3 months.
The exclusion criteria were: (1) contraindications for ozone injection, such as favism, acute myocardial infarction, and hyperthyroidism; (2) severe cardiopulmonary disease, liver and kidney dysfunction, or any severe life-threatening diseases; (3) history of psychosis.
PRF
Truncal PRF
The patient was placed in a lateral position with the affected side up. Vital signswere monitored continuously throughout the operation. Following surgical site disinfection and draping, only the injection site remained exposed. Anatomically, the location of the foraminal opening was different between the cervical vertebra, thoracic vertebra, and lumbar spine. In the cervical segment, the 7th cervical vertebra (C7) was different from other cervical levels due to its rudimentary anterior tubercles and prominent posterior tubercles. Other cervical levels were easily visualized by confirming the location of C7 and sliding the transducer cranially. As for thoracic segments, the location of the foraminal opening was in the lateral pedicle and the bottom of the superior costal transverse process ligament, which was different from the cervical segments. The dorsal root ganglion (DRG) arelocalized superolaterally in the intervertebral foramen, while they tend to be more centrally positioned at lower spinal cord levels. Additionally, lumbar DRG aremainly intraforaminal.36 An ultrasound examination was performed (Vivid-i; GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) once the target vertebral levelwas identified. The 11-L transducer probe was covered with a sterile sheath and placed at the lateral aspect, approximately parallel to the long axis of the spine, at the spinal level correlating with the skin lesion. To enhance the efficacy in PHN, the DRG primarily involved or target DRG as well as one DRG below and above were used as treatment targets. For example, if the pain location was at the T4 level, the DRGs from T3 (upper) to T5 (lower) were selected as PRF targets (Figure 2A–C).
Figure 2.
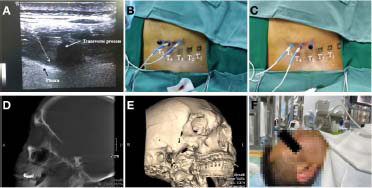
The process flows of PRF.
Note: (A) The transverse ultrasound image of T4. (B) The upper and target DRGs (T3 and T4 DRGs) were stimulated for 15 minutes. (C) The target and lower DRGs (T4 and T5 DRGs) were stimulated for 15 minutes. The T4 DRG was the primarily involved DRG and was stimulated for 30 minutes. (D) A lateral 3D C-arm reconstruction image showing the position of the needle in foramen rotundum. (E) A lateral C-arm image showing the position of the needle in foramen ovale. (F) PRF was ongoing in the patient. DRG: Dorsal root ganglion; PRF: pulsed radiofrequency.
The skin was anesthetized by injecting 2 mL of 1% lidocaine (Jinxin Double-Crane Pharmaceutical Co., Ltd., Yuncheng, China) after the target level was confirmed. Two 18-gauge RF cannulas with a 10-mm exposed tip were carefully inserted under ultrasound guidance at the foraminal opening between the two anterior tubercles until the needle approached the upper and target DRGs (the target DRG was the primarily involved DRG). Subsequently, the stylet was removed and the RF electrode was inserted (Cosman Medical Inc., Burlington, VT, USA). The sensation test (50 Hz, 0.4–0.6 V) was performed to induce abnormal sensations such as numbness, soreness, or thermalgia. When the abnormal sensations developed over the skin area currently affected by PHN, PRF treatment was performed using the following settings: 42°C, 2 Hz, 20 ms, and 900 s. The initial stimulus voltage was set at 40 V and was gradually increased until patients developed intolerance (nearly all individuals were able to sustain 99 V). The total stimulation lasted for 15 minutes and following that, the target and lower DRGs were also stimulated using this same method. This means that the primarily involved DRG (target) was stimulated for 30 minutes, and the upper and lower DRGs were stimulated for 15 minutes each.
Facial PRF
When treating trigeminal PHN, the involved Gasserian ganglion would be stimulated using the monopolar automatic mode following placement using C-arm guidance. Briefly, a 21-gauge RF trocar with a 5-mm exposed tip was inserted through the Hartel anterior approach. A C-arm was used to confirm the positional relation between foramen ovale and RF trocar tip when the tip seems to be directed to the foramen ovale. We should make sure that the needle tip does not enter the subarachnoid space. The remaining steps were identical to those described in the truncal treatment section (Figure 2D–F).
Both facial and truncal PRF were administered only once throughout the inpatient stay.
Ozone therapy
Truncal ozone therapy
The procedural technique was operated as previously described15 apart from the concentrations and forms of ozone. In the present study, ozonized water (11.5 µg/mL; SYZ-80A Therapy System, Jinan Sanyang Technology Company, Jinan, China) was used. Briefly, the needle core was gently removed and the ozonated water (10 mL) mixture was injected through the inner cannula at a concentration of 11.5 µg/mL after PRF. As for the ozone treatment alone, the physician should use the ultrasound probe to locate the target vertebral level. Subsequently, three 22-guage needles were carefully inserted under ultrasound guidance around the three adjacent DRGs (the target, upper and lower DRG) after local anesthesia. And each site was injected with 10 mL ozonized water (11.5 µg/mL). The infusion speed was approximately 3 mL/min. Of note, we aimed to let the ozonated water spread around the nerve root.
Facial ozone therapy
The operating steps of ozonized water injection around Gasserian ganglion were also consistent with our previous studies.13,14 The needle core was gently removed and the ozonated water (7 mL) was injected through the inner cannula at a concentration of 11.5 µg/mL after PRF as described above. For those patients who underwent ozone treatment alone, the puncture procedure was similar to that of PRF. A 22-guage needle was introduced into the foramen ovale after local anesthesia under C-arm guidance. Care was taken not to go into the subarachnoid space to avoid the ozonized water entering the intracranial space and damaging the nerve or brain tissue. Then, the ozonized water (7 mL) was injected at a concentration of 11.5 µg/mL. The infusion speed was approximately 2 mL/min.
Ozone therapy was administered once a day, during weekdays (Monday to Friday) for 1–2 weeks. All operative procedures were performed by the same investigator (JXA). The duration of each facial and truncal ozone therapy was approximately 30 minutes. And patients were observed for at least half an hour after treatment.
