Abstract
Background
Robotic assistance has been increasingly employed to improve the operative precision in modern knee surgery. The purpose of the study was to evaluate the trauma effect of one of the first domestically developed orthopedic surgical robots in China in a clinical trial of robot-assisted total knee arthroplasty (RA-TKA).
Methods
A total of 33 patients who underwent unilateral TKA for end-stage osteoarthritis were randomized to receive RA-TKA (17 cases) or conventional manual TKA (CM-TKA) in our institution in 2020. The trauma effects of the 4 main indicators with 48 sub-indicators in terms of subsectional operative time, inflammation and coagulation markers, physical and radiographical analyses of osteotomy deviation, and postoperative comfort were analyzed.
Results
Subsectional operative time analysis showed that the times for bone cutting and gap balancing with RA-TKA were 5.3 and 2.2 min shorter than those with CM-TKA (p = 0.010, p = 0.02), respectively. Arterial blood gas indicators (partial pressure of carbon dioxide, partial pressure of oxygen and SO2) 24 h after RA-TKA, as well as the white blood cell count and neutrophil ratio, were significantly lower than those after CM-TKA (p < 0.05). Inflammatory markers at 72 h after surgery showed the increments of C-reactive protein, erythrocyte sedimentation rate and D-dimer of RA-TKA declined by 180.7, 22.0 and 1050.0% (p < 0.05), respectively, referenced to the preoperative baseline values, as compared to CM-TKA. Mechanical deviation distribution exhibited percentages of region I errors for RA-TKA and CM-TKA of 76.5% and 27.1% (p = 0.000), respectively, and the success rates of one-time osteotomy were 94.1% and 62.5% (p = 0.039), respectively. Radiographical verification showed RA-TKA was more conducive to achieving mechanical alignment and ideal tibial component azimuths. Postoperative efficacy showed that patients were more comfortable after RA-TKA in terms of reduced administration of tranexamic acid, hydrocortisone and the utilization rate of temporary intensive opioid analgesics. No statistical difference in patient-reported outcome measures and complications were recorded between the two groups during continuous observation.
Conclusions
Compared with CM-TKA, RA-TKA decreases rather than increases trauma. It might shorten the time required for bone cutting and gap balancing, reduce mechanical errors related to the osteotomy and prosthesis position, and improve the accuracy of the mechanical alignment reconstruction. RA-TKA is also favorable in promoting postoperative comfort and minimizing inflammatory response and drug consumption.
Trial registration
The Chinese Clinical Trial Registry (ChiCTR2000031282) approved registration on 26 March 2020.
Keywords: Robot, Knee osteoarthritis, Arthroplasty, Trauma, Error, Surgery, Robot-assist, Total keen arthroplasty
Highlights.
Robotic assistance acts as a trauma savior in TKA, by reducing time consumption required for bone cutting and gap balancing, as well as blood inflammatory and coagulatory reaction.
The protective role of bone and soft tissue is also manifested as improved mechanical deviation distribution into region I, and the success rates of one-time osteotomy in robot-assisted TKA.
Patients treated with robot-assisted TKA present enhanced postoperative comfort as evidenced by reduced analgesic, anti-inflammatory and hemostatic agents.
Background
Total knee arthroplasty (TKA) is a successful surgical method for the treatment of end-stage knee arthritis. Aaccording to official statistics, ~370,000 primary TKAs were completed in China in 2019. With the acceleration of population aging, the number of TKA surgeries performed is increasing by nearly 20% per year [1]; nevertheless, the total number of TKAs performed each year in China is <50% of the number performed in the USA (where >1 million TKAs were performed in 2020 [2]). China has the largest population in the world, and a 2021 census showed that ~260 million people over the age of 60 years were living in the country. Given the projected annual population increase of ~10 million, the number of people undergoing TKA in China will reach 3 million within 5 years [3].
Despite the clinical success of the procedure, postoperative dissatisfaction with TKA is still as high as 20–30% [4]. One reason for this dissatisfaction is surgical deviation, which manifests as the total error of the angle, rotation and offset of the prosthesis along the coronal, sagittal and rotational planes, which can be as high as 30%. The resulting pain, instability and prosthesis failure are important reasons for revision surgery [5]. The high correlation between malalignment/malpositioning and postoperative failure has been met with industrywide consensus [6]. Mason et al. found that with conventional manual TKA (CM-TKA), the incidence of malalignment (>3°) was as high as 31.8%, the incidence of femoral coronal malposition (±2°) was 34.1% and the incidence of tibial coronal malpositioning (±2°) was 20.3% [7], which suggests the incidence of surgical deviation is far higher than expected.
Another factor accounting for operative error is the surgeon’s subjective determination of the gap width and tension in CM-TKA. Lee et al. compared the difference between the medial and lateral gaps in navigation-assisted TKA and CM-TKA and found that the proportion of medial and lateral gaps differing by >3 mm was 12% when the navigation-assisted TKA was bent at 90° and extended at 0°, while the proportion when CM-TKA was used was as high as 25% [8]. These errors might not only seriously weaken the long-term survival of the implant but would increase surgical trauma and patient’s dissatisfaction, leading to delayed postoperative recovery and even disability. Thus, we believe that an important strategy to improve postoperative dissatisfaction with TKA is to effectively improve the accuracy of the operation, harmonize soft tissue tension and increase postoperative comfort. This is one of the reasons why enhanced recovery after surgery (ERAS) plays an important role after TKA. However, ERAS is primarily focused on the overall effect and does not provide guidance on how to optimize surgical technology and reduce surgical trauma [9,10].
The most effective way to address the errors that occur with CM-TKA is to rely on artificial intelligence technologies, such as navigation and robotics, to improve the accuracy and intelligence of TKA. Computer navigation can allow accurate preoperative planning based on computed tomography (CT) data and relies on wireless tracking technology to provide real-time guidance during surgery. However, it is still unable to reproduce the preoperative plan with 100% accuracy or to prevent errors caused by doctors’ manual manipulations. The performance of the first-generation fully active orthopedic surgical robots (such as ROBODOC and CASPAR) did not yield optimistic results due to design defects and a lack of clinical fit [11,12]. Therefore, robot-assisted total knee arthroplasty (RA-TKA) was widely perceived as a trauma creator with a high level of complication [13].
Our team has focused on navigation and RA joint surgery for many years. We hypothesized that RA-TKA can achieve trauma control and enhanced recovery through the advantageous approaches of optimized subsectional operative time, alleviated hematological inflammation and coagulation markers, reduced intraoperative osteotomy deviation and complication, and enhanced postoperative comfort. To examine this proposal, 48 assessment indicators were included in this study to compose a comprehensive evaluation of the trauma effect of RA-TKA. To the best of our knowledge, this is the first prospective clinical trial to quantify the scope and extent of TKA-associated trauma.
Methods
Study design
This study reports the TKA-associated trauma response derived from our single institute, which is a participant of a multicenter, prospective, randomized controlled study (ChiCTR2000031282) investigating the clinical safety and efficacy of the first domestically developed RA system for joint surgery in China. The clinical trial is also the largest one in China at present, and is sponsored by the General Hospital of PLA, Xiangya Hospital, Ruijin Hospital, West China Hospital and Xinqiao Hospital of the Army Medical University. The sample size was predicted on the main variables, including hematological indicators and physical measurements and radiological verification of osteotomy deviation, from our previous work and reference reports [14–19]. The sample size was then calculated according to the following parameters: α = 0.05 and β = 0.20 (2-tailed test), p1 = 10.1, p2 = 8.6, δ (p1 − p2) = 1.5. The calculated sample size was 15 in each group. To allow for a possible 5% missing data, the final sample size was determined as 16 patients in each group.
Patients
The inclusion criteria were: (1) age between 18 and 80 years old, with no sex limitation; (2) a diagnosis of end-stage osteoarthritis, Kellgren–Lawrence (KL) staging III–IV and no response to standard conservative treatment for >6 months; (3) no intra-articular puncture and drug injection and no peri-articular drug application in the past 3 months; and (4) full awareness of the benefits and risks of this trial, a willingness to participate and signing of an informed consent form. The exclusion criteria were: (1) routine surgical contraindications; (2) a history of open knee surgery; (3) severe osteoporosis around the knee joint; (4) severe valgus knee deformity; (5) a high infection risk; (6) the need to remove internal implants during the operation; and (7) severe patellar instability.
The randomization strategy was as follows: 15 min before surgery all patients were randomly assigned to RA-TKA and CM-TKA according to the random number table method using random numbers generated by a RA system. Of the 33 patients who met the inclusion criteria, 17 received RA-TKA and 16 received CM-TKA. All the surgeries were completed by the same senior surgeon, and the perioperative management and rehabilitation were conducted by the medical staff in the same group according to the ERAS specifications. This study was approved by the institute’s ethics committee and all patients signed the informed consent form.
