Abstract
Background
Cancer and ischemic stroke are two common diseases that threaten human health and have become the main causes of death in the world. It is estimated that one‐in‐ten patients with ischemic stroke have concomitant cancer, and this incidence is expected to increase as improvements in medical technology extends the life expectancy of cancer patients.
Discussion
Cancer‐related stroke (CRS) refers to unexplained ischemic stroke in patients with active cancer that cannot be explained by current stroke mechanisms. Available evidence suggests that CRS accounts for 5–10% of embolic stroke of undetermined source (ESUS). Although the incidence of CRS is gradually increasing, its underlying pathogenesis remains unclear. Also, there is no consensus on acute treatment and secondary prevention of stroke.
Conclusion
In this review, we retrospectively analyzed the incidence, mechanisms of CRS, its potential as a new stroke subtype, options for acute treatment, secondary prevention strategies, and disease progression, with the aim of attempting to explore personalized therapy strategies.
Keywords: cancer‐related stroke, embolic stroke of undetermined source, hypercoagulability, individualized therapy, pathogenesis, stroke subtype
We discussed the possible pathogenesis of cancer‐related stroke and explored tailored treatment options based on differences in pathogenesis.
1. INTRODUCTION
Cancer and stroke are common diseases that are currently threatening human health. Evidence has shown that the risk of ischemic stroke begins to increase in the early stages of cancer diagnosis (Jang et al., 2019; Navi et al., 2018, 2015, 2018, 2017, 2019). In a health insurance‐based study, 6.9% of elderly lung cancer patients had an ischemic stroke one year after cancer diagnosis, versus 3.2% in a matched control group (Navi et al., 2017). With the aging population worldwide (Tu et al., 2022) and the advancement of medical treatment standards, the incidence of cancer‐related stroke (CRS) is expected to increase (Strongman et al., 2019). One‐quarter to one‐third of ischemic strokes have no established mechanism after standard diagnostic evaluation and are classified as embolic stroke of undetermined source (ESUS). CRS is a unique and important subgroup in ESUS, accounting for 5–10% of ESUS. Compared with traditional stroke, CRS is unique in clinical characteristic, underlying pathophysiologies, and treatment and prognostics, and is anticipated to be as common as traditional stroke subtypes such as large atherosclerosis, small artery occlusion, and cardiac embolism. Recently, Bang et al. (2020) and Navi & Iadecola (2018) proposed that CRS is an emerging subtype of ischemic stroke, with many potential mechanisms for the occurrence of heterogeneity. Of these mechanisms, cancer‐related hypercoagulability (CRH) is probably the most important. Therefore, CRS may be treated with anticoagulation therapy theoretically. However, the neutral result of TEACH pilot trial (Navi, Marshall, et al., 2018) suggested that compared with antiplatelet therapy, anticoagulation did not show obvious advantages in preventing stroke recurrence and bleeding. Unfortunately, the results of other clinical trials of anticoagulant therapy in CRS patients are not very optimistic, either (Nam, Kim, Kim, An, Oh, et al., 2017; Yamaura et al., 2021). Therefore, the effectiveness of anticoagulation therapy needs to be evaluated further. In the future, it is necessary to deeply explore the underlying pathophysiological mechanism of this disease and formulate unified management practices. In this article, we will discuss the possibility of CRS as a stroke subtype, review the latest developments in the mechanism of CRS and related treatment strategies, and try to summarize how to devise current treatment plans based on individual pathogenic mechanisms in Table 1. We hope to provide references for the formulation of personalized therapy strategies.
TABLE 1.
Exploration of individualized therapy based on underlying mechanisms
| Mechanisms where anticoagulation therapy may be effective | Possible reasons | Alternative treatment |
|---|---|---|
| Intravascular coagulopathy | TF activation, elevated thrombin; elevated D‐dimer | DOAC, heparin anticoagulant |
| NBTE | Intravascular coagulopathy, endothelial damage | Heparin anticoagulant, heart valve surgery |
| Paradoxical embolism | Intravascular coagulopathy, VTE, PFO | DOAC, heparin anticoagulant, foramen ovale closure |
| Mechanisms where anticoagulation therapy may be ineffective | ||
| Atherosclerosis | Vascular plaque, vascular injury | Antiplatelet, thrombolysis and thrombectomy (cannot be excluded), avoid offending radiotherapy |
| Abnormal platelet aggregation | Elevated platelet activation markers | Antiplatelet, surgical resection, chemotherapy or radiotherapy |
| Cancer thrombus and cancer comorbidities | Cancer thrombus, infection, radiotherapy damage blood vessels | Surgical resection, avoid offending radiotherapy, comprehensive care |
Abbreviations: TF, tissue factor; DOAC, direct oral anticoagulants; NBTE, non‐bacterial thrombotic endocarditis; VTE, venous thromboembolism; PFO, patent foramen ovale.
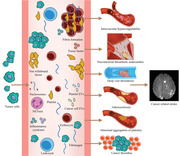
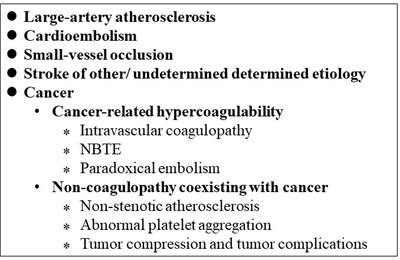
2. CRS CHALLENGES TOAST SUBTYPES
Stroke can be caused by different reasons, which can affect the prognosis, outcome, and management of each case. The etiology classification based on the various causes of stroke, namely the TOAST classification (Adams et al., 1993), is currently the most widely used stroke classification system. The TOAST classification etiologically divides the causes of strokes into five possibilities: (i) large atherosclerosis, (ii) small vessel occlusion, (iii) cardiogenic, (iv) other causes, and (v) unexplained types. In this classification system, cancer is not mentioned as a potential cause. However, with the increase in the incidence of CRS and the research progress on the pathogenesis of this stroke, the existing arrangement may not meet the needs of today's clinical medicine. Therefore, a more complete classification of the etiology of stroke is needed to facilitate faster selection of appropriate treatment methods for patients and improve their prognosis (Figure 1). It has been mentioned in the TOAST trial that if a factor is used as a unique cause of stroke, it should be based on the risk factors, clinical characteristics (laboratory, imaging), prognosis, and treatments that are linked to the cause.
FIGURE 1.
CRS challenges TOAST subtypes
Growing data suggests a potential relationship between stroke and cancer. A national study in Sweden found that the overall risks of patients developing ischemic and hemorrhagic stroke within 6 months of cancer diagnosis were 2.2 (95% confidence interval [CI] = 2.0–2.3) and 1.6 (CI = 1.5–1.6), respectively (Zöller et al., 2012). Previous studies have shown that approximately 4–12% of patients with ischemic stroke have active cancer (Gon et al., 2016; Grazioli et al., 2018) and that the link may be on the rise. Among different types of the disease, lung cancer and digestive system cancer are the ones that are more closely related to the incidence of stroke (Chen et al., 2015; Kato et al., 2016; Lin et al., 2019; Navi, Howard, et al., 2018). It was found in a large population‐based cohort that the risk of ischemic stroke began to increase 5 months before cancer diagnosis, suggesting that some cryptogenic stroke may be caused by occult cancer (Navi et al., 2019). Cancer is often underestimated as a potential risk factor for stroke, so some CRS patients are often discovered at autopsy. It is reported in an autopsy study that 15% of cancer patients have cerebrovascular disease (Rogers, 2010). These evidences all indicate that cancer is a risk of stroke.
When CRS occurs, it is often accompanied by unique clinical features. In the laboratory, it is manifested as hypercoagulability (el‐Shami et al., 2007; S. G. Kim et al., 2010; Seok et al., 2010). In imaging, it is manifested as multiple infarcts on the diffusion‐weighted image (DWI) involving multiple vascular areas. On magnetic resonance image (MRI), CRS often appears as a ring‐shaped area of restricted diffusion with a diameter of 0.5–2 cm, which is mainly located in areas with large blood vessels and usually does not accumulate cortex or deep gray matter (Finelli & Nouh, 2016). Existing evidence suggests that multivessel artery infarction and elevated D‐dimer levels are independent predictors of stroke associated with malignant tumors (S. G. Kim et al., 2010; S. J. Kim et al., 2012; Wang et al., 2018).
Studies have shown that patients with cancer complicated with ischemic stroke have a high risk of short‐term stroke recurrence and a poor prognosis. These surveys found that in patients diagnosed with cancer, the cumulative recurrence rate of stroke 1 month after the first episode is 7% (Chow et al., 2018; Navi et al., 2014), and the recurrence rate at 6 months reached 16% (Soda et al., 2004). Patients with malignant tumors also have a higher risk of venous and arterial embolism, and if cancer patients develop these clinical conditions after a stroke, they can have a significant negative impact on the patient's 1‐year survival rate (Ha et al., 2019). Due to the unclear pathogenesis and lack of effective secondary prevention strategies, even with the use of anticoagulants, 17.2% of patients with active cancer experienced a recurrence of stroke during a median observation period of 62 days after the first stroke occurred (Ohara et al., 2020). So patients with CRS usually have a poor prognosis and a higher risk of death. In a survey involving 14,358 tumor participants, the follow‐up analysis pointed out that 224 patients had a stroke after being diagnosed. Among the 2636 deaths, the all‐cause mortality rate without stroke was 0.70(95% CI, 0.68∼0.73). The mortality rate after the first stroke was 1.03(95% CI, 0.73–1.46), and the all‐cause mortality rate after stroke recurrence was 2.42(95% CI, 1.48–3.94) (Nam, Kim, Kim, An, Demchuk, et al., 2017).
A recent prospective study of 50 CRS patients found that these patients have higher coagulation, platelet, and endothelial dysfunction markers, and more circulating micro‐emboli. Compared with patients with only acute ischemic stroke or only active cancer, these differences are statistically significant. The biological markers found in this study (D‐dimer, thrombin‐antithrombin, P‐selectin, thrombomodulin, soluble intercellular adhesion molecule‐1[sICAM‐1], soluble vascular cell adhesion molecule‐1[sVCAM‐1]) has high practicability in clinical practice and is of great significance in predicting the occurrence of CRS. This can also improve our understanding of the mechanism of ischemic stroke in cancer patients. In this context, the occurrence of stroke is considered to be closely related to hypercoagulability (Navi et al., 2021). Therefore, different from the pathogenesis of traditional cerebral infarction, the choice of antithrombotic drugs for this type of patient may differ from traditional options.
