ABSTRACT
Background:
Estimating seroepidemiolgical prevalence of SARS-CoV-2 antibody is an essential public health strategy. There is insufficient evidence of prevalence among those belonging to young age population in India.
Objective:
To compare the SARS-CoV-2 seropositivity rate between children and adults in selected sites from India.
Materials and Methods:
This was a multicentric population-based seroepidemiological study conducted in selected urban and rural areas of five sites selected from four states (Delhi, Odisha, Uttar Pradesh, Tripura) of India. Participants aged ≥1 year were included from different clusters of each area. Total serum antibody against SARS-CoV-2 virus was assessed qualitatively by using a standard enzyme-linked immunosorbent assay (ELISA) kit.
Results:
Data collection period was from 15 March 2021 to 10 June 2021. Total available data was of 4509 participants, of whom 700 were <18 years of age and 3809 were ≥18 years of age. The site-wise number of available data among those aged 2–17 years was 92, 189, 165, 146 and 108 for the sites of Delhi urban, Delhi rural, Bhubaneswar rural, Gorakhpur rural and Agartala rural area, respectively. The seroprevalence was 55.7% in the <18 years age group and 63.5% in the ≥18 years age group. The prevalence among female children was 58% and among male children was 53%.
Conclusion:
SARS-CoV-2 seropositivity rate among children was high and comparable to that of the adult population. Hence, it is unlikely that any future third wave by prevailing SARS-CoV-2 variant would disproportionately infect children 2 years or older.
Keywords: Antibody, children, India, SARS-CoV-2, serology
Introduction
Seroepidemiological assessment is one of the most important aspects of policymaking and public health intervention of the coronavirus disease 2019 (COVID-19) pandemic. This helps to know the proportion of the population infected, symptomatic and asymptomatic fractions, distribution of the infected as well as susceptible population.[1,2] Therefore, the vulnerable group of population can be identified, which helps in day-to-day practice for early identification and containment of the pandemic.[3] It also indicates the section of the population that is protected to subsequent infection to some extent. In case of symptoms persisting for long, where molecular tests may not be useful, serological tests can give an idea of past infection.[4]
The clinical manifestation and immune reaction to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus are different in children compared to adults.[5] Though children get less severe infection, they are susceptible in all age groups.[6] The reported cases in the young age group give an underestimation of the infection level compared to adult age group.[7] On the other side, there is evidence indicating a lower infection rate among children.[8–10] Therefore, the role of children in the transmission of SARS-CoV-2 is not clearly understood.
Children, particularly those aged 5–18 years, attend schools. It is commonly believed that classrooms could become outbreak clusters.[11] It is further assumed that those children could then bring the infection home and pass it on to their elderly grandparents who are at a higher risk of dying due to COVID-19. Because of their reasoning, globally, there has been closure of schools, thereby disadvantaging children in receiving education.[12,13]
The available seroepidemiological evidence in India shows variable levels of prevalence in the young age groups. As per a nationwide second serosurvey done in August–September 2020 in India, the prevalence among those in the 10–17 years age group was 10.4%.[14] Another study conducted in a hospital setup in June–September 2020 showed a prevalence of 19.6%.[15] Detailed serological evidence in different age groups among children is lacking.
We, therefore, undertook a community-based serosurvey for SARS-CoV-2 among a population older than 2 years. The objective of the study was to compare the SARS-CoV-2 seropositivity rate between children and adults.
Materials and Methods
Ethical approval and consent to participate
The study received ethical clearance from all five participating institutes (letter no. for AIIMS, New Delhi: IEC-959/04.09.2020; AIIMS, Bhubaneswar: T/EMF/CM&FM/20/44; JIPMER, Puducherry: JIP/IEC/2020/248; AIIMS, Gorakhpur: IHEC/AIIMS-GKP/BMR/01/22; Agartala: F.4 (5-234)/AGMC/ACADEMIC/IEC MEETING). Written informed consent, assent and consent from the parents/guardians for participants under the legal age of giving consent was obtained as per the Indian medical research council (ICMR) guidelines.
