ABSTRACT
Context:
Viral hepatitis caused 1.34 million deaths in 2015, a number comparable to the deaths caused by tuberculosis and higher than that caused by human immunodeficiency virus (HIV). Hepatitis A virus (HAV) and hepatitis E virus (HEV) are important causes of acute viral hepatitis (AVH) and acute liver failure (ALF). Due to the paucity of data, the exact burden of the disease in western India is not established.
Objective:
Considering this background, the present study aims to determine the prevalence, epidemiology, and biochemical correlation in AVH due to HAV and HEV.
Setting and Design:
It was a retrospective observational study conducted over 3 years from January 2018 to December 2020 in a tertiary care hospital of Western India.
Material and Methods:
The study population included 1,807 patients (outdoor and hospitalized) having clinical features of AVH. All serum samples from these patients were tested in duplicate for immunoglobulin M (IgM) anti-HAV and IgM anti-HEV antibodies using commercially available enzyme-linked immunosorbent assay (ELISA) kits. The liver function tests (LFTs) were also monitored.
Results:
Of the 1,807 specimens processed from the patients with AVH, 120 (6.70%) were positive for IgM anti-HAV antibodies and 154 (8.5%) were positive for IgM HEV antibodies. A total of 11 patients (0.60%) were positive for both anti-HAV IgM and anti-HEV IgM antibodies indicating HAV-HEV coinfection. Our study shows that the HAV infection was more prevalent in the pediatric age group. The HEV infection was seen in all age groups and more prevalent in the age group of 20–30 years. The infection was more prevalent from June to October, that is, during monsoon and post-monsoon seasons. Total serum bilirubin, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvic transaminase (SGPT), and alkaline phosphatase (ALP) were elevated at 85.84, 86.79, 91.5, and 83.96%, respectively, in HAV-infected and elevated at 78.12, 93.75, 67.18, and 57.03%, respectively, in HEV-infected patients. The patients with HAV-HEV coinfection had all deranged LFTs indicating more severe disease.
Conclusion:
The present study emphasizes the importance of screening all hepatitis viral markers (A, B, C, E) for early diagnosis and curtailment of outbreaks and epidemics by the public health sector reducing morbidity and mortality.
Keywords: Acute viral hepatitis, ELISA, hepatitis A virus, hepatitis E virus, liver function test
Introduction
Viral hepatitis is now recognized as a major public health challenge that requires an urgent response. As per the global hepatitis report 2017 by the world health organization (WHO), viral hepatitis caused 1.34 million deaths in 2015, a number comparable to deaths caused by tuberculosis and higher than that caused by HIV. Hepatitis A virus (HAV) and Hepatitis E virus (HEV) are important causes of acute viral hepatitis (AVH) and acute liver failure (ALF). Due to the paucity of data, the exact burden of the disease in India is not established. However, the available literature indicates a wide range and suggests that HAV is responsible for 10–30% of acute hepatitis and 5–15% of ALF cases in India. It is further reported that HEV accounts for 10–40% of acute hepatitis and 15–45% of ALF (1). There is an increased prevalence of cases and outbreaks due to HAV, HEV, and HAV-HEV coinfections throughout the country.[1,2] HAV-HEV coinfections can lead to serious complications and high mortality due to ALF both in children and adults.[1,2] The enterically transmitted viral hepatitis due to HAV and HEV continues to be one of the major communicable diseases in India reflecting poor sanitization and hygiene practices, especially in rural and semi-urban areas. Given this scenario, the National viral hepatitis control program (NVHCP) was launched by the Ministry of Health and Family Welfare, Government of India, to reduce the associated morbidity and mortality by implementing preventive and promotive measures like prompt diagnosis and management of viral hepatitis caused by hepatitis A, B, C, E viruses.[1]
