ABSTRACT
Background:
Vascular endothelial growth factor (VEGF) stimulates angiogenesis, increases vascular permeability and seems to correlate to aggressiveness of tumors. Thyroid cancer has been found to have higher levels of VEGF expression. Thyroid stimulating hormone (TSH) is the most important thyroid hormone, yet few researches have been done on its relationship with VEGF.
Aim:
To study the clinical and demographic profile of thyroid lesions (benign and malignant) and to explored the relationship between VEGF expression (using immunochemistry) and serum TSH level.
Methods:
This prospective, observational study includes 61 patients of thyroid lesions who underwent partial, hemi, subtotal or total thyroidectomy as the primary treatment from June 2014 and July 2016. Tissue specimens of thyroid lesions for immunohistochemistry study of VEGF expression were done. Serum TSH was done using Chemiluminiscence technique and correlated to VEGF expression.
Results:
The mean age of patient was 36.26 ± 11.53 years (range 20-50 years) with female preponderance. Swelling was the most common presenting symptom. Of 61 patients, 37 (60.65%) patients were benign and 24 (39.35%) were malignant thyroid lesions. The mean TSH level in benign group was 1.92 ± 0.94 mIU/liter and malignant group was 2.73 ± 1.74 mIU/liter which was statistically significant (P = 0.023). VEGF expression was strongly positive (3+) in 26 (42.62%) patients and negative/equivocal (1+ & 2+) in 35 (57.38%) patients. In benign group, 10 (27.0%) patients were strongly positive for VEGF whereas in malignant group, 16 (66.7%) patients were strongly positive for VEGF showed significant association (P = 0.002). On comparing TSH level of benign, malignant and total patients separately with VEGF expression, significant association were also observed (P < 0.001, P = 0.004 and P < 0.001, respectively).
Conclusion:
VEGF was strongly expressed in malignant thyroid lesions which are having high serum concentration of serum TSH level. Serum TSH levels reveal a significant correlation with VEGF expression.
Keywords: Thyroid lesion, thyroid stimulating hormone, vascular endothelial growth factor
Introduction
Thyroid problems are the most frequent endocrine problem on the planet.[1] This phenomena occurs all across the world, and India is no exception. Thyroid diseases are projected to affect 42 million Indians.[2] Thyroid disorders are distinct from other diseases in terms of ease of diagnosis, medical treatment quality, and the degree of exposure that even a slight thyroid swelling provides to the treating physician. There are various well-established malignancy predictors in thyroid nodules, including finding hard and fixed lesions, rapid progression of nodule, dysphagia, lymphadenopathy or associated hoarseness, although all of these are relatively uncommon in diagnosis.[3] Certain risk factors include age (<20 years or >70 years), male sex and previous history of exposure to irradiation.[4]
Thyroid stimulating hormone (TSH) is a glycoprotein produced by anterior pituitary gland thyrotropic cells that binds to particular cell surface receptors in the thyroid gland and increases thyroid hormone synthesis.[5] TSH is also thought to be a key factor in stimulating the number, size, and activity of thyrocytes cells, that are the origin for differentiated thyroid cancers.[6] Higher TSH values in patients with thyroid nodules, even if under the limit, have been linked to a subsequent diagnosis of thyroid cancer in several studies.[7,8,9] Furthermore, increased serum TSH levels have been linked to advanced stage thyroid carcinoma.[10] TSH may play a substantial role in thyroid cancer formation and/or progression, based on these findings. TSH has also been found to promote thyroid cancer growth, invasion, and angiogenesis.
The role of angiogenesis in cancer formation, growth, and metastasis is well understood, and VEGF has been identified as a key angiogenesis mediator in the thyroid gland.[11,12] Upregulation of VEGF in human thyroid carcinoma is linked to malignancy and a poor prognosis.[13,14,15] Several studies have found a substantial link between VEGF expression and serum TSH levels in vitro.[16,17] Nonetheless, there is a scarcity of information on the relationship between VEGF expression and serum TSH content in human tissue, which was the focus of this study.
Methods
This is a prospective, observational hospital-based study that was conducted between June 2014 and July 2016 after receiving ethical approval from the Institute of Medical Sciences, Banaras Hindu University’s Ethical Committee. A total of 61 patients with metastatic and non-metastatic thyroid cancer as well as benign thyroid lesions (Grave’s thyroiditis, Hashimoto’s thyroiditis, and Colloid goiter) were included in the study. Patients with concomitant malignancies, those taking thyroxin, antithyrod medicines or steroids, and those who were pregnant were all excluded from the study. Prior to the evaluation, each patient signed a written informed consent form.
All of the patients underwent a thorough medical history, clinical examination, routine blood tests, thyroid function tests (T3, T4, and TSH), X-ray neck, thyroid ultrasonography, and fine needle aspiration cytology (FNAC). Patients underwent surgery after giving written informed consent (hemithyroidectomy or partial, subtotal and total thyroidectomy). In the University Hospital’s Endochrinology Lab, the serum TSH was measured using the Chemiluminiscence technique.