Type of study
This is part of an ongoing multicentric population-based, age-stratified, prospective SARS-CoV-2 seroprevalence study under the World Health Organisation (WHO) Unity studies.[16]
Study setting and patient selection
The study is being conducted in five sites selected in India. The sites selected are field practice areas of tertiary care medical colleges in Delhi, Bhubaneswar, Gorakhpur, Puducherry and Agartala [Figure 1]. In each site, both urban and rural area populations have been planned to be included. In Delhi, the urban area is a resettlement colony in the South Delhi district, where the majority of the population are from lower socioeconomic strata. The rural site for Delhi is in the villages of Ballabgarh block in Faridabad district of Haryana, which comes under Delhi National Capital Region (NCR). The site of Bhubaneswar is situated in the state capital of Odisha. The Gorakhpur area is near the city Gorakhpur, Uttar Pradesh, which is a transit point of international surface transport to Nepal. The Agartala site is in the northeastern state of Tripura. The Puducherry site is a Union Territory situated in South India.
Figure 1.
Geolocation of the study sites
Sample size and sampling strategy
For each study site, the sample size was 2000; thus, the total aggregate was 10,000. In each study site, 1000 participants were selected from urban areas and 1000 from rural areas. The rural and urban areas were selected purposively in each study site, out of which 25 clusters were chosen randomly from each of the urban and rural areas. In urban areas, individual municipality ward was considered as a cluster, whereas in rural areas, village was considered as a cluster. The field team visited the meeting point of multiple lanes, preferably at the centre of each cluster. The starting lane was identified using the rotating pencil method. From the first family, the recruitment process started and all family members of ≥2 years of age were included. By these methods, at least 10 consecutive families were visited, assuming four participants per family. If we could not get 40 participants in 10 houses, we kept on going until a sample of 40 in that cluster was achieved. At the end of a lane, the team moved to the left side to approach the next family.
Outcome measures
The primary outcome variable was the participants’ serum antibody reactivity to SARS-CoV-2 virus. Five millilitres of venous blood sample was collected from each participant in a clot activator vial with gel separator. The total antibodies in serum to SARS-Co-V-2 were assessed using enzyme-linked immunoassay (ELISA; WANTAI SARS-CoV-2 Ab ELISA kit, Wantai SARS-CoV-2 Diagnostics) as per the manufacturer’s protocol. WANTAI SARS-CoV-2 Ab ELISA is used for the qualitative detection of total antibodies against S-RBD SARS-CoV-2 virus in human serum or plasma specimens. It has a sensitivity of 94.4% and a specificity of 100%.[17] Specimens with an absorbance to cut-off ratio of ≥1.0 were considered as positive.
Data collection tool
Data collection was done using electronic tablet based EpiCollect5 mobile and web-based application which was filled for each participant. Information on exposure variables like age, sex, blood group, history of any symptoms experienced in the past 3 months from the date of recruitment, complications due to the symptoms, contact history, vaccination status and use of mask was collected. Once uploaded, the form was downloaded in Microsoft Excel data format and merged with registration forms filled at the time of sample collection based on unique identification numbers.
Study period
Data were collected from 15 to 31 March 2021 in Delhi urban site, from 12 April to 22 May 2021 in Delhi rural (NCR), from 22 March to 7 May 2021 in Bhubaneswar rural and from 26 March to 1 June 2021 in Agartala.
Data analysis
Data were extracted in Microsoft Excel and analysed in Stata V12. Categorical variables were expressed as proportions, whereas the continuous variables were expressed as median, mean and 95% confidence interval (CI). To find the statistical difference between categorical variables, the Chi-squared test was used. The level of significance was taken at 0.05. The seroprevalence was expressed as a proportion with 95% CI, according to the study site, rural–urban area, age groups, sex and presence of any symptoms. The age group was divided in two ways: the first type was less than 18 years and 18 years or older, whereas the second type was 1–4, 4–9 and 10–17 years. The corrected estimate was calculated by adjusting the test kit accuracy using the following formula:[18]
adjusted prevalence = (crude prevalence + specificity – 1)/(sensitivity + specificity – 1).