HEV was first identified as a non-A, non-B hepatitis virus in 1980. In 1983, Russian virologist Mikhail Balayan visualized the virus through electron microscopy while examining his feces after self-administration of the contaminated material.[3,4] The seroprevalence of HEV is high in developing countries like India and Southeast Asia ranging from 27 to 80%. Surprisingly, studies from developed countries, such as the United States of America and the United Kingdom, have shown an unexpectedly high seroprevalence (of 21–25%) and possibly due to reasons such as subclinical infection, animal exposure, cross-reactivity with other agents, or false-positive test results. The acute disease mortality is 1–4%, with the risk being higher in pregnant women and immunodeficient patients.[1,3,4] Currently, HEV is included as a member of the family Herpesviridae, which includes two genera: Orthohepevirus and Piscihepevirus. The genus Orthohepevirus encompasses all mammalian and avian HEV variants and is subdivided into four species: A–D. Moreover, among the Orthohepevirus A species, eight genotypes are recognized: HEV 1–8. Following an incubation period of 2–6 weeks, an initial IgM response is followed by the longer-lasting IgG antibodies. The presence of IgM anti-HEV is a marker of acute infection, and a four-fold rise in titer of IgG anti-HEV between the paired acute and convalescence sera can indicate a recent HEV infection.[3,4]
HAV was first identified by Feinstone et al.[5] in 1973. HAV is a non-enveloped, 27 nm, heat-, acid- and ether-resistant ribonucleic acid (RNA) virus belonging to genus Hepatovirus of the family Picornaviridae. HAV exists as a single serotype. Due to the advances of molecular technology, seven unique genotypes (I–VII) of HAV are known.[6] The antibodies to HAV (anti-HAV) can be detected during acute illness when the serum aminotransferase levels are elevated and there is fecal shedding of the virus. The initial antibody response is predominantly of the IgM class which usually persists for 6 months. During convalescence, the anti-HAV of the IgG class becomes the predominant antibody which persists for life, providing protection against reinfection.[7,8] The infection is self-limited and does not progress to chronic liver disease.[7,9] With the development of safe and effective hepatitis A vaccines in the early 1990s, understanding hepatitis An epidemiology has taken on a new importance because this information is needed to make well-informed decisions about the prevention strategies and appropriate vaccine use.[10]
Due to the paucity of data, the exact burden of the disease in this part of India is not established. The present study was, thus, planned to assess the infection rate, epidemiology, and biochemical profile of HAV, HEV, and their coinfections in AVH cases in our hospital set up to plan preventive measures by the public health sector like planning vaccine strategies for HAV, better sanitation program, and help clinicians in prompt diagnosis and management of AVH cases, especially in severe coinfections and immunosuppressed patients.
Material and Methods
This study was a retrospective observational study conducted over 3 years from January 2018 to December 2020. Institutional Ethics committee approval was obtained for same on 30-04-2021. The study population included 1,807 patients (outdoor and hospitalized) having clinical features of AVH such as fatigue, anorexia, nausea, vomiting, jaundice, and elevated liver enzymes. After obtaining informed consent, 5 mL of blood was collected from each patient, centrifuged, and the serum was stored at − 20°C till analysis. All samples were tested in duplicate for IgM anti-HAV and IgM anti-HEV antibodies using commercially available enzyme-linked immunosorbent assay (ELISA) kits (IgM HAV, IgM HEV Diapro Italy, HAV IgM ELISA Kinesis Dx, HEV IgM ELISA Kinesis Dx). Each serum sample was also tested for hepatitis B surface antigen (HBsAg) and IgM anti-hepatitis C virus (HCV) antibodies by ELISA (using Merilisa HBsAg and Merilisa HCV kits, Meryl Diagnostics Pvt., Ltd.) to rule out hepatitis B (HBV) and HCV infections. Biochemical parameters such as liver function tests (LFTs) comprising total bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) levels were also determined. The data collected were analyzed using SPSS version 11.0 for windows (SPSS, Inc., Chicago, IL, USA). The Chi-square test was used for analyzing the qualitative variables.
Results
A total of 1,807 serum samples from clinically-suspected cases of AVH were received over 3 years from January 2018 to December 2020. Of the 1,807 specimens, 120 (6.70%) were positive for IgM anti-HAV antibodies and 154 (8.5%) were positive for IgM HEV antibodies. Eleven cases (0.60%) were positive for both anti-HAV IgM and anti-HEV IgM antibodies indicating HAV-HEV coinfection. All the patients were tested negative for HBV and HCV infections. Of the 120 patients positive for HAV IgM antibodies, 57 (47.5%) were males and 63 (52.5%) were females. Of the 154 patients positive for HEV IgM antibodies, 71 (46.10%) were males and 83 (53.89%) were females.