Efficacy evaluation and follow-up observation
Baseline data were recorded prior to the first treatment, including age, sex, disease course, affected side, pain location, history of underlying diseases, VAS score, and mechanical allodynia thresholds. Follow-up examinations were performed at 3 months, 6 months, and 1 year following the procedure by an alternate blinded researcher. The therapeutic effect was evaluated based on the following parameters:
-
(1)
VAS: The VAS is a widely used scale to measure pain intensity, with a range from painless (0 points) to severe pain (10 points). Clinically meaningful pain intensity is conventionally considered as VAS < 3,37 so we opted to use a pain intensity of less than 3 points as the measure of treatment success in relieving PHN.15
-
(2)
Tactile sensation: Tactile assessments were performed via a series of calibrated von Frey filaments (Stoelting Co., Wood Dale, IL, USA). The von Frey filaments with 0.008 g/mm2 were applied perpendicular to the affected skin area several times. The von Frey response was recorded if the patients responded to this degree of mechanical stimulus. The next largest monofilament was used if the response was absent. This test was performed prior to the first treatment as well as the time periods described previously.15 All VAS and tactile sensation testing were performed by the same investigator.
-
(3)
Total efficacy rate: The efficacy rate was dichotomized into two grades: effective and ineffective. Effective: VAS < 3 points; ineffective: VAS ≥ 3. The total efficacy rate (%) = effective/n × 100.
Statistical analysis
Sample size was calculated based on preliminary data. The effective rate of ozone therapy for treating PHN was approximately 26%. While the effective rate of PRF was approximately 52%. To achieve a statistical power of 80% and a 2-sided type I error of 5%, 34 patients were needed in each group. With an assumption of an approximate 20% dropout rate, we aimed to enroll 80 patients in total.
All data collection and analytical processes were carried out by an independent researcher who was not involved in the clinical procedure. The data were analyzed using SPSS 23.0 software (IBM Corporation, Armonk, NY, USA). Except for age (independent samples t-test), all other numerical variables (disease course, VAS, and tactile sensation results at different time points) were assessed by the Shapiro-Wilk test and conformed to non-normal distribution. Therefore, age is presented as mean ±standard deviation (SD) while the other numerical variables are presented as median and range. The changes in VAS and the von Frey results for all time points were compared using the Friedman test. The Mann–Whitney U test was utilized to compare the VAS and von Frey results between the two groups. The Chi-squared test and Fisher’s exact test were used for demographic data (sex, history of underlying disease, and treatment location). By using univariate and multivariate regression, the factors associated with successful responses 1, 3, 6 months, and 1 year after treatment were analyzed. The most relevant factors associated with successful responses were included in the univariate logistic regression analysis. The inclusion of variables in the final multivariate logistic regression analysis to evaluate independent factors associated with successful responses was based on clinical importance, and statistical considerations. P < 0.05 was considered statistically significant.
RESULTS
Participant demographic and clinical characteristics
In total, we enrolled 131 patients with PHN for this evaluation. Twenty-eight patients were excluded either because of psychiatric comorbidities, the course of their illness being less than 3 months or only mild-moderate pain (VAS < 5) before treatment. Twelve patients were also excluded due to the lack of follow-up data (patients and families could not be reached by phone). Thus, 91 patients were included in the final analysis. There were 44 patients in the PRF + ozone group and 47 patients in the ozone group (Figure 1). The baseline data, including age, sex, VAS, pain intensity, affected side, pain location, history of underlying disease, and disease course, were comparative between the groups (Table 1). In addition, there was no significant difference in the usage of gabapentinoids and methylcobalamin during hospital stay and follow-up period (data not shown). No procedurally related complications were noted.
Table 1.
Participant demographic and clinical characteristics
| PRF + ozone group (n=44) | Ozone group (n=47) | F/χ2 | P-value | |
|---|---|---|---|---|
| Age (yr) | 67.11±8.74 | 66.53±11.19 | 0.275 | 0.784 |
| Sex | 0.286 | 0.676 | ||
| Male | 20 | 24 | ||
| Female | 24 | 23 | ||
| Disease course (mon) | 12 (3–120) | 8 (3–360) | – | 0.314 |
| Affected side | 0.017 | 0.897 | ||
| Left | 24 | 25 | ||
| Right | 20 | 22 | ||
| Pain location | 5.057 | 0.281 | ||
| Face | 14 | 9 | ||
| Neck and upper limb | 5 | 5 | ||
| Chest and back | 21 | 21 | ||
| Abdomen | 2 | 7 | ||
| Waist | 2 | 5 | ||
| Number of ozone treatments | 7 (1–10) | 5 (1–16) | – | 0.068 |
| Pre-treatment VAS | 8 (4–10) | 8 (5–10) | – | 0.302 |
| Pre-treatment von Frey | 0.008 (0.008–60) | 0.008 (0.008–60) | – | 0.647 |
| History of underlying disease | ||||
| Hypertension | 14 | 17 | 0.192 | 0.662 |
| Type 2 diabetes | 11 | 5 | 3.235 | 0.072 |
Notes: Data in age are expressed as mean ± SD and were analyzed by independent samples t-test. Data in sex, affected side, pain location and history of underlying disease are expressed as number and were analyzed by Shapiro-Wilk test. Data in disease course, number of ozone treatments, pre-treatment VAS and pre-treatment von Frey are expressed as median (range) and were analyzed by Mann-Whitney U test. PRF: Pulsed radiofrequency; VAS: visual analog scale.
The changes of VAS for all time points among the groups
As shown in (Figure 3), the VAS scores in both treatment groups decreased 3, 6 months, and 1 year post-treatment and were significantly different from pre-treatment values (PTF + ozone group: Ppost-treatment = 0.000, P1-month = 0.000, P3-month = 0.000, P6-month = 0.000, P1-year = 0.000; ozone group: Ppost-treatment = 0.000, P1-month = 0.000, P3-month = 0.000, P6-month = 0.000, P1-year = 0.000). Despite VAS decreases compared to baseline, the differences were not statistically significant until the 3-month evaluation. Moreover, this difference persisted to the 1-year time point (P3-month = 0.010, P6-month = 0.030, P1-year = 0.017).
Figure 3.
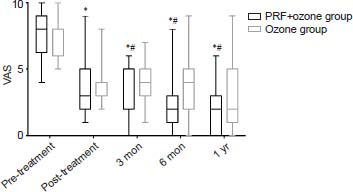
The changes of VAS at each evaluative time point in PRF + ozone and ozone groups
Note: Results are presented as median with range. *P < 0.05, vs. pre-treatment; #P < 0.05, vs. ozone group (Friedman test). PHN: Postherpetic neuralgia; PRF: pulsed radiofrequency; VAS: visual analog scale.
