Abstract
To evaluate the relationship between infant age of egg introduction and malnutrition‐related growth outcomes in the United States, we analysed secondary data of 1716 mother–child dyads in the Infant Feeding Practices Study II and its Year 6 Follow‐Up Study. Malnutrition‐related growth outcomes included body mass index z‐score (BMIZ), obesity (weight‐for‐height z‐score [WHZ] ≥3 or BMIZ ≥ 2), WHZ, wasting (WHZ < −2), height‐for‐age z‐score (HAZ), and stunting (HAZ < –2). Infant age at egg introduction was analysed as a continuous variable. We used generalised estimating equations to estimate the mean difference in continuous outcomes and relative risk [RR]) for binary outcomes, adjusting for related maternal and child confounders. We also explored interactions with child sex, maternal race/ethnicity, maternal educational level, ever breastfeeding, and formula feeding. In the total sample, a later infant age at egg introduction was associated with a lower mean difference in HAZ (confounder‐adjusted mean difference = −0.08, 95% confidence interval [CI]: −0.12 to −0.03 per month) and a higher risk of stunting (confounder‐adjusted RR = 1.17, 95% CI: 1.03–1.33 per month) at 6 years. The associations between infant age at egg introduction and 12‐month growth outcomes differed by child sex. Among females but not among males, later introduction of eggs was associated with a lower mean WHZ (−0.06 [−0.12 to 0.00] per month) at 12 months. Later egg introduction during infancy was associated with a lower mean HAZ and a higher risk of stunting in 6‐year‐old children. Besides this, it was associated with a lower WHZ among females at 12 months.
Keywords: child growth, complementary feeding, eggs, infant growth, malnutrition, obesity, stunting
Later egg introduction during infancy was associated with a lower mean height‐for‐age z‐score (HAZ) and a higher risk of stunting in 6‐year‐old children. Besides this, it was associated with a lower weight‐for‐height z‐score (WHZ) among females at 12 months.

Key messages
This secondary data analysis evaluated the relationship between infant age at egg introduction and malnutrition‐related growth outcomes among young US children within the Infant Feeding Practices Study II and its Year 6 Follow‐up Study.
In the total sample, a later infant age at egg introduction was associated with a lower mean 6‐year height‐for‐age z‐score and a higher risk of stunting at 6 years.
The associations between infant age at egg introduction and 12‐month growth outcomes differed by child sex. Later introduction of eggs was associated with a lower mean weight‐for‐height z‐score among females. However, this association was not observed among males.
1. INTRODUCTION
Malnutrition refers to deficiencies, excesses, or imbalances in energy intake and nutrients (World Health Organization, WHO, 2020a). Inadequate nutrition in early infancy may associate with poor intelligence quotient levels and academic skills in school‐age and further increase child mortality (Langley‐Evans, 2015; Reinbott & Jordan, 2016). Expected malnutrition‐related child growth outcomes include obesity, wasting, and stunting. In the United States, the overall prevalence of stunting and wasting was 2.9% and 0.2%, respectively, among children under 5 years (UNICEF, 2021). The corresponding prevalence of stunting and wasting was 3.0% and 0.4% among children under 24 months, and 2.8% and 0.1% among children from 24 to 59 months (UNICEF, 2021). Besides this, the prevalence of these malnutrition‐related outcomes differs by child sex. Both prevalence of stunting (3.2% vs. 2.6%) and wasting (0.4% vs. 0.1%) were higher in males than in females under 5 years (UNICEF, 2021). There are considerable disparities in these outcomes among vulnerable subpopulations. For example, stunting is higher among Hispanic (6.1%) than Non‐Hispanic White (2.6%) children aged 2–19 years in the United States (Iriart et al., 2013).
