Abstract
Many lactobacilli from various origins were found to apparently lack cholic acid extrusion activity. Cholic acid was accumulated spontaneously, driven by the transmembrane proton gradient. Accumulation is a newly identified kind of interaction between intestinal microbes and unconjugated bile acids and is different from extrusion and modification, which have been described previously.
Bile salts, which are the salts of bile acids conjugated with glycine or taurine, play an essential role in the emulsification and digestion of fats in vertebrates (13). They are synthesized from cholesterol in the liver and secreted into the duodenum and undergo enterohepatic circulation (7). During the enterohepatic circulation, the bile salts undergo two major modifications by the intestinal microflora. Deconjugation by bile salt hydrolases results in the formation of bile acids, which are subsequently 7α-dehydroxylated into secondary bile acids (1). In general, these bile acids produced by microbial conversion are toxic to intestinal bacteria, inhibiting their growth (2). In this article, we show a new possible fate for bile acids in the intestines, namely their uptake and accumulation by members of the genus Lactobacillus, as demonstrated with cholic acid (CA). This phenomenon appeared to be driven by the transmembrane proton gradient (ΔpH).
Accumulation of CA by lactobacilli.
CA transport experiments with lactobacilli were carried out as follows. Cells grown anaerobically with N2 gas in half-strength MRS broth (Difco Laboratories, Detroit, Mich.) at an appropriate temperature (Table 1) were harvested in the mid-exponential phase and then were washed once with buffer 1 (50 mM potassium phosphate [pH 7.0] containing 1 mM MgSO4 and 0.1 U of horseradish peroxidase [Wako Pure Chemical Industries, Ltd., Osaka, Japan] per ml [11]). The cells were resuspended in buffer 2 (supplemented with 1.0 U of peroxidase/ml, as for buffer 1) and de-energized by incubation in the presence of 10 mM 2-deoxyglucose for 30 min at an appropriate temperature (Table 1). Then the cells were washed three times with buffer 1 and resuspended in buffer 2 at a cell concentration equivalent to 3 mg of protein/ml. Ninety-six microliters of cell suspension plus 2 μl of 5.8 mM (16 mCi/mmol) [14C]CA (NEN Life Science Products Inc., Boston, Mass.) (final CA concentration, 0.116 mM) per time point was dispensed into test tubes and incubated with stirring under anaerobic conditions, introducing N2 gas into the test tubes at an appropriate temperature (Table 1). Fifteen minutes after the addition of [14C]CA, 1 μl of 1 M glucose was added to the reaction mixtures to energize the cells, and the mixture was incubated under the same conditions. At intervals, samples were added to 3 ml of ice-cold stop buffer consisting of 100 mM potassium phosphate buffer (pH 7.0) containing 100 mM LiCl. The mixture was quickly filtered through a 0.45-μm-pore-size cellulose acetate filter (Schleicher and Schuell GmbH, Dassel, Germany), which was washed once with 3 ml of stop buffer. The radioactivities on the filters were measured in a liquid scintillation counter. Background counts obtained from filtering the reaction mixture without the cells were subtracted from all readings. The protein content of the cell suspensions was determined with a DC Protein Assay kit (Bio-Rad Laboratories, Hercules, Calif.), by using supernatants obtained by boiling cell suspensions for 5 min in 1 N NaOH.
TABLE 1.