Tissue specimens of thyroid lesions for immunohistochemistry study of VEGF expression were done using antibodies in formalin fixed and paraffin embedded histological sections. Rabbit anti-human polyclonal antibodies to both receptors obtained from purified immunoglobin fractions, diluted in PBS, pH 7.6, 1% BSA and. 09% sodium azide was used as a primary antibody. The secondary antibody was biotinylated rabbit anti-goat IgG. Antibody and detection kit was obtained from “BIOGENEX” through a proper agency. Tissue sections were cut into slices at approximately 0.5 cm and the specimen were kept in 10% buffered formalin for 18-24 hours for proper fixation, following which the specimen were grossed by trained pathologist to obtain representative tissue sections which were processed routinely in the conventional way for embedding in paraffin wax. 4 mm section were cut and placed on glass slide, one slide of each tissue was stained with Hematoxylin and Eosin (H & E). After H & E staining these sections were evaluated under light microscopy for histopathological details. Blocks of the viable tumor representative area were selected for immunohistochemistry staining with antibodies for the VEGF. A block of angiocarcinoma tissue provided by Biogenex served as positive control for VEGF staining.
After proper staining, VEGF stained slides were subjected to microscopic evaluation. Only invasive tumor cells were considered and the cytoplasmatic staining intensity was scored using a semi-quantitative scale. In statistical analysis, strong immunoreactivity (3+ or more) was taken as positive results whereas weak or absent staining (0 to 2+) was considered negative.
Interpretation of immunohistochemical results was made without knowledge of clinical outcome and the status of other prognostic variables.
The statistical analysis was done using SPSS for Windows version 16.0 software (IBM Inc.). For categorical data Chi-square and Fischer’s Exact test was used. For comparing two groups of mean Students ‘t’ test was used. The critical value of ‘p’ indicating the probability of significant difference was taken as < 0.05 for comparison.
Results
The mean age of patients was 36.26 ± 11.53 years (range 20-50 years) with female preponderance (n = 50). Swelling was the most common presenting symptom. Euthyroid was the most common sign present in 54 (88.52%) patients followed by hypothyroidism in 6 (9.83%) and hyperthyroidism only 1 (1.63%) case. Solitary thyroid nodules were present in 30 (49.18%) patients while multi-nodular goiter in 31 (50.82%).
Of 61 patients, 37 (60.65%) patients were benign and 24 (39.35%) were malignant thyroid lesions on histopathology. In benign group, multi-nodular goiter was present in majority of patients (n = 12) followed by follicular adenoma (n = 10), colloid goiter (n = 6), adenomatous goiter (n = 5), thyroiditis (n = 3) and hurthle cell adenoma (n = 1). In malignant group, majority of patients were papillary carcinoma (n = 17) followed by follicular carcinoma (n = 5) and medullary carcinoma (n = 2). In benign group, solitary thyroid nodules was present in 12 (32.4%) patients and 25 (67.6%) patients were multi-nodular goiter while in malignant group, solitary thyroid nodules were present in 18 (75.0%) patients and multi-nodular in 6 (25.0%) patients which showed significant association (P < 0.001). The relationship between demographic, clinical, laboratory and radiological characteristics of thyroid lesions in both benign and malignant is shown in Table 1.
Table 1.
Relationship between demographic, clinical, laboratory and radiological characteristics of thyroid lesions
| Benign (n=37) n (%) | Malignant (n=24) n (%) | P | |
|---|---|---|---|
| Age group (years) | |||
| 30-40 | 6 (16.2%) | 13 (54.2%) | 0.004 |
| 41-50 | 23 (62.2%) | 10 (41.7%) | |
| >50 | 8 (21.6%) | 1 (4.2%) | |
| Mean±SD | 47.27±6.35 | 41.42±6.21 | 0.001 |
| Gender | |||
| Male | 10 (27.0%) | 1 (4.2%) | 0.023 |
| Female | 27 (73.0%) | 23 (95.8%) | |
| Duration of disease | 0.374 | ||
| <12 months | 14 (37.8%) | 12 (50.0%) | |
| 12-36 months | 10 (27.0%) | 3 (12.5%) | |
| >36 months | 13 (35.1%) | 9 (37.5%) | |
| Mean±SD | 38.84±47.65 | 49.75±67.654 | 0.463 |
| Symptoms | |||
| Swelling | 36 (97.3%) | 24 (100.0%) | 0.416 |
| Pain | 14 (37.8%) | 3 (12.5%) | 0.031 |
| Fever | 1 (2.7%) | 2 (8.3%) | 0.320 |
| Dyspnoea | 2 (5.4%) | 0 (0.0) | 0.515 |
| Palpitation | 1 (2.7%) | 2 (8.3%) | 0.320 |
| Pallor | 0 (0.0%) | 1 (4.2%) | 0.211 |
| Lymphadenopathy | 0 (0.0%) | 1 (4.2%) | 0.211 |
| Functional status | |||
| Hyperthyroidism | 1 (2.7%) | 0 (0.0%) | 0.266 |
| Hypothyroidism | 2 (5.4%) | 4 (16.6%) | |
| Euthyroidism | 34 (91.9%) | 20 (83.3%) | |
| Size of nodule (cm) | |||
| <5 | 12 (32.4%) | 18 (75.0%) | 0.001 |
| >5 | 25 (67.6%) | 6 (25.0%) | |
| Mean±SD | 6.14±1.97 | 5.11±1.14 | 0.023 |
| Nodularity | |||
| STN | 12 (32.4%) | 18 (75.0%) | 0.001 |
| Multi-nodular | 25 (67.6%) | 6 (25.0%) | |
| Hemoglobin | 11.94±1.46 | 11.6250±1.28376 | 0.380 |
| T3 | 60.304±61.65 | 67.1071±79.02013 | 0.708 |
| T4 | 13.42±15.22 | 12.7971±15.25253 | 0.877 |
| TSH (Mean±SD) | 1.92±0.94 | 2.73±1.74 | 0.023 |
| Calcium level | 8.94±0.94 | 9.33±1.10 | 0.145 |
| Ultrasound findings | |||
| Probably benign | 32 (86.5%) | 1 (4.2%) | <0.001 |
| Suspiciously malignant | 5 (13.5%) | 23 (95.8%) |
The mean hemoglobin, T3, T4 and serum calcium level were comparable in both benign and malignant group (P = 0.380, P = 0.708, 0.877 and 0.145 respectively). The mean TSH level was significantly raised in malignant group (2.73 ± 1.74 mIU/liter) as compared to 1.92 ± 0.94 mIU/liter in benign group (P = 0.023) [Table 1]. On ultrasonography, 32 (86.5%) patients were probably benign and 5 (13.5%) patients were suspiciously malignant in benign group whereas, in malignant group, 23 (95.8%) patients were suspiciously malignant and only 1 case was probably benign which showed significant association (P < 0.001) [Table 1].