Results
Currently, we are reporting the available data of the 2–17 years age group from four sites, that is, urban and rural sites of NCR of Delhi, Bhubaneswar, Gorakhpur and Agartala.
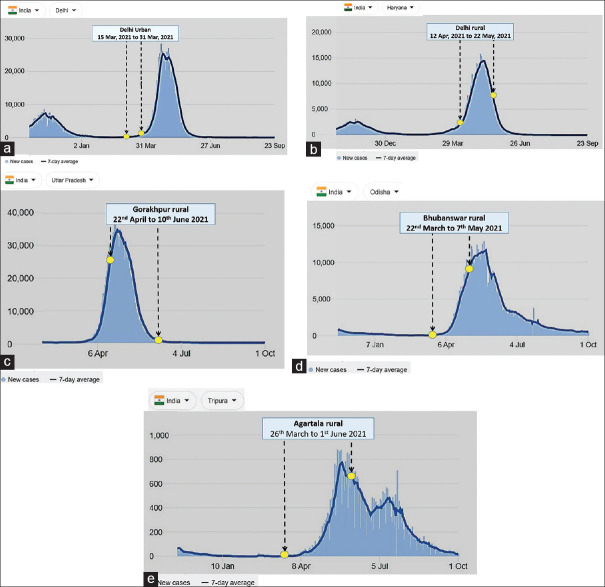
The data collection period was from 15 March to 31 March 2021 (the second wave started on 1 April, and the peak date was 23 April) in Delhi urban site, from 12 April to 22 May 2021 (the second wave started on 1 April, and the peak date was 10 May) in Delhi rural site (Faridabad, Haryana), from 22 March to 7 May 2021 (the second wave started on 1 April, and the peak date was 24 May) in Bhubaneswar site, from 22 April to 10 June 2021 (the second wave started on 1 April, and the peak date was 27 April) in Gorakhpur site and from 26 March to 1 June 2021 (the second wave started on 15 April, and the peak date was 23 May) in Agartala site [Figure 2a–e].[19]
Figure 2.
(a) Data collection period in an urban area of Delhi site in the context of ongoing COVID-19 pandemic; (b) data collection period in a rural area of Delhi site in the context of ongoing COVID-19 pandemic; (c) data collection period in a rural area of Gorakhpur site in the context of ongoing COVID-19 pandemic; (d) data collection period in a rural area of Bhubaneswar site in the context of ongoing COVID-19 pandemic; (e) data collection period in a rural area of Agartala site in the context of ongoing COVID-19 pandemic. COVID-19 = coronavirus disease 2019
Till 11 June 2021, the number of recruited participants was 1001 from Delhi urban, 1059 from Delhi rural, 448 from Gorakhpur rural, 1000 from Bhubaneswar rural and 1001 from Agartala rural. The total number of recruited participants was 4509. Out of them, 700 participants were <18 years of age and 3809 were adults (≥18 years) [Table 1]. Number of participants in <18 years age group was 92, 189, 165, 146 and 108 for the sites of Delhi urban, Delhi rural, Bhubaneswar, Gorakhpur and Agartala, respectively [Table 2]. The median age of the child participants was 11, 12, 11, 13 and 14 years for the sites of Delhi urban, Delhi rural, Bhubaneswar, Gorakhpur and Agartala, respectively, whereas the median age of the adult participants was 46, 40, 45, 40 and 41 years for the sites of Delhi urban, Delhi rural, Bhubaneswar, Gorakhpur and Agartala, respectively.
Table 1.
Total prevalence of COVID-19 seropositive participants of age <18 and ≥18 years
| Variables | Total N=4509 | |||
|---|---|---|---|---|
|
| ||||
| n | % | |||
|
|
|
|||
| Participants | Seropositive | Prop seropositive | Adjusted prevalence | |
| <18 years | 700 | 390 | 55.7 (52.0-59.4) | 59.0 (55.4-62.6) |
| ≥18 years | 3809 | 2421 | 63.5 (62.0-65.1) | 67.3 (65.8-68.8) |
| Total | 4509 | 2811 | 62.3 (60.9-63.8) | 65.9 (64.6-67.4) |
COVID-19=coronavirus disease 2019
Table 2.