Proper complementary feeding is important for healthy growth and attaining a child's human potential (Wang et al., 2021). On the basis of WHO infant feeding recommendations, children should transit from exclusive breastfeeding to sharing household meals, beginning at the age of 6–24 months (Cheikh Ismail et al., 2022). During this transition period, nutrient‐dense foods are needed to complement breast milk and support healthy growth and development (Prado et al., 2020). For example, the egg is nutrient‐rich (Iannotti et al., 2014; McKune et al., 2020), protein‐dense (Drewnowski, 2010), and cost‐efficient food for infant feeding (Papanikolaou & Fulgoni, 3rd, 2020). Evidence showed that subjects with a relatively high‐protein diet had a higher sense of satiety and the effect of decreasing appetite than those under isoenergetically fed conditions in ad libitum feeding conditions (Weigle et al., 2005; Westerterp‐Plantenga et al., 2012). Besides, a 50 g egg can provide 57% of the recommended dietary allowance for protein to a healthy infant aged 7–12 months (Lutter et al., 2018).
However, there is only limited research on the effects of infant egg intake on other health outcomes, such as infant growth. An observational study reported that egg intake was positively related to weight‐for‐age z‐score (WAZ) among children older than 60 months in Nepal from 2013 to 2016 (Miller et al., 2020; Pimpin et al., 2016). Another cohort of infants born from 1959 to 1961 in Copenhagen reported that the age at egg introduction had no long‐term associations with overweight and obesity in adults aged 42 years (Schack‐Nielsen et al., 2010). Besides this, a study from six waves of National Health and Nutrition Examination Survey (NHANES) datasets from 2001 to 2012 indicated that egg consumers were taller than non‐egg consumers among 6‐to‐24‐month‐old children (Papanikolaou & Fulgoni, 3rd, 2018). A randomised controlled trial (RCT) revealed that the introduction of eggs reduced the risk of stunting by 47% and increased linear growth among 6‐to‐9‐month‐old children enroled in 2015 from Ecuador (Iannotti Lutter, Stewart, et al. 2017; Iannotti, Lutter, Waters, et al., 2017). However, this effect on linear growth was attenuated to null at the follow‐up 2 years later (Iannotti et al., 2020). Another RCT among 6‐to‐9‐month‐old infants enroled in 2018 from Malawi found a null association between egg consumption and length (Stewart et al., 2019). Research has shown sex differences in the associations of early nutrition with later growth (Thone‐Reineke et al., 2006) and developmental outcomes (Harding et al., 2017).
Therefore, based on a US national cohort study, we aimed to 1) investigate the prospective associations between infant age at egg introduction and later malnutrition‐related growth outcomes at 12 months (short‐term) and 6 years (long‐term) and 2) explore potential sex differences in these associations.
2. METHODS
2.1. Study design and participants
We analysed a subsample (N = 1716) from the Infant Feeding Practices Study II (IFPS II, 2005–2007, N = 4902 pregnancies and 3033 newborns) and its Year 6 Follow‐Up study (Y6FU, 2012, N = 1542 children) in the United States (Fein et al., 2008; Grummer‐Strawn et al., 2014). Given that we aimed to assess the associations between infant egg introduction and malnutrition‐related child growth outcomes, we restricted the eligible samples to those mother–child dyads with complete data on 1) age at egg introduction by 12 months (exposure) and 2) child weight and height outcomes at 12 months and/or 6 years (outcomes). After excluding some mother–child dyads due to missing data and attrition, the final analytic sample consisted of 1716 eligible mother–child dyads. Figure 1 shows the analytic sample flowchart.
Figure 1.