3. MECHANISMS WHERE ANTICOAGULATION THERAPY MAY BE EFFECTIVE
3.1. Intravascular hypercoagulability
Existing studies on CRS suggest that there are various underlying mechanisms between cancer and stroke (Figure 2). Intravascular hypercoagulability has become the core of the discussion on the pathogenesis of CRS. The center of thrombus formation is thrombin, which is responsible for activating platelets and converting fibrinogen to a fibrin clot. In tumor patients, the level of thrombin is high (Abu Saadeh et al., 2016). Tumor cells increase the potential for thrombin generation both directly, through the expression and release of procoagulant factors, and indirectly, through signals that activate other cell types and components including platelets, leukocytes, erythrocytes, extracellular vesicles (EVs), and neutrophil extracellular traps (NETs) (Reddel et al., 2019). The Vienna Prospective Study of Cancer and Thrombosis (CATS) has found that patients with elevated thrombin levels are at increased risk of cancer‐related thrombosis (Ay et al., 2011).
FIGURE 2.
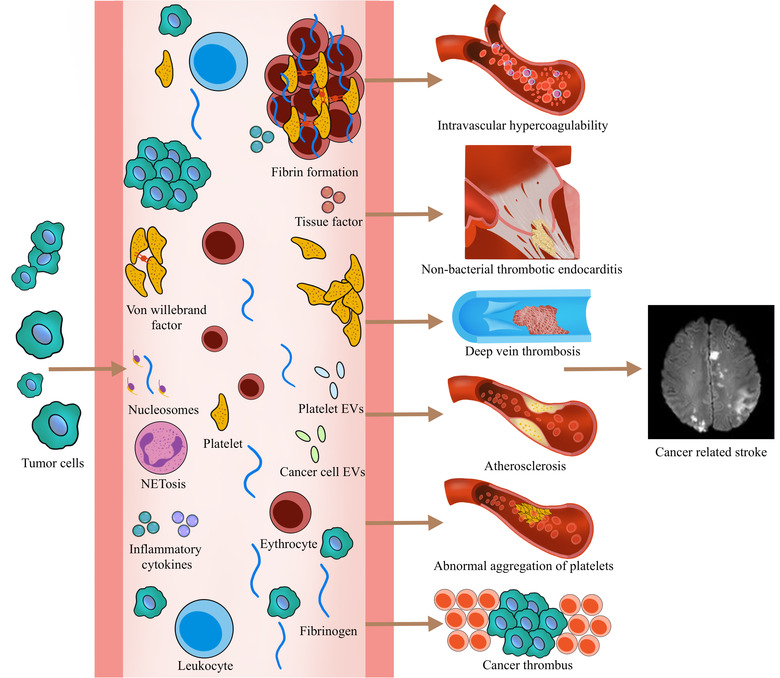
Possible mechanisms between stroke and tumor. EVs, extracellular vesicles; NET, neutrophil extracellular trap
In CRS patients, elevated plasma DNA levels were found to be associated with CRS by evaluating NET‐specific biomarkers—plasma DNA and nucleosomes—and it was concluded that NETosis is one of the molecular mechanisms of CRS (Bang et al., 2016). This finding was confirmed in pancreatic cancer patients (a cancer type that often accompanies hypercoagulability) (Yu et al., 2020). In the study of the pathological mechanism of stroke in cancer patients, it was found that cancer cells of patients with CRS were able to release higher levels of EVs, and EVs were associated with D‐dimer levels. Further investigation revealed that such EVs were not associated with TF levels (Bang et al., 2019).
Riewald & Ruf (2001) observed that the high expression of tissue factor (TF) is related to cancer‐related thromboembolism. This 47 kDa transmembrane glycoprotein is up‐regulated in cancer cells, and its soluble form also activates the coagulation pathway (Sun et al., 2015). In cancer tissues, TF is expressed in both tumor cells and endothelial cells and is the main inducer of blood coagulation (Hisada & Mackman, 2019). TF can form a complex with factor VII and activate factor VIIa, which further leads to the activation of coagulation factor X and the production of thrombin required for physiological hemostasis. When tumor cells metastasize, the hypercoagulability in the patient can be further activated (L. Liu et al., 2014; Mego et al., 2015).
Higher D‐dimer level is a manifestation of hypercoagulability in cancer patients. It is known that D‐dimer is a direct indicator of blood coagulation function and has been used as a means to assess hypercoagulability (Sun et al., 2015). Compared with stroke patients without a history of tumors, CRS patients have higher level of D‐dimer (Shen et al., 2020). Authors found that the incidence of microembolic signals of transcranial Doppler thrombosis in cancer patients that had cryptogenic strokes is linearly related to the level of D‐dimer (Seok et al., 2010). The D‐dimer assay is most useful in patients with active cancer and stroke because it can determine its cause, predict the risk of stroke recurrence, and help make precise CRS treatment decisions (Ohara et al., 2020).
There are still gaps in the understanding of the mechanisms of CRH, and it is hoped that ongoing or anticipated trials will provide a better understanding of the pathogenesis of CRS. The realization of personalized therapy for CRS should be guided by mechanism‐based diagnostics and molecular targeting.
3.2. Non‐bacterial thrombotic endocarditis (NBTE)
NBTE is considered to be the most common source of embolism in CRS (Patel & Elzweig, 2020). It is caused by circulating cytokines that lead to endothelial damage, which in turn initiates the coagulation process. Fibrin and platelets accumulate at the injury, causing thrombus formation (Dearborn et al., 2014; el‐Shami et al., 2007). NBTE is most common in malignant tumors and hypercoagulable states and can occur in 4% of patients with advanced malignancies (Singh et al., 2007; Vlachostergios et al., 2010). Nguyen and DeAngelis (2006) reported that in about 60% of NBTE patients, malignant tumors were found during the autopsy.
3.3. Paradoxical embolism
The typical manifestation of cancer hypercoagulability is vascular embolism, most commonly found in the venous system. The risk of venous thromboembolism (VTE) in patients with active cancer is four to eight times that of normal people (Salazar‐Camelo et al., 2021). Áinle & Kevane (2020) and Porfidia et al. (2019) have shown that after VTE, the risk of stroke and other related arterial embolism is greatly increased (. In the early stages of cancer diagnosis and VTE, there is a high risk of suffering from arterial embolism again. In addition, it has also been reported that the embolism of CRS may be related to a patent foramen ovale (PFO). PFOs are present in 25% of the general population, and higher in the cryptogenic stroke population. The putative mechanism of ischemic stroke in patients with PFO is the migration of emboli from the right to the left atrium. A PFO may be the initial manifestation of paradoxical embolism or cancer (Potugari et al., 2019; Tadokoro et al., 2013). In patients with a history of VTE, the emboli can enter the artery through the orifice of the PFO, leading to cerebrovascular embolism.
4. MECHANISMS BY WHICH ANTICOAGULATION THERAPY MAY NOT BE EFFECTIVE
In theory, anticoagulation therapy should be effective if we take into account the hypercoagulable mechanism of cerebral infarction in cancer patients. The neutral results of anticoagulation therapy trials indicate that the effectiveness of anticoagulation therapy in the above subgroups is affected by other mechanisms in the CRS population.
4.1. Atherosclerosis
Traditional stroke mechanisms, such as atherosclerosis, are a prevalent cause of stroke in cancer (Karlińska et al., 2015; Navi et al., 2014; Y. Zhang et al., 2007). Past autopsy studies have shown that the most common cause of ischemic stroke in cancer patients is still atherosclerosis (Adams, 2019). In addition, cancer‐related treatments, such as radiotherapy, may increase vascular damage and further increase the risk of developing this condition (Chow et al., 2018; Halle et al., 2010). S. G. Kim et al. (2010) found that among patients with traditional stroke risk, the distribution of stroke subtypes of cancer patients in the traditional stroke group was similar to that of stroke patients without cancer. Therefore, it is speculated that tumor‐specific mechanisms are unlikely to play a role in the development of strokes in patients at risk of having a traditional stroke. In a word, after a comprehensive evaluation in CRS patients, a careful vascular examination is necessary to determine the presence or absence of plaque in stroke patients.
4.2. Abnormal aggregation of platelets
Previous studies have shown that platelet activation markers are elevated in patients with malignant tumors, including soluble P‐selectin, soluble CD40 ligand, integrin αⅡbβIII, platelet activation receptors, platelet‐derived growth factors, and chemokines (Contursi et al., 2017; Foss et al., 2020; Wojtukiewicz et al., 2017). In patients with thrombosis, tumor‐derived thrombin generation leads to platelet activation through the cleavage of platelet thrombin receptors PAR1and PAR4, and the formation and aggregation of platelets lead to embolism (Tesfamariam, 2016; Q. Zhang et al., 2017). Researches on platelet subgroups (Dale, 2017; Heemskerk et al., 2013; Hua et al., 2015; Kempton et al., 2005) point out that aggregatory platelets contribute to the formation of platelet aggregation through the activation of GPIIb‐IIIa. It was also found that aggregatory platelets participate in the coagulation process to generate the thrombin and that interaction between platelets and thrombin further enhances the blood clotting activity in tumor patients. In addition, activated platelets can make circulating tumor cells escape immune attack and destruction, promoting tumor cell proliferation and metastasis (Bruno et al., 2018; Catani et al., 2020; Patmore et al., 2020). The positive role of platelets in cancer patients provides a theoretical basis for the use of antiplatelet drugs. Therefore, detecting the levels of platelets and platelet activation markers is beneficial for selecting personalized treatment plans for CRS patients.
4.3. Cancer thrombus and cancer comorbidities
Tumor ruptures during the growth process, and the thrombus can directly invade the blood vessel, leading to stroke (Bonnet et al., 2015). For these patients, intravascular interventional therapy may be their preferred choice (Byon et al., 2016; Hughes et al., 2015; Uneda et al., 2016). From being diagnosed with cancer, the patient has to undergo surgery, chemotherapy, or radiotherapy and these treatments might increase the risk of thromboembolism. Radiation therapy can cause vascular damage and increase the risk of cardiovascular diseases (Delanian, 2021; van Aken et al., 2021). Related studies have confirmed that the incidence of carotid artery stenosis and ischemic stroke increased after radiotherapy in patients with head and neck tumors (Laugaard Lorenzen et al., 2020; Makita et al., 2020). The effects of long‐term consumption and anti‐cancer treatments make patients immunodeficient, which increases their odds of getting an infection. Patients with a history of infection have a higher risk than those without any episodes in their clinical background.