Distribution of participants by age group and COVID-19 seropositivity at different study sites
| Variables | Delhi urban n=1001 | Delhi rural n=1059 | Bhubaneswar rural n=1000 | Agartala rural n=1001 | Gorakhpur rural n=448 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||
| Participants | Seropositive | Participants | Seropositive | Participants | Seropositive | Participants | Seropositive | Participants | Seropositive | |
|
| ||||||||||
| n | n (%) (95% CI)# | n | n (%) (95% CI)@ | n | n (%) (95% CI)$ | n | (95% CI)€ | n | (95% CI)€ | |
| <18 years | 92 | 68 (73.9) (63.7-82.5) |
189 | 116 (61.4) (54.0-68.3) |
165 | 75 (45.4) (37.7-53.4) |
146 | 45 (30.8) (23.4-38.9) |
108 | 87 (80.6) (71.8-87.5) |
| ≥18 years | 909 | 680 (74.8) (71.8-77.6) |
870 | 512 (58.8) (55.5-62.1) |
835 | 451 (54.0) (50.5-57.4) |
855 | 475 (55.6) (52.1-58.9) |
340 | 307 (90.3) (86.6-93.2) |
| Total | 1001 | 748 (74.7) (71.9-77.4) |
1059 | 628 (59.3) (56.3-62.3) |
1000 | 526 (52.6) (49.4-55.7) |
1001 | 520 (51.9) (48.8-55.1) |
448 | 394 (87.9) (84.6-90.8) |
CI=confidence interval, COVID-19=coronavirus disease 2019, NCR=National Capital Region. *Villages at Ballabgarh in district Faridabad, Delhi NCR*. #The adjusted prevalence for<18 years is 78.3 (95% CI: 68.4- 86.2), for ≥18 years is 79.2 (95% CI: 76.4-81.8) and for total is 79.1 (95% CI: 76.5-81.6). @The adjusted prevalence for <18 years is 65 (95% CI: 57.8-71.8), for ≥18 years is 62.3 (95% CI: 58.9-65.5) and for total is 62.8 (95% CI: 59.8-65.7). $The adjusted prevalence for <18 years is 48.1 (95% CI: 40-55.8), for≥18 years is 57.2 (95% CI: 53.8-60.6) and for total is 55.7 (95% CI: 52.5-58.8). ≤The adjusted prevalence for <18 years is 32.6 (95% CI: 25.3-41.1), for ≥18 years is 58.9 (95% CI: 55.6-62.3) and for total is 55 (95% CI: 51.8–58.1). €The adjusted prevalence for <18 years is 85.4 (95% CI: 77-91.3), for≥18 years is 95.7 (95% CI: 92.8-97.5) and for total is 93.1 (95% CI: 90.3-95.2)
The total number of participants in the <18 years age group positive for SARS-CoV-2 antibody was 390/700 (55.7%). The prevalence of adult participants was 2421/3809 (63.5%) [Table 1]. The site-wise prevalence in these two age groups was similar, except in Agartala site [Figure 3].
Figure 3.

Bar diagram of seropositivity (%) among participants < 18 years and 18 years or older, according to the study sites
Irrespective of the age groups, rural sites had lower seropositivity compared to the urban site (at Delhi). Within the rural sites, children had slightly lower seropositivity compared to adults. However, this differential prevalence was not observed in the urban site [Table 2 and 3].
Table 3.