This study shows that the HAV infection was more prevalent in the age group of 6–10 years (35/120—29%), followed by the age group of 0–5 years (16/120—13.3%), and 11–15 years (16/120—13.3%). The HEV infection was seen in all age groups and more prevalent in the age group of 21–25 years (23/154—14.9%) and 26–30 years (22/154—14.28%) [Figure 1].
Figure 1.
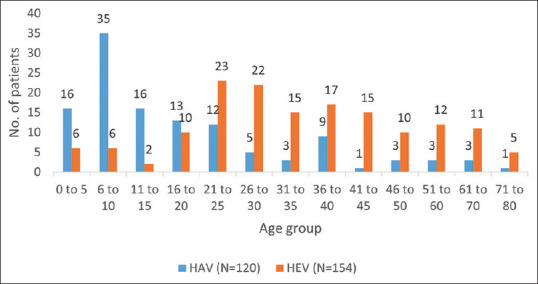
Age distribution of HAV and HEV patients
The HAV and HEV infections were prevalent all around the year with the maximum number of cases from June to October, that is, during the monsoon and post-monsoon seasons [Figure 2].
Figure 2.
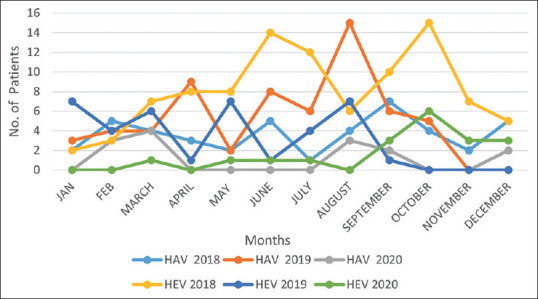
Seasonal distribution of HAV and HEV infections
In the HAV-positive patients, the total serum bilirubin, SGOT, SGPT, and ALP were elevated at 85.84, 86.79, 91.5, and 83.96%, respectively. In the HEV-positive patients, the total serum bilirubin, SGOT, SGPT, and ALP were elevated at 78.12, 93.75, 67.18, and 57.03%, respectively [Tables 1–3]. Of the 11 patients with HAV and HEV coinfections, LFT was available in eight patients. All eight patients had deranged bilirubin (100%), SGOT (100%), and SGPT (100%). All seven out of the eight (87.5%) patients had ALP levels.
Table 1.
Derangement of liver function tests in patients with acute viral hepatitis due to hepatitis A virus and hepatitis E virus
| Parameter | Deranged LFT in HAV Patients (n=106) | Deranged LFT in HEV Patients (n=128) |
|---|---|---|
| Bilirubin | 91/106 (85.84%) | 100/128 (78.12%) |
| SGOT | 92/106 (86.79%) | 120/128 (93.75%) |
| SGPT/ALT | 97/106 (91.5%) | 86/128 (67.18%) |
| Alkaline phosphatase | 89/106 (83.96%) | 73/128 (57.03%) |
*Note: LFTs were available in 106 of the total 120 HAV patients and 128 of the total 154 HEV patients
Table 3.
SGPT and SGOT in patients with acute viral hepatitis due to HAV and HEV
| SGOT/AST | HAV (106) (Mean-388.52) | HEV (128) (Mean- 241.66) | SGPT/ALT | HAV (106) (Mean-336.57) | HEV (128) (Mean-295.51) |
|---|---|---|---|---|---|
| 1-40 (Normal) | 14 (13.20%) | 8 (6.25%) | 1-40 (Normal) | 9 (8.49%) | 42 (32.8%) |
| 41-1000 | 81 (76.4%) | 117 (91.4%) | 41-1000 | 93 (87.73%) | 82 (64.06%) |
| 1001-2000 | 10 (9.43%) | 3 (2.34%) | 1001-2000 | 3 (2.83%) | 3 (2.34%) |
| 2001-3000 | 0 | 0 | 2001-3000 | 0 | 1 (0.78%) |
| >3000 | 1 (0.94%) | 0 | >3000 | 1 (0.94%) | 0 |
*SGOT/AST - Serum glutamic oxaloacetic transaminase /aspartate aminotransferase (AST), SGPT/ALT - Serum glutamic pyruvic transaminase/ alanine aminotransferase (ALT)
Table 2.
