Abstract
The challenge of pregnancy can be significant to the point of being life-threatening in a woman with a bleeding disorder. Additionally there can be a risk to the fetus and the neonate. A hemostatic defect can affect the course of the pregnancy, but the impact is most feared around delivery in the immediate and the extended post partum period, requiring rapid identification and prompt referral to a hematologist for assistance in management. Identifying the type of congenital bleeding disorder and knowing its inheritance pattern is crucial during counseling prior to conception and in preparation for delivery. A comprehensive approach by a specialized and experienced team in a tertiary care center with access to adequate laboratory monitoring and therapies can facilitate the process. The multidisciplinary team should include a hematologist, an obstetrician, a pediatric hematologist, an anesthesiologist, and in select cases a clinical geneticist and a maternal fetal medicine specialist. In this review article, we will detail the diagnostic path and management of pregnancy and delivery in women with some inherited bleeding disorders, in particular those affected by hemophilia A (HA), hemophilia B (HB), and von Willebrand disease (VWD).
Keywords: pregnancy, hemophilia A and B carriers, von Willebrand disease, delivery
Introduction
Inherited bleeding disorders represent a risk of bleeding during pregnancy and delivery for the carrying mother and the newborn that might inherit the disorder. Knowing the inheritance pattern of the disease is essential for prenatal counseling and development of an adequate management plan.
Hemophilia is an X linked disorder, where women are usually carriers of the mutation but can have reduced factor levels <40% in approximately 30% of patients.1 The male newborn receiving the affected X-chromosome, and therefore having hemophilia, is of specific concern during and after delivery. VWD, the most common inherited bleeding disorder, is an autosomal disorder with a heterogeneous presentation, various clinical subtypes, and different inheritance patterns.
Pregnancy and Physiologic Changes in Hemostasis
In healthy women, various changes affect the hemostatic system, starting with the beginning of pregnancy and becoming more pronounced around delivery. There is a gradual increase in some coagulation factors, especially factor VIII (FVIII) and von Willebrand factor (VWF) levels with a peak shortly before delivery, followed by a post partum decrease that might take 3 days to 3 weeks to achieve baseline levels.2–4 In addition, there is a decrease in anticoagulant proteins and fibrinolysis during pregnancy, followed by a gradual recovery in the post partum period. The resulting net shift toward a procoagulant state represents a physiologic preparation for blood loss during and after delivery. This naturally occurring protection might unfortunately not last long enough to prevent post partum hemorrhage (PPH) in pregnant carriers of hemophilia or women with VWD, and therefore should be taken into consideration in the management plan. Parallel to that, a good understanding of the normal level of different procoagulant and anticoagulant factors in newborns is also important in developing these delivery and post-partum care plans.
Pregnancy and Hemophilia
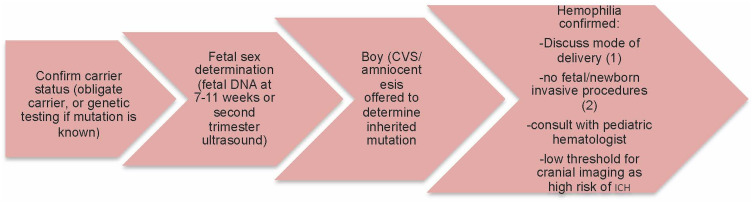
Some procoagulant factors (esp FVIII) increase physiologically at variable degrees throughout pregnancy. This increase, however, is inconsistent among all women with the same disorder and might not be enough to prevent bleeding complications. Therefore, an early approach handled by a multidisciplinary team at a tertiary care center, preferably affiliated with a hemophilia treatment center (HTC), is essential to prevent undesirable complications (Figure 1).
Figure 1.
Diagnostic approach from conception to delivery. Chorionic villus sampling (CVS), intracranial hemorrhage (ICH), (1) unassisted vaginal, no prolonged labor or C-section, (2) invasive procedures include fetal scalp sampling, scalp electrodes, intramuscular injection.
