ABSTRACT
Purpose:
Over-the-counter (OTC) sale of antibiotics have contributed to the growing threat of antibiotic resistance. The Government of India has instituted regulatory measures, such as Schedule H1 and public campaigns such as Red Line Campaign, to limit such sales. This study was conducted to assess the perceptions of stakeholders regarding their effectiveness.
Methods:
To assess Schedule H1, pharmacists who own retail pharmacies in the state of Kerala, India, were interviewed using a pre-prepared question guide. In the next phase, healthcare professionals and patients in a hospital were shown the Red Line on an antibiotic blister pack and asked about its significance. Finally, 100 patients were shown a blister pack of Amoxicillin, and asked to identify the medicine or its use.
Results:
It was observed that there is poor awareness about antibiotic regulations and a perception of laxity in enforcement. Regarding the Red Line, only 7% of healthcare professionals could describe its significance and none among patients. Among the 100 patients who were shown Amoxicillin, only 42 could identify it as an antibiotic or describe its use.
Conclusions:
There is a general perception that regulations are poorly enforced and all are not aware of the Red Line campaign including healthcare professionals. Greater awareness at all levels about appropriate antibiotic use through prescriptions followed by greater efforts towards regulatory implementation and compliance should form parts of a multi-modal strategy to contain OTC sales of antibiotics. This will greatly help to aid physicians in improving overall healthcare through safe and effective prescribing.
Keywords: AMR, antibiotic resistance, healthcare, rational drug use, regulation
Introduction
Antibiotic misuse in various sectors has hastened the rate of development of antibiotic resistance (ABR).[1] ABR has increased morbidity and mortality in patients; and affected the success of treatment of several conditions, including cancer chemotherapy and organ transplantation.[2] It is expected that Low-Middle Income Countries (LMICs) will be affected by ABR much more than High Income Countries.[3]
Curbing the misuse of antibiotics in human and animal health has been a recognized strategy to contain the increase in ABR. Right from the Jaipur Declaration (2011) which brought together members states of the World Health Organization’s (WHO) South East Asian region, India has made important commitments to regulate and rationalize the production, sale, and use of antibiotics.[4] The Global Action Plan on Antimicrobial Resistance of the WHO and the subsequent National Action Plan (2017) in India have recognized inappropriate use and sales of antibiotics as a challenge.[5,6]
In India, antibiotics fall under Schedule H of the Drugs and Cosmetics Act, 1940 and require a valid prescription for sale from retail pharmacies.[7] Studies have, however, shown extensive over-the-counter (OTC) sale of antibiotics across the country, primarily due to patient preferences, and challenges in the health system and regulatory oversight.[8] To contain OTC sale of antibiotics, the Government of India revised the Drugs and Cosmetics Rules, 1945, and notified Schedule H1 in 2014. It initially contained 24 antibiotics, primarily third and fourth generation cephalosporins, carbapenems, antituberculosis drugs, and newer fluoroquinolones, in addition to certain habit-forming agents. The retail pharmacies were supposed to maintain a register of prescription and sale, for a period of 3 years, for the medicines listed in Schedule H1.[9] Another key initiative by the government was to improve awareness among the public and healthcare professionals about the importance of appropriate use of antibiotics through the ‘Red Line campaign’. In this initiative launched in 2016, antibiotics and certain other prescription-only medicines had a bold red coloured line on the blister pack, to indicate that these drugs were to be consumed on advice of qualified prescribers only.[10]
The multiple initiatives taken by the government show the importance given to containing the threat of ABR. It is important, however, to understand whether these measures are effective, whether the public has improved its awareness and whether there is scope to further improve and strengthen regulations. Keeping these in mind, we conducted a study in the state of Kerala, India to determine how well the Schedule H1 and Red Line campaign have been understood by stakeholders and implemented in the community setting.
Since primary care physicians see a lot of patients with infectious diseases and thereby prescribe antibiotics, the threat of ABR will directly impact their treatment strategies and effective management of each patient. The unique position that primary care physicians hold as a patient’s entry point into the healthcare system facilitates an effective communication pathway by which the public can be made aware of ABR.[11] It is, therefore, important that primary care physicians be kept abreast of the ABR landscape and be informed about the important measures by the government systems in curbing ABR. Most importantly, the success of Schedule H1 and the Red Line campaign will also have a direct bearing on decreasing self-medication adopted by patients and enhance compliance with the prescribing advice given by physicians. It therefore hoped that the findings from this study will help to re-energize the focus on improving antibiotic use and further think on strategies to sustain the measures introduced by the government.