Baseline characteristics, preoperative assessment and radiographic measurement
The baseline characteristics included sex, age, occupation, region, operation side, body mass index and bone mineral density T value. The osteoarthritis severity assessment included the course of disease, Kellgren-Lawrence staging, genu varus grading and the pain score assessed using a visual analogue scale (VAS). The osteoarthritis functional assessment included the Keen Society Score (KSS), Hospital for Special Surgery score (HSS) and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Radiographical measurements included the hip–knee–ankle angle (HKA), lateral distal femoral angle (LDFA), medial proximal tibial angle (MPTA), posterior tibial slope (PTS), posterior condylar offset (PCO), Insall–Salvati index, joint line height and patellofemoral offset (PFO). The risk assessment addressed pre-existing diseases, laboratory blood testing, the American Society of Anesthesiologists score, the Nutrition Risk Screening of 2002, the Caprini score for venous thromboembolism, the self-care ability score (Barthel Index for Activities of Daily Living), and the Nursing Delirium Screening Scale (Nu-DESC). No significant difference in the baseline data or the variables included in the preoperative evaluation (p > 0.05) was noted between the two groups, as shown in Table 1.
Table 1.
Baseline characteristics, preoperative osteoarthritis severity, functional and risk assessment in this study, mean (SD)
| Indicators |
CM-TKA
(n=16) |
RA-TKA
(n=17) |
P value |
| Baseline characteristics | |||
| Gender (case, male/female) | 3/13 | 3/14 | 1.000 |
| Age [years, mean (SD)] | 67.3 (3.5) | 66.6 (3.7) | 0.768 |
| Body mass index [mean (SD)] | 25.5 (2.9) | 25.6 (3.3) | 0.321 |
| Occupation (I/II/III)a | 14/0/2 | 14/2/1 | 0.773 |
| Legion (I/II/III/IV)b | 7/4/3/2 | 8/6/2/1 | 0.590 |
| Operational side (left/right) | 11/5 | 6/11 | 0.084 |
| Osteoarthritis severity and functional assessment | |||
| Disease duration [years, mean (SD)] | 6.8 (4.7) | 6.2 (4.2) | 0.655 |
| Kellgren–Lawrence stage (III/IV) | 2/14 | 3/14 | 1.000 |
| Bone density [T value, mean (SD)] | −3.1 (0.8) | −3.1 (0.7) | 0.694 |
| Varus severity(I/II/III)c | 10/4/2 | 11/4/2 | 0.899 |
| VAS, mean (SD) | 6.1 (1.0) | 5.7 (1.1) | 0.184 |
| KSS, mean (SD) | 101.1 (32.2) | 109.3 (25.8) | 0.426 |
| HSS, mean (SD) | 58.4 (16.8) | 58.4 (16.8) | 0.581 |
| WOMAC, mean (SD) | 152.6 (46.1) | 148.9 (26.7) | 0.786 |
| Risk assessment | |||
| ASA score, mean (SD) | 1.6 (0.7) | 1.4 (0.6) | 0.525 |
| Preoperative comorbiditiesd, case | 6/1/2 | 6/0/2 | 0.809 |
| NRS2002, mean (SD) | 1.6 (0.8) | 1.5 (1.0) | 0.776 |
| Caprini scoree, mean (SD) | 1.9 (1.1) | 2.2 (1.0) | 0.420 |
| ADL-Barthel scoref, mean (SD) | 91.2 (4.9) | 90.0 (6.3) | 0.552 |
| Mental status (Nu-DESC)g, mean (SD) | 0.35 (0.5) | 0.42 (0.5) | 0.625 |
ASA American Society of Anesthesiologists, NRS2002 nutrition risk screening of 2002, VTE venous thrombus embolism, ADL activities of daily living, Nu-DESC Nursing Delirium Screening Scale, VAS visual analogue scale, KSS Keen Society Score, HSS Hospital for Special Surgery score, WOMAC Western Ontario and McMaster Universities Osteoarthritis Index
aOccupation: I, farmer; II, worker; III, office clerk (teacher, civil servants etc.)
bLegion: I, Chongqing; II, Sichuan; III, Guizhou; IV, other provinces
cVarus severity: I, mild (0~5°); II, moderate (5~10°); III, severe (>10°)
dPreoperative comorbidities: cardio-vascular/respiratory/metabolic diseases. They are expressed as classified variables as follows: cardiovascular diseases (coronary and cerebrovascular diseases, hypertension, etc.), respiratory diseases (chronic obstructive pulmonary disease, hypostatic pneumonia, etc.), metabolic diseases (diabetes, hyperthyroidism, etc.)
eCaprini score: VTE risk rating score. 0~1, low risk; 2, medium risk; 3~4, high risk; ≥5, extremely high risk
fADL-Barthel score: ≤40, severe dependence; 41-60, moderate dependence; 61-99, mild dependence; 100, no dependencies
gMental status (Nu-DESC): 0~1, normal; ≥2, delirium
Preoperative planning, surgical procedure and medication
The standard posterior substitution (PS) prosthesis [Unique Knee, IRENE, Tianjin, China] was installed under general anesthesia. The PS surgical technique was performed via the medial parapatellar approach through an anterior median longitudinal incision under general anesthesia. The entire operation was tourniquet free, and an inflatable tourniquet was applied only during the application of bone cement to the prosthesis to ensure satisfactory bone cement penetration and initial stability of the prosthesis. For all surgeries, an indwelling drainage tube was placed until 24 h after the operation. None of the patients received a patellar prosthesis replacement.
CM-TKA was based on the traditional manual instrumentation as recommended by the manufacturer. An extramedullary reference was used to guide the tibial osteotomy (the osteotomy plane was perpendicular to the tibial mechanical axis and the posterior slope was 3°). An intramedullary reference was adopted to guide the femoral osteotomy (5–7° distal femoral valgus osteotomy). The osteotomy and soft tissue balancing were completed by a combination of measured resection and gap balancing. The osteotomy data, prosthesis type and position information of all patients were recorded.
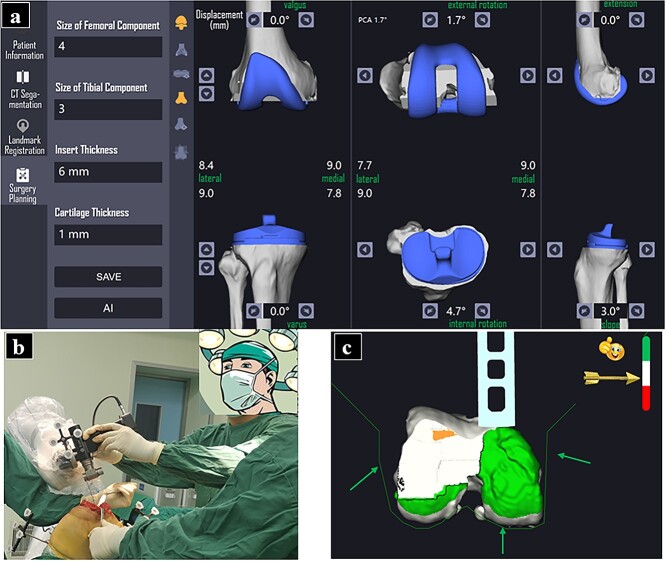
RA-TKA was facilitated by the YUANHUA robot (Yuanhua Robotics Perception and AI Technologies Co., Ltd, Shenzhen, China). This semiautomatic robot was the first orthopedic surgical robot to be developed in China and is composed of a navigator, manipulator and main control console. The manipulator is the first cooperative manipulator with 7 degrees of freedom to be developed anywhere in the world. CT plain scan data for the patients’ lower limbs (from the hip joint to the ankle joint) were obtained before the operation at a thickness of 1.0 mm. The CT data were imported into the robot system for 3D segmentation and reorganization of the cross-sectional, coronal and sagittal planes. Before the operation, the surgical plan, the osteotomy reference, the prosthesis model and the prosthesis position were jointly planned by the software engineer and the surgeon, and the reference location for the osteotomy was determined according to the navigator (Figure 1a).
Figure 1.
