ABSTRACT
Introduction:
With an increase in number of cases of relapsed or refractory multiple myeloma (RRMM), scientist have discovered various combination of medications among which one is daratumumab, Daratumumab is a mono-clonal antibody which attacks CD-38 markers present in abundance on the surface of myeloma cells and is used universally for the treatment of primary newly diagnosed multiple myeloma patients.
Methods and Methodology:
This meta-analysis was conducted according to Cochrane Collaboration guidelines in which initially 679 articles were evaluated for relevance on abstract level followed by full text screening of final list of 45 articles. Out of the 45 articles, only 10 articles qualified for selection criteria for eligibility. Three Phase 3 randomized control clinical trials which includes primary outcomes of progression free span and secondary outcomes including complete response, partial response or very good partial response and adverse effects reported were included in this study.
Results:
A total of three studies including 1533 patients (849 in Daratumumab treatment group while 684 patients in control group) were included in the study. All three of these studies were phase 3 clinical trial conducted to observe the role of daratumumab in relapsed and refractory multiple myeloma. Mean age reported was 65 years in both treatment and control groups. This study showed that daratumumab improves primary and secondary outcomes including progression free span, overall response rate, very good partial response, and complete response. However, daratumumab increases drug induced adverse effects.
Conclusion:
Our study confirmed that daratumumab in combination therapy improved primary and secondary outcomes when compared with platinum-based chemotherapy, but more adverse effects were reported in the combination group. So, we recommend that combination therapy should include daratumumab in treatment of relapsed and refractory multiple myeloma patients.
Keywords: Daratumumab, literature, multiple myeloma, refractory, relapsed, role
Introduction
Multiple myeloma (MM) is a neoplastic disease of the B cell lineage caused by accelerated monoclonal expression of plasma cells in the bone marrow resulting in anemia, hypercalcemia, renal failure, and bone destruction.[1,2] Relapsed multiple myeloma is characterized as previously treated multiple myeloma that has progressed and requires salvage therapy, whereas relapsed and refractory multiple myeloma (RRMM) is described as a disease that is not responsive to salvage therapy, or that exacerbates within 60 days of the last treatment in patients who previously achieved a minimal response or better on prior therapy. Standard treatment regimens for RRMM include proteasome inhibitors such as bortezomib, and immunomodulatory drugs such as lenalidomide alone or supplemented with glucocorticoids.[3] Unfortunately, most patients who relapse have limited treatment options after exposure to above-mentioned classes of agents. Therefore, the diseased patients that are refractory to both proteasome inhibitors and immunomodulatory drugs have poor prognoses, the estimated median overall survival is 9 months, and the estimated event free survival is 5 months at best.[4] Most cancer cells are limited to the bone marrow and only about 1–7% of patients possess extramedullary disease at the time of diagnosis and around 8% of people will progress into extramedullary disease later in life.[5] Although there have been notable advancements in the pharmacological agents along with autologous hematopoietic stem cell transplantation, MM remains to be a fatal disease.[1,6]
In November 2015, the U.S. Food and Drug Administration granted daratumumab approval, based on two phase II studies, as monotherapy (16 mg/kg in heavily treated patients) for MM patients who have received at least three prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or patient’s double refractory to these agents.[7] Daratumumab is a human IgG1 monoclonal antibody targeting CD38, 46-kDa type II transmembrane glycoprotein, expressed at high levels on malignant cells in MM.[5,8] CD38 is a transmembrane glycoprotein that is expressed on lymphoid and myeloid cells as well as on non-hematopoietic tissues, with multiple functions, including ecto-enzymatic activity and receptor-mediated regulation of cell adhesion and signal transduction.[6] Daratumumab induces tumor cell death via several CD38 immune-mediated actions, including complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, apoptosis, and modulation of CD38 enzymatic activity.[9]