5. MULTIPLE MECHANISMS WORK TOGETHER
The above‐mentioned mechanisms of CRS may not be isolated, and common risk factors might often coexist. In some cases, these coexisting mechanisms may reinforce each other and produce a synergistic effect, leading to thromboembolism. For example, NBTE works through a hypercoagulable system that can lead to embolism. Tumor‐related treatments could cause strokes by enhancing traditional stroke mechanisms. Thrombin can promote the activation and aggregation of platelets and cause vascular embolism.
6. CURRENT EXPLORATION OF PERSONALIZED THERAPY STRATEGIES
6.1. Secondary prevention of CRS
Currently, there are no relevant regulations on the treatment of CRS based on the evidence of the guidelines. Experimental evidence has shown that the use of anticoagulants in patients with high D‐dimer levels can reduce the incidence of arterial infarction events and VTE events (Castellucci et al., 2013; Oldgren et al., 2013). For example, in the prospective OASIS‐Cancer study, the 1‐year survival rate of cancer and stroke patients whose D‐dimer decreased after anticoagulant treatment was improved (M. Lee et al., 2017). Studies show P‐selectin and L‐selectin mediate coagulation activation. The anticoagulant effect of heparin through the interaction between both is the first choice in the treatment of patients with cancer‐related thromboembolism (Wahrenbrock et al., 2003). Kawano et al. (2019) believe that long‐term subcutaneous heparin treatment may prevent the recurrence of CRS. Current international guidelines all recommend LMWH as the initial and long‐term treatment for cancer‐related venous thrombosis (Farge et al., 2016; Kearon et al., 2016; Streiff et al., 2018). However, since the effectiveness of the treatment depends on the drug being injected subcutaneously, compliance with long‐term treatment is poor. A study conducted in 2017 showed that DOAC and LMWH have similar clinical efficacy and safety in the treatment of cryptogenic ischemic stroke in patients with active cancer (Nam, Kim, Kim, An, Oh, et al., 2017). Factor Xa inhibitors are routinely used in the secondary prevention of atrial fibrillation stroke. However, study of anticoagulant therapy for stroke in atrial fibrillation and CRS has found that they respond differently to anticoagulants (H. Kim et al., 2021). The coagulation mechanism of CRS may be mediated by a factor Xa‐independent pathway. There are many complex and undiscovered mechanisms of CRS; it is necessary to further explore the molecular mechanism of CRS hypercoagulability and determine the best strategies for stroke prevention in cancer patients. Experimental data on secondary prevention comparison of CRS in recent years are summarized in Table 2.
TABLE 2.
Comparison of secondary prevention strategies for CRS patients
| Year of publication | Compared drugs | Test population (n) | Observation time (months) | Results | |||||
|---|---|---|---|---|---|---|---|---|---|
| Initial/Recurrence | P value | Major bleeding | P value | Death | P value | ||||
| 2017(Nam, Kim, Kim, An, Oh, et al., 2017) | LMWH vs. NOAC | 41 vs. 7 | 3 | 49.0% vs. 57.0% | 0.846 | 39.0% vs. 29.0% | 0.696 | 59.0% vs. 57.0% | 1.000 |
| 2018(Navi, Marshall, et al., 2018) | Enoxaparin vs. Aspirin | 10 vs. 10 | 12 | 5.0% vs. 7.0% | 0.302 | 10.0% vs. 30.0% | 0.582 | N/A | N/A |
| 2020(Martinez‐Majander et al., 2020) | Rivaroxaban vs. Aspirin | 254 vs. 289 | 11 | 7.7% vs. 5.4% | 0.275 | 2.9% vs. 1.1% | 0.950 | 3.7% vs. 3.3 | 0.780 |
| 2021(Yamaura et al., 2021) | UFH vs. DiXals | 24 vs. 29 | 1 | 4.0% vs. 31.0% | 0.015 | 4.0% vs. 10.0% | 0.617 | 17.0% vs. 17.0% | 1.000 |
Abbreviations: LMWH, low‐molecular weight heparin; NOAC, new oral anticoagulant; UFH: unfractionated heparin; DiXal: direct factor Xa inhibitor.
Several randomized trials have shown that oral factor Xa inhibitors are equivalent to subcutaneous LMWH in terms of safety and efficacy in preventing recurrent VTE or massive bleeding in cancer patients, making them an attractive choice for cancer‐related ESUS(Agnelli et al., 2020; Carrier et al., 2019; Khorana et al., 2019; Raskob et al., 2018). Also, the latest guidelines recommend that DOAC be used for long‐term maintenance treatment of certain cancer patients to prevent VTE (Key et al., 2020). Since the occurrence of CRS may be more than a mechanism of hypercoagulability, the relevant treatment options for venous thrombosis can be used for reference, but it cannot be directly transferred.
For embolism caused by NBTE, anticoagulation therapy is still the basis. Current guidelines recommend heparin as the first‐line treatment for NBTE (Whitlock et al., 2012). In addition, for patients with large vegetations, valve dysfunction, or repeated embolism, if anticoagulation therapy is ineffective, valve repair or valve replacement can also be performed (Habib, 2015; J. Liu & Frishman, 2016).
In the TEACH Pilot Randomized Clinical Trial, Navi, Marshall, et al. (2018) tried to compare the effects of aspirin and heparin in treating malignant tumors with cerebral infarction. After 1‐year follow‐up, they have found no significant difference in the cumulative incidence of significant bleeding, thromboembolic events, and survival rates between the two groups of patients (p > 0.05). The subgroup analysis of the NAVIGATE ESUS randomized trial (Martinez‐Majander et al., 2020) found that patients with and without a history of cancer had a similar incidence of recurrent ischemic stroke and all‐cause mortality during aspirin and rivaroxaban treatment. It was stated that aspirin was safer than rivaroxaban for significant bleeding. Considering the above test results, we can conclude that for the secondary prevention of CRS patients, anticoagulation therapy should be feasible, but it has not shown evident benefits as we expected. More clinical trials are needed to confirm the best anticoagulant therapy for patients in these conditions.
When it comes to anticoagulant therapy for cancer patients, we must further discuss the risk of bleeding. A risk of bleeding may be even higher in cancer patients with stroke and other brain pathologies (Kamphuisen & Beyer‐Westendorf, 2014; Mantia et al., 2017). Major bleeding in cancer patients is associated with an increased risk of death, with the highest rates of major bleeding found in the elderly and people with medical comorbidities, gastrointestinal or urogenital cancers, and metastatic diseases, which are common in cancer‐related ESUS (A. Lee, 2017).
There are many potential causes of ESUS; both white thrombus and red thrombus are involved in the process of thrombus formation. Therefore, some scholars have hypothesized that the combined use of anticoagulants and antiplatelet drugs in ESUS patients can significantly reduce the overall burden of thrombosis, thereby reducing the risk of recurrent stroke. This hypothesis is supported by results from COMPASS (Cardiovascular Outcomes in People Using Anticoagulation Strategies), in which the combination of low‐dose rivaroxaban and aspirin was associated with a substantially lower risk of stroke compared with aspirin as monotherapy (Eikelboom et al., 2017; Sharma et al., 2019). Therefore, we speculate whether antiplatelet combined with anticoagulation strategies will be suitable for secondary prevention strategies of CRS, which requires clinical trials to verify.
In the traditional secondary prevention strategies for stroke, besides anti‐platelet aggregation, statins are also used. The paper of Boulet aimed to explore whether statins reduce radiation‐induced vascular complications in cancer patients postradiotherapy to the thorax, head, and neck. The results showed that statin use post radiation therapy was associated with a significant reduction in stroke, with a trend toward significantly reducing cardiovascular and cerebrovascular events (Boulet et al., 2019). Therefore, the use of statins in patients with a history of radiation therapy may be effective in preventing stroke risk.
6.2. Treatment of acute ischemic stroke in CRS
Many scholars have discussed whether thrombolysis is suitable for patients with malignant tumours associated with episodes of acute stroke, but the conclusions are not consistent. Murthy et al. (2013) observed 32,576 stroke patients, who received thrombolytic therapy. After adjusting for confounding factors, there was no significant difference in discharge rate and in‐hospital mortality in patients with malignant tumour compared with those with non‐malignant clinical cases. Still, the incidence of all cerebral hemorrhages in both groups was similar. However, the multivariate analysis presented in a retrospective study of 13,993 patients with acute ischemic stroke treated by thrombolysis revealed that the final mortality rate of patients with malignant tumours did not significantly increase compared with other patients, but the incidence of bleeding was higher (Weeda & Bohm, 2019). In another large clinical study, it was found that the presence or absence of a history of cancer had no significant effect on intracranial hemorrhage, all‐cause hemorrhage, or hospital mortality after thrombolytic therapy (Owusu‐Guha et al., 2021).
The results of the above data confirm the position of thrombolytic therapy in patients with CRS, but bleeding after thrombolysis is inevitable, and patients with primary or metastatic central nervous system malignant tumours may increase the risk of post‐thrombolysis bleeding due to vascular changes or damage (Fugate & Rabinstein, 2015; Masrur et al., 2011). Therefore, it is inferred that the deviation of the bleeding risk in the above experimental results may be due to the inconsistent number of patients with metastatic cancer among the groups. A retrospective study showed that if a cancer patient concurrently metastasizes to other sites, especially stomach, esophagus, hepatocellular carcinoma, or pancreatic cancer, the survival time will be greatly shortened; even if reperfusion treatment is performed, 80% to 100% of people die within 6 months after stroke (Yoo et al., 2019).
At present, mechanical thrombectomy is considered a first‐line treatment method for acute anterior circulating large vessel occlusive stroke (Berkhemer et al., 2015; Campbell et al., 2015; Goyal et al., 2015; Jovin et al., 2015; Saver et al., 2015). Several trials have explored the applicability of thrombectomy for patients diagnosed with tumours (refer to Table 3). After analyzing the results of these trials, we propose that when acute cerebral infarction occurs in such patients, immediate thrombectomy therapy should be feasible. However, for patients with later‐stage tumors, a more careful approach is necessary since they may have a poor prognosis.
TABLE 3.