Ideally, the woman comes from a family that is well known to the HTC, where her father, brother, or other members are being taken care of. Her genetic mutation is well known and she has been counseled prior to conception about genetic transmission and the different methods to determine the sex of the fetus and whether he is affected with hemophilia. These women are usually referred early on to the care of hemostasis experts and initial communication with their care team of obstetrician, anesthesiologist and pediatrician is started.
However, approximately a third of newborns with hemophilia are de novo cases.5,6 A new occurring mutation in the fetus is completely unpredictable and increases the risk of bleeding in the newborn only. Additionally, one may identify a newly-occurring mutation in the mother through a thorough hemostatic investigation, and a questionnaire of her bleeding history can help with accurate diagnosis of her carrier status and, therefore, development of an appropriate delivery plan.
As stated previously, genetic counseling and determination of the gender of the fetus is important in developing the perinatal care plan. A male fetus carries a 50% risk of having the disease, while the female fetus has a 50% chance of being a carrier with only mildly decreased factor levels and, therefore, a very low risk of neonatal bleeding.7 Mothers of male fetuses should be offered chorionic villus sampling (CVS) or amniocentesis to analyze the fetal DNA, especially if the causative mutation is well known. These procedures carry a very low risk of miscarriage of <0.5%, with CVS allowing earlier diagnosis at 10–13 weeks.8
Factor levels should be checked as soon as possible (if no prior to pregnancy baseline levels are known) and are then followed during pregnancy with at least another level between 28–34 weeks.9 This will help in determining the correction dose if needed prior to delivery and the post partum treatment. FVIII levels increase two-fold to three-fold, while FIX levels only slightly increase during pregnancy.10,11 Desmopressin (DDAVP), which increases levels of circulating vWF by releasing it from endogenous stores in endothelial cell, or factor replacement therapy at the time of active labor should be offered to patients with levels less than 50% (0.50 IU/mL) by week 34 of gestation, with a targeted peak at delivery around 100–150%. Desmopressin use during pregnancy is safe, especially during the first and second trimester, and can be recommended prior to any invasive procedure. However, at active labor, theoretically, if DDAVP is given before clamping the umbilical cord, there could be a risk of neonatal hyponatremia.12 However, a systematic review of 30 studies of the use of DDAVP for treatment and prophylaxis of bleeding disorders in pregnancy demonstrated efficacy and safety.13 On the other hand, there is the practical concern of maternal hyponatremia that has dissuaded some practitioners from using DDAVP peri-partum due to aggressive fluid resuscitation. Nonetheless, in select cases, DDAVP is an option, but usually factor replacement is the first choice in increasing the FVIII level peri-partum if it is <50% at the time of delivery. However, it should be kept in mind that the far majority of carriers though will have a FVIII level >50% at the time of delivery.
Regarding the factor cut-off level at time of delivery warranting intervention, historically guidelines have considered a factor level ≥50% sufficient for delivery and neuraxial anesthesia unless otherwise contraindicated.7,14–16 The Dutch guidelines increased their cut-off value to 80% and targeted level at delivery to 150% to reduce the risk of post-partum hemorrhage (PPH).17–19
In those carriers with FVIII level ≤50% in the third trimester, FVIII replacement therapy should be continued in the post partum period to target trough levels of 50% for 3–5 days if vaginal delivery and 5–7 days if C-section. In addition, the use of tranexamic post-partum can be considered in carriers with a past history of post-partum hemorrhage (PPH) or baseline FVIII level <50%.
In addition to bleeding during delivery, the most feared complication is intracranial hemorrhage (ICH), that is clearly higher in newborns with hemophilia when compared to newborns with no hemophilia. In a recent study, 2.4% were reported after planned vaginal delivery (including instrumental deliveries) and 1.7% after cesarean section (planned or urgent).20 Some guidelines favor cesarean over vaginal delivery, yet the best mode of delivery is still controversial. In fact, the risk of ICH is still present with C-section, adding to that the increased risk of maternal bleeding, hospitalization days, and the need for prolonged post partum treatment. Therefore, both delivery options should be considered and discussed with the obstetrician. The best mode should be adopted on a case-by-case basis with a focus on avoiding use of instruments and prolonged labor.
