Short abstract
While investigating the mechanisms that could mediate the significant burden of cardiovascular complications observed in persons with chronic kidney disease (CKD) and end stage renal disease (ESRD), we identified a previously unknown protein, which we named renalase (RNLS). Over the past 15 years, our understanding of the biology, physiology, and pathophysiology of RNLS has matured. Here we aim to highlight that RNLS is a bifunctional protein. It metabolizes intracellular nicotinamide adenine dinucleotide (NADH), modulates mitochondrial function, and protects energy metabolism. When secreted outside the cell, independent of its enzymatic properties, it functions as a signaling molecule that mediates resistance to stressful stimuli and promotes cell and organ survival. RNLS has been shown to modulate the severity of acute injury to the pancreas, liver, kidney, and heart. It also protects against the development of chronic injury, and here we highlight the potential use of exogenous RNLS peptide agonists to prevent cisplatin-mediated CKD (CP-CKD).
INTRODUCTION
We discovered the protein we named renalase (RNLS) while testing the hypothesis that the burden of cardiovascular complications and mortality observed in persons with CKD and ESRD was caused by the deficiency of a hitherto unknown protein. We had further hypothesized that this protein was predominantly synthesized by the kidney and secreted in the extracellular compartment, and that the secreted protein interacted with the heart and endothelium to promote cardiovascular health. This hypothesis emerged from discussions I was having with Jianchao Xu, a nephrology fellow, as we were caring for U.S. military veterans suffering from CKD and ESRD at the VA Hospital in West Haven, Connecticut. To test this hypothesis, we utilized newly developed molecular biology tools to clone kidney-expressed genes encoding likely secreted proteins. We also supplemented our cloning data using computer-aided analysis of gene sequences available in the Mammalian Gene Collection Project database (1). We obtained promising preliminary data and were successful in securing initial funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through the R21 grant mechanism. We named the newly discovered protein renalase to reflect our initial understanding that the kidney was its major source, it was reduced in CKD and ESRD, it functioned primarily as a secreted catecholamines-metabolizing enzyme, and its deficiency could lead to cardiovascular complications. The initial description of renalase was published in 2006 (1).
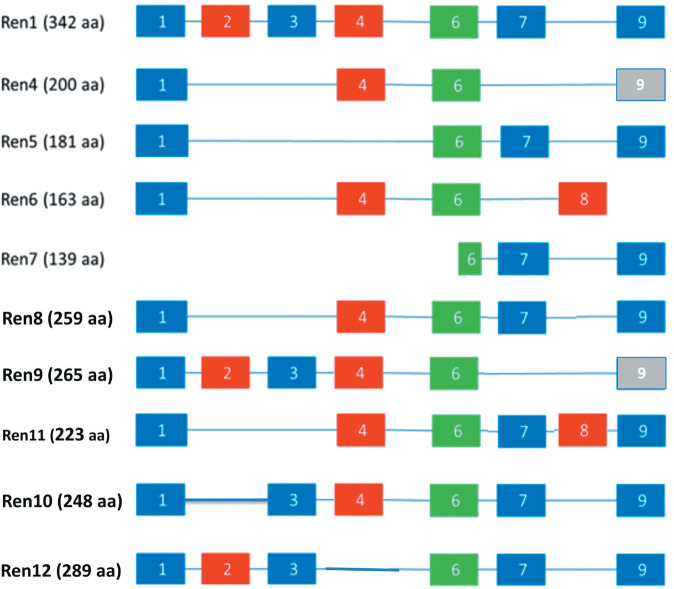
The human RNLS gene (hRNLS) resides on chromosome 10 and contains 300,000 nucleotides and 11 exons. Two major isoforms, RNLS1 and RNLS2, are found in humans. RNLS1 (mRNA: 1477 nucleotides) has 7 exons, 342 amino acids, and a predicted molecular mass of 37.85 kD. RNLS2 (mRNA: 2107 nucleotides) contains 5 exons and 315 amino acids and has a molecular mass of 34.95 kD (14). Additional splice variants have also been identified in humans (Figure 1) (2).
