Abstract
Wolbachia species are intracellular bacteria known to cause reproductive abnormalities in their hosts. In this study, we identified Wolbachia genes encoding homologs to the type IV secretion system by which many pathogenic bacteria secrete macromolecules. The genes identified encoded most of the essential components of the secretion system and were cotranscribed as an operon.
The genus Wolbachia comprises a group of maternally inherited intracellular bacteria that have been identified in a variety of arthropod hosts. Wolbachia organisms cause various sexual alterations in their hosts, such as cytoplasmic incompatibility (CI), thelytokous parthenogenesis, feminization, and male killing (20), whose mechanisms are yet unknown. CI is the most commonly expressed phenotype in a wide range of insect species. This phenotype results in reduced egg viability in crosses between Wolbachia-infected males and uninfected females in the simplest case (16, 20, 23). Since Wolbachia organisms are not present in mature sperm (3, 12), it is believed that the bacteria modify sperm during their development, possibly by secreting some proteins. Based on this assumption, we directed our attention to the macromolecule secretion system in Wolbachia.
Many gram-negative bacteria have conserved macromolecule secretion systems. Type IV secretion systems have been mainly found in pathogenic bacteria, such as Agrobacterium tumefaciens (25), Bordetella pertussis (22), Helicobacter pylori (6), Brucella suis (15), Legionella pneumophila (21), and Rickettsia prowazekii (1). The secreted proteins and nucleoproteins play important roles in the virulence of these bacteria (4, 7). In this study, we tested the presence of the type IV secretion system in Wolbachia.
The Taiwan cricket, Teleogryllus taiwanemma, and the Mediterranean flour moth, Ephestia kuehniella (Yokohama strain), were used as the sources of Wolbachia. Wolbachia strains infecting arthropods are divided into A and B groups (23). The Wolbachia strain carried by T. taiwanemma (wTai) and the strain carried by E. kuehniella (wKueYO) belong to the B and A group, respectively (12, 14, 18). Both wTai and wKueYO cause CI in each host (12, 18). Tetracycline-treated strains of these insects were used as the uninfected control strains.
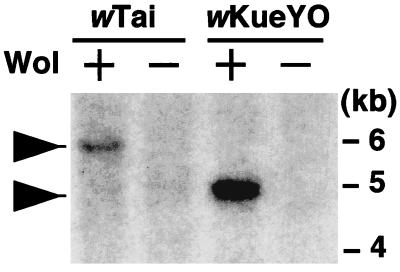
Genes for a type IV secretion system have often been found as an operon that consists of several virB genes and a virD4 gene (4, 5). The VirD4 protein is believed to bind the molecule to be secreted and deliver it to the pore complex on the bacterial membrane formed with VirB proteins (4, 9, 10). To test the presence of genes for a type IV secretion system in Wolbachia, we performed Southern hybridization using the virD4 gene of R. prowazekii (lambda clone F958) as a probe. Unless otherwise mentioned, standard molecular methods were used (17). As shown in Fig. 1, positive signals were observed in the lanes with samples from Wolbachia-infected insect hosts but not in those with samples from uninfected insect hosts. The signal from wKueYO was more intensive than that from wTai. This was probably because E. kuehniella contained Wolbachia at a higher density than T. taiwanemma. virD4 and its flanking regions of wTai were cloned and sequenced by screening the genomic library of wTai (13). It turned out that the sequenced region of wTai contained virB8, -B9, -B10, -B11, and -D4 (Fig. 2A) and that the predicted amino acid sequences of their products showed significant similarity to those homologs of A. tumefaciens and R. prowazekii (Table 1). This region did not contain virB4, which encodes the integral cytoplasmic membrane protein that plays a critical role in the type IV secretion system (2, 8). The presence of virB4 in a different region of the wTai genome was confirmed by Southern hybridization using R. prowazekii virB4 (lambda clone P438) as a probe (data not shown). It is postulated that the minimum components of the typical type IV secretion system consist of VirB4, VirB7 (or VirB8), VirB9, VirB10, VirB11, and VirD4 homologs (7, 24). Thus, the genes identified from wTai contained most of the minimum sets for the typical type IV secretion system.