Pregnancy and VWD
The management of women with VWD during pregnancy is similar in some aspects and very different in other aspects from that of women carriers of hemophilia. While VWF levels increase during pregnancy, the antenatal changes vary according to the type of VWD. Indeed, VWD is a more heterogeneous disease, with a wide spectrum of clinical presentations dictating different management strategies.11
With its autosomal inheritance, mainly dominant, the management does not vary much with the determination of the fetal gender. In general there is a 50% risk that the fetus inherits the mutation and, therefore, the disease. The genetic counseling, however, becomes relevant in families with other children with type 3 VWD, the most severe form with autosomal recessive inheritance in general.
Women with type 1 disease note an improvement in their bleeding profile as they progress with pregnancy, parallel to the increase in their factor level.
Those with type 2 disease experience an increase in their defective endogenous VWF. This translates in an increase in VW Ag and FVIII levels but not in the VW activity. Levels of VWF and FVIII remain low throughout pregnancy in women with type 3 VWD, sometimes requiring factor replacement therapy to be started antenatally. In all types of VWD, due to the unpredictable increase in factor levels, frequent monitoring, with at least one level obtained between 28–34 weeks of gestation, helps in determining the treatment delivery plan.
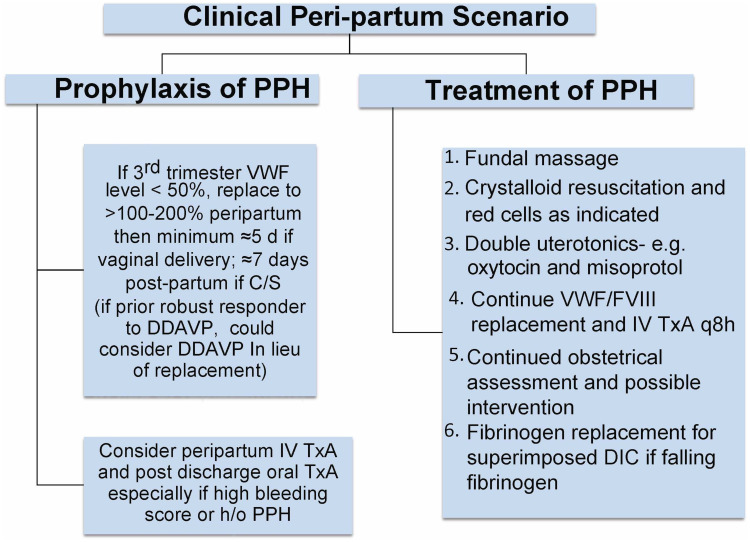
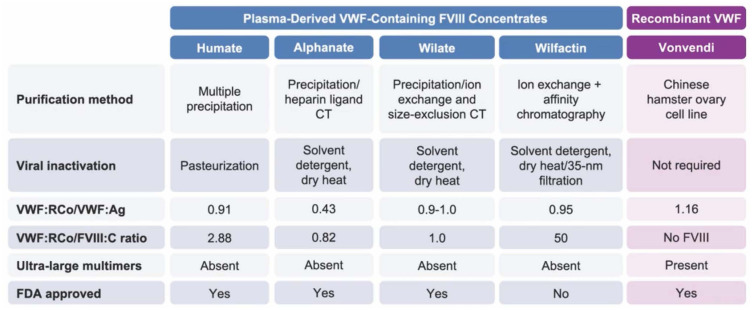
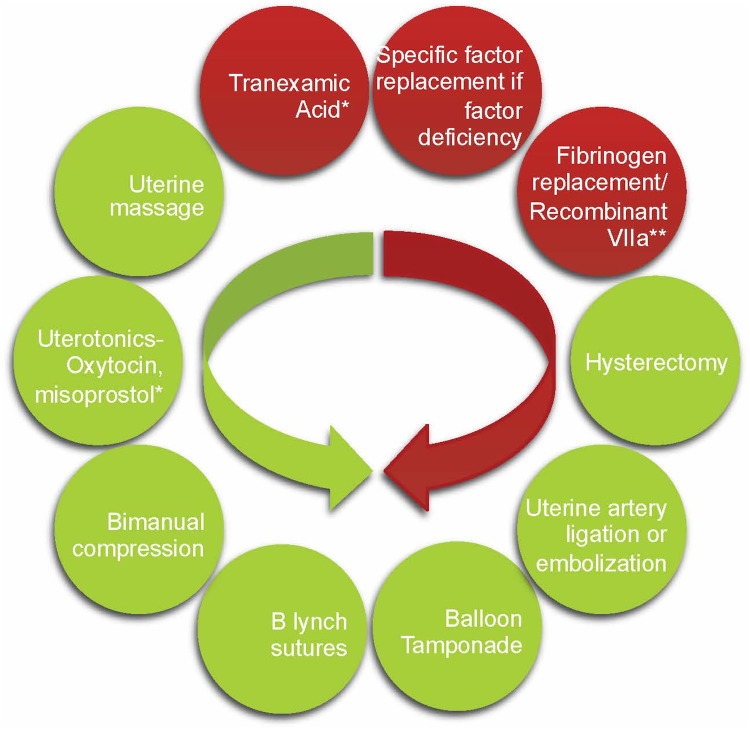
While von Willebrand factor (VWF) and factor VIII (FVIII) levels increase in a healthy pregnancy by 200–250% there is a much less pronounced increase in pregnant women with VWD.21 This results in a 5–40% prevalence of PPH compared to the 2–10% prevalence in the general population.22 In light of this relatively poor physiological response and several studies showing increased rate of PPH if the target VWF/FVIII factor level is at a minimum of 50% and not higher, factor replacement therapy should be given to those with levels <50% and targeting levels of 100–150% at delivery and troughs of 50% for 7–10 days after delivery.4,23 Figure 2 depicts an algorithmic approach to peri-partum management of VWD, while Figure 3 depicts the various products for VWF replacement peri-partum.24 The largest experience