The changes in tactile sensation at different time points
Regarding tactile sensation data, the von Frey measurements between pre- and post-treatment were significantly decreased in the PRF + ozone group (pre-treatment: mean 2.167, 95% confidence interval (CI) 0.622-4.955; post-treatment: mean 0.132, 95% CI 0.003-0.260, P = 0.000) and in the ozone group (pre-treatment: mean 5.227, 95% CI 0.796-9.658; post-treatment: mean 1.884, 95% CI 0.739-4.508, P = 0.003). However, there were no statistical differences between the two groups at two time points (pre-treatment: P = 0.647, post-treatment: P = 0.259).
Total efficacy rate
Similar to our previous research,15 we opted to use VAS < 3 points as the standard for effective treatment. The total efficacy rate of the PRF + ozone and ozone groups were 36% and 21% at post-treatment, 52% and 45% at 3 months, 72% and 45% at 6 months, and 73% and 57% at 1 year. The total efficacy rate of the PRF + ozone group was significantly higher than that of the ozone group at 3 (P = 0.002) and 6 months (P = 0.012). The observed differences at 1 year were not statistically significant (P = 0.185). We also analyzed the percentage of moderate (VAS 3-6) and severe pain (VAS ≥ 7) in the ineffective treatment groups; however, there were no statistically significant differences.
Analysis of possible outcome predictors
The patients in both groups were sorted according to age, disease course, and the number of ozone injections to analyze the short- and long-term therapeutic effects. According to the age, the subjects were dichotomized into young (< 65 years old) and elderly (≥ 65 years old) cohorts. Meanwhile, the disease course was dichotomized as < 1 year and ≥ 1 year, while the number of ozone injections was dichotomized as < 10 times and ≥ 10 times similar to our previous research.15 The results of univariate logistic regression analysis indicated that a history of diabetes [odds ratio (OR): 0.171, 95% CI: 0.280-1.050, P = 0.046[ was significantly associated with a negative effective response during 1-year follow-up in the PRF + ozone group. However, multivariate regression analysis showed that an association between a diabetic history and successful outcome was no longer significant.Age had a significant effect at the post-treatment evaluation (OR: 0.868, 95% CI: 0.778-0.970, P = 0.012) in the PRF + ozone group. More details are provided in Additional Table 1 and Table 2.
Additional Table 1.
Univariate analysis of possible outcome predictors for treatment effects at four time points in PRF + ozone group
| Predictor | Pre-treatment | 3 mon | 6 mon | 1 yr | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||||||||
| Effective | Ineffective | OR | 95% CI |
P-value | Effective | Ineffective | OR | 95% CI |
P-value | Effective | Ineffetive | OR | 95% CI |
P-value | Effective | Ineffective | OR | 95% CI |
P-value | |
| Age (yr) | 0.2 | 0.0 | 0.4 | 1.5 | 0.4 | 0.4 | 1.5 | 0.3 | 0.5 | 1.2 | 0.2 | 0.7 | ||||||||
| <65 | 3 | 14 | 31 | 54- | 10 | 10 | 7 | 38 | 52- | 90 | 13 | 4 | 00 | 41- | 90 | 10 | 4 | 50 | 36- | 93 |
| ≥65 | 13 | 14 | 0.9 | 13 | 14 | 5.2 | 13 | 6 | 6.5 | 8 | 4 | 6.6 | ||||||||
| 91 | 42 | 92 | 33 | |||||||||||||||||
| Gender | 1.3 | 0.3 | 0.6 | 1.2 | 0.3 | 0.7 | 1.3 | 0.3 | 0.6 | 1.6 | 0.3 | 0.5 | ||||||||
| Male | 8 | 12 | 33 | 89- | 47 | 11 | 9 | 22 | 72- | 41 | 12 | 4 | 33 | 89- | 47 | 9 | 3 | 67 | 03- | 55 |
| Female | 8 | 16 | 4.5 | 12 | 12 | 4.0 | 14 | 6 | 4.5 | 9 | 5 | 9.1 | ||||||||
| 76 | 18 | 76 | 57 | |||||||||||||||||
| Disease | 1.0 | 0.3 | 0.9 | 1.4 | 0.4 | 0.5 | 0.4 | 0.1 | 0.3 | 0.8 | 0.1 | 0.7 | ||||||||
| course | 37 | 00- | 54 | 90 | 48- | 15 | 89 | 11- | 41 | 00 | 51- | 93 | ||||||||
| (yr) | 3.5 | 4.9 | 2.1 | 4.2 | ||||||||||||||||
| < 1 | 7 | 12 | 81 | 11 | 8 | 56 | 11 | 6 | 59 | 8 | 4 | 45 | ||||||||
| ≥1 | ||||||||||||||||||||
| 9 | 16 | 12 | 13 | 15 | 4 | 10 | 4 | |||||||||||||
| Diabete | 0.3 | 0.0 | 0.1 | 1.1 | 0.2 | 0.8 | 0.9 | 0.0 | 0.3 | 0.1 | 0.2 | 0.0 | ||||||||
| s | 02 | 56- | 48 | 29 | 87- | 62 | 60 | 95- | 10 | 71 | 80- | 46* | ||||||||
| With | 2 | 9 | 1.6 | 6 | 5 | 4.4 | 6 | 4 | 2.1 | 4 | 5 | 1.0 | ||||||||
| Without | 14 | 19 | 19 | 17 | 16 | 41 | 20 | 6 | 41 | 14 | 3 | 50 | ||||||||
| Hyperte | 0.9 | 0.2 | 0.9 | 0.8 | 0.2 | 0.8 | 0.7 | 0.1 | 0.7 | 0.3 | 0.0 | 0.1 | ||||||||
| nsion | 60 | 56- | 51 | 75 | 46- | 37 | 94 | 77- | 63 | 00 | 53- | 65 | ||||||||
| With | 5 | 9 | 3.5 | 7 | 7 | 3.1 | 9 | 4 | 3.5 | 6 | 5 | 1.7 | ||||||||
| Without | 11 | 19 | 98 | 16 | 14 | 15 | 17 | 6 | 63 | 12 | 3 | 00 | ||||||||
| Treatm | 1.3 | 0.3 | 0.6 | 0.8 | 0.2 | 0.7 | 2.3 | 0.4 | 0.2 | 0.3 | 0.0 | 0.2 | ||||||||
| ent | 33 | 89- | 47 | 46 | 58- | 83 | 33 | 92- | 79 | 85 | 68- | 72 | ||||||||
| number | 4.5 | 2.7 | 11. | 2.1 | ||||||||||||||||
| < 10 | 8 | 12 | 76 | 10 | 10 | 78 | 13 | 3 | 056 | 5 | 4 | 64 | ||||||||
| ≥10 | 8 | 16 | 13 | 11 | 13 | 7 | 13 | 4 | ||||||||||||
Note: CI: Confidence interval; OR: odd ratio.