Treatment of acute ischemic stroke in CRS‐ER
| Year of publication | Test population (n) | TICI 2b or 3 | P value | ICH | P value | mRS 0−2 at 3 months | P value | Death at 3 months | P value | Intrahospital mortality | p value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2018(Jung et al., 2018) | CRS (19) vs. LAA (105) vs. CE (205) | 63.0% vs. 84.0% vs. 84.0% | 0.060 | N/A | N/A | 16.0% vs. 54.0% vs. 44.0% | 0.008 | 63.0% vs. 4.0% vs. 13.0% | < 0.001 | N/A | N/A |
| 2019(Sallustio et al., 2019) | CRS (24) vs. Non‐cancer (24) | 76.9% vs. 61.5% | 0.670 | 25.0% vs. 29.0% | 1.000 | 25.0% vs. 29.1% | 1.000 | 29.1% vs. 12.5% | 0.280 | 8.3% vs. 4.1% | 1.000 |
| 2019(D. Lee et al., 2019) | CRS (26) vs. Non‐cancer (227) | 88.5% vs. 90.7% | 0.723 | 57.7% vs. 38.7% | 0.034 | 23.1% vs. 41.9% | 0.064 | 30.8% vs. 8.8% | 0.003 | N/A | N/A |
| 2020(Cho et al., 2020) | CRS (27) vs. Non‐cancer (351) | 85.2% vs. 82.6% | 0.800 | 44.4% vs. 32.8% | 0.290 | 37.0% vs. 39.6% | 0.840 | 33.3% vs. 8.2% | < 0.001 | 3.7% vs. 2.3% | 0.490 |
| 2021(E. Lee et al., 2021) | CRS (34) vs. Non‐cancer (307) | 79.4% vs. 86.7% | 0.103 | 41.2% vs. 23.8% | 0.037 | N/A | N/A | 26.5% vs. 6.8% | < 0.001 | 20.6% vs. 5.9% | 0.009 |
| 2021(Ciolli et al., 2021) | CRS (14) vs. Non‐cancer (267) | 71.0% vs. 78.0% | 0.520 | 43.0% vs. 40.0% | 1.000 | 21.0% vs. 44.0% | 0.160 | 64.0% vs. 14.0% | < 0.010 | 43.0% vs. 6.0% | < 0.010 |
Abbreviations: ERT, endovascular recanalization therapy; ICH, intracranial hemorrhage.
7. CONCLUSIONS AND PROSPECTS
With the aging of the world population and the prolonged survival time of cancer patients, the incidence of CRS is gradually increasing and it is expected that CRS will become a common subtype of traditional stroke. Regarding CRS as a subtype of traditional stroke is conducive to the establishment of a standardized diagnosis and treatment system for such patients. Although this definition does not yet guide treatment, it is the basis for further diagnosis and can help clinicians adopt more effective and tailored therapy strategies to prevent strokes. At present, the pathogenesis of CRS is still not unclouded, the existing secondary stroke prevention and acute treatment programs for such patients are still in the exploratory stage. This article reviews the current possible mechanisms for the pathogenesis of CRS and the corresponding exploratory treatment options based on these pathogeneses. It is hoped that this article can contribute to the formulation of personalized therapy plans for CRS.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Yu‐Jie Chen, Rui‐Guo Dong, and Jie Sun conceived and designed the study, analyzed the data, interpreted the study findings, and drafted the manuscript. Jie Sun conceived and designed the study. Meng‐Meng Zhang analyzed data. Chao Sheng and Peng‐Fei Guo supervised and directed the conduct of the study. Yu‐Jie Chen critically reviewed the manuscript. All authors had full access to all the data and the accuracy of the data analysis. The authors read and approved the final manuscript.
PEER REVIEW
The peer review history for this article is available https://publons.com/publon/10.1002/brb3.2738.
ACKNOWLEDGMENTS
This study was supported by Xuzhou Medical Leading Talents Training Project grants XWRCHT20210024 and Jiangsu Province Science and Technology Project grants BE2020638.
Chen, Yu‐J. , Dong, R.‐G. , Zhang, M.‐M. , Sheng, C. , Guo, P.‐F. , & Sun, J. (2022). Cancer‐related stroke: Exploring personalized therapy strategies. Brain and Behavior, 12, e2738. 10.1002/brb3.2738
REFERENCES
- ÃInle, F. N. A. , & Kevane, B. (2020). Which patients are at high risk of recurrent venous thromboembolism (deep vein thrombosis and pulmonary embolism)? Hematology (Amsterdam, Netherlands), American Society of Hematology. Education Program, 2020(1), 201–212. 10.1182/hematology.2020002268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Saadeh, F. , Langhe, R. , Galvin, D. M. , O Toole, S. A. , O'donnell, D. M. , Gleeson, N. , & Norris, L. A. (2016). Procoagulant activity in gynaecological cancer patients; the effect of surgery and chemotherapy. Thrombosis Research, 139, 135–141. 10.1016/j.thromres.2016.01.027 [DOI] [PubMed] [Google Scholar]
- Adams, H. P. (2019). Cancer and cerebrovascular disease. Current Neurology and Neuroscience Reports, 19(10), 73. 10.1007/s11910-019-0985-0 [DOI] [PubMed] [Google Scholar]
- Adams, H. P. , Bendixen, B. H. , Kappelle, L. J. , Biller, J. , Love, B. B. , Gordon, D. L. , & Marsh, E. E. (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke; A Journal of Cerebral Circulation, 24(1), 35–41. 10.1161/01.str.24.1.35 [DOI] [PubMed] [Google Scholar]
- Agnelli, G. , Becattini, C. , Meyer, G. , Muã±Oz, A. , Huisman, M. V. , Connors, J. M. , Cohen, A. , Bauersachs, R. , Brenner, B. , Torbicki, A. , Sueiro, M. R. , Lambert, C. , Gussoni, G. , Campanini, M. , Fontanella, A. , Vescovo, G. , & Verso, M. (2020). Apixaban for the treatment of venous thromboembolism associated with cancer. The New England Journal of Medicine, 382(17), 1599–1607. 10.1056/NEJMoa1915103 [DOI] [PubMed] [Google Scholar]
- Ay, C. , Dunkler, D. , Simanek, R. , Thaler, J. , Koder, S. , Marosi, C. , Zielinski, C. , & Pabinger, I. (2011). Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: Results from the Vienna Cancer and Thrombosis Study. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 29(15), 2099–2103. 10.1200/jco.2010.32.8294 [DOI] [PubMed] [Google Scholar]
- Bang, O. H. Y. , Chung, J.‐W. , Cho, Y. H. , Oh, M. I. J. , Seo, W.‐K. , Kim, G.‐M. , & Ahn, M.‐J. U. (2019). Circulating DNAs, a marker of neutrophil extracellular traposis and cancer‐related stroke. Stroke; A Journal of Cerebral Circulation, 50(10), 2944–2947. 10.1161/strokeaha.119.026373 [DOI] [PubMed] [Google Scholar]
- Bang, O. H. Y. , Chung, J.‐W. , Lee, M. I. J. I. , Kim, S. J. , Cho, Y. H. , Kim, G.‐M. , Chung, C.‐S. , Lee, K. H. , Ahn, M.‐J. U. , & Moon, G. J. (2016). Cancer cell‐derived extracellular vesicles are associated with coagulopathy causing ischemic stroke via tissue factor‐independent way: The OASIS‐CANCER study. Plos One, 11(7), e0159170. 10.1371/journal.pone.0159170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang, O. H. Y. , Chung, J.‐W. , Lee, M. I. J. I. , Seo, W.‐K. , Kim, G.‐M. , & Ahn, M.‐J. U. (2020). Cancer‐related stroke: An emerging subtype of ischemic stroke with unique pathomechanisms. Journal of Stroke, 22(1), 1–10. 10.5853/jos.2019.02278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhemer, O. A. , Fransen, P. S. S. , Beumer, D. , Van Den Berg, L. A. , Lingsma, H. F. , Yoo, A. J. , Schonewille, W. J. , Vos, J. A. , Nederkoorn, P. J. , Wermer, M. J. H. , Van Walderveen, M. A. A. , Staals, J. , Hofmeijer, J. , Van Oostayen, J. A. , Lycklama à Nijeholt, G. J. , Boiten, J. , Brouwer, P. A. , Emmer, B. J. , De Bruijn, S. F. , & Dippel, D. W. J. (2015). A randomized trial of intraarterial treatment for acute ischemic stroke. The New England Journal of Medicine, 372(1), 11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- Bonnet, L. , Raposo, N. , Blot‐Souletie, N. , Faruch Bilfeld, M. , Chollet, F. §. O. , Maziã¨Re, J. , Olivot, J.‐M. , & Albucher, J.‐F. §. O. (2015). Stroke caused by a pulmonary vein thrombosis revealing a metastatic choriocarcinoma. Circulation, 131(23), 2093–2094. 10.1161/circulationaha.114.011429 [DOI] [PubMed] [Google Scholar]
- Boulet, J. , Peã±A, J. , Hulten, E. A. , Neilan, T. G. , Dragomir, A. , Freeman, C. , Lambert, C. , Hijal, T. , Nadeau, L. , Brophy, J. M. , & Mousavi, N. (2019). Statin use and risk of vascular events among cancer patients after radiotherapy to the thorax, head, and neck. Journal of the American Heart Association, 8(13), e005996. 10.1161/jaha.117.005996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno, A. , Dovizio, M. , Tacconelli, S. , Contursi, A. , Ballerini, P. , & Patrignani, P. (2018). Antithrombotic agents and cancer. Cancers, 10(8). 10.3390/cancers10080253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byon, J. H. , Kwak, H. S. , Chung, G. H. O. , & Jang, K. Y. (2016). Acute stroke from tumor embolus in a patient with cardiac sarcoma: Aspiration thrombectomy with Penumbra catheter. Interventional Neuroradiology: Journal of Peritherapeutic Neuroradiology, Surgical Procedures and Related Neurosciences, 22(1), 88–90. 10.1177/1591019915609782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, B. C. V. , Mitchell, P. J. , Kleinig, T. J. , Dewey, H. M. , Churilov, L. , Yassi, N. , Yan, B. , Dowling, R. J. , Parsons, M. W. , Oxley, T. J. , Wu, T. Y. , Brooks, M. , Simpson, M. A. , Miteff, F. , Levi, C. R. , Krause, M. , Harrington, T. J. , Faulder, K. C. , Steinfort, B. S. , & Davis, S. M. (2015). Endovascular therapy for ischemic stroke with perfusion‐imaging selection. The New England Journal of Medicine, 372(11), 1009–1018. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- Carrier, M. , Abou‐Nassar, K. , Mallick, R. , Tagalakis, V. , Shivakumar, S. , Schattner, A. , Kuruvilla, P. , Hill, D. , Spadafora, S. , Marquis, K. , Trinkaus, M. , Tomiak, A. , Lee, A. Y. Y. , Gross, P. L. , Lazo‐Langner, A. , El‐Maraghi, R. , Goss, G. , Le Gal, G. , Stewart, D. , & Wells, P. S. (2019). Apixaban to prevent venous thromboembolism in patients with cancer. The New England Journal of Medicine, 380(8), 711–719. 10.1056/NEJMoa1814468 [DOI] [PubMed] [Google Scholar]