Preoperative planning and intraoperative implementation of RA-TKA. Preoperative planning of osteotomy thickness and angle, prosthesis azimuth. size, and prosthesis position were determined in robotic system under surgeon and engineers' coordination (a); A seven-degree robotic arm was employed to assist osteotomy for accuracy (b); The entire procedure of bone-cutting could be monitored within a preset safe zone, the green area represents under-cut bone, while red area represents over-cut bone. The green arrows show the boundary for safe zone (c). RA-TKA robot-assisted total knee arthroplasty
After surgical drapes were laid, a sliding leg frame was installed along the ankle plane on the operation side, the marks on the medial and lateral malleolus were exposed and the frame was fixed with a sterile elastic bandage. Two tracer fixation nails (4.0 mm in diameter) were implanted in the femoral and tibial anterior cortices 10 cm above and below the patella, respectively, and one tracer was installed for each nail. The skin and subcutaneous tissue were opened sequentially and the synovial lesions were moderately cleaned. After the distal femoral and tibial plateaus were fully exposed, a checkpoint pin was placed. Then, knee joint registration was completed according to the system instructions, including 5 femoral planes (the anterior condyle, anterior oblique, distal, posterior oblique and posterior condyle) with 6 registration points in each plane, for a total of 30 femoral registration points, and 2 tibial planes (the tibial plateau and tibial metaphysis), for a total of 36 tibial registration points. Subsequently, the osteotomy parameters, flexion gap and extension gap were confirmed, and the osteotomy parameters were adjusted when the gap between the internal and external gaps at 90° knee flexion and 0° extension was >1.0 mm. Then, the manipulator was guided to automatically adjust the saw blade position to align with the bone-cutting plane and complete the osteotomy (Figure 1b). All of the osteotomy operations were completed within the preset safety zone (Figure 1c). Once the safety boundary was touched, the system sounded an alarm and suspended the osteotomy manipulator. After the osteotomy, the limbs were actively flexed and extended to ensure the balance of the internal and external flexion and extension gaps. The prosthesis test model was installed, and the flexion and extension angles and mechanical axis and soft tissue tension were detected again. The total knee prosthesis was fixed with bone cement after confirmation. The joint capsule, subcutaneous tissue and skin layer were closed using conventional methods, a pressurized elastic bandage was applied and an indwelling drainage tube was left in place for 24 h after the operation.
All patients received postoperative antibiotics (cefuroxime, 1.5 g intravenously quaque 12 h, Zhuhai Jinwan, China) for 24 h and anticoagulation treatment (enoxaparin, 40 mg postoperatively quaque 24 h, Sanofi Synthelabo, USA) for 14 days. Nonsteroidal anti-inflammatory drugs (NSAIDs) (celecoxib, 200 mg postoperatively quaque 12 h, Pfizer, USA) were routinely used for analgesia for 1 month. Patients with severe pain (VAS ≥ 5 points) were given opioids (aminophenol tramadol tablets, 362.5 mg postoperatively quaque 24 h, Xian Janssen, China) on demand for temporary pain relief. Tranexamic acid (1.0 g intravenously, Chongqing Lummy, China) was used for anti-fibrinogen and hemostasis effects 15 min before the operation, and 1.0 g was added at 3 and 6 h after the operation. Whether to proceed with administration was determined according to the drainage (> 50 ml/12 h), swelling, ecchymosis and other aggravating circumstances. All patients received an intravenous injection of 100 mg of hydrocortisone (Tianjin Biochem, China) during the operation and 6 h after the operation according to the trauma response and inflammatory indicators.
Comprehensive assessment of the trauma response
Trauma assessment by subsectional operative time
The total operative time was divided into the following segments according to different key time sections of surgical advancement: (1) Preoperative planning time. For CM-TKA, the preoperative planning time is the time required for the surgeon to correct the mechanical axis on full-length X-rays of the knee joint taken in the lateral and standing positions, determine the thickness and angle of the proximal tibia and distal femur osteotomy, and determine the appropriate size of the prosthesis. For RA-TKA, the preoperative planning time is the time required for the doctor and software engineer to approve the key registration points for the presegmented 3D CT data in the robot’s main control system, optimize the osteotomy volume of each bone surface, and plan the azimuth angle and model of the prosthesis. (2) Intraoperative registration time. For CM-TKA, the intraoperative registration time is the time required for the surgeon to determine the reference position of the osteotomy according to the femoral intercondylar fossa, trochlea, tibial intercondylar eminence, tibial tubercle and other bone markers and to determine the reference surface for the femoral rotation osteotomy according to the femoral condyle line, transepicondylar axis, Whiteside’s line and posterior condylar line. For RA-TKA, it is the time required for the surgeon to complete the registration of the probes and saw blades and then complete the registration of the 30 bone markers on the femoral side and the 36 bone markers on the tibial side. The time required to achieve satisfactory matching between the intraoperative data and the preoperative plan is recorded. (3) Bone cutting time. This is the time required for the surgeon to complete the osteotomies of the proximal tibia, distal femur and anteroposterior condyles of the femur and to perform the double-chamfer osteotomy and intercondylar box osteotomy either manually, by using the osteotomy guide plate, or by guiding the mechanical arm. (4) Gap balancing time. This is the time required to complete the 4-in-1 osteotomy of the distal femur and the horizontal osteotomy of the proximal tibia. The gap test model is used to evaluate the tension of the medial and lateral soft tissues until a balance of the extension/flexion gap and a balance of the medial and lateral soft tissue gap are achieved through a limited medial and lateral soft tissue release technique. (5) The time for implantation of the prosthesis components. This is the time required for bone cementing of the tibia, femur and pads to complete installation of all components. (6) Wound closure time. The time from the wound stitching to the end of suturing. (7) Total surgery time. The time from the initial incision to the completion of wound suturing. (8) Total procedure time. The time from the beginning of disinfection and draping to wound suturing.
Trauma assessment based on hematological biochemical analysis
The following stress (inflammation) indicators were used to determine the degree of postoperative trauma. (1) The total perioperative blood loss volume was calculated according to the hemoglobin balance equation, and its calculation method, advantages and significance are shown in our previous work [20]. (2) Arterial blood gas analysis results, the white blood cell (WBC) count and classification 24 h after surgery are regarded as the core indicators of the acute postoperative trauma response. Differences in key arterial blood gas indicators (partial pressure of carbon dioxide (PCO2), partial pressure of oxygen (PO2) and SO2) and WBC counts (total number + neutrophil ratio) were compared. (3) The 72-h-postoperative inflammatory markers C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) and the coagulation markers D-dimer and fibrinogen reflect early postoperative stress-related inflammation.
Trauma assessment based on physical measurements of the osteotomy error and radiographical verification
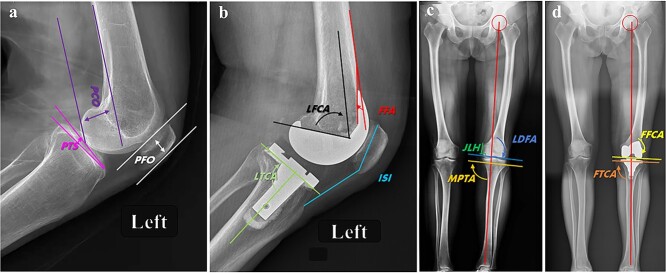
(1) Intraoperative osteotomy deviation is the primary indicator of mechanical trauma. During RA-TKA, the data for 102 cases from 6 groups (17 patients each) based on the actual osteotomy thicknesses of the medial condyle of the distal femur, lateral condyle of the distal femur, posterior medial femoral condyle, posterior lateral femoral condyle, medial tibial plateau and lateral tibial plateau, and on the osteotomy volume of each bone surface planned by the robotic system before the operation, were measured. Data from 48 patients in 3 groups (16 patients per group) who underwent conventional TKA and received a traditional TKA prosthesis (involving the distal femoral condyle, posterior lateral femoral condyle and lateral tibial plateau) were compared with the standard osteotomy volume (9 mm) required by CM-TKA using the equivalent osteotomy rule. The actual error was the absolute values of the actual and planned osteotomy volumes; the distribution of region I (< 1 mm), region II (1–3 mm) and region III (> 3 mm) errors was analyzed. (2) The repeated osteotomy rate is a secondary indicator used to measure the degree of mechanical trauma. In the distal femur, proximal tibia and femoral condyle, a secondary or multiple osteotomy on three surfaces (the distal femur, proximal tibia and posterior lateral femoral condyle) used to determine the extension and flexion gap is defined as repeated osteotomy, and the ratio of the number of cases with repeated osteotomy to the total number of cases is defined as the repeated osteotomy rate. (3) Postoperative radiographical results supported the use of the following angles as the objective basis for osteotomy deviation and mechanical axis recovery: the HKA, frontal femoral component angle (FFCA), frontal tibial component angle (FTCA), lateral tibial component angle (LTCA), femoral flexion angle (FFA), PTS, PCO, patella height (Insall-Salvati index), joint line deviation (JLD) and PFO. Among them, the HKA, FFCA and FTCA are the determinants of the mechanical axis of the lower limb in the coronal plane. The FFA, PTS, PCO and JLD are considered the key factors affecting the anatomical location of the joint line and the joint flexion and extension after TKA. The Insall–Salvati index and PFO are patellar factors that affect the moment arm of the quadriceps femoris (Figure 2).
Figure 2.