Table 2.
Multiple logistic regression analysis of possible outcome predictors for treatment effects at four time points after treatment in PRF + ozone group
| Predictor | Post-treatment | 3 mon | 6 mon | 1 yr | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age | 0.868 | 0.778–0.970 | 0.012* | 1.010 | 0.937–1.088 | 0.803 | 1.016 | 0.914–1.129 | 0.766 | 0.902 | 0.773–1.054 | 0.195 |
| Sex | 2.830 | 0.410–10.570 | 0.376 | 1.127 | 0.323–3.930 | 0.852 | 1.474 | 0.128–8.109 | 0.655 | 3.128 | 0.295–33.170 | 0.344 |
| Disease course | 1.000 | 0.970–1.027 | 0.996 | 0.980 | 0.980–1.019 | 0.929 | 1.004 | 0.977–1.031 | 0.792 | 0.947 | 0.876–1.023 | 0.164 |
| Diabetes | 9.065 | 0.837–98.213 | 0.07 | 0.157 | 0.157–4.065 | 0.788 | 2.348 | 0.379–14.547 | 0.359 | 5.943 | 0.450–78.539 | 0.176 |
| Hypertension | 0.160 | 0.019–1.335 | 0.09 | 0.276 | 0.276–6.811 | 0.695 | 1.053 | 0.162–6.850 | 0.957 | 2.020 | 0.148–27.535 | 0.598 |
| Treatment number | 1.223 | 0.928–1.610 | 0.153 | 0.754 | 0.754–1.138 | 0.468 | 1.019 | 0.769–1.349 | 0.898 | 0.884 | 0.605–1.292 | 0.524 |
Notes: CI: confidence interval; OR: odds ratio.
Similarly, age (OR: 0.227, 95% CI: 0.066-1.780, P = 0.014) had a significant positive effect at the 1-year evaluation in the ozone group according to the results of univariate logistic regression analysis. Moreover, multivariate regression analysis showed that the association between a history of diabetes (OR: 0.033, 95%CI: 0.308-0.963, P = 0.036) and a successful outcome was significant at the post-treatment period. More details are provided in Additional Table 2 and Table 3.
Additional Table 2.
Univariate analysis of possible outcome predictors for treatment effects at four time points in ozone group
| Predictor | Pre-treatment | 3 mon | 6 mon | 1 yr | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||||||||
| Effective | Ineffective | OR | 95% CI | P-value | Effective | Ineffective | OR | 95% CI | P-value | Effective | Ineffective | OR | 95% CI | P-value | Effective | Ineffective | OR | 95% CI | P-value | |
| Age (yr) | 1.4 | 0.3 | 0.5 | 0.8 | 0.2 | 0.8 | 0.7 | 0.2 | 0.5 | 0.2 | 0.0 | 0.0 | ||||||||
| < 65 | 5 | 15 | 67 | 61- | 91 | 4 | 16 | 75 | 11- | 54 | 8 | 12 | 18 | 23- | 79 | 7 | 13 | 27 | 66- | 14* |
| ≥65 | 5 | 22 | 5.9 63 | 6 | 21 | 3.629 | 13 | 14 | 2.315 | 19 | 8 | 0.780 | ||||||||
| Gender | 0.5 | 0.1 | 0.4 | 0.5 | 0.1 | 0.4 | 2.2 | 0.6 | 0.1 | 0.6 | 0.2 | 0.4 | ||||||||
| Male | 4 | 20 | 67 | 37- | 30 | 4 | 20 | 67 | 37- | 30 | 13 | 11 | 16 | 84- | 81 | 12 | 12 | 43 | 92- | 54 |
| 6 | 17 | 2.2 | 6 | 17 | 2.3 | 8 | 15 | 7.1 | 14 | 9 | 2.0 | |||||||||
| Female | 46 | 46 | 77 | 47 | ||||||||||||||||
| Disease | 0.0 | 0.3 | 0.7 | 0.7 | 0.1 | 0.7 | 0.8 | 0.2 | 0.7 | 0.6 | 0.1 | 0.4 | ||||||||
| course | 49 | 08- | 37 | 5 | 21 | 60 | 88- | 03 | 11 | 15 | 07 | 54- | 16 | 13 | 13 | 15 | 91- | 14 | ||
| (yr) | 5.2 | 5 | 16 | 3.0 | 10 | 11 | 2.5 | 13 | 8 | 1.9 | ||||||||||
| < 1 | 6 | 20 | 79 | 89 | 66 | 81 | ||||||||||||||
| ≥1 | 4 | 17 | ||||||||||||||||||
| Diabete | 2.8 | 0.4 | 0.2 | 2.8 | 0.4 | 0.2 | 0.2 | 0.0 | 0.2 | 1.2 | 0.1 | 0.8 | ||||||||
| s | 2 | 3 | 33 | 04- | 79 | 2 | 3 | 33 | 04- | 79 | 1 | 4 | 75 | 28- | 40 | 3 | 2 | 39 | 87- | 24 |
| With | 8 | 34 | 19.873 | 8 | 34 | 19.873 | 20 | 22 | 2.671 | 23 | 19 | 8.199 | ||||||||
| Without | ||||||||||||||||||||
| Hyperte | 0.9 | 0.5 | 0.3 | 1.2 | 0.2 | 0.7 | 0.8 | 0.2 | 0.7 | 1.2 | 0.3 | 0.7 | ||||||||
| nsion | 5 | 12 | 60 | 05- | 05 | 4 | 13 | 31 | 93- | 76 | 7 | 10 | 00 | 40- | 16 | 10 | 7 | 50 | 75- | 16 |
| With | 5 | 25 | 8.601 | 6 | 24 | 5.163 | 14 | 16 | 2.664 | 16 | 14 | 4.163 | ||||||||
| Without | ||||||||||||||||||||
| Treatm | 0.4 | 0.0 | 0.2 | 1.4 | 0.2 | 0.6 | 0.7 | 0.2 | 0.6 | 0.8 | 0.2 | 0.8 | ||||||||
| ent | 14 | 93- | 37 | 81 | 68- | 51 | 50 | 01 | 68 | 48 | 25- | 08 | ||||||||
| number | 6 | 29 | 1.8 | 8 | 27 | 8.1 | 15 | 20 | 2.7 | 19 | 16 | 3.1 | ||||||||
| <10 | 4 | 8 | 32 | 2 | 10 | 99 | 6 | 6 | 93 | 7 | 5 | 96 | ||||||||
| ≥10 | ||||||||||||||||||||
Note: CI: Confidence interval; OR: odd ratio.