Fig. 1.
Renalase isoforms expressed in human cells. Sequences amplified by PCR from human cancer cell lines. The exonic composition of Ren4–12 is compared to that of Ren1. The part of exon 6 that contains the agonist peptide RP-220 is conserved among all isoforms.
Fig. 2.
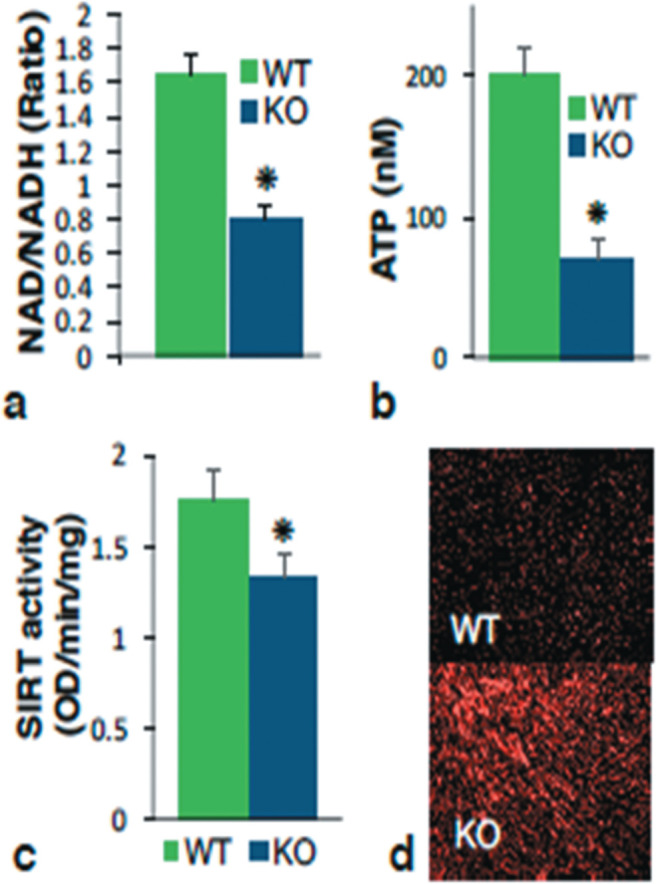
Metabolic changes in RNLS KO hearts. (a) NAD/NADH ratio; (b) ATP content (nM normalized to weight); (c) universal sirtuin activity in nuclear extracts (per mg tissue); (d) dihydroethidium staining (marker of superoxide) of myocardium six hours after induction of pressure overload with transverse aortic banding.
RNLS is an ancient protein. Bacterial proteins with up to 20% amino acid identity with human RNLS have been identified. Examination of cyanobacteria-originated metazoan/fungi proteins suggests that RNLS originated from nuclear-localized plastid-like DNA (nupDNA) fragments from cyanobacteria (3). The horizontal gene transfer event of RNLS is estimated to have taken place around 700 million years ago (18, 19). While RNLS would have diverged from its original form, the conservation of the RNLS sequence domains is suggested by the significant similarity between plant gene products and mammalian RNLS (13). While the function(s) of RNLS has likely evolved and changed over time, its existence dating so far back in the tree of life suggests that it possesses cardinal function(s).
The three-dimensional structure of RNLS1 has been solved using X-ray crystallography. The data confirm that RNLS is a flavin adenine dinucleotide (FAD) containing flavoprotein with a binding site for NADH (24,25). The association of FAD with RNLS is non-covalent, and unexpectedly, analysis of RNLS’s crystal structure revealed no disulfide bonds, although the protein contains 12 cysteine residues (15).