Illustrative index of radiographical measurement before and after TKA. a, b are the preoperative and postoperative lateral radiographs of the knee X-ray at 30° of flexion for evaluation of components position. PCO is the distance between the posterior femoral condyle and the tangent line of the posterior femoral cortex; PTS is the angle between the tibial plateau and the horizontal plane; PFO is the distance between the trochlear groove of the femur and the straight line of the anterior patellofemoral cortex; LFCA is the angle between the longitudinal axis of the femur and the prosthesis in sagittal plane; LTCA is the angle between the longitudinal axis of the tabia and the prosthesis in sagittal plane; FFA is the angle between the tangent line of the anterior femoral cortex and the anterior condyle of the prosthesis; ISI is the ratio of patellar height and patellar tendon length; JLH was determined by the distance from the prominence of medial condyle to the distal medial condyle of femur. c. the preoperative standing full-length radiographs of left lower extremity showed a moderate genu varus deformity with degenerated LDFA (91.5°), and MPTA (90.2°). d. postoperative standing full-length radiographs showed the lower limb alignment was ideally corrected as 180.0° in mechanical axis, with a FFCA 90.0°, FTCA 87.0°. TKA total knee arthroplasty, PCO posterior condylar offset, PTS posterior tibial slope, PFO patellofemoral offset, LFCA lateral femoral component angle, LTCA lateral tibial component angle, FFA femoral flexion angle, ISI Insall-Salvalti index, JLH joint line height, LDFA lateral distal femoral angle, MPTA medial proximal tibial angle, FFCA frontal femoral component angle, FTCA frontal tibial component angle
Trauma assessment based on patient comfort assessment and complications
(1) Perioperative use of key drugs. The consumption of three drugs (tranexamic acid, hydrocortisone and aminophenol tramadol) within 3 days after the operation was compared. (2) Patient-reported outcome measures (PROMs). All patients’ data were recorded at outpatient visits by independent observers in January, March, June and December after surgery, and differences in the VAS, KSS, HSS and WOMAC scores before and after surgery were compared. The total VAS score is 10 points, with higher scores indicating more severe pain; responses were categorized as mild pain (1–3 points), moderate pain (4–6 points) and severe pain (7–10 points). The KSS is divided into a clinical score (100 points) that measures pain, stability, range of activity and point-deduction items, and a functional score (100 points) that measures walking and traveling upstairs and includes a point-deduction item. The total KSS score is classified as follows: 170–200 points, excellent; 140–170 points, good; 120–140 points, fair; and <120 points, poor. The HSS includes seven items, including measurements of pain, function, activity, muscle strength, flexion deformity and stability and a point-deduction item. The total score is 100 points, with higher scores indicating milder symptoms; <59 points is classified as poor, 59–69 points as tolerable, 70–84 points as good and 85–100 points as excellent. The WOMAC is scored according to patients’ subjective feelings of pain and the degree of function; it is composed of three subscales of pain, stiffness and difficulty with daily activities. The total score is 240 points, with a higher score corresponding to more severe knee joint symptoms. (3) The incidence of complications included abnormal incision events (fat liquefaction, superficial infection, poor healing, wire reaction, incision dehiscence, etc.), ecchymosis (>1% of the body surface area), thrombosis (femoral vein and popliteal vein trunk thrombosis; intermuscular vein thrombosis with a diameter >3 mm), limited joint function (extension angle >10° and flexion function <90°) and joint instability (gap width discrepancy >3 mm in extension, flexion and mid-flexion; anterior drawer test (+++), neuropraxia, readmission, etc.)
Statistical analysis
Statistical analyses were performed using SPSS 25.0 (IBM, USA). Statistical analysis of the baseline demographics and study outcomes were performed using the independent-samples t-test for continuous variables with normal distributions and the Mann–Whitney U test for continuous variables that were not normally distributed. Categorical data were compared using the chi-squared test with Fisher’s exact test because n < 40. Two-sided tests were performed for all statistical analyses. P values of 0.05 were considered indicative of statistical significance.
Results
Trauma assessment based on subsectional operative time
The preoperative planning and registration times for RA-TKA were 4.1 min (p = 0.000) and 10.1 min (p = 0.000) longer on average than those for CM-TKA, while the bone cutting and gap balancing times for RA-TKA were 5.3 min (p = 0.010) and 2.2 min (p = 0.021) shorter than those for CM-TKA, on average. The total surgery time for RA-TKA was 36.8 min (p = 0.001) longer than that for CM-TKA, and the total operative time was 44.1 min (p = 0.000) longer than that for CM-TKA. No significant difference in the prosthesis installation time and wound suturing time was identified between the two groups (Figure 3). Notably, with the increase in the number of surgical operations and the surgeon’s mastery of the learning curve, the surgery-related time for RA-TKA progressively decreased. In this study, starting with the eighth case, the total surgery time for RA-TKA was 109.2 min, which was not significantly different from that for CM-TKA (99.8 min, p = 0.104).
Figure 3.
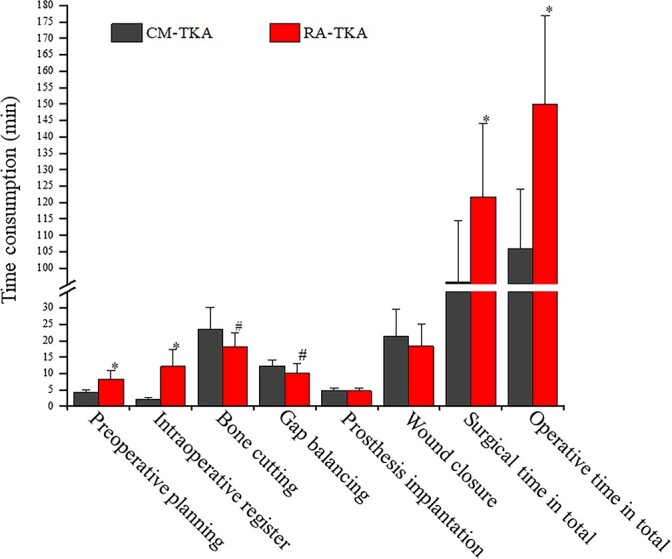
Illustration of subsectional operative time analysis in this study. The general procedure of TKA was divided into eight continuous sections in order, and a targeted analysis for time consumption to reveal trauma effect was made. *, P<0.001, #, P<0.05. CM-TKA conventional manual total knee arthroplasty, RA-TKA robot-assisted total knee arthroplasty
Trauma assessment based on biochemical analysis
Perioperative blood loss
The intraoperative blood loss volume, postoperative drainage volume and hidden blood loss volume with CM-TKA were 178.1, 49.7 and 450.4 ml, respectively, while those with RA-TKA were 160.0, 39.6 and 401.0 ml, respectively (p = 0.670, p = 0.558 and p = 0.665, respectively). The total perioperative blood loss volume with RA-TKA was lower than that with CM-TKA, but the difference was not statistically significant (p = 0.454).
Arterial blood gas analysis at 24 h after the operation
With CM-TKA, PCO2 was 16.7% higher after surgery than before the operation, and PO2 and SO2 were 8.7% and 3.5% lower than the preoperative baseline values, respectively. PCO2 at 24 h after RA-TKA was 10.8% higher than the preoperative baseline value, and PO2 and SO2 were 3.8% and 2.4% lower than those before the operation. The difference between the two groups was significant (p = 0.014, p = 0.042 and P = 0.011, respectively).
WBC count and classification 24 h after the operation
With CM-TKA, the WBC count and neutrophil percentage were increased by 63.7% and 33.6%, respectively, compared with the preoperative baseline values, while those for RA-TKA were 46.8% and 28.2%, respectively. The differences between the two groups were significant at p = 0.020 and p = 0.019, respectively. The WBC and neutrophil percentage returned to the normal range 72 h after operation, and the difference was not statistically significant (p > 0.05).
Inflammatory markers at 72 h after the operation
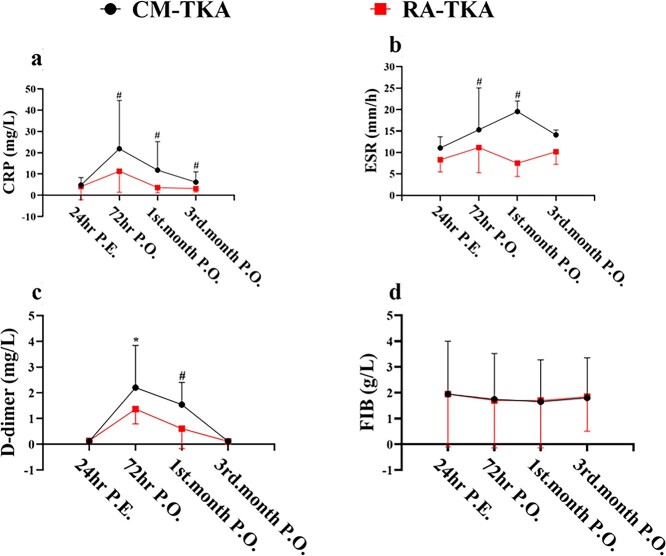
With CM-TKA, CRP increased by 356.3% compared with the preoperative baseline values, and the ESR increased by 70.5% compared with the preoperative baseline values, while with RA-TKA, CRP increased by 175.6% and the ESR increased by 48.5%; the differences between the groups were significant at p = 0.036 and p = 0.018, respectively. In the CM-TKA group, CRP and the ESR decreased by 46.1% and 4.1% at the outpatient revisit in January, while CRP and the ESR decreased by 68.1% and 36.6% in the RA-TKA group at the same time point; the differences between the groups were significant at p = 0.029 and p = 0.023, respectively (Figure 4a and b).