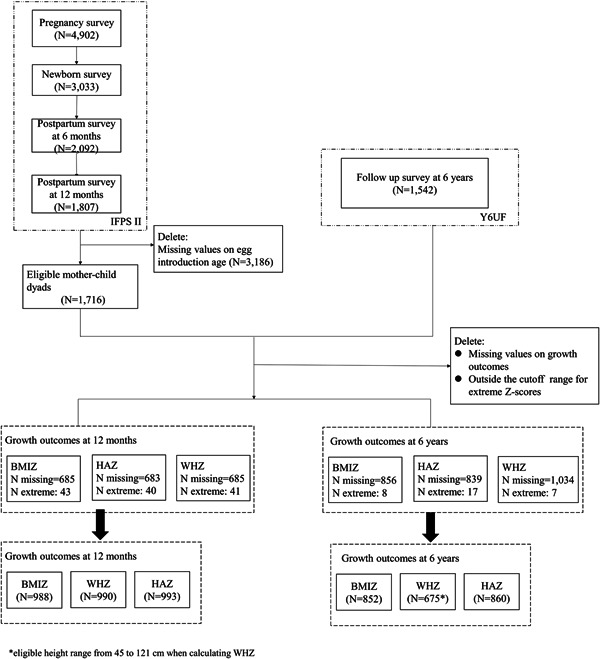
Participant flowchart. Eligible mother–child dyads had complete data on 1) age at egg introduction by 12 months (exposure) and 2) weight and height outcomes of children at 12 months and/or 6 years (outcomes). BMIZ, body mass index z‐score; HAZ, height‐for‐age z‐score; WHZ, weight‐for‐height z‐score. *Eligible height range from 45 to 121 cm when calculating WHZ.
The Center for Disease Control and Prevention (CDC) approved this analytic plan and provided access to the deidentified and public use data of IFPS II and its Y6FU. This study was approved by the University at Buffalo Institute Review Board and the Human Research Ethics Committee of Xi'an Jiaotong University.
2.2. Exposure measures
Infant age at egg introduction was our key exposure measure. In the nearly monthly surveys taken from 2 to 12 months post‐partum (2, 3, 4, 5, 6, 7, 9, 10, and 12 months), mothers reported their infant's intake of 18 types of foods in the past 7 days (Wen et al., 2014). The question that assessed infant food intake was—“In the past 7 days, how often was your baby fed each food listed below? Include feedings by everyone who feeds the baby and includes snacks and night‐time feedings.” Infant food intake was reported as the number of feedings per day or week. “Eggs” were 1 of the 18 types of foods. Accordingly, we derived the infant age at egg introduction from mothers' monthly responses to infant egg consumption questions. The final variable for infant age at egg introduction was continuous in months. To retain sample size and reduce potential bias, we used the multiple imputation approach (see more later) to impute the missing values of “the child age at egg introduction for those infants whose mothers reported “not introduced yet by 12 months” at the 12‐month post‐partum survey, with the allowable child age range being from 13 to 72 months (i.e., between the last infancy follow‐up and the 6‐year follow‐up).
2.3. Child growth outcome measures
In IFPS II and its Y6FU, mothers reported the child's weight, height, and actual age at their most recent doctor's visit at the 3‐month, 5‐month, 7‐month, 12‐month, and 6‐year surveys. Our primary growth outcomes were 12‐month (short‐term) and 6‐year (long‐term) body mass index z‐score (BMIZ), weight‐for‐height z‐score (WHZ), and height‐for‐age z‐score (HAZ) as well as their corresponding binary outcomes (obesity, wasting, and stunting). According to CDC recommendation, WHO growth charts were suitable for infants and children at the age of 0 to 2 in the United States, and CDC growth charts were recommended for monitoring growth for children aged 2 years or older in the United States (Grummer‐Strawn et al., 2010; WHO Multicenter Growth Reference Study Group, 2006). We calculated child BMIZ, WHZ/weight‐for‐length z‐score (eligible height range, 45‐121 cm), and HAZ by sex and age. The short‐term infant growth outcomes of our interest included obesity (WHZ ≥ 3), wasting (WHZ < ‐2), and stunting (HAZ < –2) at 12 months (the ending point of infancy). The corresponding long‐term child growth outcomes are defined as obesity (BMIZ ≥ 2), wasting (WHZ < ‐2), and stunting (HAZ < –2) at 6 years (WHO, 2019, 2020b). Note that the BMIZ instead of WHZ score was recommended to define obesity for children older than 2 years (Grummer‐Strawn et al., 2010; WHO, 2021). Extreme z‐score values were removed according to CDC and WHO standards (Center for Disease Control and Prevention Division of Nutrition Physical Activity and Obesity, 2019a, 2019b).