Since 2015, there is evident data for daratumumab efficacy in pretreated MM patients both as monotherapy and in combination with other agents. The GEN501 and SIRIUS trials provided the earliest reports on the efficacy and safety of daratumumab as monotherapy in heavily pretreated RRMM patients.[2] Currently, with evidence based on the present clinical trials, the optimal dose of daratumumab as a single agent has been established at 16 mg/kg as an intravenous infusion, administered weekly during the first 8 weeks, every 2 weeks for the following 16 weeks, and every 4 weeks thereafter.[10] Data on daratumumab therapy in renal failure patients requiring dialysis are insufficient, even though pharmacokinetic data suggest that it can be safely administered without dose modification in patients with creatinine clearance <30 mL/min.[3] The complement system may be compromised in MM due to decreased levels of components of the classical and alternative complement pathways. In an in vitro setting daratumumab was able to demonstrate the potential to induce maximal complement-mediated lysis of MM cells in a medium containing only 10% human serum. On the other hand, in conditions with C1q-depleted in the serum, daratumumab-induced lysis could be revived by the addition of low amounts of C1q. This proposes that daratumumab is still effective under complement-limiting conditions occurring in MM patients. Nevertheless, it will be important to supervise these aspects in the clinical setting.[1] Common (≥20%) adverse events from treatments included fatigue, nausea, anemia, back pain, cough, upper respiratory tract infection (URTI), thrombocytopenia, and neutropenia. Any new safety signals were not determined for daratumumab monotherapy in the recently updated analysis of the combined data set of the GEN501 part 2 and SIRIUS studies.[11]
In conclusion, Phase III trials of daratumumab both in the relapsed/refractory setting as well as in newly diagnosed patients will help to illuminate the role of daratumumab in the treatment of MM. Given the significant efficacy that has been seen with daratumumab in early clinical trials, daratumumab as well as other mono antibodies are likely to change the landscape of myeloma treatment.[6]
Methods and Methodology
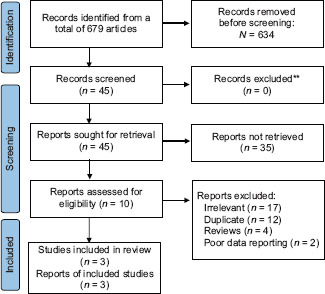
This meta-analysis was conducted according to Cochrane Collaboration guidelines and reported as per preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines and its summary is given in [Figure 1]. Two authors (AA and MZ) performed a systematic literature search using databases such as MEDLINE (via PubMed), Embase, and Cochrane library using the medical search terms and their respective entry words with the following search strategy: “daratumumab, refractory, relapsed, multiple myeloma”. Additionally, unpublished trials were identified from the clinicaltrials.gov website and references of all pertinent articles were also scrutinized to ensure the inclusion of all relevant studies. The search was completed on February 27th, 2021 and we used articles only in English language. Two authors (SA and NL) independently screened the search results in a two-step process based on predetermined inclusion/exclusion criteria. First, 679 articles were evaluated for relevance on the title and abstract level, followed by a full-text screening of the final list of 45 articles. Any disagreements were resolved by discussion or third-party review and a total of three articles were included in the study. The following eligibility criteria were used: original articles reporting the significance of daratumumab in relapsed or refractory multiple myeloma patients as compared to the controls. All articles with subjective data on clinical outcomes in patients with the significance of daratumumab in relapsed or refractory MM patients. Only 10 articles qualified the aforementioned selection criteria for eligibility. All qualifying studies were nationwide inpatient or pooled clinical trials data. The reasons of exclusion for other 35 articles were as follows: irrelevant (n = 17), duplicate (n = 12), reviews (n = 4), and poor data reporting (n = 2). Out of the 10 included studies, 3 clinical studies reported progression-free span, complete response, partial response, or very good partial response including adverse outcomes such as thrombocytopenia, neutropenia, lymphopenia, URTI, and fatigue hence, included in our study.
Figure 1.
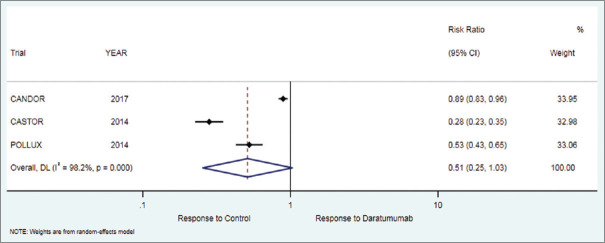
Forest plot for progression free survival (PFS)
The primary endpoint was a progression free span of 18 months. Secondary endpoints were overall response rate (ORR), complete response, partial response, minimal response, or very good partial response, also including various adverse effects including thrombocytopenia, anemia, neutropenia, lymphopenia, URTI, diarrhea, dyspnea, pneumonia, and fatigue. Data on baseline characteristics and clinical outcomes were then extracted and summary tables were created. Summary estimates of the clinical endpoints were then calculated with risk ratio (RR) and 95% confidence intervals using the random-effects model. Heterogeneity between studies was examined with Cochran’s Q-based I2 statistic which can be defined as low (25% to 50%), moderate (50% to 75%), or high (>75%). Statistical analysis was performed using Comprehensive Meta-analysis software (CMA version 3.0, Biostat Inc) [PRISMA FLOW CHART].