Transport of CA in Lactobacillus strains and Lactococcus lactis
| Strain | Origin | Culture temp (°C) | Accumulated CA (nmol mg of protein−1) in energizeda/nonenergizedb cells | Time (min)c | Accumulation factore | Internal pHf |
|---|---|---|---|---|---|---|
| L. delbrueckii subsp. bulgaricus JCM 1002 | Bulgarian yogurt | 37 | 4.86/0.7 | 30 | 14.0 | —g |
| L. helveticus SBT-2161 | Dairy products | 37 | 0.97/0.4 | 30 | 2.8 | — |
| L. helveticus JCM 1062 | Dairy products | 37 | 1.33/0.3 | 20 | 3.8/3.2 | 7.57 |
| L. helveticus JCM 1120 | Emmental cheese | 37 | 1.70/0.4 | 20 | 4.9 | — |
| L. delbrueckii subsp. lactis JCM 1248 | Emmental cheese | 37 | 2.05/0.2 | 10 | 5.9 | — |
| L. lactis MG 1363 | Dairy products | 30 | 0.0/0.6 | 10d | 0.0 | — |
| L. acidophilus JCM 1028 | Human intestine | 37 | 1.85/0.3 | 15 | 5.3/3.4 | 7.60 |
| L. salivarius subsp. salicinius JCM 1040 | Human intestine | 37 | 2.90/0.3 | 8 | 8.3/4.9 | 7.77 |
| L. salivarius subsp. salicinius JCM 1044 | Human intestine | 37 | 4.30/0.3 | 10 | 12.4/6.5 | 7.90 |
| L. acidophilus JCM 1034 | Human intestine | 37 | 1.00/0.3 | 35 | 2.9/1.8 | 7.30 |
| L. acidophilus JCM 1132 | Human | 37 | 2.46/0.6 | 25 | 7.1 | — |
| L. delbrueckii 18 | Mouse small intestine | 37 | 1.40/0.3 | 70 | 4.0 | — |
| L. fermentum 20 | Mouse small intestine | 37 | 2.20/0.4 | 35 | 6.3 | — |
| L. delbrueckii 21 | Mouse small intestine | 37 | 1.83/0.4 | 25 | 5.3 | — |
| L. mali JCM 1116 | Apple juice/cider press | 30 | 2.40/0.2 | 20 | 6.9 | — |
| L. sakei subsp. sakei JCM 1157 | Starter of sake | 30 | 2.60/0.3 | 20 | 7.5/5.2 | 7.80 |
| L. curvatus GD 12 | Indonesian ragi tape | 30 | 1.91/0.4 | 25 | 5.5 | — |
The maximum level of CA accumulation obtained in energized cells.
CA equilibration level in nonenergized cells.
The maximum level of CA accumulation was detected at the indicated time after the addition of glucose.
CA extrusion was detected until the indicated time (min) after the glucose addition.
The ratio of the internal and external CA concentrations. The external CA concentration was 0.116 mM. The internal CA concentration was determined assuming 3 μl mg of protein−1 internal volume (10). Values after the shill are the predicted accumulation factors calculated with the Henderson-Hasselbalch equation using the measured internal pH values.
Determined with a fluorescent pH probe, 5 (and 6-)-carboxyfluorescein succinimidyl ester.
—, not determined.
The experimental results showed that all lactobacilli from various environmental niches accumulated CA intracellularly in the presence of glucose (Table 1). After the initial period of CA accumulation, the intracellular CA concentration in the cells began to decline due to the depletion of glucose from the assay system (data not shown). The maximum level of CA accumulation was detected at the time point indicated in Table 1. The amounts of accumulated CA differed depending on the Lactobacillus strain used, with accumulation factors ranging from 2.8 to 14.0 (Table 1). In sharp contrast, Lactococcus lactis MG 1363 (wild type) extruded CA in an energy-dependent manner (Table 1) and accumulated CA only after glucose had been depleted from the medium (data not shown). This is in good agreement with our previous findings that this strain possesses an ATP-dependent cholate extrusion system (14). Equilibration of CA between the buffer and the de-energized Lactobacillus and Lactococcus cells was already observed before the addition of glucose (Table 1, see footnote b) as a result of free diffusion of the highly hydrophobic undissociated CA through the cell membrane (8).
Driving force of CA accumulation.