Serum bilirubin and alkaline phosphatase in patients with acute viral hepatitis
| Serum Bilirubin (mg/dL) | HAV (n=106) (Mean -5.16) | HEV (128) (Mean 7.5) | Alkaline Phosphatase | HAV (106) (Mean-619.75) | HEV (128) (Mean- 491.70) |
|---|---|---|---|---|---|
| 0-1.2 mg/dL (Normal) | 15 (14.15%) | 28 (22.22%) | 25-145 (Normal) | 17 (16.19%) | 55 (42.9%) |
| 1.3-3 mg/dL Anicteric | 23 (21.69%) | 26 (19.04%) | 146-500 | 36 (34.28%) | 48 (37.5%) |
| Hyperbilirubinemia | |||||
| 3.1-10 mg/dL | 57 (53.77%) | 37 (29.36%) | 500-1000 | 41 (39.04%) | 21 (16.40%) |
| 10.1-20 mg/dL | 7 (6.60%) | 26 (20.63%) | 1000-2000 | 12 (11.42%) | 4 (3.80%) |
| 20.1-30 mg/dL | 4 (3.77%) | 10 (7.93%) | >2001 | 0 | 0 |
| >30 mg/dL | 0 | 1 (0.10%) | - | - | - |
Discussion
In the present study, 14.55% of the AVH patients had a reactive viral marker (HAV 6.70%, HEV 8.5%, and coinfection 0.60%) comparable to a study by Samaddar et al.[7] from Mumbai reporting the AVH (HAV, HEV) infection rate of 18.7% with HAV, HEV, and coinfection rates as 6.96,9.63, and 2.07%, respectively. However, it is lower than that reported in other AVH studies from Mangalore by Joon et al.[10] as 29.9% (HAV 19.3%, HEV 10.54%, coinfection 11.55%), and as 31.5% (HAV 13.3%, HEV 17.3%, coinfection 0.8%) in a study by Radhakrishnan et al. from South India.[11] In a study from Uttarakhand by Kalita et al., 37.6% (HAV 14.7%, HEV 28.0% Combined 5.2%) of the AVH patients had a reactive viral marker.[12] Several studies from India and abroad have reported a varying prevalence ranging from 1.7 to 67% for HAV and 12.6 to 78.6% for HEV.[7,10,11,12,13,14,15,16] Such wide variation in positivity could be due to the differences in the study population, living standard, sanitation, environmental hygiene, underreporting, and the reluctance of patients seeking medical care and type of samples—whether sporadic or from outbreaks. Most of the seroprevalence studies included both IgM and IgG antibodies kits for HAV and HEV, thus, considering both acute and chronic hepatitis. In the present study, the detection of only IgM antibodies was performed, thus, indicating the prevalence of HAV and HEV in AVH cases only. In the present study, HEV (8.5%) was identified as the major cause of AVH and was more common than HAV (6.70%), which is in concordance with the other studies from different parts of the country.[1,7,10,11,12] The decline in HAV has generally been explained by improvements in socioeconomic status, improved access to clean water, and sanitation. The HAV infection generally confers lifelong immunity to all strains of HAV infection.[13,14] The increased HEV prevalence may be due to conflicting reports regarding the persistence and protective role of anti-HEV antibodies, reinfection by divergent strains of HEV. It is unclear for how long HEV-specific anamnestic B- and T-cell responses exist and whether they have a role against reinfection.[2,13,14,15]
India is hyper-endemic for HAV infection. Studies conducted in the 2000s observed that nearly 90% of the adolescents, adults, and most children acquired immunity to HAV infection in their preschool years. Recent Indian studies have indicated a shift in the epidemiology of the HAV infection in India over the past decade due to improved economy, hygiene, and sanitation. The overall seroprevalence of HAV as 29% (35/120) in the group aged 6–10 years was higher than in the age group of 0 to 5 years (13.3% [16/120]) and was comparable to a study by Arankalle V et al.[17] As the age at the time of infection increases, symptomatic cases become more common, and chances of severe infections and ALF also increase. Hence, there is a need for obtaining such nationwide data to better understand the epidemiology of hepatitis A in India to plan the strategy for hepatitis A immunization.[2,12,18,19] The HEV infection was more prevalent in young adults, that is, 21–25 years (14.9% [23/154]) and 26–30 years (14.28% [22/154]). This finding correlates with the trend and other Indian studies (7, 10, 11, 12). It is possible that the HEV infection is usually anicteric and goes unnoticed in children.[12] Also, reinfection with hepatitis E in adults may occur as HEV infection does not provide lifelong immunity due to reinfection with divergent strains of HEV.[2,13,14,15]
It was observed that the HAV and HEV infections occurred throughout the year, although seasonal variation in the incidence was evident with the maximum number of cases reported from June to October, that is, monsoon and post-monsoon seasons. This finding is consistent with other Indian studies.[7,10,11,12] The HAV and HEV infections are transmitted by the feco-oral route, shortage of drinking water supply in summer, and cross-contamination of drinking water with sewage during the monsoons. These lead to more chances of acquiring these infections.