FIG. 1.
Detection of the virD4 gene in two Wolbachia strains by Southern hybridization using virD4 of R. prowazekii as a probe. Wol+, Wolbachia-infected insects; Wol−, uninfected insects. Total DNA samples from insects were digested with EcoRI prior to agarose gel electrophoresis.
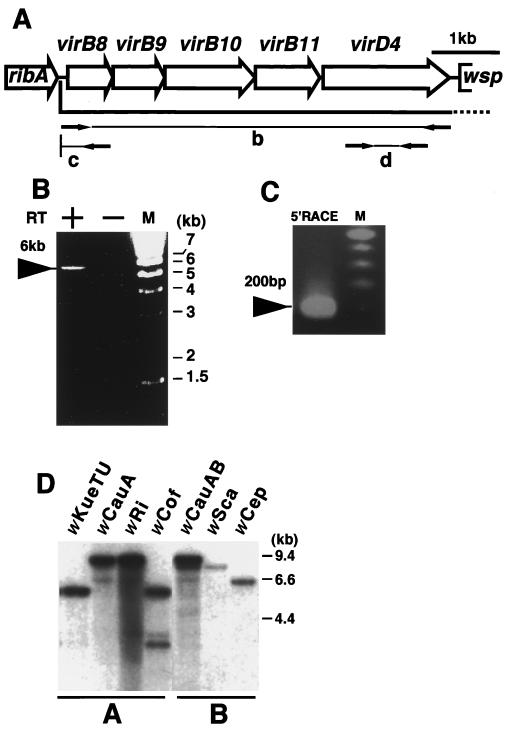
FIG. 2.
Transcriptional analysis of the vir operon and its distribution in Wolbachia strains. M, DNA size marker. (A) Open reading frame map of the wTai vir operon. Open arrows show open reading frames. Arrows in the regions b, c, and d indicate the primers used in the experiments shown in panels B, C, and D, respectively. ribA, GTP cyclohydrolase II; wsp, a surface protein. (B) RT-PCR of the entire region of the vir operon. RT+ and RT− indicate the presence and absence of reverse transcriptase in the reaction mixture, respectively. (C) 5′RACE analysis. (D) Southern hybridization of different Wolbachia strains using virD4 of wTai. wKueTU, wCauA, wRi, and wCof are A group strains, and their insect hosts are E. kuehniella (Tsuchiura strain), the almond moth E. cautella, the fruit fly Drosophila simulans Riverside, and D. simulans Coffs, respectively. wCauAB indicates A and B group strains (wCauA and wCauB, respectively) doubly infecting E. cautella. Two bands were observed in wCauAB, at about 9.4 and 9 kb, while only a single band was observed in wCauA, at 9.4 kb, indicating that wCauB contained virD4 distinct from that of wCauA. wSca and wCep are B group strains infecting the moth Ostrinia scapulalis and Corcyra cepharonica, respectively. All DNA samples were digested with EcoRI.
TABLE 1.
Identities of amino acid sequences of wTai vir gene products to those of wKueYO and other known type IV secretion systemsa
| wTai (AB045234) | wKueYO (AB045235) | R. prowazekii (RPXX02) | A. tumefaciens Ti plasmid (AF242881) | E. coli pKM101 (U09868, AF109305) |
|---|---|---|---|---|
| VirB8 | 86 | 38 | 31 | 33 |
| VirB9 | 88 | 40 | 28 | 29 |
| VirB10 | 75 | 35 | 30 | 29 |
| VirB11 | 95 | 67 | 33 | 36 |
| VirD4 | 89 | 59 | 35 | 26 |
Numbers are percent identities calculated by DNASIS-MAC (Hitachi). GenBank accession numbers are in parentheses.