VEGF expression was strongly positive (3+) in 26 (42.62%) patients and negative/equivocal (1+ & 2+) in 35 (57.38%) patients. 15 (42.9%) patients in TSH range from <1.39 mIU/liter were weak to moderately positive for VEGF and strongly positive was seen in 3 (11.5%) patients. In TSH range 1.4-2.49 mIU/liter, 18 (51.4%) patients were weak to moderately positive for VEGF and 6 (23.1%) were strongly positive. While in TSH level >2.5 mIU/liter, only 2 (5.7%) patients were weak to moderately positive and 17 (65.4%) patients were strongly positive for VEGF showed significant association (P < 0.001) [Table 2]. On comparing TSH level of benign and malignant patients individually with VEGF expression, significant association were also observed (P < 0.001 and P = 0.004 respectively) [Table 2].
Table 2.
Correlation between age, nodule size, nodularity and TSH with VEGF expression
| VEGF | P | ||
|---|---|---|---|
|
| |||
| 1+ & +2 (Negative/Equivocal) (n=35) | +3 (Positive) (n=26) | ||
| Age group (years) | |||
| 30-40 | 11 (31.4%) | 8 (30.8%) | 0.992 |
| 41-50 | 19 (54.3%) | 14 (53.8%) | |
| >50 | 5 (14.3%) | 4 (15.4%) | |
| Size of nodule (cm) | |||
| <5 | 18 (51.4%) | 12 (46.2%) | 0.683 |
| >5 | 17 (48.6%) | 14 (53.8%) | |
| Nodularity | |||
| STN | 14 (40.0%) | 16 (61.5%) | 0.096 |
| Multi-nodular | 21 (60.0%) | 10 (38.5%) | |
| TSH (Benign n=37) | |||
| <1.39 | 11 (40.7%) | 2 (20.0%) | <0.001 |
| 1.4-2.49 | 14 (51.9%) | 1 (10.0%) | |
| >2.5 | 2 (7.4%) | 7 (70.0%) | |
| TSH (Malignant n=24) | |||
| <1.39 | 4 (50.0%) | 1 (6.2%) | 0.004 |
| 1.4-2.49 | 4 (50.0%) | 5 (31.2%) | |
| >2.5 | 0 (0.0%) | 10 (62.5%) | |
| TSH (Total n=61) | |||
| <1.39 | 15 (42.9%) | 3 (11.5%) | <0.001 |
| 1.4-2.49 | 18 (51.4%) | 6 (23.1%) | |
| >2.5 | 2 (5.7%) | 17 (65.4%) | |
Of 37 patients in benign group, follicular adenoma [Figure 1a and b] was the most common (n = 4) lesion strongly positive for VEGF followed by solitary thyroid nodule, thyroiditis and adenomatous goiter [Figure 2a and b] of 2 patients each. In malignant group (n = 24), papillary carcinoma [Figure 3a and b] was the most common (n = 12) lesion strongly positive for VEGF followed by follicular and medullary carcinoma of 2 patients each. On comparing VEGV expression between benign and malignant group, 10 (27.0%) patients were strongly positive for VEGF whereas in malignant group, 16 (66.7%) patients were strongly positive for VEGF showed significant association (P = 0.002) [Table 3].
Figure 1.
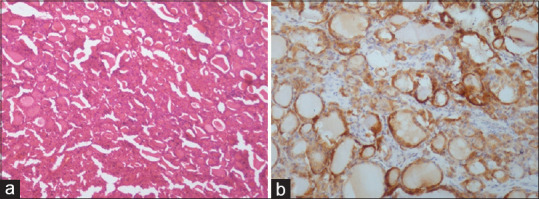
[a] H&E staining - Follicular adenoma. [b] IHC - Follicular adenoma showing moderate to strong cytoplasmic positivity (+3) for VEGF
Figure 2.