Distribution of participants by age group and type of study site
| Age group | Location | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Urban | Rural | |||||
|
|
|
|||||
| Total participants (n) | Total seropositive (n) | Adjusted prevalence (%) 95% CI | Total participants (n) | Total seropositive (n) | Adjusted prevalence (%) 95% CI | |
| <18 years | 92 | 68 (73.9) (63.7-82.5) | 78.3 (69.8-86.7) | 608 | 322 (52.9) (49.0-56.9) | 55.9 (52.0-59.9) |
| ≥18 years | 909 | 680 (74.8) (71.8-77.6) | 79.2 (76.6-81.8) | 2900 | 1741 (60.0) (58.3-61.8) | 63.5 (61.8-65.3) |
| Total | 1001 | 748 (74.7) (71.9-77.4) | 79.1 (76.5-81.6) | 3508 | 2063 (58.8) (57.2-60.4) | 62.2 (60.7-63.9) |
CI=confidence interval
The prevalence in children was slightly higher among female participants compared to males (58.6% vs. 53.0%). However, there was no statistically significant difference (P-value 0.140) in seropositivity between males and females. Among 700 children aged 2–17 years, 362 (51.7%) were males. The number of participants aged 2–4 years was 33 (4.8%), 5–9 years was 153 (21.8%) and 10–17 years was 512 (73.1%). Children aged 2–4 and 5–9 years had almost identical seropositivity rates (42.4% and 43.8%, respectively), which were lower than the rate observed for children aged 10–17 years (60.3%) [Table 4]. The overall proportion of seropositivity in the <18-year age group was also similar across different symptom categories [Table 5].
Table 4.
Distribution of child participants by selected variables and seropositivity rate
| Variables | Total (N=700) | |||
|---|---|---|---|---|
|
| ||||
| Participants (n) | Seropositive (n) | Proportion of seropositive (%) (95% CI) | Adjusted prevalence (%) 95% CI | |
| Male | 362 | 192 | 53.0 (47.7-58.3) | 56.1 (50.8-61.3) |
| Female | 338 | 198 | 58.6 (53.1-63.8) | 62.1 (56.7-67.3) |
| 1-4 years | 33 | 14 | 42.4 (25.5-60.8) | 45.0 (28.1-63.6) |
| 5-9 years | 153 | 67 | 43.8 (35.8-52.0) | 46.4 (38.3-54.6) |
| 10-17 years | 512 | 309 | 60.3 (55.9-64.6) | 63.9 (59.5-68.0) |
| Symptomatic | 131 | 90 | 68.7 (60.0-76.5) | 72.8 (64.0-79.9) |
| Asymptomatic | 569 | 300 | 52.7 (48.5-56.9) | 55.9 (51.7-60.0) |
| Total | 700 | 390 | 55.7 (51.9-59.4) | 59.0 (55.2-62.7) |
CI=confidence interval
Table 5.
Distribution of participants <18 years of age by symptoms and seropositivity rate
| Symptoms | Total number of participants with any one symptom (n=131) | ||
|---|---|---|---|
|
| |||
| Total number of symptoms reported n | Seropositive n | Proportion seropositive (95% CI) % | |
| Fever | 67 | 45 | 67.2 (54.6-78.1) |
| Respiratory symptoms (sore throat, runny nose, cough, shortness of breath, conjunctivitis) | 67 | 44 | 65.7 (53.1-76.8) |
| Abdominal symptoms (vomiting, nausea and diarrhoea) | 15 | 10 | 66.7 (38.4-88.2) |
| Neurological symptoms (loss of smell and loss of taste) | 4 | 3 | 75 (19.4-99.4) |
CI=confidence interval
Discussion
The seroprevalence of SARS-CoV-2 antibody among children was similar to that of the adult population across the study sites, except at Agartala rural. Though there was urban–rural difference in terms of total prevalence, it was similar between the children and adult populations. There was a slightly higher seropositivity rate observed among female children. This finding is in contrast to the meta-analysis where it was shown that the prevalence is higher in men.[20] This may be a chance finding due to a small number of data available at the time of midterm analysis. The higher seropositivity rate in children aged 10–17 years may be reflective of their higher mobility and independence compared to the younger children. As reported in the literature, a large proportion of children (50.9%) had asymptomatic SARS-CoV-2 infection.[21]
In India, seroprevalence among children and younger age group was estimated as a part of a larger nationwide survey on adult age group. The second nationwide seroprevalence study done in August–September 2020 had reported 9.0% seropositivity among 3021 children aged 10–17 years,[14] while in our study, it was 60.3%. One hospital-based study in Chennai had reported a prevalence of 19.6% in the age group of 1 month to 17 years.[15] Another recent study done in Chandigarh among children reported a prevalence of 71% in urban, rural and slum areas.[22] As per a large nationwide cross-sectional study performed in 21 states, the seroprevalence among children aged 6–9 years was 57.2%, whereas it was 61.6% in the age group of 10–17 years.[23]
Urban area
During the first wave of the pandemic in India, the worst affected areas were the large urban areas, including Delhi. We collected data during the second fortnight of March 2021. This was the time when the first wave was subsiding and the second wave had not yet started. Results show that a large majority of the population had already been infected by the time we conducted the study at Delhi urban site, which predominantly had lower and middle socioeconomic strata people and had a very congested neighborhoods. The obliteration of any difference in seropositivity rate between children and adults suggests that as the disease become generalised, it affects all age groups equally.