Expression of the genes identified above was examined by reverse transcription (RT)-PCR. Total RNA from the testis of T. taiwanemma was reverse transcribed by SuperScript II RT (GIBCO BRL) using the primer virR1 (5′-TTAACCTCTATCCTCGAT-3′). PCR was performed using LA Taq (TAKARA) with the primer set of virLF1 (5′-ATTGGAATTCAAGTCGCTATAGCACAGTTG-3′) and virRL1 (5′-TCCTCATCGTCAAATTCATCCTTACTGTC-3′). A positive signal at about 6 kb was detected only in the lane RT+ (Fig. 2B), indicating that vir genes of Wolbachia were cotranscribed as an operon. The transcription start site of the vir operon was then determined by 5′RACE analysis using the 5′/3′ RACE kit (Boehringer). The transcript of the vir operon was reverse transcribed with the specific primer virR11 (5′-CCCTTGCTTTTATATACTCTG-3′), and a polymeric dA tail was added to the 3′ end of the cDNA by terminal transferase. A DNA fragment containing the poly(dA) addition site was amplified by nested PCR. Primers used were the oligo(dT) anchor primer and vir5R1 (5′-CGTTCAGCGTTTCATTTGCAG-3′) in the first PCR and the anchor primer and vir5R2 (5′-CAAATGGCTCAATAGTGCTACTTGTGC-3′) in the second PCR. As a result, a PCR product of about 200 bp was obtained (Fig. 2C). Sequence analysis of the PCR product revealed that the cDNA was extended to about 50 bp upstream from the virB8 start codon. Thus, the transcription of the vir operon was started from virB8 and extended to virD4, though we could not exclude the possibility that the operon may include additional downstream genes. The vir operon of wKueYO was also cloned and sequenced. This operon contained the same 5 vir homologs as those of wTai (Table 1) and was transcribed in the same manner as the operon of wTai (data not shown).
To estimate the distribution of the type IV secretion system among Wolbachia strains, the presence of virD4 was examined by Southern hybridization for seven other strains, four from group A and three from group B. The probe used was the virD4 fragment amplified from the lambda clone of wTai by PCR using the primers virD4F (5′-ATCAGAGAAAGACATACGAAAAGCAGG-3′) and virD4R (5′-CAATGGCTTACCCCATCTGGC-3′). Positive signals were detected in all seven strains tested (Fig. 2D).
In this study, it was demonstrated that wTai has the genes for the type IV secretion system and that the vir operon containing these genes is expressed, suggesting that this system is functional in wTai. The vir operon was highly conserved between A and B group Wolbachia. In addition, many Wolbachia strains were shown to share virD4. These observations imply that the type IV secretion system is ubiquitous and may have important roles in Wolbachia. Although the function of this system in Wolbachia is not clear, the type IV secretion systems in other pathogenic bacteria are known to secrete various macromolecules that affect the physiology of the host cells. Examples include the T-DNA complex of A. tumefaciens for inducing tumors in plant cells (5, 25), pertussis toxin of B. pertussis for ADP-ribosylation of the G protein of various cells (11), and CagA of H. pylori for pseudopodium formation to facilitate phagocytosis of epithelial cells (19). It is conceivable that Wolbachia organisms secrete certain molecules that may participate in the expression of CI through the type IV secretion system.
Nucleotide sequence accession numbers.
The sequence of virD4 and its flanking regions in wTai obtained in this study was submitted to GenBank and assigned accession no. AB045234. The sequence of the vir operon of wKueYO obtained in this study was also submitted to GenBank and was assigned accession no. AB045235.
Acknowledgments
We thank Charles Kurland and Siv Andersson for providing the probes of vir genes of Rickettsia prowazekii.