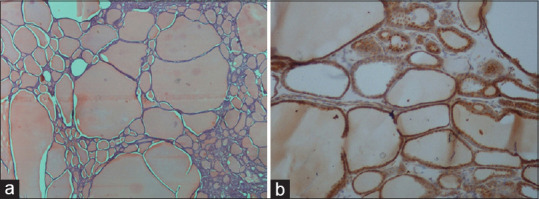
[a] H&E - Adenomatous goiter showing enlarged thyroid follicles filled with colloid. [b] IHC - Adenomatous goiter showing moderate to strong cytoplasmic positivity (+3) in many follicles
Figure 3.
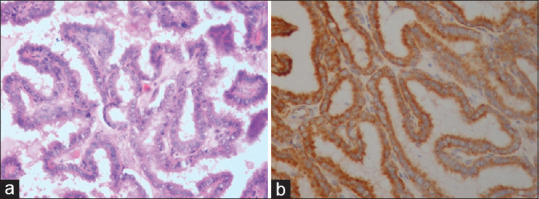
[a] H&E – Papillary thyroid carcinoma (PTC) showing papillae and nuclear features of PTC. [b] IHC – Papillary thyroid carcinoma showing strong diffuse cytoplasmic positivity (+3) for VEGF
Table 3.
Correlation between benign and malignant histopathological features with VEGF expression
| VEGF | P | ||
|---|---|---|---|
|
| |||
| 1+ & +2 (Negative/equivocal) (n=35) | +3 (Positive) (n=26) | ||
| Histopathology Benign (n=37) | |||
| STN | 10 | 2 | 0.325 |
| MNG | 1 | 0 | 0.999 |
| Colloid Goitre | 1 | 0 | 0.999 |
| Hurthle cell Adenoma | 1 | 0 | 0.999 |
| Thyroiditis | 1 | 2 | 0.106 |
| Adenomatous goiter | 3 | 2 | 0.482 |
| Follicular adenoma | 10 | 4 | 0.868 |
| Histopathology Malignant (n=24) | |||
| Papillary carcinoma | 5 | 12 | 0.525 |
| Follicular carcinoma | 3 | 2 | 0.155 |
| Medullary carcinoma | 0 | 2 | 0.869 |
| Histopathology Total (n=61) | |||
| Benign | 27 | 10 | 0.002 |
| Malignant | 8 | 16 | |
Discussion
The purpose of this study is to see if there was a relationship between VEGF expression and serum TSH levels in thyroid lesions (benign and malignant). The average age of the patients in our study was 36.26 ± 11.53 years (range 20-50 years), with a female preponderance (n = 50). Patients in the malignant group were younger than those in the benign group (41.42 ± 6.21 vs 47.27 ± 6.35 years; P = 0.001). It agrees with the findings of a study conducted in India by Nagarkar et al.[18]
In studies, the average incidence of thyroid cancer in individuals with hyperthyroidism ranged from 1.6 to 21.1% during surgery, as previously stated.[19] It differs significantly from our findings, which show that there are no patients with hyperthyroidism in the malignant group, whereas 4 (16.6%) patients have hypothyroidism symptoms and around 20 (83.3%) are euthyroid. TSH levels above the average population are also related with a significantly increased risk of thyroid cancer than TSH levels below the average.[10] Most patients with thyroid nodules are regarded to be euthyroid, according to Welker et al.,[20] with less than 1% of nodules causing hyperthyroidism or malignant thyrotoxicosis.
In our study, solitary thyroid nodules was found in 12 (32.4%) of benign patients and 25 (67.6%) of patients with multi-nodular goiter, while solitary thyroid nodules were found in 18 (75.0%) of malignant patients and 6 (25.0%) of patients with multi-nodular goiter. The association between benign and malignant was found to be statistically significant (P < 0.001). It agrees with a study conducted by Lema et al.[21]
In our study, 12 (32.4%) patients had nodule size <5 cm and 25 (67.6%) patients had nodule size >5 cm in benign group while 18 (75%) patients were nodule size <5 cm and 6 (25.0%) patients were >5 cm in malignant group. It was found that there was a statistically significant correlation between the size of the thyroid nodule and its existence (P < 0.001) being benign or malignant. According to a study conducted by Nam-Goong et al.,[22] 8% of the 25 patients with incidentally observed thyroid nodules <5 mm, 15% of the 153 patients with 5–10 mm nodules and 13% of the 139 patients with 10–15 mm nodules had cancer. The thyroid nodule size does not predict the risk of differentiated thyroid carcinoma.
High-resolution ultrasound with color Doppler is key to thyroid lesion diagnosis. Over the past decade or two, a number of ultrasonographic features have been established which help predict the risk of malignancy in a nodule. In our study, we found that in the histologically proven benign group, 32 (86.5%) patients were probably benign, and 5 (13.5%) patients were ultrasonographically suspiciously malignant, while in the histologically proven malignant group, 23 (95.8%) were suspiciously malignant, and only 1 case was probably benign. The correlation is considered to be statistically significant (P < 0.001). According to Moon et al.[23] the statistically significant features for the depiction of a benign nodule were an ovoid to round shape, a well-defined smooth margin, isoechogenicity, and a spongiform appearance while for a malignant nodule were a taller than wide shape, a spiculated margin, marked hypoechogenicity, microcalcification, and macrocalcification. Despite statistical significance, hypoechogenicity was found in 33.7% of benign nodules and 46.1% of malignant nodules.