has been with the VWF containing plasma derived FVIII concentrate, Humate P, while the recombinant VWF product Vonvendi may theoretically be more effective with added 3–6 hours to the half-life, requiring less doses and theoretically less risk of post-partum thrombosis as it does not contain FVIII.25 Ongoing studies are comparing a conservative management with a more aggressive one aiming to higher target levels and taking into consideration the expansion in blood volume that occurs during pregnancy.26 Figure 4 depicts management when PPH continues, despite adequate factor replacement usually when there is super-imposed uterine atony or placental abnormalities or uterine, cervical, or vaginal trauma.
Figure 2.
Peri-partum management of VWD.
Abbreviations: PPH, Post Partum Hemorrhage; C/S, C Section; DDAVP, Desmopressin; IV TxA, Intravenous Tranexamic Acid; VWF, von Willebrand Factor; FVIII, Factor VIII; DIC, Disseminated Intravascular Coagulopathy.
Figure 3.
Products for VWF replacement peri-partum.24
Figure 4.
Interventions in post partum hemorrhage. Direction of arrow depicts escalation of therapy if worsening PPH with obstetrical interventions in green and hemostatic interventions in red (Fibrinogen replacement in case of low fibrinogen levels and DIC, recombinant VIIa used as last resort to control hemostasis). *Denotes consideration in prevention of PPH if underlying bleeding disorder and/or placental previa, twin gestation, or antepartum hemorrhage. **Fibrinogen to be replaced if low, recombinant VIIa to be considered if bleeding is still not controlled before moving to hysterectomy.
Contrary to hemophilia A carriers where the risk of PPH is not as high as the majority have adequate peri-partum normalization of their factor levels, antifibrinolytics have a role in prophylactic therapy along the lines of the 2021 American Society of Hematology/International Society of Haemostasis and Thrombosis/National Hemophilia Foundation/World Federation of Haemophilia guidelines, wherein the use of post-partum tranexamic acid is a conditional recommendation.16 Dosing is typically 1,000–1,300 mg 3-times per day for 10 to 14 days or longer if blood loss remains heavy. It is also safe if breastfeeding. Presently, there is still a wide treatment variation in the use of tranexamic acid post-partum in VWD patients.27 Table 1 reviews the various guidelines for post-partum tranexamic acid use.