Figure 4.
Dynamic change of hematological inflammation (CRP, ESR) and coagulation markers (D-dimer, Fibrinogen) of the two groups in this study. The elevation of CRP in RA-TKA was eliminated by 48.4% at 72hrs P.O. compared to CM-TKA, and its recovery into normal range can be found as early as 1st-month after RA-TKA (a); The increasement of ESR in RA-TKA was eliminated by 31.1% at 72hrs P.O., 54.5% at 1st-month P.O., 17.4% at 3rd month P.O., as compared to CM-TKA (b); The elevated D-dimer in RA-TKA was eliminated by 47.3% at 72hrs P.O., 46.0% at 1st-month P.O., compared to CM-TKA (c); There was no significant difference in content change of fibrinogen between CM-TKA and RA-TKA after TKA (d). *, P < 0.001, #, P < 0.05. CM-TKA conventional manual total knee arthroplasty, 1RA-TKA robot-assisted total knee arthroplasty. CRP C-reactive protein, ESR erythrocyte sedimentation rate, FIB fibrinogen, hrs hours, P.E. Preoperatively, P.O. postoperatively
Coagulation markers at 72 h after the operation
In the CM-TKA and RA-TKA groups, D-dimer increased by 1985.1% and 935.1% compared with the preoperative baseline values, respectively, and the difference between the groups was significant at p = 0.000. At 1 month after CM-TKA, D-dimer was still 1233.5% higher than the value before the operation, whereas in the RA-TKA group, D-dimer at 1 month was 578.9% higher than that before the operation, and the difference between the groups was significant at p = 0.003. At 3 months after the operation, D-dimer levels had returned to the normal range in both groups. No significant difference in fibrinogen changes was observed between the two groups before and after the operation (p > 0.05); Table 2; Figure 4c and d.
Table 2.
Hematological analysis of traumatic and inflammatory indicators in this study, mean (SD)
| Assessment | Phase |
CM-TKA
(n=16) |
RA-TKA
(n=17) |
P value |
| Blood loss (ml) | ||||
| Total blood loss | 678.2 (306.7) | 600.6 (281.0) | 0.454 | |
| Intraoperative blood loss | 178.1 (151.6) | 160.0 (82.5) | 0.670 | |
| Drainage | 49.7 (53.5) | 39.6 (44.4) | 0.219 | |
| Hidden blood loss | 450.4 (339.6) | 401.0 (308.9) | 0.665 | |
| Arterial blood gas | ||||
| PCO2 (mm/Hg) | 24 h P.E. | 40.7 (2.6) | 40.7 (3.6) | 0.987 |
| 24 h P.O. | 47.5 (3.4) | 45.1 (1.5) | 0.014 | |
| PO2 (mm/Hg) | 24 h P.E. | 76.2 (8.3) | 78.1 (9.0) | 0.527 |
| 24 h P.O. | 69.6 (5.9) | 75.1 (8.8) | 0.042 | |
| SO2(%) | 24 h P.E. | 97.6 (6.1) | 97.8 (5.6) | 0.630 |
| 24 h P.O. | 94.2 (1.2) | 95.4 (1.4) | 0.011 | |
| Blood cell count | ||||
| WBC (109/l) | 24 h P.E. | 6.29 (1.49) | 6.34 (1.44) | 0.922 |
| 24 h P.O. | 10.3(0.9) | 9.3(1.3) | 0.020 | |
| NEUT% | 24 h P.E. | 66.4 (7.9) | 67.3 (5.6) | 0.717 |
| 24 h P.O. | 88.7(2.3) | 86.3(3.2) | 0.019 | |
| RBC (1012/l) | 24 h P.E. | 4.43 (0.49) | 4.42 (0.46) | 0.990 |
| 72 h P.O. | 3.55 (0.49) | 3.62 (0.38) | 0.649 | |
| HgB (g/l) | 24 h P.E. | 129.6 (16.1) | 131.9 (13.7) | 0.658 |
| 72 h P.O. | 115.6 (12.4) | 114.9 (10.7) | 0.867 | |
| HCT (%) | 24 h P.E. | 39.4 (3.8) | 40.3 (3.9) | 0.524 |
| 72 h P.O. | 31.6 (2.9) | 33.4 (3.4) | 0.110 | |
P.E. preoperatively, P.O. postoperatively, WBC white blood cells, NEUT% neutrophil percentage, HCT hematocrit, RBC red blood cell, HgB hemoglobin
Physical measurements of the osteotomy error and radiographical verification
Physical measurement of osteotomy deviation
A total of 27.1% (26/96) of the osteotomy deviation in the CM-TKA group was located in region I, 53.1% (51/96) was in region II and 19.8% (19/96) was in region III; the proportions of the osteotomy deviations in the RA-TKA group that fell within regions I, II and III were 76.5% (78/102), 20.6% (21/102) and 2.9% (3/102), respectively (Figure 5). The results of the χ2 test showed that the accuracy of CM-TKA was significantly lower than that of RA-TKA (p = 0.000). In addition, the success rate of one-time osteotomy with CM-TKA was 62.5% (4 cases underwent secondary osteotomy, 2 cases underwent tertiary osteotomy), while the success rate of one-time osteotomy with RA-TKA was 94.1% (only 1 case underwent secondary osteotomy, p = 0.039) (Table 3).
Figure 5.
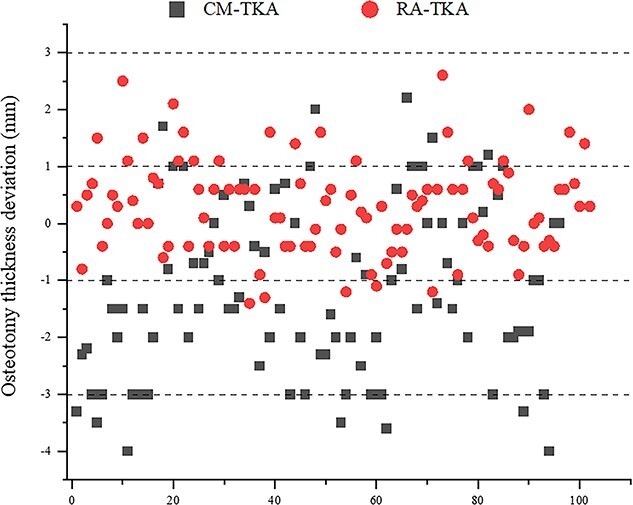
Scattering distribution of mechanical deviation of osteotomy thickness of 106 bone-cuts in RA-TKA compared to 96 bone-cuts in CM-TKA by physical measurement in this study. CM-TKA conventional manual total knee arthroplasty, RA-TKA robot-assisted total knee arthroplasty
Table 3.
Radiographical measurement of preoperative and postoperative index indicating lower limb alignment, prosthesis azimuths and anatomical reconstruction of the knee in this study, mean (SD)
| Variables | Phase |
CM-TKA
(n=16) |
RA-TKA
(n=17) |
P value |
| HKA (°) | P.E. | 175.0 (5.1) | 175.2 (3.2) | 0.888 |
| P.O. | 177.8 (1.7) | 179.1 (0.5) | 0.010 | |
| FFCA (°) | P.O. | 90.8 (2.4) | 89.7 (2.2) | 0.168 |
| LDFA (°) | P.E. | 88.8 (4.4) | 88.3 (2.9) | 0.685 |
| FTCA (°) | P.O. | 88.9 (1.3) | 90.1 (1.4) | 0.019 |
| MPTA (°) | P.E. | 86.6 (2.9) | 86.3 (2.4) | 0.763 |
| PTS (°) | P.E. | 7.4 (2.4) | 8.7 (3.3) | 0.382 |
| P.O. | 4.4 (1.6) | 4.5 (1.4) | 0.870 | |
| LFCA (°) | P.O. | 71.0 (2.9) | 70.4 (1.8) | 0.526 |
| LTCA (°) | P.O. | 91.2 (2.6) | 88.8 (3.4) | 0.034 |
| FFA (°) | P.O. | 7.4 (4.0) | 6.7 (2.5) | 0.558 |
| ISI | P.E. | 0.98 (0.1) | 0.99 (0.1) | 0.788 |
| P.O. | 1.0 (0.1) | 1.00 (0.1) | 0.991 | |
| PCO (mm) | P.E. | 26.8 (3.6) | 25.7 (3.8) | 0.362 |
| P.O. | 28.1 (2.6) | 28.2 (2.8) | 0.901 | |
| PFO (mm) | P.E. | 17.3 (1.0) | 16.8 (0.7) | 0.108 |
| P.O. | 18.9 (1.2) | 18.4 (0.9) | 0.142 | |
| JLH (mm) | P.E. | 32.2 (4.2) | 32.1 (4.2) | 0.924 |
| P.O. | 34.9 (3.5) | 34.9 (3.9) | 0.891 |
P.E. preoperatively, P.O. postoperatively, FFCA frontal femoral component angle, FTCA frontal tibia component angle, LTCA lateral tibia component angle, MPTA medial proximal tibial angle, PTS posterior tibial slope, HKA hip–knee–ankle angle, LDFA lateral distal femoral angle, PCO posterior condylar offset, PFO patelofemoral offset, FFA femoral flexion angle, ISI Insall-Salvalti index, JLH joint line height, LFCA lateral femoral component angle
Radiographical results validation
No significant differences in FFCA and FFA were noted between the CM-TKA and RA-TKA groups in the coronal and sagittal angles of the femoral prosthesis (p > 0.05), but an outlier of the azimuthal FTCA of the tibial prosthesis in the coronal plane away from the target value of 90° was 0.1° (vs 1.1° in CM-TKA, p = 0.019), and an outlier of the azimuthal LTCA of the tibial prosthesis in the sagittal plane away from the target value of 87° was 1.8° (vs 4.2° in CM-TKA, p = 0.034). The resulting HKA angles with CM-TKA and RA-TKA were 177.8° and 179.1° (p = 0.010), respectively. RA-TKA produced values closer to the ideal result. Four cases in the CM-TKA group exceeded the recognized cutoff value of mechanical alignment (± 3°) compared to 0 cases in the RA-TKA group.