Angiogenesis plays a key role in the development of both benign thyroid tissue and cancer of the thyroid. Numerous growth factors such as VEGF, FGF, PDFG derived from cancer cells and much more stimulate angiogenesis of the tumor by paracrine action. Between them, VEGF is the most essential and strongest growth factor for developing and metastasizing thyroid cancer. VEGF expression levels are often higher than normal in malignant tumors. The rate of VEGF expression in malignant lesions is associated with cancer incidence and progression.[24,25] The expression VEGF may be an indicator of the metastasis of localized and distant PTC.[24]
Our study has shown that VEGF is overexpressed in malignant thyroid lesions compared with benign lesions (P = 0.002), and is implicated in tumor growth and metastasis. Findings in the VEGF expression were consistent with other multiple studies.[25,26] Nevertheless, this study proposed that between benign and malignant lesions there was a difference in the expression of VEGF. Following our observations of overexpression of VEGF in thyroid disease including follicular adenoma (4/14 patients) and papillary thyroid cancer (12/17 patients), Jebreel et al.[26] found both PTC (7/7 patients) and 94% (n = 16) of multinodular goiter to be immunopositive to VEGF. In our study, we observed that VEGF was not significantly overexpressed in patients with age (>50 years), nodule size (>5 cm) and nodularity (multinodular) (P > 0.05) that is consistent with a research conducted by Haytaoglu et al.[27]
The expression of VEGF and VEGF receptors (VEGFR) in thyroid nodular hyperplasia and PTC was evaluated in a study conducted by Mohamad Pakarul Razy[28] in 2019. VEGF, VEGFR-1, and VEGFR-2 were shown to be overexpressed in both nodular hyperplasia and PTC in his study. PTC has higher levels of VEGFR-1 and VEGFR-2 expression, as well as VEGF and VEGFR-1 co-expression. As a result, inhibiting VEGFR could be a promising strategy for treating thyroid cancer. Ceric et al.[29] clearly establish that VEGF-C expression, in addition to the classic prognostic markers such as tumor size, tumor margin involvement, extrathyroid extension, i.e., local aggressiveness, is an obvious negative prognostic factor in patients with papillary thyroid cancer.
TSH is a primary thyroid stimulating hormone that controls a variety of thyrocyte biological processes and is regarded as a distinct growth factor. The possibility of serum TSH playing a role in thyroid cancer has piqued our interest. Serum TSH levels that are elevated in the presence of undetected nodules are indicative of malignancy, and serum TSH levels and tumor progression in PTC have a close correlation.[10,30] In summary, serum TSH plays a major role in thyroid cancer.
The mean levels of T3, T4 and serum calcium in both benign and malignant groups were comparable while the mean level of TSH in malignant group was significantly higher than in benign group (P = 0.023) in our study. In a study conducted by Gao et al.[31] in 2019 found TSH expression levels in serum of the thyroid cancer group were significantly higher than those of the benign thyroid disease group (P < 0.05). There is substantial evidence that the serum TSH level is an independent marker for the diagnosis of thyroid cancer in patients with nodular thyroid disease, according to other studies. Furthermore, preoperative serum TSH levels are higher in patients with more aggressive tumors, suggesting that TSH may play a role in the progression of differentiated thyroid cancer.[30] Several other investigations have found that thyroid nodules that are autonomously functioning are less likely to host cancer.[32,33] As a result, Boelaert et al.[30] and others[8,10] found the lowest incidence of thyroid cancer among people with TSH levels below the normal reference range, indicating the presence of thyroid autonomy.[34]
The relationship between serum TSH levels and VEGF expression, on the other hand, has received little attention. TSH increases VEGF mRNA in human thyroid follicles in response to TSH, and TSH promotes VEGF secretion in human thyroid cell cultures as well as in vitro thyroid cancer cells.[35,36]
We found a strong positive relationship between serum TSH levels and VEGF expression in thyroid carcinoma (P < 0.001). Similar findings of serum TSH levels, which were favorably linked with VEGF expression (P < 0.01), were reported by Li et al.[37] Thyroid cancer prevalence and growth are correlated with serum TSH levels, according to our findings. Thyroid cancers with high serum TSH levels, particularly those greater than 2.5 mIU/L, may be more aggressive, with a higher risk of local infiltration and metastasis. According to Li et al.[37] a positive PTC association between preoperative serum TSH and tumor VEGF expression implies that greater serum TSH is associated with a more aggressive tumor phenotype.
The findings of our study mainly indicate that TSH and VEGF correlation has a more positive association in malignant thyroid lesions rather than benign lesions (P < 0.001), which is consistent with Lin et al.,[38] who described in primary cultures which included normal human thyroid, medullary thyroid cancer, and papillary, follicular, and hurthle cell thyroid cancer cell lines, VEGF was secreted in significantly higher concentrations in all thyroid cancers compared to normal thyroid cells.
TSH stimulates both normal and cancerous thyroid cells, and we have found that TSH stimulation improves serum VEGF levels.[39] TSH and thyroid stimulating antibodies, according to Sato et al.[36] promoted the expression of VEGF messenger ribonucleic acid in thyroid epithelial cells in vitro. Thyroid endothelium vascular cells were then stimulated by VEGF, resulting in more blood vessels and thyroid volume.