Table 3.
Multiple logistic regression analysis of possible outcome predictors for treatment effects at four time points after treatment in ozone group
| Post-treatment | 3 mon | 6 mon | 1 yr | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Predictor | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Age | 0.353 | 0.124–1.010 | 0.052 | 0.96 | 0.836–1.103 | 0.567 | 1.004 | 0.914–1.103 | 0.928 | 0.928 | 0.858–1.004 | 0.062 |
| Sex | 0.563 | 0.078–4.073 | 0.57 | 0.672 | 0.146–3.101 | 0.611 | 1.687 | 0.458–6.217 | 1.687 | 0.693 | 0.193–2.494 | 0.575 |
| Disease course | 2.179 | 0.989–4.799 | 0.053 | 1.034 | 0.942–1.134 | 0.483 | 0.971 | 0.910–1.037 | 0.791 | 1.011 | 0.982–1.041 | 0.446 |
| Diabetes | 0.033 | 0.001–0.911 | 0.044* | 0.356 | 0.049–2.572 | 0.306 | 3.533 | 0.344–36.311 | 3.533 | 0.927 | 0.131–6.589 | 0.94 |
| Hypertension | 0.283 | 0.040–1.987 | 0.202 | 0.9 | 0.203–3.982 | 0.889 | 1.251 | 0.333–4.705 | 1.251 | 1.162 | 0.319–4.239 | 0.82 |
| Treatment number | 0.545 | 0.308–0.963 | 0.036* | 0.947 | 0.700–1.289 | 0.723 | 0.942 | 0.716–1.238 | 0.942 | 0.881 | 0.669–1.159 | 0.365 |
Notes: CI: confidence interval; OR: odds ratio.
DISCUSSION
PHN is refractory neuropathic pain that occurs as a complication of varicella-zoster virus reactivation. When patients are diagnosed with PHN, treatment should be initiated immediately with one of several available interventions. Specific treatments for PHN can be categorized as non-pharmacological therapies and pharmacological treatments. However, each treatment approach has limitations and associated repercussions. PRF and ozone injection are two recent and novel therapeutic approaches applied to PHN therapy. There have been several studies demonstrating the therapeutic effects of both treatments.13,14,14,24,25,26 However, the effects of the combined therapy of PRF and ozone injection around DRG and trigeminal ganglion have heretofore been largely unrecognized. In this study, we contrasted the treatment effects, total efficacy rate, as well as possible outcome predictors of combined therapy of PRF + ozone injection and ozone therapy alone.
Although a synergistic interaction between PRF and ozone has not been reported, we can speculate on the potential mechanisms that account for this phenomenon. The pathophysiology of PHN is still not clearly elucidated; however, what is known is that herpes zoster subsequently causes the occurrence of PHN by affecting both the central and peripheral nervous systems.38 Studies have suggested that replication of latent varicella-zoster virus in cranial or spinal ganglia contributes to an inflammatory neural disruption following resolution of the primary infection.39,40 Aside from an inflammatory response, a deafferentation process caused by the destruction of sensory neurons in the DRG infected by varicella-zoster virus is another factor known to contribute to the pain of PHN.41 In addition, an increasing number of studies have indicated that the DRG has become a driver of spontaneous pain in PHN42 as it can be a second major locus of ectopic spontaneous and evoked electrogenesis in severe neuropathic pain.43,44,45 That is why the DRG has become a priority target for intervention in PHN treatment.
PRF is a common minimally invasive therapy for treating PHN, and it can alleviate both acute and chronic pain by interrupting the signals in A-delta and C fibers. Animal studies have shown that the application of PRF to DRGs can relieve the mechanical pain thresholds in rats with neuropathic pain provoked by peripheral nerve injury. However, there are also many studies demonstrating PRF exposure, particularly prolonged exposure, results in nerve injury,46 severe nerve degeneration of myelinated axons,47 interrupted myelin coverage in the DRG, as well as injuries relatively selective to small fibers.48 Therefore, the optimal treatment parameters and analgesic mechanisms of PRF remain poorly defined.
Ozone treatment has been used to treat different diseases for over a century.49 Ozone has anti-inflammatory and anti-infective properties, and accelerates blood metabolism and immunomodulatory effects.50 Besides, other potential mechanisms of the analgesic effects of ozone therapy include activating the descending antinociceptive system and promoting endorphin release.9 Thus, we surmise that the neuromodulatory effects of PRF coupled with the strong virus inhibitory and anti-inflammatory effects of ozone should achieve a more pronounced analgesic effect in patients with PHN and possibly other forms of neuropathic pain. Furthermore, ozone, via its effects on tissue repair and regeneration,18 may be protective from the potential injury induced by PRF. This synergistic effect is in line with and similar to the majority of previous studies.51,52,53,54 That is, the effects of combination therapy are more satisfactory than either method administered alone, thereby increasing the desired effects and reducing side effects of each treatment approach.55,56 In our previous clinical studies about ozone therapy for treating neuropathic pain, the concentration of 30 μg/mL of gaseous ozone was used. However, compared with the ozonized water, the patients with gaseous ozone therapy have higher risks of complications such as gas embolism and pneumocephalus. Such complications, although rare, did occur in clinical practice.57 Subsequently, we found that the ozonized water therapy could achieve the same therapeutic efficacy as gaseous ozone therapy, but ozonized water was safer than gaseous one according to our clinical observations (unpublished data). Moreover, 12 μg/mL of ozonized water was sufficient. This was the reason for choosing 12 μg/mL of ozonized water in the present study.