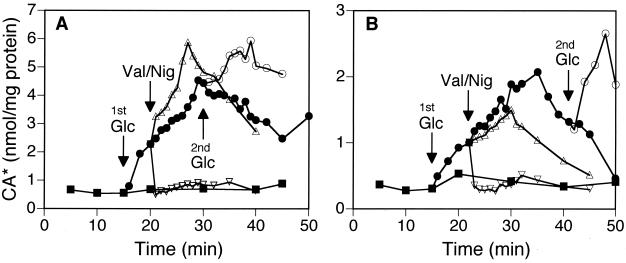
The bioenergetics of CA accumulation was investigated by the addition of 2 μM valinomycin (which dissipates transmembrane electrical potential [ΔΨ]) or 1 μM nigericin (which dissipates ΔpH). In the energized cells of Lactobacillus salivarious subsp. salicinius JCM 1044 (Fig. 1A) and Lactobacillus acidophilus JCM 1028 (Fig. 1B), the addition of nigericin resulted in the immediate loss of the accumulated CA, down to the equilibration level. These results indicate the involvement of ΔpH as a driving force of CA accumulation. Addition of valinomycin increased the amounts of accumulated CA in JCM 1044 even further than in the control, while no such effect was observed in the case of JCM 1028. This increase of CA accumulation in the presence of valinomycin was due to the increase of ΔpH as a result of the dissipation of ΔΨ, which was verified by the measurement of the internal pH of this strain with a method described below (data not shown).
FIG. 1.
Transport of CA by L. salivarius subsp. salicinius JCM 1044 (A) and L. acidophilus JCM 1028 (B) in the presence of ionophores. De-energized, washed cells were incubated at pH 7.0 with [14C]CA in the absence (■) and presence (●) of glucose and after the addition of valinomycin (Val; ▵) or nigericin (Nig; ▿). Ionophores were added 5 min (A) or 7 min (B) after the first glucose addition (1st Glc). A second addition of glucose (2nd Glc; ○) was performed 15 min (A) or 25 min (B) after the first addition of glucose. CA* denotes both cholic acid and cholate anion.
Passive equilibration of CA across the membrane.
These results proved that the driving force for the CA accumulation is the ΔpH. However, we hypothesized that the CA accumulation observed in many Lactobacillus strains is not catalyzed by a transporter protein but rather is a result of passive equilibration of a hydrophobic weak acid through the membrane, as follows. After energization of the cells, which leads to the formation of ΔpH, the protonated CA molecules that have passed through the membrane bilayer are trapped in the cytoplasm, because the intracellular pH is more alkaline than the extracellular pH. Under these conditions, more deprotonation of CA, which is a weak acid with a pKa of 6.4, occurs inside the cells, and the resulting cholate anions cannot diffuse out of the cells across the membrane because of their polarity. The equilibration is reached when the concentrations of protonated CA become equal on both sides of the membrane. Extension of the energization period by a second addition of glucose prolonged the ΔpH formation, leading to an additional accumulation of CA (Fig. 1A and B). The results described below support this working hypothesis.
(i) Effect of external pH.
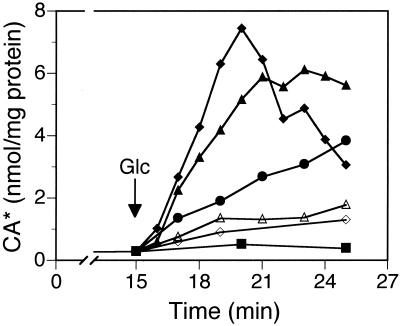
Accumulation of CA in JCM 1044 cells was measured at the external pH values of 7.6, 7.3, 7.0, 6.6, and 6.2. The Henderson-Hasselbalch equation predicts that lowering the external pH values from 7.6 to 6.2 will allow the external concentration of protonated, uncharged CA to increase about 10-fold. Indeed, the accumulation of CA, which should be proportional to the concentration of external, protonated CA, increased at acidic pH values and showed an around eightfold increase from the external pH value of 7.6 to that of 6.2 (Fig. 2, compare the data at around 5 min after the glucose addition), as was expected based on the theory.
FIG. 2.
Accumulation of CA by JCM 1044 at different external pH values. The experiments were carried out at different pH values: 7.6 (◊), 7.3 (▵), 7.0 (●), 6.6 (▴), and 6.2 (⧫). For energization of the cells, glucose was added at t = 15 min. CA amounts in the nonenergized cells are demonstrated by only one line (■), since these were very similar at all pH values tested. CA* denotes both cholic acid and cholate anion.