In patients with untreated goitrous HT, Iitaka et al.[40] found substantial association between serum TSH levels and the thyroid vascular index. Multiple studies showed an increase in thyroidal blood flow following endogenous or exogenous stimulation of TSH.[41,42] These findings indicate that TSH plays a significant role in thyroid angiogenesis.
VEGF expression will be significantly higher in patients with chronic or recurring thyroid cancer than in those who have been cured. TSH stimulation may also increase circulating serum VEGF levels in individuals with chronic or recurring thyroid cancer, because TSH promotes the development of thyroid cancer cells and appears to directly enhance the level of VEGF in preclinical models.[39]
As a result, the correlation between TSH and VEGF is more positive in malignant patients than in benign patients; for example, papillary carcinoma of the thyroid has a significant positive correlation, whereas follicular adenoma has a greater positive correlation in benign individuals. The reason for the discrepancy in our results is that the number of patients was too small, necessitating the performance of much larger local population-based research.
Soh, et al.[35] suggested that constitutive secretion of VEGF in some thyroid cancers is a result of TSH stimulation; thus TSH may promote the thyroid tumor growth by stimulating VEGF secretion and angiogenesis.[15] More interestingly, some investigations show that the endogenous and exogenous TSH stimulation can significantly decrease the serum levels of VEGF, suggesting that TSH may exert its regulatory effects via receptors located outside the thyrocytes and may exert its effects on VEGF production from tissues other than the thyroid gland, respectively.[43]
Thyroid cancer angiogenesis is aided by a variety of causes. Some factors, however, have a greater impact on this form of cancer’s angiogenic activity than others. As a result, VEGFs and their receptors appear to play a key role in thyroid tumor angiogenesis. As a result, other players appear to regulate this important element upstream (s). All factors, however, have the potential to be used as diagnostic, therapeutic, and prognostic indicators, but due to a lack of evidence on their determining role in these processes, it is not possible to have a more thorough discussion of their function in thyroid cancer formation and progression. As a result, additional relevant and thorough research to discover many aspects of thyroid cancer angiogenesis, with a focus on various angiogenic and antiangiogenic factors, appear to be required.
The current study included a number of limitations. First, this study was carried out in a single center and did not collect samples together with other centers. Second, the sample size was small. Hence, sample sizes should be increased in future studies to better understand the association between VEGF and TSH expression in thyroid disease and to confirm current findings.
VEGF is overexpressed in malignant thyroid lesions compared to benign thyroid lesions (P = 0.002), and is implicated in tumor growth and metastasis, according to our findings. TSH levels were substantially greater in the malignant group than in the benign group (P = 0.023). We found a strong positive relationship between serum TSH levels and VEGF expression in thyroid carcinoma (P < 0.001). Thyroid cancers with high serum TSH levels, particularly those greater than 2.5 mIU/L, may be more aggressive, with a higher risk of local infiltration and metastasis. The results of our study show that the TSH and VEGF correlation is more positive in malignant thyroid lesions than benign thyroid lesions (P < 0.001).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Crafa A, Calogero AE, Cannarella R, Mongioi' LM, Condorelli RA, Greco EA, et al. The burden of hormonal disorders:A worldwide overview with a particular look in Italy. Front Endocrinol (Lausanne) 2021;12:694325. doi: 10.3389/fendo.2021.694325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Unnikrishnan AG, Menon UV. Thyroid disorders in India:An epidemiological perspective. Indian J Endocrinol Metab. 2011;15:S78–81. doi: 10.4103/2230-8210.83329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi L, Li Y, Guan H, Li C, Shi L, Shan Z, et al. Usefulness of serum thyrotropin for riskprediction of differentiated thyroid cancers does not apply tomicrocarcinomas:Result os 1,870 Chinese patients with thyroid nodules. Endocr J. 2012;59:973–80. doi: 10.1507/endocrj.ej12-0154. [DOI] [PubMed] [Google Scholar]
- 4.Siderova M. Thyroid Cancer:Diagnosis, Treatment and Follow-Up. In: Raman PG, editor. Thyroid Disorders [Internet] London: IntechOpen; 2018. [Last accessed on 2021 Jun 02]. Available from:https://www.intechopen.com/chapters/61460 doi:10.5772/intechopen.77163 . [Google Scholar]