We found that the seropositivity rate in our study was higher (74.7%) than the fifth serosurvey (conducted in January 2021) which reported an overall 56.1% for Delhi and 62.8% for South Delhi district.[24,25] However, information on prevalence in children and young age groups was lacking.
Rural areas
We had included four rural sites. Two of the sites (Bhubaneswar and Agartala) were state capitals, one site (Faridabad under the rural site of AIIMS, Delhi) was in NCR and one site (Gorakhpur) was a major transit point for surface transportation to another country (Nepal). Thus, these sites were more vulnerable to a pandemic. The data were collected during the second wave. Gorakhpur site was the worst affected (seropositivity rate of 87.9%), while Faridabad was the least affected (seropositivity rate of 58.8%). Data collection in all the sites was done just before the second wave hit the country, except the site of Gorakhpur. This may be the reason for the highest prevalence at the Gorakhpur site among all the sites. Overall, more than half (62.3%) of those surveyed showed evidence of past infection. Agartala site included some tribal populations as well. In general, the tribal population might have lower mobility, resulting in lower vulnerability to SARS-CoV-2 infection. This might explain the comparatively low prevalence in children at this site.
In a rapidly evolving pandemic, individuals who have been recently infected (<14 days) may not have developed antibodies. They would have been reported negative in serosurvey. Hence, our findings are likely to be an underestimate.
We observed that children had a slightly lower seropositivity rate compared to adults (55.7% vs. 63.5%). These findings are similar to the previously reported evidence which found that children are less affected than the adult age group.[26,27] During the pandemic, schools were closed and children were more likely to have remained indoors compared to adults.[28] For children, the source of infections is likely to be household adults who bring the infection from outside during livelihood activities. Hence, we can expect some lag in seropositivity among children. Evidence suggests that children might produce different levels of antibodies than adults when infected.[29] In that case, the antibody might not be detectable by the existing laboratory tests. So, the observed seropositivity rate would reflect the laboratory test’s proficiency rather than any true difference between the infection rates of children and adults. Overall, the results suggest that children and adults are equally susceptible to SARS-CoV-2 infection.
Overall, our study found more than 50% of both young and adult populations had already been infected with SARS-CoV-2. Out of all seropositive participants, around two thirds had no symptoms. The seroprevalence was similar in males and females, and the populations of rural and urban areas had been almost equally got infected across the sites.
Strengths
This study included participants from four different states representing different geographical locations of India. We, for the first time, provide a seroprevalence estimate for children aged 2–17 years in India. Data from urban slum areas, rural areas and some tribal populations at one site further increase the generalisability. Including a large number of clusters within each of the study sites makes our findings more representative.
Limitations
In Delhi urban, we had purposively selected an urban resettlement colony. This area had a high population density inhabited mostly by lower socioeconomic populations. Therefore, it is not representative of Delhi urban population as a whole. At all other sites, clusters were selected randomly. Secondly, we did not perform neutralising antibody assay for all these samples, which would have helped to interpret the significance of seropositivity.