2.4. Confounders
On the basis of literature (Alman et al., 2021; Burgess et al., 2019) and prior knowledge, we considered demographic, pregnancy, and child characteristics as potential confounders. To facilitate choosing confounders for adjustment, we constructed a conceptual framework to visualise relationships among the exposure, outcome, and confounders by using directed acyclic graphs (DAGs) (Supporting Information: Figure 1) with the DAGitty program (http://dagitty.net/, version 2.3) (Textor et al., 2011, 2017). The final set of confounders that we chose to adjust contained: age at introduction of other complementary foods (continuous), baseline BMIZ, WHZ, or HAZ score at birth (continuous), household income (≤185% vs. >185% of the US federal poverty level), maternal marital status (married vs. unmarried), age (18–24, 25–29, 30–34, and ≥35 years), education (3 years college or lower vs. college graduate or higher), preconception body mass index (BMI) (normal/underweight, overweight, and obesity), preterm, weight gain during pregnancy (inadequate, adequate, and excessive), and child sex (female vs. male).
2.5. Statistical analysis
Continuous variables were summarised using the mean ± standard deviation and frequency (proportion) for categorical variables. Analysis of variance and χ 2 tests were used to test differences across infants who introduced eggs less than or equal to 12 months and those who were not introduced to eggs by 12 months.
To examine the associations of infant age at egg introduction with malnutrition‐related growth outcomes at 12 months and 6 years, we used generalised estimating equations to estimate the mean difference in continuous outcomes (BMIZ, WHZ, and HAZ) and relative risk (RR) for binary outcomes (obesity, wasting, and stunting) with 95% confidence interval (CI). Model 1 included exposure only. In addition, Model 2 was adjusted for potential confounders including sociodemographic factors (e.g., maternal age, education level, and family income) and health‐related behaviours/status during pregnancy (e.g., preconception BMI). Model 3 was further adjusted for child sex and the corresponding infant growth measurement (BMIZ, WHZ, or HAZ) at birth to address the influences of child sex and baseline growth status. Model 4 was additionally adjusted for infant age at introducing complementary foods other than eggs to assess the independent effect of egg introduction.
On the basis of previous research (Harding et al., 2017), we further explored the potential interaction with child sex (male vs. female), maternal race (Non‐Hispanic White vs. Others), maternal educational level (3 years college or lower vs. college graduate or higher), ever breastfeeding (yes vs. no), and formula feeding (yes vs. no) by adding the two‐way interaction term, such as “child sex × infant age at egg introduction,” into multivariable regression models.
The missing proportions of adjusted confounders ranged from 0.0% to 5.8% (Supporting Information: Table 1). To reduce selection bias due to missing data on confounders, we applied multivariate imputation (MI) by MI by chained equations (van Buuren et al., 1999) under the assumption of missing at random. The number of burn‐in iterations before each imputation was set to 10 (see more details in Supporting information). As suggested in the literature (Glynn et al., 1993), we included all the relevant variables that were likely to be used in the subsequent analyses. The confounders with missing values and all other variables influencing the processor causing the missing data (e.g., exposure, outcomes, and other confounders) were included in the imputation models to generate 20 completed datasets. We chose to use the MI method for several reasons: (1) the missing proportions of confounders were not too large (<40%) or small enough to ignore (>5%), (2) the missing not at random and the missing completely at random assumption seemed implausible for our dataset, and (3) missing values on a confounder was likely to be influenced by the exposure, outcome, and other confounders (Jakobsen et al., 2017). Standard errors calculated based on Rubin's rules were used to take account of variability in results between the imputed datasets, reflecting the uncertainty associated with the missing values (White et al., 2011). Other imputation diagnostic indicators included variance total, a relative increase in variance, fraction missing information, and relative efficiency (Supporting Information: Table 2).