PRISMA flow chart:
Results
A total of three studies and 1533 patients (849 in the daratumumab treatment group while 684 patients in the control group) were included in the study [Figure 1]. All three of these studies were phase 3 clinical trial conducted to observe the role of daratumumab in relapsed or refractory MM. The mean age was 65 years in both treatment and control groups. Further details on study and participant characteristics, primary and secondary outcome, and adverse effects are summarized in [Tables 1-3] respectively. No evidence of publication bias was found [Prisma flow chart] [Table 1].
Table 1.
Study characteristics including in our meta-analysis
| Study | Trial name | Publication year | Design | Country | Treatment group | Control group |
|---|---|---|---|---|---|---|
| Dimopoulos et al. | CANDOR trial | 2020 | Phase 3 randomized control trial | North America, Europe, Australia, and Asia | Carfilzomib, dexamethasone, and daratumumab | Carfilzomib, dexamethasone |
| Spencer et al. | CASTOR trial | 2018 | Phase 3 randomized control trial | Australia | Daratumumab plus bortezomib and dexamethasone | bortezomib and dexamethasone |
| Dimopoulos, et al. | POLLUX trial | 2018 | Phase 3 randomized control trial | Canada, North America, and Australia | Daratumumab plus lenalidomide and dexamethasone | lenalidomide and dexamethasone |
Table 3.
Adverse effects included in our meta-analysis
| Study/Trial name | Treat ment/Contr ol group | Thromb ocytop enia | Anemia | Neutrop enia | Lymph openia | URTI* | Diarr hea | Fatigu e | Dyspn ea | Pneum onia |
|---|---|---|---|---|---|---|---|---|---|---|
| Dimopoulos et al./CANDOR trial | Treat ment group | 115 (36.8%) | 101 (32.3%) | 43 (13.8 %) | 27 (8.6 %) | 90 (2 8.9 %) | 97 (31 0.1%) | 75 (24 %) | 61 (19.5%) | 55 (17.3 %) |
| Contr ol group | 45 (29%) | 48 (31.2 %) | 15 (9.7%) | 12 (8%) | 35 (2 2.8 %) | 22 (14 0.3%) | 28 (18.2%) | 34 (22.1%) | 19 (12.4 %) | |
| Spencer, et al./CASTOR trial | Treat ment group | 145 (57.7%) | 69 (27.5 %) | 46 (18.3 %) | 32 (12.7 %) | 76 (3 0.3 %) | 85 (33 0.9%) | 53 (21.1%) | 46 (18.3%) | 36 (14.3 %) |
| Contr ol group | 105 (42.5%) | 75 (30.4 %) | 23 (9.3%) | 9 (3.6%) | 43 (1 7.4 %) | 53 (21 0.5%) | 58 (23.5%) | 21 (8.5 %) | 31 (12.6 %) | |
| Dimopoulos, et al./POLLUX trial | Treat ment group | 81 (28.3 %) | 104 (36.4%) | 172 (60.1 %) | 18 (6.3 %) | 105 (36.7 %) | 144 (5 0.3%) | 103 (3 6%) | 59 (20.7%) | 58 (20.3 %) |
| Contr ol group | 87 (30.7 %) | 109 (38.5%) | 127 (44.8 %) | 16 (5.6 %) | 74 (2 6.2 %) | 89 (31 0.5%) | 85 (30 %) | 35 (12.4%) | 42 (14.8 %) |
*URTI=Upper respiratory tract infection
Progression free survival (PFS)
The primary outcome of progression free survival of 18 months in patients who received daratumumab therapy was 54.4% while in the control group it was 8%. There was significant difference in favor of the treatment as compared to the control group [RR = 2.86 (95% CI: 1.05 to 7.79; P = 0.040)], [Table 2, Figure 1].