Intracellular renalase functions as a NADH oxidase. Our laboratory provided the initial evidence that recombinant RNLS oxidizes NADH (4, 5). Subsequent studies by others showed that this oxidation takes place at the 2- or 6-position of the nicotinamide ring, rather than the metabolically active 4-position (6–8). RNLS and pyridoxine 5 phosphate oxidase can convert metabolically inert 2 or 6 NADH to active 4-NAD+ and are key components of the repair pathway for intracellular NADH that prevents the buildup of unusable and/or inhibitory substrates (9). NAD metabolism is tightly linked to ATP production. In collaboration with Frank Giordano, we have observed decreased NAD/NADH ratios and ATP content, lower sirtuin activation, and increased ROS levels in RNLS KO hearts (Figure 2). These observations underscore the significant contribution of the enzymatic properties of RNLS to cellular energy metabolism and homeostasis.
Extracellular RNLS signals through the calcium ATPase PMCA4b to activate pathways that promote cell survival. We have identified the region of RNLS (aa 220 to 239 of human RNLS referred to as RP-220) that fully replicates the signaling and cell protective properties of RNLS. Although devoid of enzymatic activity, RP-220, like RNLS, signals through ERK, AKT and STAT3 and protects against acute organ injury (kidney, pancreas, liver, heart) (50,51). RP-220 was used as RP220’s amino acid sequence is highly conserved throughout evolution. The observation that monoclonal antibodies raised against the RP-220 peptide are potent inhibitors of cancer growth underscores its functional importance (10–12).
A key goal is to prevent the development of CKD, which is often progressive and significantly increases risk for heart disease, stroke, and death (13). The available data suggest that repeated episodes of acute kidney injury (AKI) can lead to the development of CKD, and a failure to repair, not fibrosis, underlies the transition from AKI to CKD. RNLS mitigates oxidative stress, minimizes injury in in vivo models of myocardial infarction (14), and prevents acute kidney injury (15, 16). Using a sensitive and specific ELISA assay for human RNLS, we showed that a decrease in RNLS level is associated with lower GFR and higher creatinine (17). Therefore, we asked if the administration of exogenous RNLS agonist peptides could prevent the development of CP-CKD.
MATERIALS AND METHODS
The materials and methods used to obtain the results summarized below are described in detail in a recent publication (18).
RESULTS
We previously showed that exogenous RNLS and RNLS peptide agonist administration protect against AKI (16). To test the hypothesis that renalase can prevent the progression of chronic kidney disease, we asked if the first dose of cisplatin (CP) in humans was associated with sustained subclinical kidney injury and changes in plasma RNLS. We measured pre and post (0, 1, 2, and 14 days) plasma samples from 11 patients who were receiving their first dose of CP for cancer treatment and found that KIM1 remained elevated at 2 weeks in all subjects, and this was associated with a fall plasma RNLS in most patients (18). These data provide evidence of subclinical injury (elevated KIM1 and decreased plasma RNLS, and no change in plasma creatinine) in human subjects following the first cisplatin dose.
We then used a mouse model of CP-CKD that we had developed (19) to investigate and establish the therapeutic efficacy of RP81-MNP, a kidney-targeted RNLS peptide agonist related to, but more potent than, RP-220. We utilized meso-particles for kidney-specific delivery (20) of RP81 in vivo because cisplatin preferentially collects in the proximal tubules. We observed a stepwise and sustained decrease in kidney RNLS protein and gene expression in CP-CKD (18). We found that RP81-MNP provided excellent protection in a severe model of CP-CKD. To explore the mechanisms underlying the protective action of RP81-MNP in CP-CKD, we carried out scRNA-seq analysis to determine the effect of RP81-MNP on CP-mediated transcriptional changes in the kidney. We found that RP81-MNP prevented cell death, reduced the inflammatory response, and preserved renal proximal tubular (PT) function (18).
RP81-MNP induced the expression of genes involved in ATP production: Cytb, Nd1, Nd3, Nd4L, and Nd5. Cytb is a ubiquinol-cytochrome c reductase component of respiratory chain complex III (21). Nd1, Nd3, Nd4L, and Nd5 encode subunits of NADH-ubiquinone oxidoreductase complex I, a key source of reactive oxygen species (ROS) (22). These changes suggest that RP81 increases the state of reduction of complex I.