In addition, we revealed that the administration of PRF + ozone and ozone therapy alone significantly but differentially reduced the VAS of patients with PHN compared to baseline.PRF + ozone administration had a significantly higher efficacy rate than ozone alone at 3 and 6 months post treatment. According to the observations made during the 1-year follow-up, the efficacy rate of PRF + ozone and ozone groups were 73.1% and 57.4% respectively. However, the difference did not reach statistical significance, possibly because of the small sample size. Moreover, no patients classified as “ineffective” in the PRF + ozone group suffered severe pain (VAS > 7); while up to 20% in the ozone group did. Therefore, we infer that the combined effect of PRF + ozone was better than that of ozone alone, especially for long-term effectiveness. A series of studies by Ding et al.25,58,59 showed that the application of PRF to the DRG could effectively alleviate pain symptoms in patients with PHN. These results were similar to those obtained in our present evaluation. The total efficacy rate in these papers was over 80%, which is higher than our present evaluation (73.1%); however, this difference does not unequivocally argue that PRF alone is better than that of PRF + ozone combination therapy. The standard for effective treatment was different in Ding’s studies. In our research, we opted to use a VAS < 3 points as the standard for effective treatment. Ding and colleagues divided their total efficacy rate into three different levels: excellent, effective, and ineffective. Further, there was another criterion for effective treatment, which was whether the VAS reduction is more than 50%.20 Finally, although the pain locations were different from the Ding’s work, there is no evidence suggesting that physical location on the trunk is significantly associated with treatment outcome.
Similarly, the von Frey measurements at post-treatment were significantly decreased in both groups compared with pre-treatment; however, there was no statistical difference between the groups. This finding was also similar to the results from our previous studies.13,14,15 This suggested that the damaged sensory nerves were gradually repaired in both groups. We previously documented that ozone injection around the DRG has a protective effect on mechanical pain thresholds. However, we could not verify the effects of PRF on tactile sensitivity. In the future, it will be necessary to evaluate additional methods to detect further effects of PRF such as skin temperature.
In our current evaluation, the univariate and multiple regression analyses demonstrated that the history of diabetes mellitus and age had significant negative and positive effects, respectively, on the treatment results. In the PRF + ozone group but not the ozone group, we found that a history of diabetes was significantly associated with a negative effective response at 1-year follow-up. Diabetes is a common chronic illness associated with injury to the nervous system. The possible association between a history of diabetes and PHN is still debatable. Weitzman and colleagues suggested that the development of PHN was associated with a history of diabetes mellitus.60 However, a meta-analysis suggested that there was insufficient evidence to support this association.3 According to the results of our current evaluation as well as our previous studies, we suspect that the development of diabetic peripheral neuropathy impairs the ameliorative effects of ozone and PRF. In the ozone group, only 10.74% (5 patients) of the patients with PHN had a history of diabetes. Therefore, the fact that our results did not reach statistical significance could be due to insufficient sample size. Age was also associated with a successful response in the PRF + ozone group. There are several papers indicating that the older the patient, the greater the risk of developing PHN.3 In the present evaluation, we noted that better outcomes (higher efficacy of treatment) were associated with older patients (over 65 years old). We divided patients into young and elderly groups according to the World Health Organization standard. The World Health Organization defines an elderly as one who has a chronological age of 65 years or more.
This study has several limitations. Firstly, it is a single-center retrospective study with relatively small sample size; thus additional prospective and multi-center studies are necessary. Secondly, the specific mechanism behind the synergistic interaction between PRF and ozone requires further exploration. Thirdly, we need to set up a PRF alone group to evaluate whether the combined therapy of PRF + ozone is superior to PRF alone, and then we can compare the therapeutic efficacy of PRF and ozone. Fourthly, some patients who complained of moderate pain or more, especially severe pain, received other treatments such as oral traditional Chinese medicine for pain relief during the follow-up period. This part of the data has yet to be quantified andstatistically analyzed. Finally, a quality-of-life analysis was not conducted during this evaluation.
In conclusion, in this single-center retrospective analysis, we found that the administration of PRF + ozone and ozone therapy alone both improved pain symptoms in patients with PHN. Moreover, treatment effects and total efficacy rates tended to be higher for the combination of PRF and ozone compared with ozone alone and this was especially true for long-term therapeutic effects.
Additioanl files:
Additioanl Table 1: Univariate analysis of possible outcome predictors for treatment effects at four time points in PRF + ozone group.
Additional Table 2: Univariate analysis of possible outcome predictors for treatment effects at four time points in ozone group.
Footnotes
Conflicts of interest
The author reports no conflicts of interest in this work.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Funding: This study was supported by National Natural Science Foundation of China, Nos. 81671076 & 82072086.
REFERENCES
- 1.Yang F, Yu S, Fan B, et al. The epidemiology of herpes zoster and postherpetic neuralgia in china: results from a cross-sectional study. Pain Ther. 2019;8:249–259. doi: 10.1007/s40122-019-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson RR, Kong CL, Porco TC, Kim E, Ebert CD, Acharya NR. Herpes zoster and postherpetic neuralgia: changing incidence rates from 1994 to 2018 in the United States. Clin Infect Dis. 2021;73:e3210–e3217. doi: 10.1093/cid/ciaa1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes HJ, Thomas SL, Smeeth L, et al. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016;157:30–54. doi: 10.1097/j.pain.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Wei L, Zeng Q, Lin K, Zhang J. The Treatment of topical drugs for postherpetic neuralgia: a network meta-analysis. Pain Physician. 2020;23:541–551. [PubMed] [Google Scholar]