Table 2.
Primary and secondary outcomes including in our meta-analysis
| Study/Trial name | Treatment /Control group | PFS of 18 months since first dose of Daratumumab | ORR* | VGPR* | CR* | PR* | MR* |
|---|---|---|---|---|---|---|---|
| Dimopoulo s et al./CANDOR Trial | Treatment group | 57 (18.3%) | 263 (84.3%) | 216 (69%) | 89 (28.5%) | 55 (17.6%) | 23 (7.4%) |
| Control group | 13 (8%) | 115 (74.6%) | 75 (49%) | 16 (10.4%) | 6 (4%) | 22 (14.3%) | |
| Spencer, et al./CASTOR trial | Treatment group | 188 (74%) | 201 (80.1%) | 149 (59.3%) | 69 (27.4%) | 52 (20.7%) | 9 (3.6%) |
| Control group | 24 (9.7%) | 148 (59.9%) | 68 (27.5%) | 23 (9.3%) | 80 (32.4%) | 20 (8.1%) | |
| Dimopoulo s, et al./POLLUX Trial | Treatment group | 203 (71%) | 261 (91.2%) | 221 (77.2%) | 144 (50.3%) | 40 (14%) | 5 (1.7%) |
| Control group | 127 (45%) | 211 (74.5%) | 132 (46.6%) | 58 (20.5%) | 79 (28%) | 26 (9.2%) |
*PFS: Progression free span, ORR=overall response rate, VGPR=Very good partial response, CR=Complete response, PR=Partial response, MR=Minimal Response
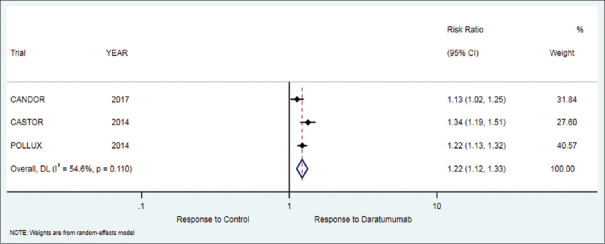
Overall response rate
The secondary outcome of the ORR in patients who received daratumumab therapy was 85.2% while in the control group it was 69.7%. There was significant difference in favor of treatment as compared to control group [RR = 1.22 (95% CI: 1.12 to 1.33; P < 0.001)], [Table 2, Figure 2].
Figure 2.
Forest plot for overall response rate (ORR)
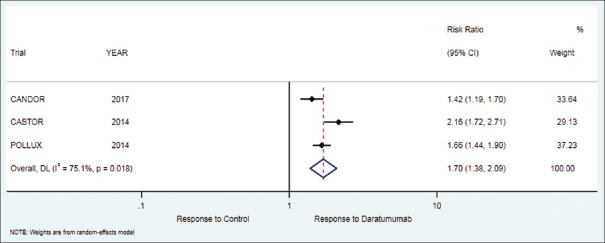
Very good partial response
The secondary outcome of very good partial response in patients who received daratumumab therapy was 68.5% while in the control group it was 41%. There was a significant difference in favor of treatment as compared to control group [RR = 1.70 (95% CI: 1.38 to 2.09; P < 0.001)], [Table 2, Figure 3].
Figure 3.
Forest plot for very good partial response (VGPR)
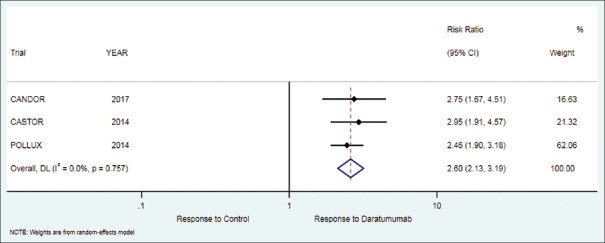
Complete response
The secondary outcome of complete response in patients who received daratumumab therapy was 35.4% while in the control group it was 13.4%. There was significant difference in favor of treatment as compared to control group [RR = 2.60 (95% CI: 2.13 to 3.19; P < 0.001)], [Table 2, Figure 4].
Figure 4.