This study was supported in part by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
REFERENCES
- 1.Andersson S G, Zomorodipour A, Andersson J O, Sicheritz-Ponten T, Alsmark U C, Podowski R M, Naslund A K, Eriksson A S, Winkler H H, Kurland C G. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 2.Berger B R, Christie P J. The Agrobacterium tumefaciens virB4 gene product is an essential virulence protein requiring an intact nucleoside triphosphate-binding domain. J Bacteriol. 1993;175:1723–1734. doi: 10.1128/jb.175.6.1723-1734.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bressac C, Rousset F. The reproductive incompatibility system in D. simulans: DAPI-staining analysis of the Wolbachia symbionts in sperm cysts. J Invertebr Pathol. 1992;61:226–230. doi: 10.1006/jipa.1993.1044. [DOI] [PubMed] [Google Scholar]
- 4.Burns D L. Biochemistry of type IV secretion. Curr Opin Microbiol. 1999;2:25–29. doi: 10.1016/s1369-5274(99)80004-6. [DOI] [PubMed] [Google Scholar]
- 5.Christie P J. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covacci A, Falkow S, Berg D E, Rappuoli R. Did the inheritance of a pathogenicity island modify the virulence of Helicobacter pylori? Trends Microbiol. 1997;5:205–208. doi: 10.1016/S0966-842X(97)01035-4. [DOI] [PubMed] [Google Scholar]
- 7.Covacci A, Telford J L, Giudice G D, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 8.Dang T A T, Christie P J. The VirB4 ATPase of Agrobacterium tumefaciens is a cytoplasmic membrane protein exposed at the periplasmic surface. J Bacteriol. 1997;179:453–462. doi: 10.1128/jb.179.2.453-462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das A, Xie Y-H. Construction of transposon Tn3phoA: its application in defining the membrane topology of the Agrobacterium tumefaciens DNA transfer proteins. Mol Microbiol. 1998;27:405–414. doi: 10.1046/j.1365-2958.1998.00688.x. [DOI] [PubMed] [Google Scholar]
- 10.Disque-Kochem C, Dreiseikelmann B. The cytoplasmic DNA-binding protein TraM binds to the inner membrane protein TraD in vitro. J Bacteriol. 1997;179:6133–6137. doi: 10.1128/jb.179.19.6133-6137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamoda S, Masui S, Ishikawa H, Sasaki T. Wolbachia infection and cytoplasmic incompatibility in the cricket, Teleogryllus taiwanemma. J Exp Biol. 2000;203:2503–2509. doi: 10.1242/jeb.203.16.2503. [DOI] [PubMed] [Google Scholar]
- 13.Masui S, Kamoda S, Sasaki T, Ishikawa H. The first detection of the insertion sequence ISW1 in the intracellular reproductive parasite Wolbachia. Plasmid. 1999;42:13–19. doi: 10.1006/plas.1999.1407. [DOI] [PubMed] [Google Scholar]
- 14.Masui S, Sasaki T, Ishikawa H. GroE-Homologous operon of Wolbachia, an intracellular symbiont of arthropods. Zool Sci. 1997;14:701–706. doi: 10.2108/zsj.14.701. [DOI] [PubMed] [Google Scholar]
- 15.O'Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli M L, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 16.O'Neill S L, Hoffmann A A, Werren J H, editors. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford, United Kingdom: Oxford University Press; 1997. [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 18.Sasaki T, Ishikawa H. Wolbachia infections and cytoplasmic incompatibility in the almond moth and the Mediterranean flour moth. Zool Sci. 1999;16:739–744. [Google Scholar]
- 19.Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stouthamer R, Breeuwer J A J, Hurst G D D. Wolbachia pipientis: microbial manipulation of arthropod reproduction. Annu Rev Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. [DOI] [PubMed] [Google Scholar]
- 21.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 22.Weiss A A, Johnson F D, Burns D L. Molecular characterization of an operon required for pertussis toxin secretion. Proc Natl Acad Sci USA. 1993;90:2970–2974. doi: 10.1073/pnas.90.7.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werren J H. Biology of Wolbachia. Annu Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 24.Winans S C, Burns D L, Christie P J. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 1996;4:64–68. doi: 10.1016/0966-842X(96)81513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zambryski P. Basic processes underlying Agrobacterium-mediated DNA transfer to plant cells. Annu Rev Genet. 1988;22:1–30. doi: 10.1146/annurev.ge.22.120188.000245. [DOI] [PubMed] [Google Scholar]