- 5.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014;371:1526–1533. doi: 10.1056/NEJMcp1403062. [DOI] [PubMed] [Google Scholar]
- 7.Vlassi E, Vlachos P, Kornaros M. Effect of ozonation on table grapes preservation in cold storage. J Food Sci Technol. 2018;55:2031–2038. doi: 10.1007/s13197-018-3117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowen RJ. Remission of aggressive autoimmune disease (dermatomyositis) with removal of infective jaw pathology and ozone therapy: review and case report. Auto Immun Highlights. 2018;9:7. doi: 10.1007/s13317-018-0107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bocci V, Borrelli E, Zanardi I, Travagli V. The usefulness of ozone treatment in spinal pain. Drug Des Devel Ther. 2015;9:2677–2685. doi: 10.2147/DDDT.S74518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowen RJ, Robins H. Ozone therapy for complex regional pain syndrome: review and case report. Curr Pain Headache Rep. 2019;23:41. doi: 10.1007/s11916-019-0776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biazzo A, Corriero AS, Confalonieri N. Intramuscular oxygen-ozone therapy in the treatment of low back pain. Acta Biomed. 2018;89:41–46. doi: 10.23750/abm.v89i1.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliviero A, Giordano L, Maffulli N. The temporal effect of intra-articular ozone injections on pain in knee osteoarthritis. Br Med Bull. 2019;132:33–44. doi: 10.1093/bmb/ldz028. [DOI] [PubMed] [Google Scholar]
- 13.An JX, Liu H, Chen RW, et al. Computed tomography-guided percutaneous ozone injection of the Gasserian ganglion for the treatment of trigeminal neuralgia. J Pain Res. 2018;11:255–263. doi: 10.2147/JPR.S140369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao L, Chen RW, Williams JP, et al. Efficacy and safety of percutaneous ozone injection around gasserian ganglion for the treatment of trigeminal neuralgia: a multicenter retrospective study. J Pain Res. 2020;13:927–936. doi: 10.2147/JPR.S232081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin SY, Zhang SZ, An JX, et al. The effect of ultrasound-guided percutaneous ozone injection around cervical dorsal root ganglion in zosterassociated pain: a retrospective study. J Pain Res. 2018;11:2179–2188. doi: 10.2147/JPR.S163340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanpolat Y, Savas A, Bekar A, Berk C. Percutaneous controlled radiofrequency trigeminal rhizotomy for the treatment of idiopathic trigeminal neuralgia: 25-year experience with 1,600 patients. Neurosurgery. 2001;48:524. doi: 10.1097/00006123-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Yao P, Hong T, Wang ZB, et al. Treatment of bilateral idiopathic trigeminal neuralgia by radiofrequency thermocoagulation at different temperatures. Medicine (Baltimore) 2016;95:e4274. doi: 10.1097/MD.0000000000004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang B, Yao M, Feng Z, et al. CT-guided percutaneous infrazygomatic radiofrequency neurolysis through foramen rotundum to treat V2 trigeminal neuralgia. Pain Med. 2014;15:1418–1428. doi: 10.1111/pme.12440. [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Park SJ, Yoon DM, Yoon KB, Kim SH. Predictors of the analgesic efficacy of pulsed radiofrequency treatment in patients with chronic lumbosacral radicular pain: a retrospective observational study. J Pain Res. 2018;11:1223–1230. doi: 10.2147/JPR.S164414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo F, Wang T, Shen Y, Meng L, Lu J, Ji N. High voltage pulsed radiofrequency for the treatment of refractory neuralgia of the infraorbital nerve: a prospective double-blinded randomized controlled study. Pain Physician. 2017;20:271–279. [PubMed] [Google Scholar]
- 21.Gupta A, Huettner DP, Dukewich M. Comparative effectiveness review of cooled versus pulsed radiofrequency ablation for the treatment of knee osteoarthritis: a systematic review. Pain Physician. 2017;20:155–171. [PubMed] [Google Scholar]
- 22.Shi Y, Wu W. Treatment of neuropathic pain using pulsed radiofrequency: a meta-analysis. Pain Physician. 2016;19:429–444. [PubMed] [Google Scholar]
- 23.Snidvongs S, Mehta V. Pulsed radio frequency: a non-neurodestructive therapy in pain management. Curr Opin Support Palliat Care. 2010;4:107–110. doi: 10.1097/SPC.0b013e328339628a. [DOI] [PubMed] [Google Scholar]
- 24.Han Z, Hong T, Ding Y, Wang S, Yao P. CT-guided pulsed radiofrequency at different voltages in the treatment of postherpetic neuralgia. Front Neurosci. 2020;14:579486. doi: 10.3389/fnins.2020.579486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding Y, Li H, Hong T, Yao P. Efficacy of pulsed radiofrequency to cervical nerve root for postherpetic neuralgia in upper extremity. Front Neurosci. 2020;14:377. doi: 10.3389/fnins.2020.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan CF, Liu Y, Dong DS, et al. Bipolar high-voltage, long-duration pulsed radiofrequency improves pain relief in postherpetic neuralgia. Pain Physician. 2016;19:E721–728. [PubMed] [Google Scholar]
- 27.Zhu J, Fei Y, Deng J, Huang B, Yao M. Application and therapeutic effect of puncturing of the costal transverse process for pulsed radiofrequency treated T1-T3 herpes zoster neuralgia. J Pain Res. 2020;13:2519–2527. doi: 10.2147/JPR.S266481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu B, Yang Y, Zhang Z, Wang H, Fan B, Sima L. Clinical study of spinal cord stimulation and pulsed radiofrequency for management of herpes zoster-related pain persisting beyond acute phase in elderly patients. Pain Physician. 2020;23:263–270. [PubMed] [Google Scholar]
- 29.Kim ED, Lee YI, Park HJ. Comparison of efficacy of continuous epidural block and pulsed radiofrequency to the dorsal root ganglion for management of pain persisting beyond the acute phase of herpes zoster. PLoS One. 2017;12:e0183559. doi: 10.1371/journal.pone.0183559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K, Jo D, Kim E. Pulsed radiofrequency to the dorsal root ganglion in acute herpes zoster and postherpetic neuralgia. Pain Physician. 2017;20:E411–E418. [PubMed] [Google Scholar]
- 31.Pi ZB, Lin H, He GD, Cai Z, Xu XZ. Randomized and controlled prospective trials of Ultrasound-guided spinal nerve posterior ramus pulsed radiofrequency treatment for lower back post-herpetic neuralgia. Clin Ter. 2015;166:e301–305. doi: 10.7417/T.2015.1882. [DOI] [PubMed] [Google Scholar]