Forest plot for complete response (CR)
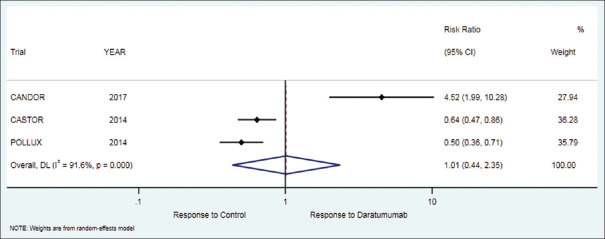
Partial response
The secondary outcome of partial response in patients who received daratumumab therapy was 17.4% while in the control group it was 21.5%. There was no significant difference between the treatment and control group [RR = 1.01 (95% CI: 0.44 to 2.35; P = 0.977)], [Table 2, Figure 5].
Figure 5.
Forest plot for partial response (PR)
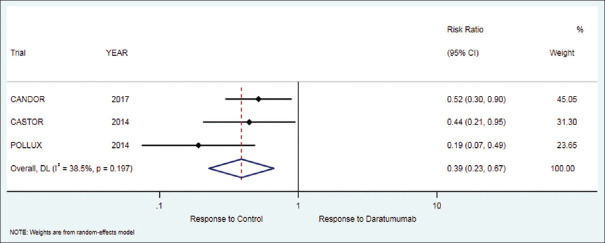
Minimal response
The secondary outcome of minimal response in patients who received daratumumab therapy was 4.23% while in the control group it was 10.5%. There was a significant difference between treatment and control group but in the favor of control [RR = 0.39 (95% CI: 0.23 to 0.67; P = 0.001)], [Table 2, Figure 6].
Figure 6.
Forest plot for minimal response (MR)
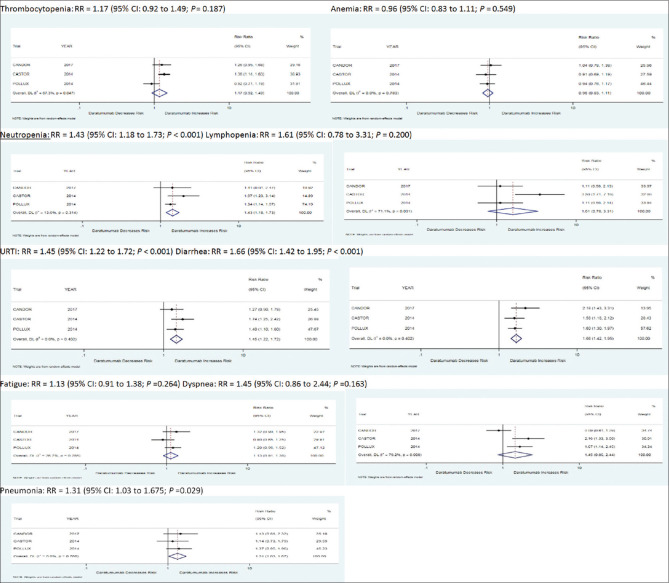
Adverse outcomes
Few adverse effects are associated with both daratumumab and control group in all three studies included in our study, including thrombocytopenia, anemia, neutropenia, lymphopenia, URTI, diarrhea, fatigue, dyspnea, and pneumonia. A total of 41% of people in the daratumumab group developed thrombocytopenia as compared to 34.1% in the control group. A total of 32.1% of the population in the daratumumab group developed anemia as compared to 33.4% of the population in the control group. A total of 30.73% of the population in the daratumumab group developed neutropenia as compared to 21.3% of the population in the control group. A total of 9.2% of the population in the treatment group developed lymphopenia as compared to 5.7% in the control group. A total of 32% of the population in the treatment group developed URTI as compared to 22.1% in the control group. A total of 38.43% of the population in the treatment group developed diarrhea as compared to 22.4% in the control group. A total of 27% of the population in the treatment group developed fatigue as compared to 23.9% in the control group. A total of 19.5% of the population in the treatment group developed dyspnea as compared to 14.3% in the control group. A total of 17.3% of the population developed pneumonia as compared to 13.3% in the control group as shown in [Table 3 and Figure 7], respectively.
Figure 7.
Forest Plots for adverse effects with Daratumumab when compared with control group
Discussion
Despite the advancement in MM treatment, the management of RRMM still poses a challenge.[12] Thus, the efficacy and safety of novel therapeutics need to be studied. Daratumumab is a monoclonal antibody leading the pack with regards to RRMM management. This meta-analysis aims to contribute to existing evidence supporting the use of daratumumab in the RRMM treatment.