Table 1.
Guidelines on Post-Partum Tranexamic Acid for PPH Prevention in VWD
| 2017 Royal College of Obstetrics and Gynecology | 2018 Canadian Hemophilia Society (Reaffirmed from 2005) | 2021 ASH/ISTH/NHF/WFH Guidelines | |
|---|---|---|---|
| Prophylactic use of Tranexamic acid? |
Yes, qualified Women with VWD should be considered for tranexamic acid for the post partum period. A standard dose is 1 g three to four times a day for 7–14 days. In some cases prolonged use for 2–3 weeks or more may be necessary. |
No Should late postpartum hemorrhage occur, tranexamic acid and oral contraceptives are first-line therapy for its management. The risk of thrombosis might be a concern if antifibrinolytics agents are used post partum, but the risk is probably reasonable in women without other risk factors. |
Yes The guideline panel suggests the use of tranexamic acid over not using it in women with type 1 VWD or low VWF levels (and this may also apply to types 2 and 3 VWD during the post partum period) (conditional recommendation based on low certainty in the evidence of effects) |
Abbreviations: PPH, post partum hemorrhage; VWD, von Willebrand disease; ASH, American Society of Hematology; ISTH, International Society of Thrombosis and Hemostasis; NHF, National Hemophilia Foundation; WFH, World Federation of Hemophilia; VWF, Von Willebrand Factor.
Concerns about the use of desmopressin during pregnancy apply in the same way to women with VWD (please refer to the hemophilia carrier section). Its use should only be reserved for women in whom a positive challenge test was already done prior to pregnancy with a low perceived peri-partum risk of maternal hyponatremia in consultation with the obstetrician.
The debate on mode of delivery and safety of neuraxial anesthesia is similar to that with hemophilia carriers with minimal differences. Of note, the 2018 UK guidelines advise “that neuraxial anesthesia be avoided unless VWF activity is more than 50% and the haemostatic defect has been corrected; this may be difficult to achieve in type 2, and central neuraxial anesthesia should not be given in cases of type 3”.14,28 The 2021 ASH/ISTH/NHF/WFH guidelines state
In women with VWD for whom neuraxial anesthesia during labor is deemed suitable, the panel suggests targeting a VWF activity level of 0.50 to 1.50 IU/mL over targeting an activity level of >150 IU/mL to allow neuraxial anesthesia16,29
While the focus here is mainly on preventing PPH and bleeding during delivery, bleeding complications in the newborn are raised in particular with some type 2 and type 3 patients, as newborns of women with type 1 disease, even if affected, will have VWF levels only mildly decreased. This results from the physiologic increase in VWF levels that is seen in healthy newborns.30
In these women in particular, close monitoring of factor levels during pregnancy and after delivery, especially if receiving factor replacement therapy, is the mainstay of their management to prevent bleeding as well as thrombosis.31 Indeed, FVIII levels might be very high in this patient population, increasing their risk of thrombosis in the post partum period at the least in-hospital non-chemical thromboprophylaxis measures. Systemic thromboprophylaxis with low molecular weight heparin is rarely indicated except when post-partum factor replacement leads to excessively high levels >250%.
Patients with type 2B VWD, where there is a gain of function mutation in the A1 domain leading to an augmented affinity of VWF to platelets with subsequent increased clearance, require specific attention to their platelet levels too, with a need for transfusion if numbers are <50×109/L.
Conclusion
An accurate diagnosis and management of bleeding disorders in pregnancy must take into consideration the risks to the mother and the fetus. With individualized proper planning and care by a team of experienced specialists in a tertiary care center, these women can, in their majority, carry a pregnancy to term and deliver healthy newborns. We have not addressed here other bleeding disorders that usually present with their own challenges during pregnancy and post partum period, but benefit from an early identification and require the same team approach.