- Castellucci, L. A. , Cameron, C. , Le Gal, G. , Rodger, M. A. , Coyle, D. , Wells, P. S. , Clifford, T. , Gandara, E. , Wells, G. , & Carrier, M. (2013). Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: Systematic review and network meta‐analysis. BMJ (Clinical research ed.), 347, f5133. 10.1136/bmj.f5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani, M. V. , Savini, I. , Tullio, V. , & Gasperi, V. (2020). The “Janus Face” of platelets in cancer. International Journal of Molecular Sciences, 21(3),. 10.3390/ijms21030788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Zeng, J. , Xie, X. , Wang, Z. , Wang, X. , & Liang, Z. (2015). Clinical features of systemic cancer patients with acute cerebral infarction and its underlying pathogenesis. International Journal of Clinical and Experimental Medicine, 8(3), 4455–4463. [PMC free article] [PubMed] [Google Scholar]
- Cho, B. ‐H. , Yoon, W. , Kim, J. ‐T. , Choi, K. ‐H. o. , Kang, K.‐W. , Lee, J.‐H. , Cho, K. I. ‐H. , & Park, M. ‐S. (2020). Outcomes of endovascular treatment in acute ischemic stroke patients with current malignancy. Clinical Neurophysiology, 41(2), 379–385. 10.1007/s10072-019-04103-y [DOI] [PubMed] [Google Scholar]
- Chow, E. J. , Chen, Y. , Hudson, M. M. , Feijen, E. A. M. , Kremer, L. C. , Border, W. L. , Green, D. M. , Meacham, L. R. , Mulrooney, D. A. , Ness, K. K. , Oeffinger, K. C. , Ronckers, C. ã©C. M. , Sklar, C. A. , Stovall, M. , Van Der Pal, H. J. , Van Dijk, I. W. E. M. , Van Leeuwen, F. E. , Weathers, R. E. , Robison, L. L. , & Yasui, Y. (2018). Prediction of ischemic heart disease and stroke in survivors of childhood cancer. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 36(1), 44–52. 10.1200/jco.2017.74.8673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolli, L. , Bigliardi, G. , Ferraro, D. , Maffei, S. , Vandelli, L. , Dell'acqua, M. l. , Rosafio, F. , Picchetto, L. , Laterza, D. , Vincenzi, C. , Meletti, S. , Vallone, S. , & Zini, A. (2021). Efficacy of mechanical thrombectomy in patients with ischemic stroke and cancer. Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia, 91, 20–22. 10.1016/j.jocn.2021.06.029 [DOI] [PubMed] [Google Scholar]
- Contursi, A. , Sacco, A. , Grande, R. , Dovizio, M. , & Patrignani, P. (2017). Platelets as crucial partners for tumor metastasis: From mechanistic aspects to pharmacological targeting. Cellular and Molecular Life Sciences: CMLS, 74(19), 3491–3507. 10.1007/s00018-017-2536-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, G. L. (2017). Procoagulant platelets. Arteriosclerosis, Thrombosis, and Vascular Biology, 37(9), 1596–1597. 10.1161/atvbaha.117.309847 [DOI] [PubMed] [Google Scholar]
- Dearborn, J. , Urrutia, V. , & Zeiler, S. (2014). Stroke and cancer—A complicated relationship. Journal of Neurology & Translational Neuroscience, 2(1), 1039. [PMC free article] [PubMed] [Google Scholar]
- Delanian, S. (2021). Is radiation‐induced arteriopathy in long‐term breast cancer survivors an underdiagnosed situation? Critical and pragmatic review of available literature. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology, 157, 163–174. 10.1016/j.radonc.2021.01.009 [DOI] [PubMed] [Google Scholar]
- Eikelboom, J. W. , Connolly, S. J. , Bosch, J. , Dagenais, G. R. , Hart, R. G. , Shestakovska, O. , Diaz, R. , Alings, M. , Lonn, E. M. , Anand, S. S. , Widimsky, P. , Hori, M. , Avezum, A. , Piegas, L. S. , Branch, K. R. H. , Probstfield, J. , Bhatt, D. L. , Zhu, J. , Liang, Y. , & Yusuf, S. (2017). Rivaroxaban with or without aspirin in stable cardiovascular disease. The New England Journal of Medicine, 377(14), 1319–1330. 10.1056/NEJMoa1709118 [DOI] [PubMed] [Google Scholar]
- El‐Shami, K. , Griffiths, E. , & Streiff, M. (2007). Nonbacterial thrombotic endocarditis in cancer patients: Pathogenesis, diagnosis, and treatment. The Oncologist, 12(5), 518–523. 10.1634/theoncologist.12-5-518 [DOI] [PubMed] [Google Scholar]
- Farge, D. , Bounameaux, H. , Brenner, B. , Cajfinger, F. , Debourdeau, P. , Khorana, A. A. , Pabinger, I. , Solymoss, S. , Douketis, J. , & Kakkar, A. (2016). International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. The Lancet. Oncology, 17(10), e452–e466. 10.1016/s1470-2045(16)30369-2 [DOI] [PubMed] [Google Scholar]
- Finelli, P. F. , & Nouh, A. (2016). Three‐territory DWI acute infarcts: diagnostic value in cancer‐associated hypercoagulation stroke (Trousseau syndrome). American Journal of Neuroradiology, 37(11), 2033–2036. 10.3174/ajnr.A4846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss, A. , Muñoz‐Sagredo, L. , Sleeman, J. , & Thiele, W. (2020). The contribution of platelets to intravascular arrest, extravasation, and outgrowth of disseminated tumor cells. Clinical & Experimental Metastasis, 37(1), 47–67. 10.1007/s10585-019-10009-y [DOI] [PubMed] [Google Scholar]
- Fugate, J. E. , & Rabinstein, A. A. (2015). Absolute and relative contraindications to IV rt‐PA for acute ischemic stroke. The Neurohospitalist, 5(3), 110–121. 10.1177/1941874415578532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gon, Y. , Okazaki, S. , Terasaki, Y. , Sasaki, T. , Yoshimine, T. , Sakaguchi, M. , & Mochizuki, H. (2016). Characteristics of cryptogenic stroke in cancer patients. Annals of Clinical and Translational Neurology, 3(4), 280–287. 10.1002/acn3.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal, M. , Demchuk, A. M. , Menon, B. K. , Eesa, M. , Rempel, J. L. , Thornton, J. , Roy, D. , Jovin, T. G. , Willinsky, R. A. , Sapkota, B. L. , Dowlatshahi, D. , Frei, D. F. , Kamal, N. R. , Montanera, W. J. , Poppe, A. Y. , Ryckborst, K. J. , Silver, F. L. , Shuaib, A. , Tampieri, D. , & Hill, M. D. (2015). Randomized assessment of rapid endovascular treatment of ischemic stroke. The New England Journal of Medicine, 372(11), 1019–1030. 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- Grazioli, S. , Paciaroni, M. , Agnelli, G. , Acciarresi, M. , Alberti, A. , D'amore, C. , Caso, V. , Venti, M. , Guasti, L. , Ageno, W. , & Squizzato, A. (2018). Cancer‐associated ischemic stroke: A retrospective multicentre cohort study. Thrombosis Research, 165, 33–37. 10.1016/j.thromres.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Habib, G. et al. (2015). The 2015 ESC Guidelines for the management of infective endocarditis. European Heart Journal, 36(44), 3036–3037. 10.1093/eurheartj/ehv488 [DOI] [PubMed] [Google Scholar]
- Ha, J. , Lee, M. i. J. i. , Kim, S. J. , Park, B. ‐. Y. , Park, H. , Cho, S. , Chung, J.‐W. , Seo, W.‐K. , Kim, G.‐M. , Bang, O. h. Y. , & Chung, C.‐S. (2019). Prevalence and impact of venous and arterial thromboembolism in patients with embolic stroke of undetermined source with or without active cancer. Journal of the American Heart Association, 8(21), e013215. 10.1161/jaha.119.013215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle, M. , Gabrielsen, A. , Paulsson‐Berne, G. , Gahm, C. , Agardh, H. E. , Farnebo, F. , & Tornvall, P. (2010). Sustained inflammation due to nuclear factor‐kappa B activation in irradiated human arteries. Journal of the American College of Cardiology, 55(12), 1227–1236. 10.1016/j.jacc.2009.10.047 [DOI] [PubMed] [Google Scholar]
- Heemskerk, J. W. M. , Mattheij, N. J. A. , & Cosemans, J. M. E. M. (2013). Platelet‐based coagulation: Different populations, different functions. Journal of Thrombosis and Haemostasis, JTH, 11(1), 2–16. 10.1111/jth.12045 [DOI] [PubMed] [Google Scholar]
- Hisada, Y. , & Mackman, N. (2019). Cancer cell‐derived tissue factor‐positive extracellular vesicles. Current Opinion in Hematology, 26(5), 349–356. 10.1097/moh.0000000000000521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, V. u. M. , Abeynaike, L. , Glaros, E. , Campbell, H. , Pasalic, L. , Hogg, P. J. , & Chen, V. M. Y. (2015). Necrotic platelets provide a procoagulant surface during thrombosis. Blood, 126(26), 2852–2862. 10.1182/blood-2015-08-663005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, S. E. , Hunter, A. , Campbell, J. , Brady, A. , Herron, B. , Smyth, G. , Rennie, I. , & Hunt, S. J. (2015). Extraction of tumour embolus following perioperative stroke. Journal of the Neurological Sciences, 353, 172–174. 10.1016/j.jns.2015.03.034 [DOI] [PubMed] [Google Scholar]