Considering the increase in the number of relapsed or refractory multiple myeloma patients, the role of monoclonal antibodies other than daratumumab has also been studied in RRMM to date. In ELOQUENT-3 trial, the role of Elotuzumab was studied in RRMM patients which showed an ORR of 53% in the treatment group in comparison to our study which showed an ORR of 85.2% with a significant P value of < 0.001.[13] Similar kinds of results were seen with the Eloquent- 2 trial which showed an overall response rate of 79% respectively.[3] The efficacy of Isatuximab was studied in the ICARIA-MM trial showing very good partial response (VGPR) in 27% of the population as compared to 68.5% seen in the daratumumab group observed in our study.[14] Patients included in the ICARIA-MM study were refractory to Lenalidomide or Bortezomib which was similarly seen in our study. Efficacy of pomalidomide was studied in the OPTIMISSM trial, ORR of 82.2% was observed which was very similar when seen in daratumumab group in our study.[15] Other endpoints like VGPR and CR were inferior -37%, 12.5% to our 68.5%, 35.4%.[15]
With the advent of modern medicine and increased information on targeted therapy, the role of bcl-2 inhibitor, venetoclax, was also studied in RRMM patients in BELLINI trial. A total of 32% patients in the venetoclax group achieved VGPR which was approximately half as compared to 68.5% seen in our daratumumab group, complete response was also favoring daratumumab group showing 19% in the venetoclax group as compared to 35.4% seen in the daratumumab group.[16] The role of Panobinostat was studied in RRMM patients, results for overall response rate were inferior when compared with the daratumumab group (60.7% v/s 85.2%).[17] Ixazomib, an oral proteasome inhibitor role was studied in TOURMALINE-MM1 trial showing ORR in 78.3% which was very close to 85.2% as observed in our study.[18] Another oral proteasome inhibitor named carfilzomib studied in the ENDEAVOR trial showed a very good partial response of 42% which was far less as compared to the daratumumab group showing 68.5%.[19]
The safety profile of daratumumab has always been controversial, Anemia was observed in our study in 32.1% of the cases in study as compared to 99% seen in the Eloquent 2 trial which is almost three folds higher as compared to the daratumumab combination regimen. About 40.1% of the patients included in our study developed thrombocytopenia during their course of management which is close to half of 84% seen or observed in the Eloquent 2 trial. Also, neutropenia was observed in only 30.7% of the patient in the treatment group as compared to 96%, being observed in the Isatuximab group,[14] lymphopenia was observed in 10% of patients in our study which was almost 9.2% as observed in the Elotuzumab group, respectively.[13]
URTIs were observed in 32% of patients included in our study which was three-fold higher as compared to 11.6% seen in the Elotuzumab study.[13] In contrast to the Isatuximab group in which URTI was observed in 28% of the patient population very close to the treatment group in our study.[14] Diarrhea was observed in 38.4% of the patient included in the treatment group, a similar percentage of patients developed diarrhea in OPTIMISMM trial in which the role of pomalidomide was studied.[15] In contrast to the OPTIMISMM trial, diarrhea was observed in 57.5% of the subjects in the Bellini trial, a trial recently conducted a couple of years back in 2020.[16]
Fatigue was observed in 27% of subjects included in our project as compared to 21% and 37% of patients with Isatuximab and pomalidomide groups.[14,15] Dyspnea is a rare adverse effect observed in the daratumumab treatment group which was seen in only 19.5% of the entire population studied as compared to 15% and 12% observed in Elotuzumab and venetoclax treatment group.[13,16] Columba trial was conducted back in 2020 to compare the efficacy of intravenous with subcutaneous administration, which proved equivalent efficacy of drug irrespective of its administration.[20]
Key Points