This study also focused on hidden factors contributing to two types of deviation (joint line and offset) with PS TKA. The results showed that the joint line after RA-TKA was 2.8 mm higher than that before the operation and was not significantly different from the average decrease of 2.7 mm with CM-TKA (p = 0.891). The PCO was used to measure the important indicators of flexion gap tension and femoral condyle rollback; the PCO values after CM-TKA and RA-TKA were 28.2 and 28.1 mm (p = 0.901), respectively. PFO is an important indicator for measuring the moment arm of the quadriceps femoris. The results showed that the PFO after RA-TKA and CM-TKA was increased by 1.6 mm compared with that before the operation, and no significant difference was found between the two groups (p > 0.142).
Trauma assessment based on patient comfort and complications
The consumption of key drugs during the early postoperative period
During the perioperative period, the RA-TKA group used 785.4 mg less tranexamic acid (p = 0.012) and 38.3 mg less of the stress effect regulator hydrocortisone (p = 0.016) than the CM-TKA group. The utilization rate of opioid analgesics for temporary relief was 23.5% in the RA-TKA group, which was significantly lower than the 62.5% in the CM-TKA group (p = 0.037).
PROMs
The KSS, HSS, VAS and WOMAC scores at 3 months after RA-TKA and CM-TKA were 162.5 vs 151.3 (p = 0.140), 73.5 vs 73.9 (p = 0.894), 1.6 vs 1.5 (p = 0.841) and 91.1 vs 113.7 (p = 0.165), respectively. The joint function of the two groups was stable during continuous observation until 1 year after operation and the difference was not statistically significant.
Complications
One case of wound complication each occurred in the CM-TKA and RA-TKA groups (p = 1.000); both were subcutaneous wire responses combined with fat liquefaction. One case of ecchymosis occurred in the RA-TKA group, which mainly affected the posterior medial area of the thigh and calf, accounting for ~3% of the body surface area. Within 1 week after the operation, 2 cases of thrombosis were detected in the CM-TKA group on lower extremity vascular ultrasound examination, and 3 cases of thrombosis were detected in the RA-TKA group (p = 0.680). All cases were intermuscular venous thrombosis, and no trunk thrombosis in the popliteal or femoral vein occurred. Five cases and 3 cases of moderate pain (VAS score > 3 points) were noted in the CM-TKA and RA-TKA groups, respectively, at 1 month after the operation (p = 0.438). At 1 year after the operation, the incidence rates of infection, dislocation, loosening, a translucent line on imaging, periprosthetic fracture, joint stiffness, all-cause secondary admission and other common complications were 0 for both groups of patients.
Discussion
This study reported partial results from a multicenter, randomized controlled clinical trial of the largest domestically developed surgical robot (YUANHUA) in China. We intensively analyzed the performance of RA-TKA in terms of stratified trauma-related indicators to determine whether the use of RA-TKA was beneficial or harmful in terms of trauma response. The innovation in this study is that we completed the animal and cadaver experiments with the YUANHUA robot (YUANHUA, Shenzhen) in previous work and have now confirmed that the YUANHUA system was helpful to assist the surgeon in executing the operation according to the preoperative plan and has good auxiliary value for TKA in terms of safety and accuracy [18,19]. Besides, most of the observation indicators for RA-TKA used worldwide are limited to radiographic results, such as the accuracy of the mechanical axis of the lower limb and the prosthesis position, or to overall function scores based on PROMs; to the best of our understanding, systematic evaluations of the iatrogenic trauma caused by the operation itself are still lacking. Therefore, this study is believed to be the first prospective controlled study to conduct a hierarchical and quantitative evaluation on the trauma effect of RA-TKA.
We first investigated the trauma effect in terms of subsectional operative time. The reason for segmentalizing operative time is that total operative time does not provide an objective basis for measuring the severity of surgical trauma. In this study, we divided the total operative time into eight consecutive subsections according to the key time points of RA-TKA, which allowed us to accurately identify the exact point at which RA-TKA minimizes or amplifies the trauma response. Our results found that RA-TKA was conducive to controlling the trauma response at the level of the time taken for surgery. First, RA-TKA significantly shortened the time required for bone cutting and gap balancing: these durations were 5.3 min (p = 0.010) and 2.2 min (p = 0.021) shorter, respectively, with RA-TKA than with CM-TKA. The significance of this finding is that previous studies have shown that osteotomy error and repeated osteotomy during TKA can lead to excessive opening of the metaphyseal sinus, exhaustion of coagulation factors and progressive inflammatory cascade [21,22]. Second, although RA-TKA had a significantly longer total surgical time and operative time compared with CM-TKA, careful observation showed that the time increase was mainly concentrated in the preoperative planning and intraoperative registration stages. In other words, this time increase did not affect the periods associated with the opening of the joint cavity or sinus, or interference with the medullary cavity, which trigger trauma and inflammation. In addition, the above increases in total surgical time and operative time were undeniably affected by the inclusion of the authors’ earliest experiences with operating the YUANHUA robot; that is, they can be attributed to the learning curve. We found that the total surgical time for RA-TKA fell within ±10% of that for CM-TKA after completion of 7–8 RA surgeries, which is consistent with reports from Kayani et al. [23]. Therefore, this study confirms that even in terms of the learning curve, RA-TKA still has advantages over CM-TKA.
The dynamic changes of hematological biochemical markers are considered to reflect the trauma severity in TKA. Oelsner et al. pointed out that postoperative CRP peaked during the inpatient period and returned to baseline by 2 weeks. Fibrinogen peaked after CRP and returned to baseline by 6 weeks. Elevated preoperative CRP correlated with a more robust postoperative acute phase response for both total hip arthroplasty and total knee arthroplasty [16]. An et al. found D-dimer measurements peaked 2 weeks postoperatively for TKA; the peak serum D-dimer measurement decresed by 54.3% for TKA at the 6-week time point [17]. Tanavalee et al. compared inflammation in primary osteoarthritis between two groups of patients undergoing TKA with and without synovectomy. Similar changes in serial inflammatory markers were found in both groups, including mean peak levels of interleukin 6 (189 pg/ml vs 201 pg/ml, respectively) and CRP (91 mg/l vs 88 mg/l, respectively) on the first post-operative day, and return to pre-operative baseline at 2 and 6 weeks, respectively [24]. In this study, we found RA-TKA could further reduce the content of classical biochemical markers in the acute phase inflammatory response, as evidenced by significantly declined CRP (by 180.7%), ESR (by 22.0%) and D-dimer (by 1050.0%), referenced to the preoperative baseline values, as compared to CM-TKA (p < 0.05). A similar observation was made by Kayani et al. [14], whoeported that the levels of interleukin-6, tumor necrosis factor-α, the ESR, CRP, lactate dehydrogenase and creatine kinase on day 7 after surgery were 70.9%, 42.4%, 38.1%, 61.4%, 16.3% and 47.5% lower, respectively, after RA-TKA than after CM-TKA (all p < 0.05).
In this study, we also selected the white cell count and classification, blood gas analysis results and coagulation markers as auxiliary biochemical indicators, and described the postoperative expression of these markers in terms of two characteristics. First, the changes of these indicators induced by RA-TKA were much lower those induced by CM-TKA. For example, CRP, the ESR and D-dimer in the RA-TKA group at 72 h after the operation were 175.6%, 48.5% and 935.1% higher than the preoperative baseline values, respectively; these were much lower than the changes induced by CM-TKA, which were 356.3% (p = 0.036), 70.5% (p = 0.018) and 1985.1% (p = 0.000), respectively. Second, the time required for these indicators to return to the normal range during postoperative observation was much shorter with RA-TKA than with CM-TKA. For example, CRP and the ESR returned to the physiological range at 1 month after operation in 100% of the RA-TKA patients, while among the CM-TKA patients, 37.5% and 43.8%, respectively, had CRP and ESR values that had not returned to normal at 1 month after the operation.