All statistical analyses were performed using the software of SAS version 9.4 (SAS Institute Inc). Multivariable logistic and linear regression model were conducted by using SAS PROC GENMOD. Multiple imputations were performed by using SAS PROC MI and MIANALYZE. Statistical tests were two‐sided, and p < 0.05 were considered statistically significant.
3. RESULTS
Overall, the distributions of sociodemographic and pregnancy characteristics in the analytic sample (N = 1716) were considerably different from those in the excluded sample (N = 3186) (Supporting Information: Table 3). Specifically, mothers in the analytic sample had higher education levels, higher family income, older age, and higher infant birthweight and were more likely to be Non‐Hispanic White, married, and nonsmoking.
In the analytic sample of 1716 mother–child dyads, 75.52% of infants were introduced to eggs at 12 months or earlier, and the other 24.48% were not yet introduced by 12 months (Supporting Information: Figure 2). Supporting Information: Table 4 showed the distribution of sample characteristics according to infant age at egg introduction. Mothers of infants not yet introduced to eggs by 12 months had an older age at pregnancy, higher education level, higher family income, and lower preconception BMI. They were also more likely to be married and nonsmokers. In this cohort, 9.97% and 7.33% of the infants had stunting at 12 months and 6 years, respectively. Besides this, 4.05% and 10.09% of infants were obese at 12 months and 6 years, respectively (Supporting Information: Figure 3).
As shown in Table 1, the infants with a later age at egg introduction had significantly lower HAZ at 6 years (confounder‐adjusted mean difference = −0.08 [95% CI, −0.12 to −0.04] per month, p < 0.001), after adjusting for maternal sociodemographic characteristics and health‐related behaviours/status during pregnancy. This difference remained almost the same (−0.08 [95% CI, −0.12 to −0.03] per month, p < 0.001) after further adjusting for child sex, the corresponding growth measurement at birth, and infant age at introduction of other complementary foods. Consistently, the risk of stunting at 6 years was significantly higher if infants were introduced to eggs at a later age (confounder‐adjusted RR = 1.17 [95% CI, 1.03–1.33] per month, p = 0.016) (Table 2). We did not find any significant associations between infant age at egg introduction and other malnutrition‐related growth outcomes, including BMIZ, WHZ, risk of obesity, and wasting in the total sample (Tables 1 and 2).
Table 1.
Associations between infant age at egg introduction and malnutrition‐related child growth outcomes (continuous) at 12 months and 6 years
| Sample sizea | Mean (SD) | Model 1 (crude) | Adjusted model 2c | Adjusted model 3d | Adjusted model 4e | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Difference (95% CI)b | p‐Value | Mean Difference (95% CI)b | p‐Value | Mean difference (95% CI)b | p‐Value | Mean difference (95% CI)b | p‐Value | |||
| BMIZ | ||||||||||
| 12 months | 988 | 0.44 (1.42) | −0.04 (−0.08, 0.01) | 0.143 | −0.02 (−0.07, 0.03) | 0.416 | −0.02 (−0.07, 0.03) | 0.467 | −0.01 (−0.06, 0.04) | 0.664 |
| 6 years | 852 | 0.34 (1.28) | −0.02 (−0.07, 0.02) | 0.259 | 0.00 (−0.04, 0.05) | 0.848 | 0.01 (−0.03, 0.05) | 0.731 | 0.00 (−0.04, 0.04) | 0.952 |
| WHZ | ||||||||||
| 12 months | 990 | 0.47 (1.35) | −0.03 (−0.08, 0.01) | 0.180 | −0.02 (−0.06, 0.03) | 0.466 | −0.02 (−0.06, 0.03) | 0.510 | −0.01 (−0.05, 0.04) | 0.761 |
| 6 years | 675 | 0.35 (1.26) | −0.01 (−0.05, 0.04) | 0.803 | 0.02 (−0.02, 0.07) | 0.348 | 0.02 (−0.02, 0.07) | 0.296 | 0.02 (−0.03, 0.06) | 0.396 |
| HAZ | ||||||||||
| 12 months | 993 | 0.06 (1.71) | 0.03 (−0.04, 0.09) | 0.404 | 0.02 (−0.05, 0.08) | 0.602 | 0.00 (−0.06, 0.06) | 0.975 | 0.01 (−0.05, 0.07) | 0.738 |
| 6 years | 860 | −0.06 (1.37) | −0.05 (−0.09, −0.01) | 0.008 | −0.08 (−0.12, −0.04) | <0.001 | −0.08 (−0.13, −0.04) | <0.001 | −0.08 (−0.12, −0.03) | <0.001 |
Note: The significant parameters (p < 0.05) are highlighted in bold.