Our study focused on the role of daratumumab in relapsed or refractory multiple myeloma patients. It showed that when compared with the control, daratumumab is superior regarding primary or secondary outcomes including progression-free span, overall response rate, very good partial response, and complete response. It showed no difference regarding partial response while inferior when a minimal response was considered. As far as adverse effects were considered, Patients on daratumumab showed increased chances to develop thrombocytopenia, fatigue, URTI, neutropenia, lymphopenia, fatigue dyspnea, and diarrhea. In conclusion, the advent of daratumumab was a real game changer as it improves the primary and secondary outcomes in relapsed or refractory multiple myeloma patients but increases the risk for adverse effects when compared with the controls which can be managed by reducing the dose of treatment or by increasing the duration of subsequent treatment. So, we recommend that combination therapy should include daratumumab in the treatment of relapsed or refractory multiple myeloma patients and early referral of the patient to hematology/oncology clinic is essential to treat patients having multiple myeloma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011;186:1840–8. doi: 10.4049/jimmunol.1003032. [DOI] [PubMed] [Google Scholar]
- 2.Abdallah N, Kumar SK. Daratumumab in untreated newly diagnosed multiple myeloma. Ther Adv Hematol. 2019;10:2040620719894871. doi: 10.1177/2040620719894871. doi:10.1177/2040620719894871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang T, Wang S, Lin T, Xie J, Zhao L, Liang Z, et al. Systematic review and meta-analysis of the efficacy and safety of novel monoclonal antibodies for treatment of relapsed/refractory multiple myeloma. Oncotarget. 2017;8:34001–17. doi: 10.18632/oncotarget.16987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207–19. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- 5.Dima D, Dower J, Comenzo RL, Varga C. Evaluating daratumumab in the treatment of multiple myeloma:Safety, efficacy and place in therapy. Cancer Manag Res. 2020;12:7891–903. doi: 10.2147/CMAR.S212526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanchez L, Wang Y, Siegel DS, Wang ML. Daratumumab:A first-in-class CD38 monoclonal antibody for the treatment of multiple myeloma. J Hematol Oncol. 2016;9:51. doi: 10.1186/s13045-016-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, et al. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcg receptor-mediated cross- linking. J Immunol. 2016;197:807–13. doi: 10.4049/jimmunol.1501351. [DOI] [PubMed] [Google Scholar]
- 8.Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts van Bueren JJ, et al. Antibody- mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs. 2015;7:311–21. doi: 10.1080/19420862.2015.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mateos MV, Spencer A, Nooka AK, Pour L, Weisel K, Cavo M, et al. Daratumumab-based regimens are highly effective and well tolerated in relapsed or refractory multiple myeloma regardless of patient age:Subgroup analysis of the phase 3 CASTOR and POLLUX studies. Haematologica. 2020:105468–77. doi: 10.3324/haematol.2019.217448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costello C. An update on the role of daratumumab in the treatment of multiple myeloma. Ther Adv Hematol. 2017;8:28–37. doi: 10.1177/2040620716677523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128:37–44. doi: 10.1182/blood-2016-03-705210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–31. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos MA, Dytfeld D, Grosicki S, Moreau P, Takezako N, Hori M, et al. Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med. 2018;379:1811–22. doi: 10.1056/NEJMoa1805762. [DOI] [PubMed] [Google Scholar]
- 14.Attal M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA- MM):A randomized, multi-centre, open-label, phase 3 study. Lancet. 2019;394:2096–107. doi: 10.1016/S0140-6736(19)32556-5. [DOI] [PubMed] [Google Scholar]
- 15.Richardson PG, Oriol A, Beksac M, Liberati AM, Galli M, Schjesvold F, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM):A randomised, open- label, phase 3 trial. Lancet Oncol. 2019;20:781–94. doi: 10.1016/S1470-2045(19)30152-4. [DOI] [PubMed] [Google Scholar]
- 16.Kumar SK, Harrison SJ, Cavo M, de la Rubia J, Popat R, Gasparetto C, et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI):A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020;21:1630–42. doi: 10.1016/S1470-2045(20)30525-8. [DOI] [PubMed] [Google Scholar]
- 17.San-Miguel JF, Hungria VT, Yoon SS. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma:A multi- centre, randomized, double-blind phase 3 trial. Lancet Oncol. 2014;15:1195–206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 18.Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral Ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–34. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR):A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 20.Mateos MV, Nahi H, Legiec W, Grosicki S, Vorobyev V, Spicka I, et al. Subcutaneous versus intravenous daratumumab in patients with relapsed or refractory multiple myeloma (COLUMBA):A multicentre, open-label, non-inferiority, randomised, phase 3 trial. Lancet Haematol. 2020;7:e370–80. doi: 10.1016/S2352-3026(20)30070-3. [DOI] [PubMed] [Google Scholar]