RP81 delivery to the PT, the principal site of CP-induced damage, was the most prominent site of protection. Encapsulated RP81 peptide reduced PT damage, reduced oxygen stress, diminished mitochondrial damage, reduced inflammation, and restored expression of PT transport genes, all of which play a prominent role in cisplatin-induced AKI and CKD.
DISCUSSION
RNLS is an ancient protein that has existed for more than 3 billion years. About a billion years ago, transfer from cyanobacteria to metazoan and fungi took place. Fifteen years ago, while investigating the connection between kidney and heart disease, we identified a human protein of interest (we named renalase) of unknown function that had remarkable sequence and domain similarities with cyanobacterial proteins also of unknown function. We now believe that RNLS is a bifunctional protein with broad and fundamental activities that helps maintain cell and tissue homeostasis, prevent cellular injury, and facilitate cellular repair. Furthermore, we provide evidence that RNLS peptide agonists can be targeted to specific organs, display potent protective properties, and can be effective therapeutic agents to prevent the development of chronic kidney disease.
ACKNOWLEDGMENTS AND FINANCIAL SUPPORT
This work was supported in part by VA Connecticut (G. V. Desir), National Institute of Health grants RC1DK086465, RC1DK086402, and R01DK081037 (G. V. Desir).
I wish to acknowledge all who have collaborated with me over the past 15 years and helped uncover the many facets of renalase biology. Very special thanks to Robert Safirstein, Fred Gorelick, Frank Giordano, and Xiaojia Guo who have contributed the most to the work described here.
DISCUSSION
Zeidel, Boston: Gary, terrific talk—I know that you know Samir Parikh’s work. There’s a lot of mitochondrial damage that occurs in proximal tubule cells when they are injured acutely. Is this a mitochondrial protectant, and, if so, does it have something to do with the NAD+/NADH ratios that you showed at the beginning? Or are there other mechanisms involved?
Desir, New Haven: Yes, the single cell data actually showed that the peptide down regulates inflammatory genes and up regulates mitochondrial genes. Of the 13 genes exposed in the mitochondria, seven of them are off regulated including complex one, five, and three. Although the peptide itself has no enzymatic activity, we think because of the STAT3 positive feedback loop, the peptide probably increases intracellular renalase that then regulates mitochondria gene expression. In addition to protecting the energy metabolism of cells, it also activates survival pathways such as STAT3, MAPK, and AKT so I think it is two things.
Militoris, Indianapolis: Very nice presentation. I have two questions for you. Where is it produced in the kidney? I’m surprised you had to go to nanoparticles because you’re dealing with a small peptide that is rapidly filtered and going to be taken up by the proximal tubule anyway—did you try it without nanoparticles?
Desir, New Haven: It’s excreted fairly quickly, and it doesn’t get taken up that quickly by the proximal tubule. It’s made primarily in the proximal tubule and also in the distal tubule, but the majority of it comes from the proximal tubule.
Boyce, Boston: Great talk. I’m curious about how kidney-restricted the actions of renalase are. Are there receptors in other organs? If so, what do they do?
Desir, New Haven: The main receptor PMCA4b is expressed in many organs. We have examined models of acute pancreatitis and acute myocardial infarction, and it’s also protective. So it acts on many different organs. The kidney was the first one we identified.
Rubin, Chapel Hill: I was interested that the knockout had low phosphate, I wonder if you measured FGF23 and what the effects are on bone. What does the bone look like in that knockout animal?
Desir, New Haven: I’ve always wondered about that. What we do know is that the knockout has increased catecholamine production and excretion. We believe the increase in phosphate excretion can be explained by the observed increase in urinary dopamine.
Rubin, Chapel Hill: But it’s always FGF23.
Desir, New Haven: Right (laughter).
Tweardy, Houston: We really enjoyed your talk. It is not the phosphorylated form of STAT3 that actually gets into the mitochondria and has potential to benefit. Were you able to interrogate the serine 727 phosphorylated STAT3 or just total STAT3 levels in your analysis of STAT3’s role?