- Jang, H.‐S. , Choi, J. , Shin, J. , Chung, J.‐W. , Bang, O. h. Y. , Kim, G. ‐M. , Seo, W. ‐K. , & Lee, J. (2019). The long‐term effect of cancer on incident stroke: A nationwide population‐based cohort study in Korea. Frontiers in Neurology, 10, 52. 10.3389/fneur.2019.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin, T. G. , Chamorro, A. , Cobo, E. , De Miquel, M. A. A. , Molina, C. A. , Rovira, A. , San Romã¡N, L. , Serena, J. N. , Abilleira, S. , Ribã3 , M. , Millã¡N, M. ã2 , Urra, X. , Cardona, P. , LÃPez‐Cancio, E. , Tomasello, A. , Castaã±O, C. , Blasco, J. , Aja, L. A. , Dorado, L. , … DáValos, A. (2015). Thrombectomy within 8 hours after symptom onset in ischemic stroke. The New England Journal of Medicine, 372(24), 2296–2306. 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- Jung, S. , Jung, C. , Hyoung Kim, J. , Se Choi, B. , Jung Bae, Y. , Sunwoo, L. , Geol Woo, H. o. , Young Chang, J. , Joon Kim, B. , Han, M. ‐K. u. , & Bae, H. ‐J. (2018). Procedural and clinical outcomes of endovascular recanalization therapy in patients with cancer‐related stroke. Interventional Neuroradiology: Journal of Peritherapeutic Neuroradiology, Surgical Procedures and Related Neurosciences, 24(5), 520–528. 10.1177/1591019918776207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuisen, P. W. , & Beyer‐Westendorf, J. (2014). Bleeding complications during anticoagulant treatment in patients with cancer. Thrombosis Research, S49–S55. 10.1016/s0049-3848(14)50009-6 [DOI] [PubMed] [Google Scholar]
- Karliå Ska, A. G. , Gromadzka, G. , Karliå Ski, M. A. , & Czå Onkowska, A. (2015). The activity of malignancy may determine stroke pattern in cancer patients. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 24(4), 778–783. 10.1016/j.jstrokecerebrovasdis.2014.11.003 [DOI] [PubMed] [Google Scholar]
- Kato, M. , Shukuya, T. , Mori, K. , Kanemaru, R. , Honma, Y. , Nanjo, Y. , Muraki, K. , Shibayama, R. , Koyama, R. , Shimada, N. , Takahashi, F. , & Takahashi, K. (2016). Cerebral infarction in advanced non‐small cell lung cancer: A case control study. BMC cancer, 16, 203. 10.1186/s12885-016-2233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano, H. , Honda, Y. , Amano, T. , Okano, H. , Suzuki, R. , Torii, M. , Unno, Y. , Shiokawa, Y. , & Hirano, T. (2019). Subcutaneous heparin therapy for patients with cancer‐associated stroke. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 28(2), 399–404. 10.1016/j.jstrokecerebrovasdis.2018.10.012 [DOI] [PubMed] [Google Scholar]
- Kearon, C. , Akl, E. A. , Ornelas, J. , Blaivas, A. , Jimenez, D. , Bounameaux, H. , Huisman, M. , King, C. S. , Morris, T. A. , Sood, N. , Stevens, S. M. , Vintch, J. R. E. , Wells, P. , Woller, S. C. , & Moores, L. (2016). Antithrombotic therapy for VTE disease. Chest, 149(2), 315–352. 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- Kempton, C. L. , Hoffman, M. , Roberts, H. R. , & Monroe, D. M. (2005). Platelet heterogeneity. Arteriosclerosis, Thrombosis, and Vascular Biology, 25(4), 861–866. 10.1161/01.ATV.0000155987.26583.9b [DOI] [PubMed] [Google Scholar]
- Key, N. S. , Khorana, A. A. , Kuderer, N. M. , Bohlke, K. , Lee, A. Y. Y. , Arcelus, J. I. , Wong, S. L. , Balaban, E. P. , Flowers, C. R. , Francis, C. W. , Gates, L. E. , Kakkar, A. K. , Levine, M. N. , Liebman, H. A. , Tempero, M. A. , Lyman, G. H. , & Falanga, A. (2020). Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 38(5), 496–520. 10.1200/jco.19.01461 [DOI] [PubMed] [Google Scholar]
- Khorana, A. A. , Soff, G. A. , Kakkar, A. K. , Vadhan‐Raj, S. , Riess, H. , Wun, T. , Streiff, M. B. , Garcia, D. A. , Liebman, H. A. , Belani, C. P. , Oâ™Reilly, E. M. , Patel, J. N. , Yimer, H. A. , Wildgoose, P. , Burton, P. , Vijapurkar, U. , Kaul, S. , Eikelboom, J. , Mcbane, R. , … Lyman, G. H. (2019). Rivaroxaban for thromboprophylaxis in high‐risk ambulatory patients with cancer. The New England Journal of Medicine, 380(8), 720–728. 10.1056/NEJMoa1814630 [DOI] [PubMed] [Google Scholar]
- Kim, H. J. , Chung, J. ‐W. , Bang, O. h. Y. , Cho, Y. H. , Lim, Y. J. , Hwang, J. , Seo, W. ‐K. , Kim, G.‐M. , Kim, H. ‐J. , & Ahn, M. ‐ J. u. (2021). The role of factor xa‐independent pathway and anticoagulant therapies in cancer‐related stroke. Journal of Clinical Medicine, 11(1). 10.3390/jcm11010123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. G. , Hong, J. i. M. , Kim, H. Y. , Lee, J. , Chung, P.‐W. , Park, K.‐Y. , Kim, G.‐M. , Lee, K. H. o. , Chung, C.‐S. , & Bang, O. h. Y. (2010). Ischemic stroke in cancer patients with and without conventional mechanisms. Stroke; A Journal of Cerebral Circulation, 41(4), 798–801. 10.1161/strokeaha.109.571356 [DOI] [PubMed] [Google Scholar]
- Kim, S. J. , Park, J. H. , Lee, M. i‐ J. i. , Park, Y. G. , Ahn, M. ‐J. u. , & Bang, O. h. Y. (2012). Clues to occult cancer in patients with ischemic stroke. Plos One, 7(9), e44959. 10.1371/journal.pone.0044959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugaard Lorenzen, E. , Christian Rehammar, J. , Jensen, M.‐B. , Ewertz, M. , & Brink, C. (2020). Radiation‐induced risk of ischemic heart disease following breast cancer radiotherapy in Denmark, 1977â2005. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology, 152, 103–110. 10.1016/j.radonc.2020.08.007 [DOI] [PubMed] [Google Scholar]
- Lee, A. Y. Y. (2017). When can we stop anticoagulation in patients with cancer‐associated thrombosis? Blood, 130(23), 2484–2490. 10.1182/blood-2017-05-787929 [DOI] [PubMed] [Google Scholar]
- Lee, D. , Lee, D. H. , Suh, D. C. , Kwon, H. S. , Jeong, D. a. ‐E. , Kim, J. ‐G. , Lee, J. i. ‐S. , Kim, J. S. , Kang, D. ‐W. , Jeon, S. ‐B. , Lee, E. ‐J. , Noh, K. C. , & Kwon, S. U. (2019). Intra‐arterial thrombectomy for acute ischaemic stroke patients with active cancer. Journal of Neurology, 266(9), 2286–2293. 10.1007/s00415-019-09416-8 [DOI] [PubMed] [Google Scholar]
- Lee, E. ‐J. , Bae, J. , Jeong, H. ‐B. , Lee, E. J. i. , Jeong, H. ‐Y. , & Yoon, B. ‐W. (2021). Effectiveness of mechanical thrombectomy in cancer‐related stroke and associated factors with unfavorable outcome. BMC Neurology, 21(1), 57. 10.1186/s12883-021-02086-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M. i. J. i. , Chung, J. ‐W. , Ahn, M. ‐J. u. , Kim, S. , Seok, J. M. , Jang, H. M. , Kim, G. ‐M. , Chung, C.‐S. , Lee, K. H. o. , & Bang, O. h. Y. (2017). Hypercoagulability and mortality of patients with stroke and active cancer: The OASIS‐CANCER study. Journal of Stroke, 19(1), 77–87. 10.5853/jos.2016.00570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, J. , Wu, S. , Xu, R. , Shi, Q. , Tian, C. , Cui, F. , Shao, X. , & Liu, H. (2019). Clinical characteristics and risk factors of lung cancer‐associated acute ischemic stroke. BioMed Research International, 6021037. 10.1155/2019/6021037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , & Frishman, W. H. (2016). Nonbacterial thrombotic endocarditis. Cardiology in Review, 24(5), 244–247. 10.1097/crd.0000000000000106 [DOI] [PubMed] [Google Scholar]
- Liu, L. , Zhang, X. i. , Yan, B. , Gu, Q. , Zhang, X. , Jiao, J. , Sun, D. , Wang, N. , & Yue, X. (2014). Elevated plasma D‐dimer levels correlate with long term survival of gastric cancer patients. Plos One, 9(3), e90547. 10.1371/journal.pone.0090547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita, C. , Okada, S. , Kajiura, Y. , Tanaka, O. , Asahi, Y. , Yamada, N. , …, & Matsuo, M. (2020). Vascular events from carotid artery atherosclerosis after radiation therapy for laryngeal and hypopharyngeal cancer: The incidence and risk factors. Nagoya Journal of Medical Science, 82(4), 747–761. doi: 10.18999/nagjms.82.4.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantia, C. , Uhlmann, E. J. , Puligandla, M. , Weber, G. M. , Neuberg, D. , & Zwicker, J. I. (2017). Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood, 129(25), 3379–3385. 10.1182/blood-2017-02-767285 [DOI] [PubMed] [Google Scholar]
- MartinezâMajander, N. , Ntaios, G. , Liu, Y. Y. , Ylikotila, P. , Joensuu, H. , Saarinen, J. , Perera, K. S. , MartiâFabregas, J. , Chamorro, A. , Rudilosso, S. , Pratsâ Sanchez, L. , Berkowitz, S. D. , Mundl, H. , Themeles, E. , Tiainen, M. , Demchuk, A. , Kasner, S. E. , Hart, R. G. , & Tatlisumak, T. (2020). Rivaroxaban versus aspirin for secondary prevention of ischaemic stroke in patients with cancer: A subgroup analysis of the NAVIGATE ESUS randomized trial. European Journal of Neurology, 27(5), 841–848. 10.1111/ene.14172 [DOI] [PubMed] [Google Scholar]