Abbreviations: BMI, body mass index; BMIZ, body mass index z‐score; CI, confidence interval; HAZ, height‐for‐age z‐score; SD, standard deviation; WHZ, weight‐for‐height z‐score.
For some variables, the sum of categories was not equal to the total due to missing data.
Mean difference per month older age at egg introduction.
Model 2 adjusted for maternal sociodemographic characteristics (e.g., age, education level, family income, race/ethnicity, and marital status) and health behaviours/status during pregnancy (e.g., preconception BMI, gestational weight gain, and gestational duration).
Model 3 additionally adjusted for child sex and the corresponding growth measurement at birth.
Model 4 additionally adjusted for infant age at introducing other complementary foods.
Table 2.
Associations between infant age at egg introduction and malnutrition‐related child growth outcomes (categorical) at 12 months and 6 years
| Sample sizea | Prevalence n (%) | Model 1 (crude) | Adjusted model 2c | Adjusted model 3d | Adjusted model 4e | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Relative risk (RR) (95% CI)b | p‐Value | RR (95% CI)b | p‐Value | RR (95% CI)b | p‐Value | RR (95% CI)b | p‐Value | |||
| Obesity | ||||||||||
| 12 months | 988 | 40 (4.05) | 0.95 (0.81, 1.12) | 0.555 | 0.98 (0.84, 1.16)f | 0.854 | 0.99 (0.84, 1.17)f | 0.898 | 0.99 (0.84, 1.16)f | 0.868 |
| 6 years | 852 | 86 (10.09) | 0.90 (0.83, 0.99) | 0.022 | 0.85 (0.70, 1.04) | 0.109 | 0.97 (0.88, 1.05) | 0.440 | 0.96 (0.88, 1.05) | 0.388 |
| Wasting | ||||||||||
| 12 months | 990 | 21 (2.12) | 0.86 (0.73, 1.01) | 0.071 | 0.85 (0.70, 1.04)f | 0.114 | 0.85 (0.70, 1.04)f | 0.109 | 0.84 (0.68, 1.04)f | 0.105 |
| 6 years | 675 | 21 (3.11) | 0.93 (0.80, 1.08) | 0.329 | 0.94 (0.81, 1.10)f | 0.458 | 0.94 (0.81, 1.10)f | 0.469 | 0.96 (0.82, 1.11)f | 0.562 |
| Stunting | ||||||||||
| 12 months | 993 | 99 (9.97) | 0.93 (0.84, 1.02) | 0.121 | 0.95 (0.86,1.05) | 0.289 | 0.96 (0.87,1.05) | 0.370 | 0.95 (0.86, 1.05) | 0.331 |
| 6 years | 860 | 63 (7.33) | 1.12 (1.00, 1.26) | 0.059 | 1.18 (1.04, 1.34) | 0.011 | 1.18 (1.04, 1.34) | 0.010 | 1.17 (1.03, 1.33) | 0.016 |
Note: The significant parameters (p < 0.05) are highlighted in bold.
Abbreviations: BMI, body mass index; CI, confidence interval.
For some variables, the sum of categories was not equal to the total due to missing data.
RR of categorical outcome per month older age at egg introduction.
Model 2 adjusted for maternal sociodemographic characteristics (e.g., age, education level, family income, race/ethnicity, and marital status) and health behaviours/status during pregnancy (e.g., preconception BMI, gestational weight gain, and gestational duration).