In addition, a much more polar [14C]taurocholic acid (TCA) (3.2 mCi/mmol) (NEN Life Science Products Inc.) with a pKa of 1.4 was not accumulated at all by the energized cells of strain JCM 1044 (data not shown), which lacks bile salt hydrolase activity (P. Kurdi and A. Yokota, unpublished data). This result is also consistent with the diffusion of the protonated CA across the lipid bilayer.
(ii) Effect of internal pH in various strains.
The internal pH value was measured with 5 (and 6-)-carboxyfluorescein diacetate succinimidyl ester (3). This compound is membrane permeable, and upon entering cells, it is cleaved by esterases and the arising fluorescent pH probe, 5 (and 6-)-carboxyfluorescein succinimidyl ester is conjugated to cellular aliphatic amines. Washed suspensions of exponential-phase cells in buffer 2 (optical density at 660 nm of about 0.5) were incubated with 4 μM 5 (and 6-)-carboxyfluorescein diacetate succinimidyl ester (Molecular Probes Inc., Eugene, Oreg.) for 30 min at an appropriate temperature (Table 1). Subsequent incubation with 10 mM glucose for 1 h under N2 gas flow facilitated the efflux of unconjugated probes from the cells. The cells were centrifuged and resuspended in prewarmed buffer 2 to an optical density at 660 nm of about 0.5 and were transferred to a stirred and heated cuvette holder (for temperatures, see Table 1) of a luminescence spectrophotometer (LS-50B; Perkin-Elmer Instruments, Wilton, Conn.). The internal pH value was determined from fluorescence intensities of the cell suspension after energization with 10 mM glucose in the presence of 0.116 mM sodium cholate. In order to calibrate the fluorescent signal of the intracellularly conjugated probe, intra- and extracellular pH values were equalized by the addition of 2 μM valinomycin plus 2 μM nigericin, and fluorescence was measured at pH values between 4 and 10.
In this way, we found a significant difference in the internal pH values of six different strains, ranging from 7.30 to 7.90 (Table 1). The Henderson-Hasselbalch equation indicates that the concentration of uncharged protonated species of a weak acid, [HA], can be determined as follows: [HA] = C/(1+10(pH − pKa)), when C is the total concentration of this acid. Use of this equation for the measured values of internal pH shows that the total CA concentration in the cell should be 6.5-fold and 1.8-fold higher than the total external concentration of CA (i.e., the accumulation factor) at the internal pH values of 7.90 and 7.30, respectively (Table 1). As expected, the experimental levels of CA accumulation showed a correlation with the internal pH values at a constant external pH value, as can be seen in Table 1; the observed accumulation factors of 12.4 and 2.9 in strains with internal pH values of 7.90 and 7.30 (Table 1), respectively, were close to the values predicted above, assuming passive equilibration. Similar results were also obtained with the four other strains, as indicated in Table 1. However, the measured accumulation factors were somewhat higher than the predicted ones in all cases. This difference might be due to the acidification of the reaction mixture in the transport experiments upon addition of glucose, which would increase the proportion of protonated CA outside the cell and therefore the CA accumulation in the cell.
Conclusions.
To date, a few species of bacteria have been described as possessing active bile acid/salt exporters, which make them resistant to these compounds. Escherichia coli, an intestinal bacterium, possesses chenodeoxycholate and TCA export activities driven by the proton motive force (12). Similar examples are also reported even in some nonintestinal bacteria. Lactococcus lactis MG 1363 (14) and Neisseria gonorrhoeae (4, 6) thus possess multispecific efflux pumps. In a CA-resistant mutant derived from MG 1363, an increased activity of this efflux system has been observed (14). On the other hand, uptake transporters for bile acid/salt have also been reported in intestinal microbes. Eubacterium sp. strain VPI12708 actively takes up CA, which then enters the 7α-dehydroxylation pathway (9). A conjugated bile acid transporter was described for L. johnsonii 100-100 which mediates the import of TCA for deconjugation by the cytoplasmic bile salt hydrolase (5). In contrast, the present study demonstrated that under experimental conditions, CA is accumulated, apparently passively, in Lactobacillus cells according to the ΔpH. The quantitative relationship between the amount of CA and the internal pH value (Table 1) suggests that the accumulation is mediated not by a protein carrier but by the well-known spontaneous distribution of hydrophobic weak acids across the bacterial membranes. This apparent lack of CA extrusion, especially in the cells of intestinal lactobacilli, is surprising, since bile acids are generally toxic to bacterial cells (2).