- Masrur, S. , Abdullah, A. R. , Smith, E. E. , Hidalgo, R. , El‐Ghandour, A. , Rordorf, G. , & Schwamm, L. H. (2011). Risk of thrombolytic therapy for acute ischemic stroke in patients with current malignancy. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 20(2), 124–130. 10.1016/j.jstrokecerebrovasdis.2009.10.010 [DOI] [PubMed] [Google Scholar]
- Mego, M. , Zuo, Z. , Gao, H. , Cohen, E. , Giordano, A. , Tin, S. , Anfossi, S. , Jackson, S. , Woodward, W. , Ueno, N. , Valero, V. , Alvarez, R. , Hortobagyi, G. , Khoury, J. , Cristofanilli, M. , & Reuben, J. (2015). Circulating tumour cells are linked to plasma D‐dimer levels in patients with metastatic breast cancer. Thrombosis and Haemostasis, 113(3), 593–598. 10.1160/th14-07-0597 [DOI] [PubMed] [Google Scholar]
- Murthy, S. B. , Karanth, S. , Shah, S. , Shastri, A. , Rao, C. P. V. , Bershad, E. M. , & Suarez, J. I. (2013). Thrombolysis for acute ischemic stroke in patients with cancer. Stroke; A Journal of Cerebral Circulation, 44(12), 3–3576. 10.1161/strokeaha.113.003058 [DOI] [PubMed] [Google Scholar]
- Nam, K. i.‐W. , Kim, C. K. , Kim, T. J. , An, S. J. , Oh, K. , Ko, S. ‐B. , & Yoon, B. ‐W. (2017). Treatment of cryptogenic stroke with active cancer with a new oral anticoagulant. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 26(12), 2976–2980. 10.1016/j.jstrokecerebrovasdis.2017.07.029 [DOI] [PubMed] [Google Scholar]
- Nam, K.‐W. , Kim, C. K. , Kim, T. J. , An, S. J. , Demchuk, A. M. , Kim, Y. , Jung, S. , Han, M.‐K. , Ko, S.‐B. , & Yoon, B.‐W. (2017). D‐dimer as a predictor of early neurologic deterioration in cryptogenic stroke with active cancer. European Journal of Neurology, 24(1), 205–211. 10.1111/ene.13184 [DOI] [PubMed] [Google Scholar]
- Navi, B. B. , Howard, G. , Howard, V. J. , Zhao, H. , Judd, S. E. , Elkind, M. S. V. , Iadecola, C. , Deangelis, L. M. , Kamel, H. , Okin, P. M. , Gilchrist, S. , Soliman, E. Z. , Cushman, M. , & Muntner, P. (2018). New diagnosis of cancer and the risk of subsequent cerebrovascular events. Neurology, 90(23), e2025–e2033. 10.1212/wnl.0000000000005636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navi, B. B. , & Iadecola, C. (2018). Ischemic stroke in cancer patients: A review of an underappreciated pathology. Annals of Neurology, 83(5), 873–883. 10.1002/ana.25227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navi, B. B. , Marshall, R. S. , Bobrow, D. , Singer, S. , Stone, J. B. , Desancho, M. T. , & Deangelis, L. M. (2018). Enoxaparin vs aspirin in patients with cancer and ischemic stroke. JAMA Neurology, 75(3), 379–381. 10.1001/jamaneurol.2017.4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navi, B. B. , Reiner, A. S. , Kamel, H. , Iadecola, C. , Elkind, M. S. V. , Panageas, K. S. , & Deangelis, L. M. (2015). Association between incident cancer and subsequent stroke. Annals of Neurology, 77(2), 291–300. 10.1002/ana.24325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navi, B. B. , Reiner, A. S. , Kamel, H. , Iadecola, C. , Okin, P. M. , Elkind, M. S. V. , Panageas, K. S. , & Deangelis, L. M. (2017). Risk of arterial thromboembolism in patients with cancer. Journal of the American College of Cardiology, 70(8), 926–938. 10.1016/j.jacc.2017.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navi, B. B. , Reiner, A. S. , Kamel, H. , Iadecola, C. , Okin, P. M. , Tagawa, S. T. , Panageas, K. S. , & Deangelis, L. M. (2019). Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood, 133(8), 781–789. 10.1182/blood-2018-06-860874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navi, B. B. , Sherman, C. P. , Genova, R. , Mathias, R. , Lansdale, K. N. , Lemoss, N. M. , Wolfe, J. , Skakodub, A. , Kamel, H. , Tagawa, S. T. , Saxena, A. , Ocean, A. J. , Soff, G. A. , Desancho, M. T. , Iadecola, C. , Elkind, M. S. V. , Peerschke, E. , Zhang, C. , & Deangelis, L. M. (2021). Mechanisms of ischemic stroke in patients with cancer: A prospective study. Annals of Neurology, 90(1), 159–169. 10.1002/ana.26129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navi, B. B. , Singer, S. , Merkler, A. E. , Cheng, N. T. , Stone, J. B. , Kamel, H. , Iadecola, C. , Elkind, M. S. V. , & Deangelis, L. M. (2014). Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology, 83(1), 26–33. 10.1212/wnl.0000000000000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. , & Deangelis, L. M. (2006). Stroke in cancer patients. Current Neurology and Neuroscience Reports, 6(3), 187–192. 10.1007/s11910-006-0004-0 [DOI] [PubMed] [Google Scholar]
- Ohara, T. , Farhoudi, M. , Bang, O. h. Y. , Koga, M. , & Demchuk, A. M. (2020). The emerging value of serum D‐dimer measurement in the work‐up and management of ischemic stroke. International Journal of Stroke: Official Journal of the International Stroke Society, 15(2), 122–131. 10.1177/1747493019876538 [DOI] [PubMed] [Google Scholar]
- Oldgren, J. , Wallentin, L. , Alexander, J. H. , James, S. , Jonelid, B. , Steg, G. , & Sundstrom, J. (2013). New oral anticoagulants in addition to single or dual antiplatelet therapy after an acute coronary syndrome: A systematic review and meta‐analysis. European Heart Journal, 34(22), 1670–1680. 10.1093/eurheartj/eht049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu‐Guha, J. , Guha, A. , Miller, P. E. , Pawar, S. , Dey, A. K. , Ahmad, T. , Attar, H. , Awan, F. T. , Mitchell, D. , Desai, N. R. , & Addison, D. (2021). Contemporary utilization patterns and outcomes of thrombolytic administration for ischemic stroke among patients with cancer. International Journal of Stroke: Official Journal of the International Stroke Society, 16(2), 150–162. 10.1177/1747493019895709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, M. J. , & Elzweig, J. (2020). Non‐bacterial thrombotic endocarditis: A rare presentation and literature review. BMJ Case Reports, 13(12). 10.1136/bcr-2020-238585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patmore, S. , Dhami, S. P. S. , & O'sullivan, J. M. (2020). Von Willebrand factor and cancer; metastasis and coagulopathies. Journal of Thrombosis and Haemostasis, 18(10), 2444–2456. 10.1111/jth.14976 [DOI] [PubMed] [Google Scholar]
- Porfidia, A. , Porceddu, E. , Feliciani, D. , Giordano, M. , Agostini, F. , Ciocci, G. , Cammã, G. , Giarretta, I. , Gaetani, E. , Tondi, P. , & Pola, R. (2019). Differences in clinical presentation, rate of pulmonary embolism, and risk factors among patients with deep vein thrombosis in unusual sites. Clinical and Applied Thrombosis/Hemostasis: Official Journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis, 25, 1076029619872550. 10.1177/1076029619872550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potugari, B. , Priyanka, P. , Komanapalli, S. , & Mercier, R. (2019). Ovarian cancer presenting as cryptogenic stroke from patent foramen ovale. Clinical Medicine & Research, 17, 97–101. 10.3121/cmr.2019.1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskob, G. E. , Van Es, N. , Verhamme, P. , Carrier, M. , Di Nisio, M. , Garcia, D. , Grosso, M. A. , Kakkar, A. K. , Kovacs, M. J. , Mercuri, M. F. , Meyer, G. , Segers, A. , Shi, M. , Wang, T.‐F. , Yeo, E. , Zhang, G. , Zwicker, J. I. , Weitz, J. I. , & BüLler, H. R. (2018). Edoxaban for the treatment of cancer‐associated venous thromboembolism. The New England Journal of Medicine, 378(7), 615–624. 10.1056/NEJMoa1711948 [DOI] [PubMed] [Google Scholar]
- Reddel, C. , Tan, C. , & Chen, V. (2019). Thrombin generation and cancer: Contributors and consequences. Cancers, 11(1),. 10.3390/cancers11010100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riewald, M. , & Ruf, W. (2001). Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proceedings of the National Academy of Sciences of the United States of America, 98(14), 7742–7747. 10.1073/pnas.141126698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, L. (2010). Cerebrovascular complications in patients with cancer. Seminars in Neurology, 30(3), 311–319. 10.1055/s-0030-1255224 [DOI] [PubMed] [Google Scholar]
- Salazar‐Camelo, R. A. , Moreno‐Vargas, E. A. , Cardona, A. ©S. F. , & Bayona‐Ortiz, H. ¡N. F. (2021). Ischemic stroke: A paradoxical manifestation of cancer. Critical Reviews in Oncology/Hematology, 157, 103181. 10.1016/j.critrevonc.2020.103181 [DOI] [PubMed] [Google Scholar]
- Sallustio, F. , Mascolo, A. P. , Marrama, F. , Koch, G. , Alemseged, F. , Davoli, A. , Da Ros, V. , Morosetti, D. , Konda, D. , & Diomedi, M. (2019). Safety and efficacy of reperfusion therapies for acute ischemic stroke patients with active malignancy. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 28(8), 2287–2291. 10.1016/j.jstrokecerebrovasdis.2019.05.018 [DOI] [PubMed] [Google Scholar]