- 5.Prezioso G, Giannini C, Chiarelli F. Effect of thyroid hormones on neurons and neurodevelopment. Horm Res Paediatr. 2018;90:73–81. doi: 10.1159/000492129. [DOI] [PubMed] [Google Scholar]
- 6.Freudenthal B, Williams GR. Thyroid stimulating hormone suppression in the long-term follow-up of differentiated thyroid cancer. Clin Oncol (R Coll Radiol) 2017;29:325–8. doi: 10.1016/j.clon.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Jonklaas J, Nsouli-Maktabi H, Soldin SJ. Endogenous thyrotropin and triiodothyronine concentrations in individuals with thyroid cancer. Thyroid. 2008;18:943–52. doi: 10.1089/thy.2008.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polyzos SA, Kita M, Efstathiadou Z, Poulakos P, Slavakis A, Sofianou D, et al. Serum thyrotropin concentration as a biochemical predictor of thyroid malignancy in patients presenting with thyroid nodules. J Cancer Res Clin Oncol. 2008;134:953–60. doi: 10.1007/s00432-008-0373-7. [DOI] [PubMed] [Google Scholar]
- 9.Duccini K, de Souza MVL, Delfim R, Aguiar AP, Teixeira P, Vaisman M. High serum thyrotropin concentrations within the reference range:A predictor of malignancy in nodular thyroid disease. Med Princ Pract. 2018;27:272–7. doi: 10.1159/000488196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, et al. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroidcancer and advanced tumor stage. J Clin Endocrinol Metab. 2008;93:809–14. doi: 10.1210/jc.2007-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soh EY, Duh QY, Sobhi SA, Young DM, Epstein HD, Wong MG, et al. Vascular endothelial growth factor expression is higher in differentiated thyroid cancer than in normal or benign thyroid. J Clin Endocrinol Metab. 1997;82:3741–7. doi: 10.1210/jcem.82.11.4340. [DOI] [PubMed] [Google Scholar]
- 12.Rajabi S, Dehghan MH, Dastmalchi R, Mashayekhi FJ, Salami S, Hedayati M. The roles and role-players in thyroid cancer angiogenesis. Endocr J. 2019;66:277–93. doi: 10.1507/endocrj.EJ18-0537. [DOI] [PubMed] [Google Scholar]
- 13.Fenton C, Patel A, Dinauer C, Robie DK, Tuttle RM, Francis GL. The expression of vascular endothelial growth factor and the type 1 vascular endothelial growth factor receptor correlate with the size of papillary thyroid carcinoma in children and young adults. Thyroid. 2000;10:349–57. doi: 10.1089/thy.2000.10.349. [DOI] [PubMed] [Google Scholar]
- 14.Klein M, Vignaud JM, Hennequin V, Toussaint B, Bresler L, Plénat F, et al. Increased expression of the vascular endothelial growth factor is a pejorative prognosis marker in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:656–8. doi: 10.1210/jcem.86.2.7226. [DOI] [PubMed] [Google Scholar]
- 15.Melaccio A, Sgaramella LI, Pasculli A, Di Meo G, Gurrado A, Prete FP, et al. Prognostic and therapeutic role of angiogenic microenvironment in thyroid cancer. Cancers. 2021;13:2775. doi: 10.3390/cancers13112775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann S, Hofbauer LC, Scharrenbach V, Wunderlich A, Hassan I, Lingelbach S, et al. Thyrotropin (TSH)-induced production of vascular endothelial growth factor in thyroid cancer cells in vitro. J Clin Endocrinol Metab. 2004;89:6139–45. doi: 10.1210/jc.2004-1260. [DOI] [PubMed] [Google Scholar]
- 17.Klein M, Brunaud L, Muresan M, Barbé F, Marie B, Sapin R, et al. Recombinant human thyrotropin stimulates thyroid angiogenesis in vivo. Thyroid. 2006;16:531–6. doi: 10.1089/thy.2006.16.531. [DOI] [PubMed] [Google Scholar]
- 18.Nagarkar R, Roy S, Akheel M, Palwe V, Kulkarni N, Pandit P. Incidence of thyroid disorders in India:An institutional retrospective analysis. Int J Dent Med Spec. 2015;2:19–23. [Google Scholar]
- 19.Paschke R. Differenziertes Schilddrüsenkarzinom:Bildgebende Verfahren zur Nachuntersuchung richtig einsetzen [Reasonable use of imaging tests for follow-up care of thyroid cancer] Dtsch Med Wochenschr. 2016;141:1521. doi: 10.1055/s-0042-117086. [DOI] [PubMed] [Google Scholar]
- 20.Welker MJ, Orlov D. Thyroid nodules. Am Fam Physician. 2003;67:559–66. [PubMed] [Google Scholar]
- 21.Lema LEK, Aziz MR, Mbembati NA, Mwakyoma HA. The frequency of carcinoma in solitary thyroid nodules and in multinodular goiters. East Central African J Surg. 1992;2:11–4. [Google Scholar]
- 22.Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma:Correlation with pathological findings. Clin Endocrinol. 2004;60:21–8. doi: 10.1046/j.1365-2265.2003.01912.x. [DOI] [PubMed] [Google Scholar]
- 23.Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules:US differentiation--multicenter retrospective study. Radiology. 2008;247:762–70. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 24.Klein M, Picard E, Vignaud JM, Marie B, Bresler L, Toussaint B, et al. Vascular endothelial growth factor gene and protein:Strong expression in thyroiditis and thyroid carcinoma. J Endocrinol. 1999;161:41–9. doi: 10.1677/joe.0.1610041. [DOI] [PubMed] [Google Scholar]
- 25.Katoh R, Miyagi E, Kawaoi A, Hemmi A, Komiyama A, Oyama T, et al. Expression of vascular endothelial growth factor (VEGF) in human thyroid neoplasms. Hum Pathol. 1999;30:891–7. doi: 10.1016/s0046-8177(99)90241-1. [DOI] [PubMed] [Google Scholar]