Model 3 additionally adjusted for child sex and the corresponding growth measurement at birth.
Model 4 additionally adjusted for infant age at introduction of other complementary foods and maternal height (only for stunting).
Gestational duration was adjusted as a continuous variable because of the model convergence.
Tables 3 and 4 show the interaction between child sex and infant age at egg introduction for continuous and categorical outcomes, respectively. There was a significant interaction between child sex and infant age at egg introduction for BMIZ (p‐value for interaction = 0.036), WHZ (p‐value for interaction = 0.018), and risk of obesity (p‐value for interaction = 0.015) at 12 months but not at 6 years. Among females at 12 months, an older infant age at egg introduction was associated with a lower WHZ (−0.06 [95% CI, −0.12 to 0.00], p = 0.045), although the mean difference in BMIZ (−0.06 [95% CI, −0.13 to 0.00], p = 0.060) and the risk of obesity (0.83 [95% CI, −0.64 to 1.01], p = 0.067) were only marginally significant. However, among males, egg introduction later was not significantly associated with BMIZ, WHZ, HAZ, and risk of obesity at 12 months. We also explored the potential interaction between egg introduction age and other several covariates (i.e., ever formula feeding, breastfeeding, maternal race/ethnicity, and maternal education) (Supporting Information: Tables 5–10). The results indicated the following interactions were significant: between ever breastfeeding and infant age at egg introduction for HAZ at 12 months (p‐value for interaction = 0.028), between ever formula feeding and infant age at egg introduction for the risk of stunting at 12 months (p‐value for interaction = 0.012), and between maternal education level and infant age at egg introduction for HAZ at 6 years (p‐value for interaction = 0.013).
Table 3.
Interaction between child sex and infant age at egg introduction in malnutrition‐related child growth outcomes (continuous) at 12 months and 6 years
| Females | Males | p‐Value for sex interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample sizea | Mean (SD) | Adjusted mean difference | p‐Value | Sample sizea | Mean (SD) | Adjusted mean difference | p‐Value | ||
| (95% CI)b | (95% CI)b | ||||||||
| BMIZ | |||||||||
| 12 months | 507 | 0.44 (1.37) | −0.06 (−0.13, 0.00) | 0.060 | 481 | 0.43 (1.48) | 0.04 (−0.03, 0.11) | 0.281 | 0.036 |
| 6 years | 424 | 0.39 (1.21) | 0.00 (−0.05, 0.06) | 0.952 | 428 | 0.30 (1.35) | 0.00 (−0.06, 0.06) | 0.975 | 0.874 |
| WHZ | |||||||||
| 12 months | 507 | 0.46 (1.29) | −0.06 (−0.12, 0.00) | 0.045 | 483 | 0.49 (1.41) | 0.05 (−0.02, 0.11) | 0.176 | 0.018 |
| 6 years | 351 | 0.37 (1.24) | 0.01 (−0.04, 0.07) | 0.658 | 324 | 0.32 (1.28) | 0.03 (−0.04, 0.09) | 0.431 | 0.763 |
| HAZ | |||||||||
| 12 months | 515 | 0.00 (1.75) | 0.07 (−0.03, 0.16) | 0.182 | 478 | 0.13 (1.66) | −0.05 (−0.12, 0.03) | 0.198 | 0.069 |
| 6 years | 428 | −0.10 (1.39) | −0.08 (−0.14, −0.02) | 0.006 | 432 | −0.02 (1.36) | −0.07 (−0.13, −0.01) | 0.017 | 0.827 |
Note: The significant parameters (p < 0.05) are highlighted in bold.
Abbreviations: BMI, body mass index; BMIZ, body mass index z‐score; CI, confidence interval; HAZ, height‐for‐age z‐score; SD, standard deviation; WHZ, weight‐for‐height z‐score.
For some variables, the sum of categories was not equal to the total due to missing data.