Methods
This study was done in 2019 to determine the understanding and perceptions around Schedule H1 and the Red Line campaign.
Five pharmacists who own retail pharmacies in the state of Kerala, India, were interviewed using a pre-prepared question guide, so as to ascertain their perceptions and practices around Schedule H1 medicines. The retail pharmacists were identified using snowballing technique and all of them agreed to participate. The question guide looked at practices followed in dispensing antibiotics, their awareness regarding schedule H1, regulatory oversight regarding schedule H1 medicines, the general compliance of retail pharmacies to Schedule H1 regulations, and their perceptions about ABR. The pharmacists were also given hypothetical case scenarios, involving medicines mentioned in Schedule H1- Levofloxacin, Cefpodoxime, and Zolpidem. Cefpodoxime is an oral third-generation cephalosporin commonly used in Respiratory Tract Infections (RTI),[12] and Levofloxacin, an oral fluoroquinolone effective against a variety of upper and lower RTI, genitourinary, and skin infections.[13] Levofloxacin is also an effective drug for the treatment of drug-resistant Tuberculosis, and this increases its utility in the LMIC contexts.[14] The non-benzodiazepine hypnotic given as a hypothetical scenario was Zolpidem, recognized widely in India as a sleeping pill and clinically used for short-term treatment of insomnia.[15] The non-benzodiazepine hypnotic was included with the other two antibiotics to identify any difference in the way the retail pharmacies approached different classes of medicines (antibiotics vs sedatives) within the same schedule of the law. The interviews were conducted electronically or over the phone for logistic reasons and the process was stopped when data saturation was achieved. The interviews were recorded for transcription purposes, after obtaining verbal informed consent. The interviews and its transcription were conducted in the local language by one of the investigators. Manifest and latent content analysis were done to understand the practices and perceptions of the interviewees. Coding was done using Microsoft Excel by one of the authors and codes, categories, and themes were identified.
A part of the study dealing with the Red Line campaign was carried out in a tertiary care hospital in Kerala, India. In this, voluntary participants were shown blister packs of antibiotics and asked to identify the significance of the red line. A total of 100 patients, 50 doctors, 50 nurses, and 50 paramedical staff (including pharmacists) were included through non-probability sampling. No leading questions or prompts were given to the participants. The 100 patients, who were a part of the study, were asked to identify a blister pack of Cap. Amoxicillin 500 mg, regarding its type and utility. For this also, no leading questions or prompts were given to the participants. We did not collect personal, demographic, professional, or educational details of the participants.
The data was entered and univariate analysis was performed using Microsoft Excel, a proprietary spreadsheet software of Microsoft Inc. As this work did not involve any interventions and was more to gauge awareness and perceptions, permission was asked from individuals directly.
Results
Data was collected in the months of January to May 2019. The first part of the study included interviews for pharmacy owners (three males and two females). All five had bachelor’s qualifications and were between the age range of 28 to 45 years.
Awareness about the rational use of antibiotics and ABR
The interviews revealed that there was low awareness about antibiotic misuse and ABR. Most owners had heard about the issue but did not know the contributory factors of ABR in the healthcare sector. As per the participants, many pharmacies may give antibiotics when the patients ask for them. This is often because the consequences of antibiotic misuse and ABR are not perceived as a major problem by retail pharmacists or patients.
“I have heard about it (antibiotic resistance) but I don’t know much about it.”
“Some people come to buy antibiotics without prescription and without seeing a doctor, for wet cough and similar ailments that people say. If we feel they have a chest infection, then we give antibiotics.”
Capacity among regulatory agencies
Many of the drug inspector offices at the district level have less than optimal staff numbers as compared to the number of pharmacies. This capacity constraint often results in fewer inspections and prioritization of inspections for issues such as sales of psychotropic substances. This also meant that the role of regulators in sensitizing the retail pharmacists about Schedule H1 or the requirement to have a prescription register has been sub-optimal.
“Till about 3-4 years earlier they used to come regularly. Now it’s been some time.”