Disclosure
Maissaa Janbain received honoraria from Takeda, CSL Behring, Octapharma, Sanofi, Sobi, Bayer, Genetech, and Biomarin is on speaker bureau of Biomarin, research support from Genentech. Peter Kouides serves as DMSB member for FIX gene therapy/Uniqure, and Tremeau Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
- 1.Plug I, Mauser-Bunschoten EP, Brocker-Vriends AHJT, et al. Bleeding in carriers of hemophilia. Blood. 2006;108(1):52–56. doi: 10.1182/blood-2005-09-3879 [DOI] [PubMed] [Google Scholar]
- 2.Nowak-Göttl U, Limperger V, Kenet G, et al. Developmental hemostasis: a lifespan from neonates and pregnancy to the young and elderly adult in a European white population. Blood Cells Mol Dis. 2017;67:2–13. doi: 10.1016/j.bcmd.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 3.Huq FY, Kulkarni A, Agbim EC, et al. Changes in the levels of factor VIII and von Willebrand factor in the puerperium. Haemophilia. 2012;18(2):241–245. doi: 10.1111/j.1365-2516.2011.02625.x [DOI] [PubMed] [Google Scholar]
- 4.James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21(1):81–87. doi: 10.1111/hae.12568 [DOI] [PubMed] [Google Scholar]
- 5.Chalmers EA. Haemophilia and the newborn. Blood Rev. 2004;18(2):85–92. doi: 10.1016/S0268-960X(03)00062-6 [DOI] [PubMed] [Google Scholar]
- 6.Conway JH, Hilgartner MW. Initial presentations of pediatric hemophiliacs. Arch Pediatr Adolesc Med. 1994;148(6):589–594. doi: 10.1001/archpedi.1994.02170060043007 [DOI] [PubMed] [Google Scholar]
- 7.Dunkley S, Curtin JA, Marren AJ, et al. Updated Australian consensus statement on management of inherited bleeding disorders in pregnancy. Med J Aust. 2019;210(7):326–332. doi: 10.5694/mja2.50123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salomon LJ, Sotiriadis A, Wulff CB, et al. Risk of miscarriage following amniocentesis or chorionic villus sampling: systematic review of literature and updated meta-analysis. Ultrasound Obstet Gynecol. 2019;54(4):442–451. doi: 10.1002/uog.20353 [DOI] [PubMed] [Google Scholar]
- 9.Sood SL, James AH, Ragni MV, et al. A prospective study of von Willebrand factor levels and bleeding in pregnant women with type 1 von Willebrand disease. Haemophilia. 2016;22(6):e562–e564. doi: 10.1111/hae.13086 [DOI] [PubMed] [Google Scholar]
- 10.Szecsi PB, Jørgensen M, Klajnbard A, et al. Haemostatic reference intervals in pregnancy. Thromb Haemost. 2010;103(4):718–727. doi: 10.1160/TH09-10-0704 [DOI] [PubMed] [Google Scholar]
- 11.Huq FY, Kadir RA. Management of pregnancy, labour and delivery in women with inherited bleeding disorders. Haemophilia. 2011;17 Suppl 1:20–30. doi: 10.1111/j.1365-2516.2011.02561.x [DOI] [PubMed] [Google Scholar]
- 12.Svensson PJ, Bergqvist PBF, Juul KV, et al. Desmopressin in treatment of haematological disorders and in prevention of surgical bleeding. Blood Rev. 2014;28(3):95–102. doi: 10.1016/j.blre.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 13.Trigg DE, Stergiotou I, Peitsidis P, et al. A systematic review: the use of desmopressin for treatment and prophylaxis of bleeding disorders in pregnancy. Haemophilia. 2012;18(1):25–33. doi: 10.1111/j.1365-2516.2011.02573.x [DOI] [PubMed] [Google Scholar]
- 14.Pavord S, Rayment R, Madan B, et al. Management of inherited bleeding disorders in pregnancy: green-top guideline No. 71 (joint with UKHCDO). BJOG. 2017;124(8):e193–e263. doi: 10.1111/1471-0528.14592 [DOI] [PubMed] [Google Scholar]
- 15.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1–47. doi: 10.1111/j.1365-2516.2012.02909.x [DOI] [PubMed] [Google Scholar]