- 32.Ke M, Yinghui F, Yi J, et al. Efficacy of pulsed radiofrequency in the treatment of thoracic postherpetic neuralgia from the angulus costae: a randomized, double-blinded, controlled trial. Pain Physician. 2013;16:15–25. [PubMed] [Google Scholar]
- 33.Kim YH, Lee CJ, Lee SC, et al. Effect of pulsed radiofrequency for postherpetic neuralgia. Acta Anaesthesiol Scand. 2008;52:1140–1143. doi: 10.1111/j.1399-6576.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald BK, Cockerell OC, Sander JW, Shorvon SD. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain. 2000;123(( Pt 4)):665–676. doi: 10.1093/brain/123.4.665. [DOI] [PubMed] [Google Scholar]
- 35.Liu DY, Chen JS, Fang ZZ, Liu SY, Wan L. Pulsed radiofrequency of the trigeminal ganglion for treating postherpetic neuralgia of the ophthalmic branch. Pain Res Manag. 2021;2021:6638392. doi: 10.1155/2021/6638392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haberberger RV, Barry C, Dominguez N, Matusica D. Human Dorsal Root Ganglia. Front Cell Neurosci. 2019;13:271. doi: 10.3389/fncel.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanai A, Suzuki A, Kobayashi M, Hoka S. Intranasal lidocaine 8% spray for second-division trigeminal neuralgia. Br J Anaesth. 2006;97:559–563. doi: 10.1093/bja/ael180. [DOI] [PubMed] [Google Scholar]
- 38.Mallick-Searle T, Snodgrass B, Brant JM. Postherpetic neuralgia: epidemiology, pathophysiology, and pain management pharmacology. J Multidiscip Healthc. 2016;9:447–454. doi: 10.2147/JMDH.S106340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baron R. Mechanisms of postherpetic neuralgia--we are hot on the scent. Pain. 2008;140:395–396. doi: 10.1016/j.pain.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Jones J. Postherpetic neuralgia. J Pain Palliat Care Pharmacother. 2015;29:180–181. doi: 10.3109/15360288.2015.1037520. [DOI] [PubMed] [Google Scholar]
- 41.Fields HL, Rowbotham M, Baron R. Postherpetic neuralgia: irritable nociceptors and deafferentation. Neurobiol Dis. 1998;5:209–227. doi: 10.1006/nbdi.1998.0204. [DOI] [PubMed] [Google Scholar]
- 42.Devor M. Rethinking the causes of pain in herpes zoster and postherpetic neuralgia: the ectopic pacemaker hypothesis. Pain Rep. 2018;3:e702. doi: 10.1097/PR9.0000000000000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Defrin R, Devor M, Brill S. Tactile allodynia in patients with lumbar radicular pain (sciatica) Pain. 2014;155:2551–2559. doi: 10.1016/j.pain.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 44.Koplovitch P, Devor M. Dilute lidocaine suppresses ectopic neuropathic discharge in dorsal root ganglia without blocking axonal propagation: a new approach to selective pain control. Pain. 2018;159:1244–1256. doi: 10.1097/j.pain.0000000000001205. [DOI] [PubMed] [Google Scholar]
- 45.Vaso A, Adahan HM, Gjika A, et al. Peripheral nervous system origin of phantom limb pain. Pain. 2014;155:1384–1391. doi: 10.1016/j.pain.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 46.Perret DM, Kim DS, Li KW, et al. Application of pulsed radiofrequency currents to rat dorsal root ganglia modulates nerve injury-induced tactile allodynia. Anesth Analg. 2011;113:610–616. doi: 10.1213/ANE.0b013e31821e974f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tun K, Cemil B, Gurcay AG, et al. Ultrastructural evaluation of pulsed radiofrequency and conventional radiofrequency lesions in rat sciatic nerve. Surg Neurol. 2009;72:496–500. doi: 10.1016/j.surneu.2008.11.016. discussion 501. [DOI] [PubMed] [Google Scholar]
- 48.Erdine S, Bilir A, Cosman ER, Cosman ER., Jr Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009;9:407–417. doi: 10.1111/j.1533-2500.2009.00317.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Chen H, Liu XH, et al. Ozone oxidative preconditioning inhibits renal fibrosis induced by ischemia and reperfusion injury in rats. Exp Ther Med. 2014;8:1764–1768. doi: 10.3892/etm.2014.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sagai M, Bocci V. Mechanisms of action involved in ozone therapy: is healing induced via a mild oxidative stress. Med Gas Res? 2011;1:29. doi: 10.1186/2045-9912-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang Y, Luo F, He X. Clinical observations on selective dorsal root ganglion pulsed radiofrequency lesioning combined with gabapentin in the treatment of postherpetic neuralgia. Neurol India. 2018;66:1706–1710. doi: 10.4103/0028-3886.246245. [DOI] [PubMed] [Google Scholar]
- 52.Li D, Sun G, Sun H, Wang Y, Wang Z, Yang J. Combined therapy of pulsed radiofrequency and nerve block in postherpetic neuralgia patients: a randomized clinical trial. PeerJ. 2018;6:e4852. doi: 10.7717/peerj.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makharita MY, El Bendary HM, Sonbul ZM, Ahmed SES, Latif MA. Ultrasound-guided pulsed radiofrequency in the management of thoracic postherpetic neuralgia: a randomized, double-blinded, controlled trial. Clin J Pain. 2018;34:1017–1024. doi: 10.1097/AJP.0000000000000629. [DOI] [PubMed] [Google Scholar]
- 54.Saxena AK, Lakshman K, Sharma T, Gupta N, Banerjee BD, Singal A. Modulation of serum BDNF levels in postherpetic neuralgia following pulsed radiofrequency of intercostal nerve and pregabalin. Pain Manag. 2016;6:217–227. doi: 10.2217/pmt.16.3. [DOI] [PubMed] [Google Scholar]
- 55.Brown EN, Pavone KJ, Naranjo M. Multimodal general anesthesia: theory and practice. Anesth Analg. 2018;127:1246–1258. doi: 10.1213/ANE.0000000000003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hendrickx JF, Eger EI, 2nd, Sonner JM, Shafer SL. Is synergy the rule? A review of anesthetic interactions producing hypnosis and immobility. Anesth Analg. 2008;107:494–506. doi: 10.1213/ane.0b013e31817b859e. [DOI] [PubMed] [Google Scholar]
- 57.Liu H, Wang Y, An JX, Williams JP, Cope DK. Thunderclap headache caused by an inadvertent epidural puncture during oxygen-ozone therapy for patient with cervical disc herniation. Chin Med J (Engl) 2016;129:498–499. doi: 10.4103/0366-6999.176080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ding Y, Hong T, Li H, Yao P, Zhao G. Efficacy of CT guided pulsed radiofrequency treatment for trigeminal postherpetic neuralgia. Front Neurosci. 2019;13:708. doi: 10.3389/fnins.2019.00708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding Y, Yao P, Li H, et al. CT-guided stellate ganglion pulsed radiofrequency stimulation for facial and upper limb postherpetic neuralgia. Front Neurosci. 2019;13:170. doi: 10.3389/fnins.2019.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of Herpes Zoster and its complications. J Infect. 2013;67:463–469. doi: 10.1016/j.jinf.2013.06.016. [DOI] [PubMed] [Google Scholar]