Perceptions about antibiotics
Antibiotics are seen as medicines with minimal side effects (as compared to other medicine classes). There is a perception that misuse of analgesics may result in renal failure but there is no such view for misuse of antibiotics. Therefore, many pharmacists feel that it can be given without too many precautions. Many pharmacists also feel that the regulatory enforcement is stricter for medicines under Schedule X or the Narcotic Drugs and Psychotropic Substances (NDPS) Act. Therefore, they are very cautious when a medicine is under the purview of NDPS Act.
“Till now I have not noted any side effects because of antibiotic consumption.”
“Zolpidem is available but it is a narcotic. It is given only with a prescription; otherwise strictly not given.”
Health system factors which drive antibiotic use
Many people directly access retail pharmacies for their healthcare needs, as going to a healthcare facility takes time and often loss of a day’s wages. Going to a private healthcare facility may be faster, but with greater expense. Therefore, patients often prefer retail pharmacies for minor medical issues and the pharmacists oblige to their needs. The lack of an audit trail for antibiotics sales and the absence of a health information system also make it difficult for regulatory agencies to tackle antibiotic misuse.
“Some have financial difficulties like if they go to a hospital then there will be consultation charges and many others. And they feel that anyways the same medicine will be prescribed, so we will just buy it from a medical store.”
There are several linkages between the multiple themes listed above and it is evident that the implementation efforts for Schedule H1 need to be further strengthened in order to contain the sales of antibiotics from retail pharmacies [Figure 1].
Figure 1.
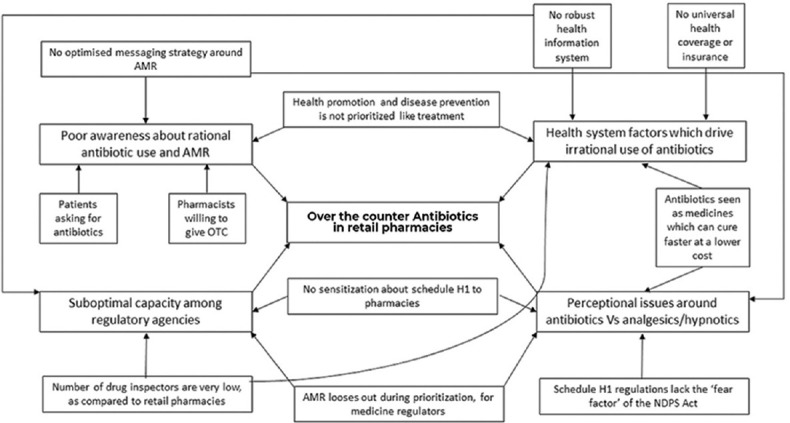
Concept map on linkages between stakeholder, health system and regulatory factors contributing to antibiotic use
Response to hypothetical scenarios
The pharmacists were unaware that Levofloxacin and Cefpodoxime were a part of Schedule H1 and therefore a valid prescription is needed. They were much more cautious however about Zolpidem, which also comes under Schedule H1, but was considered a dangerous medicine as it is a sedative-hypnotic agent. They also mentioned that the two antibiotics are usually in stock, whereas Zolpidem was often not stocked due to the fear around NDPS Act (even though the medicine is not covered under NDPS Act).
“We have stock of it (regarding Cefpodoxime and Levofloxacin). People do come and ask for it without a prescription….”
“.sleeping pills. We do have a stock of it (regarding Zolpidem). People do ask for it without a prescription but we do not give it. We are very strict about this one, only after seeing the prescription and verifying the date do we give that medicine.”
Awareness about red line campaign
In the second part of the study, on asking various occupational groups about the Red Line in the blister packs of antibiotics, only 6% of doctors and 8% of the paramedical personnel could correctly identify the purpose. None of the nurses or patients coming to a tertiary care hospital knew the meaning or utility of the Red Line [Table 1].
Table 1.
Participants who correctly identified the purpose of the Red Line in a blister pack of medicines
| Group | Identified Red Line | Did not Identify Red Line | Total |
|---|---|---|---|
| Doctors | 3 (6%) | 47 (94%) | 50 |
| Nurses | 0 (0%) | 50 (100%) | 50 |
| Paramedics | 4 (8%) | 46 (92%) | 50 |
| Patients | 0 (0%) | 100 (100%) | 100 |
When we asked 100 patients included in the study to examine a blister pack of Capsule Amoxicillin 500 mg and explain its utility, 42% mentioned that it is used in infections and identified it as an antibiotic or anti-infective. Any synonyms of ‘infection’ in the local language and terms suggestive of localized infections were also clubbed together for the study.