Conclusion
SARS-CoV-2 seropositivity rate among children was high and comparable to the adult population. Hence, it is unlikely that any future third wave by prevailing SARS-CoV-2 variant would disproportionately affect children 2 years or older.
Study protocol
The protocol is available upon reasonable request.
Financial support and sponsorship
This work was supported by a research grant (ref. no.: 2020/1085497, purchase order: 202630166) from the WHO Country Office, New Delhi 110016, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We thank the World Health Organisation (WHO) Country Office, India team, particularly Dr. Mohammad Ahmad (National Professional Officer, WHO) and Dr. Anisur Rahman (Health Emergencies and Research Officer, WHO), for continuous support. Special thanks to the participants who allowed us to investigate the extent of infection, as determined by seropositivity in the general population, in which coronavirus disease 2019 (COVID-19) virus infection has been reported.
References
- 1.Organization WH. Geneva:A Coordinated Global Research Roadmap:2019 novel coronavirus. [Last accessed on 2021 Dec 30]. Available from:https://www.who.int/publications/m/item/a-coordinated-global-research-roadmap .
- 2.Peeling RW, Olliaro PL. The time to do serosurveys for COVID-19 is now. Lancet Respir Med. 2020;8:836–8. doi: 10.1016/S2213-2600(20)30313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puschel K, Ferreccio C, Peñaloza B, Abarca K, Rojas M-P, Tellez A, et al. Clinical and serological profile of asymptomatic and non-severe symptomatic COVID-19 cases:Lessons from a longitudinal study in primary care in Latin America. BJGP Open. 2021;5:bjgpopen20X101137. doi: 10.3399/bjgpopen20X101137. doi:10.3399/bjgpopen20X101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong DSY, Fragkou PC, Schweitzer VA, Chemaly RF, Moschopoulos CD, Skevaki C. How to interpret and use COVID-19 serology and immunology tests. Clin Microbiol Infect. 2021;27:981–6. doi: 10.1016/j.cmi.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP, Connors TJ, Zhu Y, Baldwin MR, Lin WH, Wontakal S, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 7.Smith BK, Janowski AB, Danis JE, Harvey IB, Zhao H, Dai YN, et al. Seroprevalence of SARS-CoV-2 antibodies in children and adults in St. Louis, Missouri, USA. mSphere. 2021;6:e01207–20. doi: 10.1128/mSphere.01207-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children:A systematic review. Pediatr Pulmonol. 2020;55:2565–75. doi: 10.1002/ppul.24991. [DOI] [PubMed] [Google Scholar]
- 9.Jeng MJ. Coronavirus disease 2019 in children:Current status. J Chin Med Assoc. 2020;83:527–33. doi: 10.1097/JCMA.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 Infection in Children. N Engl J Med. 2020;382:1663–5. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle T, Kendrick K, Troelstrup T, Gumke M, Edwards J, Chapman S, et al. COVID-19 in primary and secondary school settings during the first semester of school reopening —Florida, August–December 2020. MMWR Morb Mortal Wkly Rep. 2021;70:437–41. doi: 10.15585/mmwr.mm7012e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klimek-Tulwin M, Tulwin T. Early school closures can reduce the first-wave of the COVID-19 pandemic development. Z Gesundh Wiss. 2020:1–7. doi: 10.1007/s10389-020-01391-z. doi:10.1007/s10389-020-01391-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bekkering G, Delvaux N, Vankrunkelsven P, Toelen J, Aertgeerts S, Crommen S, et al. Closing schools for SARS-CoV-2:A pragmatic rapid recommendation. BMJ Paediatr Open. 2021;5:e000971. doi: 10.1136/bmjpo-2020-000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murhekar MV, Bhatnagar T, Selvaraju S, Saravanakumar V, Thangaraj JWV, Shah N, et al. SARS-CoV-2 antibody seroprevalence in India, August–September, 2020:Findings from the second nationwide household serosurvey. Lancet Glob Health. 2021;9:e257–66. doi: 10.1016/S2214-109X(20)30544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkataraman A, Balasubramanian S, Putilibai S, Lakshan Raj S, Amperayani S, Senthilnathan S, et al. Correlation of SARS-CoV-2 serology and clinical phenotype amongst hospitalised children in a tertiary children's hospital in India. J Trop Pediatr. 2021;67:fmab015. doi: 10.1093/tropej/fmab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unity Studies:Early Investigation Protocols. [Last accessed on 2021 Jun 11]. Available from:https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/early-investigations .