- Saver, J. L. , Goyal, M. , Bonafe, A. , Diener, H.‐C. , Levy, E. I. , Pereira, V. M. , Albers, G. W. , Cognard, C. , Cohen, D. J. , Hacke, W. , Jansen, O. , Jovin, T. G. , Mattle, H. P. , Nogueira, R. G. , Siddiqui, A. H. , Yavagal, D. R. , Baxter, B. W. , Devlin, T. G. , Lopes, D. K. , & Jahan, R. (2015). Stent‐retriever thrombectomy after intravenous t‐PA vs. t‐PA alone in stroke. The New England Journal of Medicine, 372(24), 2285–2295. 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- Seok, J. M. , Kim, S. G. , Kim, J. i. W. , Chung, C.‐S. , Kim, G.‐M. , Lee, K. H. o. , & Bang, O. h. Y. (2010). Coagulopathy and embolic signals in cancer patients with ischemic stroke. Annals of neurology, 68(2), 213–219. 10.1002/ana.22050 [DOI] [PubMed] [Google Scholar]
- Sharma, M. , Hart, R. G. , Connolly, S. J. , Bosch, J. , Shestakovska, O. , Ng, K. K. H. , Catanese, L. , Keltai, K. , Aboyans, V. , Alings, M. , Ha, J.‐W. , Varigos, J. , Tonkin, A. , OâDonnell, M. , Bhatt, D. L. , Fox, K. , Maggioni, A. , Berkowitz, S. D. , Bruns, N. C. , & Eikelboom, J. W. (2019). Stroke outcomes in the COMPASS trial. Circulation, 139(9), 1134–1145. 10.1161/circulationaha.118.035864 [DOI] [PubMed] [Google Scholar]
- Shen, Y. , Li, Y. , Chen, C. , Wang, W. , & Li, T. (2020). D‐dimer and diffusion‐weighted imaging pattern as two diagnostic indicators for cancer‐related stroke. Medicine, 99(4), e18779. 10.1097/md.0000000000018779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, V. , Bhat, I. , & Havlin, K. (2007). Marantic endocarditis (NBTE) with systemic emboli and paraneoplastic cerebellar degeneration: Uncommon presentation of ovarian cancer. Journal of Neuro‐oncology, 83(1), 81–83. 10.1007/s11060-006-9306-y [DOI] [PubMed] [Google Scholar]
- Soda, T. , Nakayasu, H. , Maeda, M. , Kusumi, M. , Kowa, H. , Awaki, E. , Saito, J. , & Nakashima, K. (2004). Stroke recurrence within the first year following cerebral infarction—Tottori University Lacunar Infarction Prognosis Study (TULIPS)*. Acta neurologica Scandinavica, 110(6), 343–349. 10.1111/j.1600-0404.2004.00290.x [DOI] [PubMed] [Google Scholar]
- Streiff, M. B. , Holmstrom, B. , Angelini, D. , Ashrani, A. , Bockenstedt, P. L. , Chesney, C. , Fanikos, J. , Fenninger, R. B. , Fogerty, A. E. , Gao, S. , Goldhaber, S. Z. , Gundabolu, K. , Hendrie, P. , Lee, A. I. , Lee, J. T. , Mann, J. , Mcmahon, B. , Millenson, M. M. , Morton, C. , & Engh, A. M. (2018). NCCN guidelines insights: Cancer‐associated venous thromboembolic disease, version 2.2018. Journal of the National Comprehensive Cancer Network: JNCCN, 16(11), 1289–1303. 10.6004/jnccn.2018.0084 [DOI] [PubMed] [Google Scholar]
- Strongman, H. , Gadd, S. , Matthews, A. , Mansfield, K. E. , Stanway, S. , Lyon, A. R. , Dos‐Santos‐Silva, I. , Smeeth, L. , & Bhaskaran, K. (2019). Medium and long‐term risks of specific cardiovascular diseases in survivors of 20 adult cancers: A population‐based cohort study using multiple linked UK electronic health records databases. Lancet (London, England), 394(10203), 1041–1054. 10.1016/s0140-6736(19)31674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, W. , Ren, H. e. , Gao, C. ‐T. , Ma, W. ‐D. , Luo, L. , Liu, Y. , Jin, P. , & Hao, J. i. ‐H. (2015). Clinical and prognostic significance of coagulation assays in pancreatic cancer patients with absence of venous thromboembolism. American Journal of Clinical Oncology, 38(6), 550–556. 10.1097/01.coc.0000436088.69084.22 [DOI] [PubMed] [Google Scholar]
- Tadokoro, Y. , Sakaguchi, M. , Yagita, Y. , Furukado, S. , Okazaki, S. , Fujinaka, T. , Kimura, T. , Yoshimine, T. , Mochizuki, H. , & Kitagawa, K. (2013). Ischemic stroke in patients with solid gynecologic tract tumors and coagulopathy. European Neurology, 70, 304–307. 10.1159/000353799 [DOI] [PubMed] [Google Scholar]
- Tesfamariam, B. (2016). Involvement of platelets in tumor cell metastasis. Pharmacology & Therapeutics, 157, 112–119. 10.1016/j.pharmthera.2015.11.005 [DOI] [PubMed] [Google Scholar]
- Tu, W.‐J. , Zeng, X. , & Liu, Q. (2022). Aging tsunami coming: The main finding from Chinaâs seventh national population census. Aging Clinical and Experimental Research, 34(5), 1159–1163. 10.1007/s40520-021-02017-4 [DOI] [PubMed] [Google Scholar]
- Uneda, A. , Suzuki, K. , Hirashita, K. , & Yoshino, K. (2016). Tandem cervical/intracranial internal carotid artery occlusion due to cardiac myxoma treated successfully with mechanical endovascular thrombectomy. Acta Neurochirurgica, 158(7), 1393–1395. 10.1007/s00701-016-2833-1 [DOI] [PubMed] [Google Scholar]
- Van Aken, E. S. M. , Van Der Laan, H. P. , Bijl, H. P. , Van Den Bosch, L. , Van Den Hoek, J. G. M. , Dieters, M. , Steenbakkers, R. J. H. M. , & Langendijk, J. A. (2021). Risk of ischaemic cerebrovascular events in head and neck cancer patients is associated with carotid artery radiation dose. Radiotherapy and Oncology : Journal of the European Society for Therapeutic Radiology and Oncology, 157, 182–187. 10.1016/j.radonc.2021.01.026 [DOI] [PubMed] [Google Scholar]
- Vlachostergios, P. J. , Daliani, D. D. , Dimopoulos, V. , Patrikidou, A. , Voutsadakis, I. A. , & Papandreou, C. N. (2010). Nonbacterial thrombotic (marantic) endocarditis in a patient with colorectal cancer. Onkologie, 33, 456–459. 10.1159/000317342 [DOI] [PubMed] [Google Scholar]
- Wahrenbrock, M. , Borsig, L. , Le, D. , Varki, N. , & Varki, A. (2003). Selectin‐mucin i as a probable molecular explanation for the association of Trousseau syndrome with mucinous adenocarcinomas. The Journal of Clinical Investigation, 112(6), 853–862. 10.1172/jci18882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.‐y. , Zhang, G.‐j. , Zhuo, S.‐x. , Wang, K. , Hu, X.‐p. , Zhang, H. , & Qu, L.‐d. (2018). D‐dimer >2.785 μg/ml and multiple infarcts ≥3 vascular territories are two characteristics of identifying cancer‐associated ischemic stroke patients. Neurological Research, 40(11), 948–954. 10.1080/01616412.2018.1504179 [DOI] [PubMed] [Google Scholar]
- Weeda, E. R. , & Bohm, N. (2019). Association between comorbid cancer and outcomes among admissions for acute ischemic stroke receiving systemic thrombolysis. International Journal of Stroke: Official Journal of the International Stroke Society, 14(1), 48–52. 10.1177/1747493018778135 [DOI] [PubMed] [Google Scholar]
- Whitlock, R. P. , Sun, J. C. , Fremes, S. E. , Rubens, F. D. , & Teoh, K. H. (2012). Antithrombotic and thrombolytic therapy for valvular disease, 9th edn. American college of chest physicians evidence‐based clinical practice guidelines. Chest, 141, e576S–e600S. 10.1378/chest.11-2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtukiewicz, M. Z. , Sierko, E. , Hempel, D. , Tucker, S. C. , & Honn, K. V. (2017). Platelets and cancer angiogenesis nexus. Cancer Metastasis Reviews, 36(2), 249–262. 10.1007/s10555-017-9673-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaura, G. , Ito, T. , Miyaji, Y. , Ueda, N. , Nakae, Y. , Momoo, T. , Nakano, T. , Johmura, Y. , Higashiyama, Y. , Joki, H. , Doi, H. , Takeuchi, H. , Takahashi, T. , Koyano, S. , Yamaguchi, S. , Yokoyama, M. , & Tanaka, F. (2021). Therapeutic efficacy of heparin and direct factor Xa inhibitors in cancer‐associated cryptogenic ischemic stroke with venous thromboembolism. Thrombosis Research, 206, 99–103. 10.1016/j.thromres.2021.08.016 [DOI] [PubMed] [Google Scholar]
- Yoo, J. , Nam, H. S. , Kim, Y. D. , Lee, H. S. , & Heo, J. i. H. (2019). Short‐term outcome of ischemic stroke patients with systemic malignancy. Stroke, 50(2), 507–511. 10.1161/strokeaha.118.023044 [DOI] [PubMed] [Google Scholar]
- Yu, M. , Li, T. , Li, B. , Liu, Y. , Wang, L. , Zhang, J. , Jin, J. , Guan, Y. , Zuo, N. , Liu, W. , Jing, H. , Li, Y. , Du, J. , Dong, Z. , Jiang, T. , Xie, R. , Zhou, J. , & Shi, J. (2020). Phosphatidylserine‐exposing blood cells, microparticles and neutrophil extracellular traps increase procoagulant activity in patients with pancreatic cancer. Thrombosis Research, 188, 5–16. 10.1016/j.thromres.2020.01.025 [DOI] [PubMed] [Google Scholar]
- ZöLler, B. , Ji, J. , Sundquist, J. , & Sundquist, K. (2012). Risk of haemorrhagic and ischaemic stroke in patients with cancer: A nationwide follow‐up study from Sweden. European Journal of Cancer (Oxford, England : 1990), 48(12), 1875–1883. 10.1016/j.ejca.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Zhang, Q. , Liu, H. , Zhu, Q. , Zhan, P. , Zhu, S. , Zhang, J. , & Song, Y. (2017). Patterns and functional implications of platelets upon tumor “education”. The International Journal of Biochemistry & Cell Biology, 90, 68–80. 10.1016/j.biocel.2017.07.018 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. Y. , Cordato, D. , Shen, Q. , Sheng, A. i. Z. , Hung, W. T. , & Chan, D. K. Y. (2007). Risk factor, pattern, etiology and outcome in ischemic stroke patients with cancer: A nested case‐control study. Cerebrovascular Diseases (Basel, Switzerland), 23, 181–187. 10.1159/000097639 [DOI] [PubMed] [Google Scholar]