Discussion
In developing countries, there is a high rate of OTC antibiotic sales without prescription or through unregulated supply chains.[2] A study by Phalke et al.[16] reported the prevalence of self-medication in the rural population of the state of Maharashtra in India to be 81.5%. However, in Tamil Nadu, only 23% of the rural population resorted to self-medication or household remedies and almost 77% consulted a doctor for their ailment.[17] Overall, 52% of Indians were estimated to self-medicate themselves in India, according to a web portal-based survey of 20,000 people across 10 cities. The reasons given were lack of time, need to avoid doctors’ fees, and dependence on the internet.[18]
The findings from this study bear testament that OTC sales of antibiotics are a common practice. Noticeably, our study shows the stark difference in the perception around antibiotics when compared to the non-benzodiazepine hypnotic Zolpidem. While it was considered simple to obtain the antibiotics (Cefpodoxime, Levofloxacin) in Schedule H1, it was reported difficult to purchase Zolpidem. This when seen in the light of the success of Schedule X implementation in India[19] supported by the NDPS Act of 1985 brings clarity to the situation.[20] Schedule X contains mainly habit-forming and narcotics drugs and the NDPS Act mandates strict punishments for offenses relating to Schedule X drugs. Zolpidem is a drug used in the management of insomnia with some case reports of abuse, dependence, or withdrawal syndrome.[15] We assume that the occurrence of narcotic side effects though mild, and the false perception that Zolpidem comes under Schedule X and the NDPS Act might be the reason for pharmacists to insist on prescriptions.
Perceptions expressed through our study show that the regulatory implementation of Schedule H1 is considered weak and, also a sustained public campaign for Red Line is urgently needed due to the poor awareness levels among various stakeholders. A lack of continued efforts in the Red Line campaign could likely be attributed to financial or resource constraints which are commonly faced by developing nations.[2] In 2018, a shortage of drug inspectors in Kerala state made headlines. For the more than 21,000 retail pharmacies functioning in the state, there were only 47 drug inspectors available, whose duties apart from regular inspection of medical stores included collecting samples of cosmetic products sold in the state and monitoring the function of blood banks.[21] The importance of having adequate human resources in the regulatory field needs to be underlined since it impacts the enforcement of vital regulations and policies.
In India, pharmacies often function as a business or shop, rather than a healthcare-providing facility. Though the situation is improving, this factor often contributes to OTC. In a study from Nagpur, pharmacists and clients were interviewed to determine patterns of antimicrobial drug use. The study reports that only two-thirds (65.4%) of the dispensing chemists claimed to be ‘registered pharmacists’ with college-level training in pharmacy.[22] As recommended by a study done in UAE, continuing professional development of pharmacists and pharmacy assistants would be imperative to promote prudent use of antibiotics.[23] Incorporating pharmacists into the healthcare team, keeping them updated about medicines, and training them to be capable of disseminating basic medical knowledge, may greatly enhance the potential to educate the public even in the remotest corner of a country. The Pharmacy Guild of Australia’s nation-wide campaign on safe alcohol consumption with pharmacists as health educators can serve as a model for the success of such strategies.[24]
The Red Line campaign is easy to understand and implement, and therefore has garnered almost total compliance by pharmaceutical companies. It has the potential for a better outcome if the information can be relayed widely and awareness regarding ABR can be better provided to the public so that they understand the significance of complying with the message given by Red Line. A barrier observed in the Indian context is that most of the blister packs are cut before they are dispensed to the public, as many patients may not have the money with them to buy a whole strip or course at that point in time. It is a practice that is prevalent in most developing countries.[25] This defeats the purpose of the campaign. It is interesting to know that 42 out of 100 patients recognized the antibiotics but not the significance of the Red Line. This reveals that the public is relatively conscious of the nature of medicine being used by them, but initial awareness of the Red Line may have dissipated as years passed. Furthermore, it is important to always consider that the messaging adopted for public campaigns is culturally relevant and molded to the preferences of the target population.[26] In some parts of India, red is the color of auspiciousness, unlike in the Western world, where red signifies danger.[27] This could be one of the reasons why a Red Line on an antibiotic blister pack does not result in a reason for concern among the Indian population.