HAV and HEV affect hepatocytes resulting in abnormal functions of the liver. The development of jaundice is a characteristic of viral hepatitis. SGPT and SGOT are important liver enzymes that help to process the proteins. They might be raised if the liver is inflamed or injured. Alkaline phosphatase enzymes are raised when there is a blockage in the liver or bile duct. In HAV-positive patients, the total serum bilirubin (0.2–1.2 mg/dL), SGOT/AST (10–50 U/L), SGPT/ALT (7–55 U/L), and ALP (20–140 IU/L) were elevated at 85.84, 86.79, 91.5, and 83.96%, respectively. In HEV-positive patients, total serum bilirubin, SGOT, SGPT, and ALP were elevated at 78.12, 93.75, 67.18, and 57.03%, respectively. Of the 11 patients with HAV and HEV coinfections, the LFTs were available in eight patients. Of the eight patients, three patients had high levels of liver enzymes SGOT (500 U/L in all three) and specifically SGPT (1000, 1500, and 1900 U/L). This suggests that dual infection can lead to severe disease manifestations such as ALF and hepatic encephalopathy. This finding was consistent with the other studies by Samaddar et al.,[7] Mittal et al.,[20] and Park et al.[21]
Conclusion
The HEV seroprevalence (8.5%) is higher than HAV (6.70%) with the HEV predominating in relatively older patients (age group 21–30 years) compared to younger subjects (0–10 years) in HAV infections. The coinfection rate was 0.60% and all cases had severe deranged LFTs. This emphasizes regular testing of HAV and HEV in patients of AVH on a routine basis, especially for the management of severe infections in HAV-HEV coinfections, high-risk groups like pregnant females, chronic liver diseases, and immunosuppressed patients. With a feco-oral route of transmission, periodic surveillance, especially in monsoon and post-monsoon, is of utmost importance for early diagnosis and curtailment of outbreaks and epidemics by the public health sectors through proper sanitization, hygiene, and public awareness. Considering the high seroprevalence of anti-HAV antibodies in the general population, mass immunization with the hepatitis A vaccine may not be cost-effective in a country like India, however, its use in risk populations, like chronic liver disease patients, during the onset of outbreaks and epidemics, travelers to endemic areas, and in younger children, should be considered.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.National Viral Hepatitis Control Program –Operational Guidelines 2018 issued by Ministry of Health and Family welfare, Government of India [Google Scholar]
- 2.Kumar T, Shrivastava A, Kumar A, Laserson KF, Narain JP, Venkatesh S, et al. Viral Hepatitis Surveillance India, 2011–2013. Morbid Mortal Wkly Rep MMWR. 2015;64:758–62. doi: 10.15585/mmwr.mm6428a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerra JAAA, Kampa KC, Morsoletto DGB, Junior AP, Ivantes CAP. Hepatitis E:A literature review. J Clin Transl Hepatol. 2017;5:376–83. doi: 10.14218/JCTH.2017.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237–44. doi: 10.1056/NEJMra1204512. [DOI] [PubMed] [Google Scholar]
- 5.Feinstone SM, Kapikian AZ, Purceli RH. Hepatitis A:Detection by immune electron microscopy of a virus like antigen associated with acute illness. Science. 1973;182:1026–8. doi: 10.1126/science.182.4116.1026. [DOI] [PubMed] [Google Scholar]