Desir, New Haven: The renalase gene has as a STAT3 binding site. When you add the peptide to the outside of the cell, it stimulates STAT3 production, which then binds to the renalase gene and increases intercellular production of renalase. That is possibly how extracellular renalase can modify mitochondrial bioenergetics.
Tweardy, Houston: So you were really talking about phosphorylated STAT3 regulating the target—in this case the renalase gene?
Desir, New Haven: Right, exactly—that’s how extracellular renalase can regulate intracellular expression.
Tweardy, Houston: Thanks.
Footnotes
Correspondence and reprint requests: Gary Desir, MD, Section of Nephrology, Department of Internal Medicine, Yale School of Medicine, P.O. Box 208029, New Haven, CT 06520-8029; Tel: 203-785-4119; E-mail: gary.desir@yale.edu.
Potential Conflicts of Interest: Dr. G Desir is a named inventor on several issued patents related to the discovery and therapeutic use of renalase. Renalase is licensed to Bessor Pharma, and Dr. G Desir holds an equity position in Bessor and its subsidiary Personal Therapeutics.
REFERENCES
- 1.Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, Wu Y, Peixoto A, Crowley S, Desir GV. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. 2005;115((5)):1275–80. doi: 10.1172/JCI24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennebry SC, Eikelis N, Socratous F, Desir G, Lambert G, Schlaich M. Renalase, a novel soluble FAD-dependent protein, is synthesized in the brain and peripheral nerves. Mol Psychiatry. 2010;15((3)):234–6. doi: 10.1038/mp.2009.74. [DOI] [PubMed] [Google Scholar]
- 3.Yuan S, Guo JH, Du JB, Lin HH. Phylogenetic analyses of plastid-originated proteins imply universal endosymbiosis in ancestors of animals and fungi. Z Naturforsch C J Biosci. 2008;63((11-12)):903–8. doi: 10.1515/znc-2008-11-1221. [DOI] [PubMed] [Google Scholar]
- 4.Desir GV, Tang L, Wang P, Li G, Sampaio Maia B, Quelhas-Santos J, Pestana M, Velazquez H. Renalase lowers ambulatory blood pressure by metabolizing circulating catecholamines. J Am Heart Assoc. 2012;1:e002634. doi: 10.1161/JAHA.112.002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farzaneh-Far R, Desir GV, Na B, Schiller NB, Whooley MA. A functional polymorphism in renalase (Glu37Asp) is associated with cardiac hypertrophy, dysfunction, and ischemia: data from the heart and soul study. PLoS One. 2010;5((10)):e13496. doi: 10.1371/journal.pone.0013496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran GR, Hoag MR. The enzyme: Renalase. Archives of Biochem and Biophys. 2017;63:66–76. doi: 10.1016/j.abb.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Hoag MR, Roman J, Beaupre BA, Silvaggi NR, Moran GR. Bacterial renalase: structure and kinetics of an enzyme with 2- and 6-dihydro-β-NAD(P) oxidase activity from pseudomonas phaseolicola. Biochem. 2015;54((24)):3791–802. doi: 10.1021/acs.biochem.5b00451. [DOI] [PubMed] [Google Scholar]
- 8.Beaupre BA, Hoag MR, Roman J, Forsterling FH, Moran GR. Metabolic function for human renalase: oxidation of isomeric forms of beta-NAD(P)H that are inhibitory to primary metabolism. Biochem. 2015;54((3)):795–806. doi: 10.1021/bi5013436. [DOI] [PubMed] [Google Scholar]
- 9.Marbaix AY, Chehade G, Noel G, Morsomme P, Vertommen D, Bommer GT, Van Schaftingen E. Pyridoxamine-phosphate oxidases and pyridoxamine-phosphate oxidase-related proteins catalyze the oxidation of 6-NAD(P)H to NAD(P) Biochem J. 2019;476((20)):3033–52. doi: 10.1042/BCJ20190602. [DOI] [PubMed] [Google Scholar]
- 10.Guo X, Jessel S, Qu R, Kluger Y, Chen T-M, Hollander L, Safirstein R, Nelson B, Cha C, Bosenberg M, et al. Inhibition of renalase drives tumour rejection by promoting T cell activation. European J of Cancer. 2022;165:81–96. doi: 10.1016/j.ejca.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollander L, Guo X, Velazquez H, Chang J, Safirstein R, Kluger H, Cha C, Desir GV. Renalase expression by melanoma and tumor-associated macrophages promotes tumor growth through a STAT3-mediated mechanism. Cancer Research. 2016;76((13)):3884–94. doi: 10.1158/0008-5472.CAN-15-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X, Hollander L, MacPherson D, Wang L, Velazquez H, Chang J, Safirstein R, Cha C, Gorelick F, Desir GV. Inhibition of renalase expression and signaling has antitumor activity in pancreatic cancer. Scientific Reports. 2016;6:22996. doi: 10.1038/srep22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atlanta GA. U.S Department of Health and Human Services, Centers for Disease Control and Prevention. 2021.