One of the drivers for a long term sustainable solution on ABR is awareness. A possible route in achieving public awareness about ABR would be to train media professionals to disseminate scientific information in ways that the population would understand.[28] In the initial stages of any campaign, communication strategies should focus on raising awareness in specific interest groups, thereby channeling limited resources to achieve specific objectives. This can improve the chance of behavior change, with the general public targeted at a later stage with definitive strategies and messages.[26] It has already been proven by Vietnam and Thailand that a double-edged approach involving different degrees of regulation and education is effective.[29]
India spends only 4.5% of its GDP on healthcare.[30] This can be improved upon with additional funds and resources allocated towards meaningful intervention measures specific to each region or state. A more robust health system that ensures better access to resources and giving adequate health coverage to every citizen has the potential to solve many of the circumstances related to the irrational use of antibiotics.
Our findings suggest that initiatives by the government have found limited success in regulating OTC antibiotic sales and in creating awareness regarding types of medication. Financial and resource constraints may have contributed to inadequate awareness and suboptimal implementation of regulations planned by the government. Due consideration to sustainable access of antibiotics alongside regulatory measures might increase compliance from the general public. Also, it might seem prudent to evaluate the regional and cultural appropriateness of campaigns before adoption. There is no unilateral pathway to resolving the OTC issue. An insightful approach is required that combines regulatory implementation, community awareness, along with interventions at multiple levels in production, supply chain, and procurement to achieve the common goal of reducing OTC sale of antibiotics. Such an approach would greatly help the physicians in delivering safe and effective healthcare through appropriate prescriptions for the population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to acknowledge the contributions of Mr Lijo Cyril, Junior Consultant, ReAct Asia Pacific.
References
- 1.Aminov RI. A brief history of the antibiotic era:Lessons learned and challenges for the future. Front Microbiol. 2010;1:134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries:Causes and control strategies. Antimicrob Resist Infect Control. 2017;6:47. doi: 10.1186/s13756-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chandy SJ, Naik GS, Balaji V, Jeyaseelan V, Thomas K, Lundborg CS. High cost burden and health consequences of antibiotic resistance:The price to pay. J Infect Dev Ctries. 2014;8:1096–102. doi: 10.3855/jidc.4745. [DOI] [PubMed] [Google Scholar]
- 4.Asia WHORO for S-E. Jaipur declaration on antimicrobial resistance. 2011. [Last accessed on 2021 Apr 15]. Available from:https://apps.who.int/iris/handle/10665/205397 .
- 5.WHO |Global action plan on antimicrobial resistance. WHO Available. [Last accessed on 2021 Apr 20]. from:http://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/
- 6.Ranjalkar J, Chandy SJ. India's National Action Plan for antimicrobial resistance –An overview of the context, status, and way ahead. J Fam Med Prim Care. 2019;8:1828–34. doi: 10.4103/jfmpc.jfmpc_275_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marathe P, Kamat S, Tripathi R, Raut S, Khatri N. Over-the-counter medicines:Global perspective and Indian scenario. J Postgrad Med. 2020;66:28–34. doi: 10.4103/jpgm.JPGM_381_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cars O, Nordberg P. Antibiotic resistance –The faceless threat. Int J Risk Saf Med. 2005;17:103–10. [Google Scholar]
- 9.Laxminarayan R, Chaudhury RR. Antibiotic resistance in India:Drivers and opportunities for action. PLoS Med. 2016;13:e1001974. doi: 10.1371/journal.pmed.1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Srivastava R. India lauded for red line campaign on antibiotics. The Hindu. 2016. [Last accessed on 2021 Apr 10]. Available from:https://www.thehindu.com/news/national/india-lauded-for-red-line-campaign-on-antibiotics/article8622474.ece .
- 11.Primary Care. [Last accessed on 2021 Dec 14]. Available from:https://www.aafp.org/about/policies/all/primary-care.html .