- 6.Lin KY, Chen GJ, Lee YL, Huang YC, Cheng A, Sun HY, et al. Hepatitis A virus infection and hepatitis A vaccination in human immunodeficiency virus-positive patients:A review. World J Gastroenterol. 2017;23:3589–606. doi: 10.3748/wjg.v23.i20.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samaddar A, Taklikar S, Kale P, Kumar CA, Baveja S. Infectious hepatitis:A 3-year retrospective study at a tertiary care hospital in India. Indian J Med Microbiol. 2019;37:230–4. doi: 10.4103/ijmm.IJMM_19_197. [DOI] [PubMed] [Google Scholar]
- 8.Hadem J, Manns MP. Immune response to hepatitis A and E viruses. In: Gershwin ME, Vierling JM, Manns MP, editors. Liver Immunology. New Jersey: Humana Press; 2007. pp. 163–77. [Google Scholar]
- 9.Kar P. Hepatitis E virus infection during pregnancy:Why is the disease stormy? Medicine. 2012;22:459–62. [Google Scholar]
- 10.Joon A, Rao P, Shenoy SM, Baliga S. Prevalence of Hepatitis A virus (HAV) and Hepatitis E virus (HEV) in the patients presenting with acute viral hepatitis. Indian J Med Microbiol. 2015;33:102–5. doi: 10.4103/0255-0857.150908. [DOI] [PubMed] [Google Scholar]
- 11.Radhakrishnan S, Raghuraman S, Abraham P, Kurian G, Chandy G, Sridharan G. Prevalence of enterically transmitted hepatitis viruses in patients attending a tertiary –Care hospital in South India. Indian J Pathol Microbiol. 2000;43:433–6. [PubMed] [Google Scholar]
- 12.Kalita D, Paul M, Deka S, Badoni G, Gupta P. Simultaneous infection of Hepatitis A and Hepatitis E viruses among acute viral hepatitis patients:A hospital-based study from Uttarakhand. J Family Med Prim Care. 2020;9:6130–4. doi: 10.4103/jfmpc.jfmpc_1373_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen KH, Koopman JS. Declining Hepatitis A seroprevalence:A global review and analysis. Epidemiol Infect. 2004;132:1005–22. doi: 10.1017/s0950268804002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lemon SM, Ott JJ, Damme PM, Shouval D. Type A viral hepatitis:A summary and update on the molecular virology, epidemiology, pathogenesis, and prevention. J Hepatol. 2018;68:167–84. doi: 10.1016/j.jhep.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni S, Sharma M, Tripathy A. Antibody and memory B cell Responses in Hepatitis E recovered Individuals, 1-30 years post Hepatitis E Virus infection. Sci Rep. 2019;9:4090. doi: 10.1038/s41598-019-40603-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chadha MS, Walimbe AM, Chobe LP, Arankalle VA. Comparison of etiology of sporadic acute and fulminant viral hepatitis in hospitalized patients in Pune, India during 1978-81 and 1994-97. Indian J Gastroenterol. 2003;22:11–5. [PubMed] [Google Scholar]
- 17.Arankalle V, Mitra M, Bhave S, Ghosh A, Balasubramanian S, Chatterjee S, et al. Changing epidemiology of hepatitis A virus in Indian children. Vaccine Development Ther. 2014;4:7–11. [Google Scholar]
- 18.Pandit A, Mathew LG, Bavdekar A, Mehta S, Ramakrishnan G, Datta S, et al. Hepatotropic viruses as etiological agents of acute liver failure and related-outcomes among children in India:A retrospective hospital-based study. BMC Res Notes. 2015;8:381. doi: 10.1186/s13104-015-1353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naher B, Islam R, Ghosal S, Nahid KL RR. Seroprevalence and co-infection of hepatitis A and Hepatitis E viruses in children. J Neonatol Clin Pediatr. 2021;8:1–6. [Google Scholar]
- 20.Mittal A, Bithu R, Vyas N, Maheshwari RK. Prevalence of hepatitis A virus and hepatitis E virus in the patients presenting with acute viral hepatitis at a tertiary care hospital Jaipur Rajasthan. N Niger J Clin Res. 2016;5:47–50. [Google Scholar]
- 21.Park JH, Kim BS, Lee CH, Kim SY, Seo JH, Hur CJ. A case of co infection of hepatitis A and E virus with hepatic encephalopathy. Korean J Med. 2011;80(Suppl 2):S101–5. [Google Scholar]