- 26.Jebreel A, England J, Bedford K, Murphy J, Karsai L, Atkin S. Vascular endothelial growth factor (VEGF), VEGF receptors expression and microvascular density in benign and malignant thyroid diseases. Int J Exp Pathol. 2007;88:271–7. doi: 10.1111/j.1365-2613.2007.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haytaoglu G, Kuzu F, Arpaci D, Altas A, Can M, Barut F, et al. Correlation of vascular endothelial growth factor and vascular endothelial growth factor receptor-1 levels in serum and thyroid nodules with histopathological and radiological variables. J Lab Physicians. 2019;11:51–7. doi: 10.4103/JLP.JLP_41_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamad Pakarul Razy NH, Wan Abdul Rahman WF, Win TT. Expression of vascular endothelial growth factor and its receptors in thyroid nodular hyperplasia and papillary thyroid carcinoma:A tertiary health care centre based study. Asian Pac J Cancer Prev. 2019;20:277–82. doi: 10.31557/APJCP.2019.20.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceric S, Ceric T, Pojskic N, Bilalovic N, Musanovic J, Kucukalic-Selimovic E. Immunohistochemical expression and prognostic significance of VEGF-C in well-differentiated thyroid cancer. Acta Endocrinol (Buchar) 2020;16:409–16. doi: 10.4183/aeb.2020.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC, Franklyn JA. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J Clin Endocrinol Metab. 2006;91:4295–301. doi: 10.1210/jc.2006-0527. [DOI] [PubMed] [Google Scholar]
- 31.Gao Y, Sheng M, Zhao Y, Jiang H, Zhang Y. Serum Chemerin and thyrotropin expression levels in patients with thyroid cancer and roles in predicting survival. Int J Clin Exp Med. 2019;12:7561–8. [Google Scholar]
- 32.Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764–71. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 33.Hegedus L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter:Current status and future perspectives. Endocr Rev. 2003;24:102–32. doi: 10.1210/er.2002-0016. [DOI] [PubMed] [Google Scholar]
- 34.Ross DS. Predicting thyroid malignancy. J Clin Endocrinol Metab. 2006;91:4253–5. doi: 10.1210/jc.2006-1772. [DOI] [PubMed] [Google Scholar]
- 35.Soh EY, Sobhi SA, Wong MG, Meng YG, Siperstein AE, Clark OH, et al. Thyroid-stimulating hormone promotes the secretion of vascular endothelial growth factor in thyroid cancer cell lines. Surgery. 1996;120:944–7. doi: 10.1016/s0039-6060(96)80038-9. [DOI] [PubMed] [Google Scholar]
- 36.Sato K, Yamazaki K, Shizume K, Kanaji Y, Obara T, Ohsumi K, et al. Stimulation by thyroid-stimulating hormone and Grave's immunoglobulin G of vascular endothelial growth factor mRNA expression in human thyroid follicles in vitro and flt mRNA expression in the rat thyroid in vivo. J Clin Invest. 1995;96:1295–302. doi: 10.1172/JCI118164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Teng L, Jiang H. Relationship between preoperative serum TSH levels and expression of VEGF in papillary thyroid carcinoma. Asia Pac J Clin Oncol. 2014;10:149–52. doi: 10.1111/ajco.12075. [DOI] [PubMed] [Google Scholar]
- 38.Lin JD, Chao TC, Huang BY, Chen ST, Chang HY, Hsueh C. Thyroid cancer in the thyroid nodules evaluated by ultrasonography and fine-needle aspiration cytology. Thyroid. 2005;15:708–17. doi: 10.1089/thy.2005.15.708. [DOI] [PubMed] [Google Scholar]
- 39.Tuttle RM, Fleisher M, Francis GL, Robbins RJ. Serum vascular endothelial growth factor levels are elevated in metastatic differentiated thyroid cancer but not increased by short-term TSH stimulation. J Clin Endocrinol Metab. 2002;87:1737–42. doi: 10.1210/jcem.87.4.8388. [DOI] [PubMed] [Google Scholar]
- 40.Iitaka M, Miura S, Yamanaka K, Kawasaki S, Kitahama S, Kawakami Y, et al. Increased serum vascular endothelial growth factor levels and intrathyroidal vascular area in patients with Graves'disease and Hashimoto's thyroiditis. J Clin Endocrinol Metab. 1998;83:3908–12. doi: 10.1210/jcem.83.11.5281. [DOI] [PubMed] [Google Scholar]
- 41.Tegler L, Gillquist J, Anderberg B, Jacobson G, Lundstrom B, Roos P. Human thyroid blood flow response to endogenous, exogenous human, and bovine thyrotrophin measured by electromagnetic flowmetry. Acta Endocrinol (Copenh) 1981;98:540–8. doi: 10.1530/acta.0.0980540. [DOI] [PubMed] [Google Scholar]
- 42.Connors JM, Huffman LJ, Hedge GA. Effects of thyrotropin on vascular conductance of the thyroid gland. Endocrinology. 1988;122:921–9. doi: 10.1210/endo-122-3-921. [DOI] [PubMed] [Google Scholar]
- 43.Sorvillo F, Mazziotti G, Carbone A, Piscopo M, Rotondi M, Cioffi M, et al. Recombinant human thyrotropin reduces serum vascular endothelial growth factor levels in patients monitored for thyroid carcinoma even in the absence of thyroid tissue. J Clin Endocrinol Metab. 2003;88:4818–22. doi: 10.1210/jc.2003-030789. [DOI] [PubMed] [Google Scholar]