- 12.Aggarwal A, Rath S. Cefpodoxime —Utility in respiratory tract infections and typhoid fever. Indian J Pediatr. 2004;71:413–5. doi: 10.1007/BF02725629. [DOI] [PubMed] [Google Scholar]
- 13.Davis R, Bryson HM. Levofloxacin:A review of its antibacterial activity, pharmacokinetics and therapeutic efficacy. Drugs. 1994;47:677–700. doi: 10.2165/00003495-199447040-00008. [DOI] [PubMed] [Google Scholar]
- 14.Anh LTN, Kumar AMV, Ramaswamy G, Htun T, Thanh Hoang Thi T, Hoai Nguyen G, et al. High levels of treatment success and zero relapse in multidrug-resistant tuberculosis patients receiving a levofloxacin-based shorter treatment regimen in Vietnam. Trop Med Infect Dis. 2020;5:43. doi: 10.3390/tropicalmed5010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison TS, Keating GM. Zolpidem. CNS Drugs. 2005;19:65–89. doi: 10.2165/00023210-200519010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Phalke VD, Phalke DB, Durgawale PM. Self-medication practices in rural Maharashtra. Indian J Community Med. 2006;31:34. [Google Scholar]
- 17.Dutta R, Raja D, R A, Dcruze L, Jain T, P S. Self-medication practices versus health of the community. Int J Community Med Public Health. 2017;4:8. doi:10.18203/2394-6040.ijcmph20173169. [Google Scholar]
- 18.Parulekar M, Mekoth N, Ramesh CM, Parulekar A. Self medication in developing countries a systematic review. J Pharm Technol Res Manage. 2016;4:103–127. [Google Scholar]
- 19.Nafade V, Huddart S, Sulis G, Daftary A, Miraj SS, Saravu K, et al. Over-the-counter antibiotic dispensing by pharmacies:A standardised patient study in Udupi district, India. BMJ Glob Health. 2019;4:e001869. doi: 10.1136/bmjgh-2019-001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.India's Narcotics Control Bureau seizes 55,000 zolpidem tablets in drugs bust [online] |Pharmafile. [Last accessed on 2021 Apr 21]. Available from:http://www.pharmafile. com/news/516823/indias-narcotics-control-bureau-seizes55000-zolpidem-tablets-drugs-bust .
- 21.Shortage of drug inspectors hits check of medicines in Kerala. New Indian Express. [Last accessed on 2021 Apr 12]. Available from:https://www.newindianexpress.com/states/kerala/2018/dec/02/shortage-of-drug-inspectors-hits-check-of-medicines-in-state-1905995.html .
- 22.Dua V, Kunin CM, White LV. The use of antimicrobial drugs in Nagpur, India. A window on medical care in a developing country. Soc Sci Med. 1994;38:717–24. doi: 10.1016/0277-9536(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 23.Dameh M, Green J, Norris P. Over-the-counter sales of antibiotics from community pharmacies in Abu Dhabi. Pharm World Sci. 2010;32:643–50. doi: 10.1007/s11096-010-9418-5. [DOI] [PubMed] [Google Scholar]
- 24.Joyce AW, Sunderland VB, Burrows S, McManus A, Howat P, Maycock B. Community pharmacy's role in promoting healthy behaviours. J Pharm Pract Res. 2007;37:42–4. [Google Scholar]
- 25.Amin MEK, Amine A, Newegy MS. Perspectives of pharmacy staff on dispensing subtherapeutic doses of antibiotics:A theory informed qualitative study. Int J Clin Pharm. 2017;39:1110–8. doi: 10.1007/s11096-017-0510-y. [DOI] [PubMed] [Google Scholar]
- 26.Mathew P, Sivaraman S, Chandy S. Communication strategies for improving public awareness on appropriate antibiotic use:Bridging a vital gap for action on antibiotic resistance. J Fam Med Prim Care. 2019;8:1867–71. doi: 10.4103/jfmpc.jfmpc_263_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchings J. Colour in folklore and tradition?The principles. Color Res Appl. 2004;29:57–66. [Google Scholar]
- 28.Blakely JT-M, Sinkowitz-Cochran RL, Jarvis WR. Infectious diseases Physicians'preferences for continuing medical education on antimicrobial resistance and other general topics. Infect Control Hosp Epidemiol. 2006;27:873–5. doi: 10.1086/505922. [DOI] [PubMed] [Google Scholar]
- 29.Chalker J, Ratanawijitrasin S, Chuc NTK, Petzold M, Tomson G. Effectiveness of a multi-component intervention on dispensing practices at private pharmacies in Vietnam and Thailand—A randomized controlled trial. Soc Sci Med. 2005;60:131–41. doi: 10.1016/j.socscimed.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Dieleman J, Campbell M, Chapin A, Eldrenkamp E, Fan VY, Haakenstad A, et al. Evolution and patterns of global health financing 1995–2014:Development assistance for health, and government, prepaid private, and out-of-pocket health spending in 184 countries. Lancet. 2017;389:1981–2004. doi: 10.1016/S0140-6736(17)30874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