- 16.Connell NT, Flood VH, Brignardello-Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021;5(1):301–325. doi: 10.1182/bloodadvances.2020003264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paroskie A, Gailani D, DeBaun MR, et al. A cross-sectional study of bleeding phenotype in haemophilia A carriers. Br J Haematol. 2015;170(2):223–228. doi: 10.1111/bjh.13423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James PD, Mahlangu J, Bidlingmaier C, et al. Evaluation of the utility of the ISTH-BAT in haemophilia carriers: a multinational study. Haemophilia. 2016;22(6):912–918. doi: 10.1111/hae.13089 [DOI] [PubMed] [Google Scholar]
- 19.Leebeek FWG, Duvekot J, Kruip M. How I manage pregnancy in carriers of hemophilia and patients with von Willebrand disease. Blood. 2020;136(19):2143–2150. doi: 10.1182/blood.2019000964 [DOI] [PubMed] [Google Scholar]
- 20.Andersson NG, Chalmers EA, Kenet G, et al. Mode of delivery in hemophilia: vaginal delivery and Cesarean section carry similar risks for intracranial hemorrhages and other major bleeds. Haematologica. 2019;104(10):2100–2106. doi: 10.3324/haematol.2018.209619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drury-Stewart DN, Lannert KW, Chung DW, et al. Complex changes in von Willebrand factor-associated parameters are acquired during uncomplicated pregnancy. PLoS One. 2014;9(11):e112935. doi: 10.1371/journal.pone.0112935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Punt MC, Waning ML, Mauser-Bunschoten EP, et al. Maternal and neonatal bleeding complications in relation to peripartum management in women with Von Willebrand disease: a systematic review. Blood Rev. 2020;39:100633. doi: 10.1016/j.blre.2019.100633 [DOI] [PubMed] [Google Scholar]
- 23.Stoof SC, van Steenbergen HW, Zwagemaker A, et al. Primary postpartum haemorrhage in women with von Willebrand disease or carriership of haemophilia despite specialised care: a retrospective survey. Haemophilia. 2015;21(4):505–512. doi: 10.1111/hae.12635 [DOI] [PubMed] [Google Scholar]
- 24.Peyvandi F, Kouides P, Turecek PL, et al. Evolution of replacement therapy for von Willebrand disease: from plasma fraction to recombinant von Willebrand factor. Blood Rev. 2019;38:100572. doi: 10.1016/j.blre.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 25.Kouides P, Wawra‐Hehenberger K, Sajan A, et al. Safety of a pasteurized plasma-derived Factor VIII and von Willebrand factor concentrate: analysis of 33 years of pharmacovigilance data. Transfusion. 2017;57(10):2390–2403. doi: 10.1111/trf.14241 [DOI] [PubMed] [Google Scholar]
- 26.Machin N, Ragni MV. Recombinant vs plasma-derived von Willebrand factor to prevent postpartum hemorrhage in von Willebrand disease. Blood Adv. 2020;4(14):3234–3238. doi: 10.1182/bloodadvances.2020002046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavin M, Sánchez Luceros A, Kouides P, et al. Examining international practices in the management of pregnant women with von Willebrand disease. J Thromb Haemost. 2022;20(1):82–91. doi: 10.1111/jth.15561 [DOI] [PubMed] [Google Scholar]
- 28.Laffan MA, Lester W, O’Donnell JS, et al. The diagnosis and management of von Willebrand disease: a United Kingdom haemophilia centre doctors organization guideline approved by the British committee for standards in haematology. Br J Haematol. 2014;167(4):453–465. doi: 10.1111/bjh.13064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6(1):228–237. doi: 10.1182/bloodadvances.2021005589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strauss T, Elisha N, Ravid B, et al. Activity of Von Willebrand factor and levels of VWF-cleaving protease (ADAMTS13) in preterm and full term neonates. Blood Cells Mol Dis. 2017;67:14–17. doi: 10.1016/j.bcmd.2016.12.013 [DOI] [PubMed] [Google Scholar]
- 31.Castaman G, James PD. Pregnancy and delivery in women with von Willebrand disease. Eur J Haematol. 2019;103(2):73–79. doi: 10.1111/ejh.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]