The osteotomy deviation during CM-TKA leads to gap imbalance, poor recovery of the mechanical axis of the lower limb and instability, thereby increasing the incidence of postoperative polyethylene liner wear, prosthesis loosening and subsidence and reducing the long-term survival rate of the prosthesis [6]. When measured in terms of long-term efficacy, these adverse events are subtle traumatic responses. Therefore, improving the accuracy of the surgical operation is undoubtedly the basic premise for reducing such trauma, and existing mainstream studies believe that robot assistance is the optimal strategy to achieve this goal. In addition to the well-documented records of mechanical axis restoration by RA-TKA, a panel of studies investigated the value of the RA technique for eliminating the adverse structural and load effects caused by the conventional technique. Vaidya et al. [25] reported a JLD of 3.5 mm in the CM-TKA group compared to 0.9 mm in the RA-TKA group (p < 0.001). Liow [26] noted that the incidence rates of anterior notching were 10.3 and 0% in the CM-TKA and RA-TKA groups, respectively (p = 0.049). Song [27] emphasized that the ratio of instability (flexion-extension gap difference ≥ 4 mm) was 6.0% with RA-TKA compared to 20.0% with CM-TKA (p < 0.05). Moon et al. [28] confirmed that the femoral rotational alignment with RA-TKA was 0.52° compared to 2.76° with CM-TKA in relation to the transepicondylar axis. More importantly, Held [29] found that the percentage of high load compartment pressure in flexion (>40 lbs) was significantly greater for the conventional (18%) vs robotic (3%) TKA cohorts (p = 0.025); the ratio with unbalanced knees (>20-lb differential between medial and lateral compartments) in flexion was significantly greater in the conventional (24%) vs robotic (5%) TKA cohorts (p = 0.018). In this study, we found restoration of the mechanical axis of the lower limb with RA-TKA (0 case) was far superior to that with CM-TKA (4 cases). More importantly, RA-TKA was more conducive to the tibial prosthesis achieving a coronal azimuth perpendicular to the mechanical axis and an ideal PS prosthesis tilt angle (87°) on the sagittal plane.
Another potential advantage of robot assistance is the reduction of soft tissue invasion and iatrogenic wounding [30]. The range of osteotomy in RA-TKA is preset in the system according to the preoperative plan, and the soft tissue around the knee is outside the preset safety zone. Therefore, the risk of injury to the ligaments and their attachments is significantly reduced. Khlopas et al. [31] conducted a trial based on 23 cadavers and found that no visible evidence of disruption of any of the ligaments was identified with RA-TKA, but disruption of the posterior cruciate ligament (PCL) was noted in 2 of the 7 manual TKA procedures performed. A study consisting of 14 cases of knee osteoarthritis with constitutional femoral varus from southwest China revealed that 7 patients underwent computer navigation-assisted TKA, and none involved pie-crusting release of the MCL or peeling-off detachment of the posteromedial tibia; in contrast, among the 7 patients who underwent CM-TKA, 4 required releases of the superficial medial collateral ligament (MCL) and 3 required peeling off of the tibial attachment [32]. In this study, the osteotomy error with RA-TKA was 0.67 mm, the success rate of one-time osteotomy was 94.1% and the incidence of ligament and joint capsule injury was 0%, which verified the previous statements.
ERAS is the core perioperative management principle in modern TKA, and the use of key drugs such as analgesics, anti-inflammatory agents and hemostatic agents serve as the indicators of early comfort after TKA. Rice et al. [33] found that the prevalence rates of moderate to severe postoperative pain were 21% and 16% at 6 and 12 months after CM-TKA, respectively. Bhimani et al. [34] found that patients who underwent RA-TKA had lower pain levels both at rest and with activity (p < 0.05) and required 3.2 mg morphine equivalents less per day than patients who underwent CM-TKA (p < 0.001). In this study, under the premise of providing equal doses of NSAID-based analgesia, the RA-TKA patients had a 39.0% lower rate of temporary intensive opioid analgesic use than the CM-TKA patients. The roles of tranexamic acid and hydrocortisone have been properly proved, but their consumption profiles in TKA have not been investigated to date. In the present study, the RA-TKA group’s consumption levels of these drugs were 785.4 mg (p = 0.012) and 38.3 mg (p = 0.016) lower, respectively, than those of the CM-TKA group. These results preliminarily show that RA-TKA patients had better early postoperative comfort and lower incidence rates of pain, bleeding and excessive stress.
The limitation of this study is that single-center data from a multicenter prospective clinical study of YUANHUA RA-TKA in China were used. Since the overall objective of the study was to evaluate the safety and efficacy of surgical robots, quantitative trauma indicators were not uniformly included; therefore, data from other centers cannot be added to conduct a large multicenter comparative analysis of trauma effects. Second, this study aimed mainly to perform an observational analysis of clinical phenomena and did not consider the cellular and molecular mechanisms of RA-TKA that might be beneficial to trauma control. We assume that the improved trauma control may be related to the following factors: reduction of the area and duration of blood sinus opening, avoidance of medullary cavity interference and decompensation of the bone marrow microenvironment, etc. Additional basic studies are needed to verify these conjectures.
Although this study preliminarily confirms that RA-TKA offers certain advantages in terms of subsectional operative time, biochemical indicators, osteotomy success rate, radiological precision and postoperative comfort, we cannot blindly promote RA-TKA at present because the large-scale development of artificial intelligence technology is still affected by ethics, cognition, economic factors, medical insurance policies and other factors [35]. The unsignificant improvement in evaluating PROMs does not demonstrate sufficient benefits to match its clinical advantages based on the trauma control observed during continuous observation from 3 months to 1 year after surgery. The question remains as to whether this result suggests that we should start using RA-TKA in some specific situations, such as complex deformities that are prone to osteotomy deviation, soft tissue contractures requiring expansion of the soft tissue balance and situations requiring the strict management of surgical trauma.
Conclusions
Compared with CM-TKA, RA-TKA decreases rather than increases trauma. This approach might shorten the time required for bone cutting and gap balancing, reduce the incidence of mechanical errors related to the osteotomy and prosthesis position, and improve the accuracy of the mechanical alignment reconstruction. RA-TKA is also favorable in promoting postoperative comfort and minimizing inflammatory response and drug consumption.
Abbreviations
CM-TKA: Conventional manual total knee arthroplasty; CP: Computed tomography; CRP: C-Reactive protein; ERAS: Enhanced recovery after surgery; ESR: Erythrocyte sedimentation rate; FFA: Femoral flexion angle; FFCA: Frontal femoral component angle; FTCA: Frontal tibia component angle; HKA: Hip–knee–ankle angle; HSS: Hospital for special surgery score; JLD: Joint line deviation; KSS: Keen Society Score; LDFA: Lateral distal femoral angle; LTCA: Lateral tibia component angle; MPTA: Medial proximal tibial angle; PCO: Posterior condylar offset; PFO: Patellofemoral offset; PROMs: Patient-reported outcome measures; PTS: Posterior tibial slope; RA-TKA robot-assisted total knee arthroplasty: VAS: Visual analog scale; WBC: White blood cell; WOMAC: The Western Ontario and McMaster Universities Osteoarthritis Index; NEUT%: neutrophil percentage, HCT: hematocrit; RBC: red blood cell; HgB: hemoglobin; ISI: Insall-Salvalti index; JLH: joint line height; LFCA: lateral femoral component angle; PCL: posterior cruciate ligament; MCL: medial collateral ligament.
Funding
Innovative technology in military and clinical medicine (2018JSLC0035); Technological Innovation and Application Demonstration Project of Chongqing (cstc2018jscx-msyb0541); Continual Medical Education Project of Chongqing (2020-04-07-067); and Central Committee Guiding Local Technology Development Project (0028).
Authors’ contributions
All authors have made contributions to the paper and authorized the submission. YZ conceived the article and carried out all the operations. ZHX collected the data and drafted manuscript. HL contributed to preoperative planning and intraoperative coordination. ZYL, JZ and JL provided data management and analyses support. All authors contributed to manuscript preparation and critical revision. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The research protocol was approved by the clinical trial ethics of Xinqiao Hospital of Army Medical University Review Board Approval. China Clinical Trial Center Registration No.: ChiCTR2000031282. All patients signed informed consent forms.
Conflict of interest
The authors have no competing interests.
Contributor Information
Zhonghua Xu, Joint Disease & Sport Medicine Center, Department of Orthopedics, Xinqiao Hospital, Army Medical University, Chongqing, China, 400038.
Hua Li, Department of Anesthesiology and Operation Room, Xinqiao Hospital, Army Medical University, Chongqing, China, 400038 .
Zaiyang Liu, Joint Disease & Sport Medicine Center, Department of Orthopedics, Xinqiao Hospital, Army Medical University, Chongqing, China, 400038.