- 14.Wu Y, Xu J, Velazquez H, Wang P, Li G, Liu D, Sampaio-Maia B, Quelhas-Santos J, Russell K, Russell R, et al. Renalase deficiency aggravates ischemic myocardial damage. Kidney Int. 2011;79((8)):853–60. doi: 10.1038/ki.2010.488. [DOI] [PubMed] [Google Scholar]
- 15.Lee HT, Kim JY, Kim M, Wang P, Tang L, Baroni S, D’Agati VD, Desir GV. Renalase protects against ischemic AKI. J Am Soc Nephrol. 2013;24((3)):445–55. doi: 10.1681/ASN.2012090943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Velazquez H, Moeckel G, Chang J, Ham A, Lee HT, Safirstein R, Desir GV. Renalase prevents AKI independent of amine oxidase activity. J Am Soc Nephrol. 2014;25((6)):1226–35. doi: 10.1681/ASN.2013060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang J, Guo X, Rao V, Gromisch ES, Chung S, Kluger HM, Cha C, Gorelick F, Testani J, Safirstein R, et al. Identification of two forms of human plasma renalase, and their association with all-cause mortality. Kidney Int Reports. 2020;5((3)):362–8. doi: 10.1016/j.ekir.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X, Xu L, Velazquez H, Chen T-M, Williams RM, Heller DA, Burtness B, Safirstein R, Desir GV. Kidney-targeted renalase agonist prevents cisplatin-induced chronic kidney disease by inhibiting regulated necrosis and inflammation. J Am Soc Nephrol. 2022;33((2)):342–56. doi: 10.1681/ASN.2021040439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, Avanzi A, Barilla R, Daley D, Greco SH, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and mincle-induced immune suppression. Nature. 2016;532((7598)):245–9. doi: 10.1038/nature17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han SJ, Williams RM, D’Agati V, Jaimes EA, Heller DA, Lee HT. Selective nanoparticle-mediated targeting of renal tubular Toll-like receptor 9 attenuates ischemic acute kidney injury. Kidney Int. 2020;98((1)):76–87. doi: 10.1016/j.kint.2020.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blakely EL, Mitchell AL, Fisher N, Meunier B, Nijtmans LG, Schaefer AM, Jackson MJ, Turnbull DM, Taylor RW. A mitochondrial cytochrome b mutation causing severe respiratory chain enzyme deficiency in humans and yeast. FEBS J. 2005;272((14)):3583–92. doi: 10.1111/j.1742-4658.2005.04779.x. [DOI] [PubMed] [Google Scholar]
- 22.Larosa V, Remacle C. Insights into the respiratory chain and oxidative stress. Biosci Rep. 2018;38((5)) doi: 10.1042/BSR20171492. [DOI] [PMC free article] [PubMed] [Google Scholar]