The observed increases in the amounts of accumulated CA at decreasing external pH values (Fig. 2) suggest that factors which decrease the intestinal pH, e.g., the presence of short-chain fatty acids or lactic acid produced by intestinal microbes, may enhance the accumulation of CA by lactobacilli in vivo.
Acknowledgments
Gerald W. Tannock and Hendrik W. van Veen were partly supported by the Short Term JSPS (Japan Society for Promotion of Science) Fellowship for Research in Japan, S-99285 and S-99306, respectively, which is gratefully acknowledged.
REFERENCES
- 1.Baron S F, Hylemon P B. Biotransformation of bile acids, cholesterol, and steroid hormones. In: Mackie R I, White B A, editors. Gastrointestinal microbiology. I. Gastrointestinal ecosystems and fermentations. New York, N.Y: International Thomson Publishing; 1997. pp. 470–510. [Google Scholar]
- 2.Binder H J, Filburn B, Floch M. Bile acid inhibition of intestinal anaerobic organisms. Am J Clin Nutr. 1975;28:119–125. doi: 10.1093/ajcn/28.2.119. [DOI] [PubMed] [Google Scholar]
- 3.Breeuwer P, Drocourt J, Rombouts F M, Abee T. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl Environ Microbiol. 1996;62:178–183. doi: 10.1128/aem.62.1.178-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delahay R M, Robertson B D, Balthazar J T, Shafer W M, Ison C A. Involvement of the gonococcal MtrE protein in the resistance of Neisseria gonorrhoeae to toxic hydrophobic agents. Microbiology. 1997;143:2127–2133. doi: 10.1099/00221287-143-7-2127. [DOI] [PubMed] [Google Scholar]
- 5.Elkins C A, Savage D C. Identification of genes encoding conjugated bile salt hydrolase and transport in Lactobacillus johnsonii 100-100. J Bacteriol. 1998;180:4344–4349. doi: 10.1128/jb.180.17.4344-4349.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagman K E, Lucas C E, Balthazar J T, Snyder L, Nilles M, Judd R C, Shafer W M. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology. 1997;143:2117–2125. doi: 10.1099/00221287-143-7-2117. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann A F. Chemistry and enterohepatic circulation of bile acids. Hepatology. 1984;4:4S–14S. doi: 10.1002/hep.1840040803. [DOI] [PubMed] [Google Scholar]
- 8.Kamp F, Hamilton J A. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry. 1993;32:11074–11086. doi: 10.1021/bi00092a017. [DOI] [PubMed] [Google Scholar]
- 9.Mallonee D H, Hylemon P B. Sequencing and expression of a gene encoding a bile acid transporter from Eubacterium sp. strain VPI 12708. J Bacteriol. 1996;178:7053–7058. doi: 10.1128/jb.178.24.7053-7058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poolman B, Smid E J, Konings W N. Kinetic properties of a phosphate-bound-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987;169:2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakamoto M, Tano Y, Uchimura T, Komagata K. Aerobic growth of some lactic acid bacteria enabled by the external addition of peroxidase (horseradish) to the culture medium. J Ferment Bioeng. 1998;85:627–629. [Google Scholar]
- 12.Thanassi D G, Cheng L W, Nikaido H. Active efflux of bile salts by Escherichia coli. J Bacteriol. 1997;179:2512–2518. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson F A. Intestinal transport of bile acids. In: Schultz S G, Field M, Frizzell R A, Rauner B B, editors. Handbook of physiology, section 6. IV. Bethesda, Md: American Physiological Society; 1991. pp. 389–404. [Google Scholar]
- 14.Yokota A, Veenstra M, Kurdi P, van Veen H W, Konings W N. Cholate resistance in Lactococcus lactis is mediated by an ATP-dependent multispecific organic anion transporter. J Bacteriol. 2000;182:5196–5201. doi: 10.1128/jb.182.18.5196-5201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]