Jie Li, Joint Disease & Sport Medicine Center, Department of Orthopedics, Xinqiao Hospital, Army Medical University, Chongqing, China, 400038.
Jun Zhang, Joint Disease & Sport Medicine Center, Department of Orthopedics, Xinqiao Hospital, Army Medical University, Chongqing, China, 400038.
Min Wang, Joint Disease & Sport Medicine Center, Department of Orthopedics, Xinqiao Hospital, Army Medical University, Chongqing, China, 400038.
Yuan Zhang, Joint Disease & Sport Medicine Center, Department of Orthopedics, Xinqiao Hospital, Army Medical University, Chongqing, China, 400038.
References
- 1. Feng B, Zhu W, Bian YY, Chang X, Cheng KY, Weng XS. China artificial joint annual data report. Chin Med J. 2020;134:752–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Springer BD, Levine BR, Golladay GJ. Highlights of the 2020 American joint replacement registry annual report. Arthroplasty Today. 2021;9:141–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bian YY, Cheng KY, Chang X, Weng XS. Reports and analysis of amount of hip and knee arthroplasty in China from 2011 to 2019. Chin J Orthop 2020; 40: 1453–60. [Google Scholar]
- 4. Matsuda S, Kawahara S, Okazaki K, et al. . Postoperative alignment and ROM affect patient satisfaction after TKA. Clin Orthop Relat Res. 2013;471:127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ritter MA. The anatomical graduated component total knee replacement: a long-term evaluation with 20-year survival analysis. J Bone Joint Surg Br. 2009;91:745–9. [DOI] [PubMed] [Google Scholar]
- 6. Kim YH, Park JW, Kim JS, Park SD. The relationship between the survival of total knee arthroplasty and postoperative coronal, sagittal and rotational alignment of knee prosthesis. Int Orthop. 2014;38:379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplast. 2007;22:1097–106. [DOI] [PubMed] [Google Scholar]
- 8. Lee DH, Park JH, Song DI, Padhy D, Jeong WK, Han SB. Accuracy of soft tissue balancing in TKA: comparison between navigation-assisted gap balancing and conventional measured resection. Knee Surg Sports Traumatol Arthrosc. 2010;18: 381–7. [DOI] [PubMed] [Google Scholar]
- 9. Ripollés-Melchor J, Abad-Motos A, Díez-Remesal Y, Aseguinolaza-Pagola M, Padin-Barreiro L, Sánchez-Martín R, et al. . Association between use of enhanced recovery after surgery protocol and postoperative complications in Total hip and knee arthroplasty in the postoperative outcomes within enhanced recovery after surgery protocol in elective Total hip and knee arthroplasty study (POWER2). JAMA Surg. 2020;155:e196024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Z, Zhang J, He K, Zhang Y, Zhang Y. Optimized clinical practice for superaged patients with hip fracture: significance of damage control and enhanced recovery program. Burns Trauma. 2019;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siebert W, Mai S, Kober R, Heeckt PF. Technique and first clinical results of robot-assisted total knee replacement. Knee. 2002;9:173–80. [DOI] [PubMed] [Google Scholar]
- 12. Liow M, Chin PL, Pang HN, Tay DK, Yeo SJ. THINK surgical TSolution-one((R)) (Robodoc) total knee arthroplasty. SICOT J. 2017;3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karthik K, Colegate-Stone T, Dasgupta P, Tavakkolizadeh A, Sinha J. Robotic surgery in trauma and orthopaedics: a systematic review. Bone Joint J. 2015;97-B:292–9. [DOI] [PubMed] [Google Scholar]
- 14. Kayani B, Tahmassebi J, Ayuob A, Konan S, Oussedik S, Haddad FS. A prospective randomized controlled trial comparing the systemic inflammatory response in conventional jig-based total knee arthroplasty versus robotic-arm assisted total knee arthroplasty. Bone Joint J. 2021;103-B:113–22. [DOI] [PubMed] [Google Scholar]
- 15. Burbul M, Tomaszewski D, Rogalska A, Gawroński K, Literacki S, Waśko M. Thrombotic activation before and after total hip arthroplasty. A prospective cohort study. BMC Musculoskelet Disord. 2021;22:691–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oelsner WK, Engstrom SM, Benvenuti MA, An TJ, Jacobson RA, Polkowski GG, et al. . Characterizing the acute phase response in healthy patients following Total joint arthroplasty: predictable and consistent. J Arthroplast. 2017;32:309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. An TJ, Engstrom SM, Oelsner WK, Benvenuti MA, Polkowski GG, Schoenecker JG. Elevated d-dimer is not predictive of symptomatic deep venous thrombosis after Total joint arthroplasty. J Arthroplast. 2016;31:2269–72. [DOI] [PubMed] [Google Scholar]
- 18. Chai W, Xie J, Zang XG, Yan TF, Zhao YL, He CA, et al. . Animal experimental study on domestic robot-assisted total knee arthroplasty. Chin J of Reparative Reconstr Surg. 2020;34:1376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chai W, Xie J, Zang XG, He C, Yan TF, Liu L, et al. . A cadaveric experimental study on domestic robot-assisted total knee arthroplasty. Chin J of Reparative Reconstr Surg. 2021;35:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu ZY, Zhang J, Li J, He K, Zhang YM, Zhang Y. The role of continuous optimization program in damage control of perioperative blood loss during primary total knee arthroplasty. J Trauma Surg. 2020;22:94–100. [Google Scholar]
- 21. Heim CE, Yamada KJ, Fallet R, Odvody J, Schwarz DM, Lyden ER, et al. . Orthopaedic surgery elicits a systemic anti-inflammatory signature. J Clin Med. 2020;9:2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langkilde A, Jakobsen TL, Bandholm TQ, Eugen-Olsen J, Blauenfeldt T, Petersen J, et al. . Inflammation and post-operative recovery in patients undergoing total knee arthroplasty-secondary analysis of a randomized controlled trial. Osteoarthr Cartil. 2017;25:1265–73. [DOI] [PubMed] [Google Scholar]
- 23. Kayani B, Konan S, Huq SS, Tahmassebi J, Haddad FS. Robotic-arm assisted total knee arthroplasty has a learning curve of seven cases for integration into the surgical workflow but no learning curve effect for accuracy of implant positioning. Knee Surg Sports Traumatol Arthrosc. 2019;27:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanavalee A, Honsawek S, Rojpornpradit T, Sakdinakiattikoon M, Ngarmukos S. Inflammation related to synovectomy during total knee replacement in patients with primary osteoarthritis: a prospective, randomised study. J Bone Joint Surg Br. 2011;93:1065–70. [DOI] [PubMed] [Google Scholar]
- 25. Vaidya NV, Deshpande AN, Panjwani T, Patil R, Jaysingani T, Patil P. Robotic-assisted TKA leads to a better prosthesis alignment and a better joint line restoration as compared to conventional TKA: a prospective randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2020;30:621–6. [DOI] [PubMed] [Google Scholar]
- 26. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplast. 2014;29:2373–7. [DOI] [PubMed] [Google Scholar]
- 27. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471:118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moon YW, Ha CW, Do KH, Kim CY, Han JH, Na SE, et al. . Comparison of robot-assisted and conventional total knee arthroplasty: a controlled cadaver study using multiparameter quantitative three-dimensional CT assessment of alignment. Comput Aided Surg. 2012;17:86–95. [DOI] [PubMed] [Google Scholar]
- 29. Held MB, Grosso MJ, Gazgalis A, Sarpong NO, Boddapati V, Neuwirth A, et al. . Improved compartment balancing using a robot-assisted Total knee arthroplasty. Arthroplasty Today. 2021;7:130–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng B, Tian J, Peng Y, Fu X. Iatrogenic wounds: a common but often overlooked problem. Burns Trauma. 2019;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khlopas A, Chughtai M, Hampp EL, Scholl LY, Prieto M, Chang TC, et al. . Robotic-arm assisted Total knee arthroplasty demonstrated soft tissue protection. Surg Technol Int. 2017;30:441–6. [PubMed] [Google Scholar]
- 32. Xu ZH, Zhang Y. What’s New in Artificially Intelligent Joint Surgery in China? A Conference Record from the 2021 IEEE ICRA and Literature Review. Arthroplasty, 2022; 4:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rice DA, Kluger MT, McNair PJ, Lewis GN, Somogyi AA, Borotkanics R, et al. . Persistent postoperative pain after total knee arthroplasty: a prospective cohort study of potential risk factors. Br J Anaesth. 2018;121:804–12. [DOI] [PubMed] [Google Scholar]
- 34. Bhimani SJ, Bhimani R, Smith A, Eccles C, Smith L, Malkani A. Robotic-assisted total knee arthroplasty demonstrates decreased postoperative pain and opioid usage compared to conventional total knee arthroplasty. Bone Jt Open. 2020;1:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun M, Yang L, He R. Application and research progress of robotic-arm in total knee arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021;35:913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]