- 17.Wantai BioPharm:COVID 19 –Serology and Molecular Tests. [Last accessed on 2021 Dec 30]. Available from:http://www.ystwt.cn/covid-19/
- 18.Sempos CT, Tian L. Adjusting coronavirus prevalence estimates for laboratory test kit error. Am J Epidemiol. 2020;190:109–15. doi: 10.1093/aje/kwaa174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CSSEGISand Data. COVID-19 Data Repository by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2021. [Last accessed on 2021 Oct 03]. Available from:https://github.com/CSSEGISandData/COVID-19 .
- 20.Abate BB, Kassie AM, Kassaw MW, Aragie TG, Masresha SA. Sex difference in coronavirus disease (COVID-19):A systematic review and meta-analysis. BMJ Open. 2020;10:e040129. doi: 10.1136/bmjopen-2020-040129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han X, Li X, Xiao Y, Yang R, Wang Y, Wei X. Distinct Characteristics of COVID-19 Infection in Children. Front Pediatr. 2021;9:619738. doi: 10.3389/fped.2021.619738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livemint. Covid-19:71% of children in sero survey show antibodies, says PGIMER. mint. 2021. [Last accessed on 2021 Dec 28]. Available from:https://www.livemint.com/science/health/71-of-children-in-sero-survey-show-antibodies-pgimer-11631586099864.html .
- 23.Murhekar MV, Bhatnagar T, Thangaraj JWV, Saravanakumar V, Kumar MS, Selvaraju S, et al. Seroprevalence of IgG antibodies against SARS-CoV-2 among the general population and healthcare workers in India, June–July 2021:A population-based cross-sectional study. PLoS Med. 2021;18:e1003877. doi: 10.1371/journal.pmed.1003877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.A look at serological surveys conducted in Delhi. Hindustan Times. 2021. [Last accessed on 2021 Jun 08]. Available from:https://www.hindustantimes.com/cities/delhi-news/a-look-at-serological-surveys-conducted-in-delhi-101612270983224.html .
- 25.Staff S. Coronavirus:Over 56% people in Delhi have antibodies, shows fifth sero survey. Scroll.in. [Last accessed on 2021 Jun 12]. Available from:https://scroll.in/latest/985753/coronavirus-over-56-people-in-delhi-have-antibodies-shows-fifth-sero-survey .
- 26.Viner RM, Mytton OT, Bonell C, Melendez-Torres GJ, Ward J, Hudson L, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults:A systematic review and meta-analysis. JAMA Pediatr. 2021;175:143–56. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Somekh E, Gleyzer A, Heller E, Lopian M, Kashani-Ligumski L, Czeiger S, et al. The role of children in the dynamics of intra family coronavirus 2019 spread in densely populated area. Pediatr Infect Dis J. 2020;39:e202–4. doi: 10.1097/INF.0000000000002783. [DOI] [PubMed] [Google Scholar]
- 28.India coronavirus school closures:How the pandemic is endangering the future of India's most vulnerable students, especially girls. The Washington Post. [Last accessed on 2021 Oct 03]. Available from:https://www.washingtonpost.com/world/asia_pacific/india-coronavirus-school-closures/2020/12/23/7e80f628-3efc-11eb-b58b-1623f6267960_story.html .
- 29.Yang HS, Costa V, Racine-Brzostek SE, Acker KP, Yee J, Chen Z, et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw Open. 2021;4:e214302. doi: 10.1001/jamanetworkopen.2021.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]