Mean difference for continuous outcome per month older age at egg introduction adjusted for maternal sociodemographic characteristics (e.g., age, education level, family income, race/ethnicity, and marital status), health behaviours/status during pregnancy (e.g., preconception BMI, gestational weight gain, and gestational duration), child sex, the corresponding growth measurement at birth, and infant age at introduction of other complementary foods.
Table 4.
Interaction between child sex and infant age at egg introduction in malnutrition‐related child growth outcomes (categorical) at 12 months and 6 years
| Females | Males | p‐Value for sex interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample sizea | Prevalence, n (%) | Adjusted relative risk (RR) | p‐Value | Sample sizea | Prevalence, n (%) | Adjusted RR | p‐Value | ||
| (95% CI)b | (95% CI)b | ||||||||
| Obesity | |||||||||
| 12 months | 507 | 20 (3.94) | 0.83 (0.64, 1.01)c | 0.067 | 481 | 20 (4.16) | 1.24 (0.95, 1.54)c | 0.108 | 0.015 |
| 6 years | 424 | 39 (9.20) | 0.98 (0.85, 1.11) | 0.783 | 428 | 47 (10.98) | 0.94 (0.83, 1.06) | 0.336 | 0.64 |
| Wasting | |||||||||
| 12 months | 507 | 10 (1.97) | 0.86 (0.58, 1.13)c | 0.302 | 483 | 11 (2.28) | 0.82 (0.55, 1.10)c | 0.208 | 0.819 |
| 6 years | 351 | 9 (2.56) | 0.95 (0.74, 1.16)c | 0.655 | 324 | 12 (3.70) | 0.96 (0.76, 1.16)c | 0.698 | 0.997 |
| Stunting | |||||||||
| 12 months | 515 | 56 (10.87) | 0.91 (0.78, 1.03) | 0.147 | 478 | 43 (9.00) | 1.02 (0.86, 1.17) | 0.821 | 0.526 |
| 6 years | 428 | 32 (7.48) | 1.16 (0.99, 1.33) | 0.063 | 432 | 31 (7.18) | 1.18 (0.99, 1.37) | 0.064 | 0.988 |
Note: The significant parameters (p < 0.05) are highlighted in bold.
Abbreviations: BMI, body mass index; CI, confidence interval.
For some variables, the sum of categories was not equal to the total due to missing data.
RR of categorical outcome per month older age at egg introduction adjusted for maternal socio‐demographic characteristics (e.g., age, education level, family income, race/ethnicity, and marital status), health behaviours/status during pregnancy (e.g., preconception BMI, gestational weight gain, gestational duration), child sex, the corresponding growth measurement at birth, and infant age at introduction of other complementary foods.
Gestational duration was adjusted as a continuous variable because of the model convergence.
4. DISCUSSION
In a US prospective longitudinal prebirth cohort, we examined the associations between infant age at egg introduction and malnutrition‐related child growth outcomes at 12 months and 6 years. Our results showed that later egg introduction was associated with a lower HAZ score and a higher risk of stunting at 6 years old. This suggested a beneficial effect of early egg introduction on longer‐term height growth.
Most previous studies on the associations between egg introduction and growth outcomes were conducted in low‐income countries and were limited by not studying the long‐term effect (Iannotti, Lutter, Stewart, et al., 2017; Papanikolaou & Fulgoni, 3rd, 2018). In a prospective cohort study with a 5‐year follow‐up beyond infancy, our analysis added new evidence on the potential long‐term influences of infant egg introduction in the United States. Our observed associations between later egg introduction in infancy with lower HAZ at 6 years were supported by some (Iannotti et al., 2020; Iannotti, Lutter, Stewart, et al., 2017; Nachvak et al., 2020; Papanikolaou & Fulgoni, 3rd, 2018), but not all previous studies (Stewart et al., 2019). For example, an egg intervention project reported that length‐for‐age z‐scores (LAZ) increased by 0.63 units among infants who have introduced to eggs at 6–9 months of age from March to December 2015 in Ecuador where they were experiencing the nutrition transition with a disease burden of both obesity and stunting (Iannotti et al., 2020, Iannotti, Lutter, Stewart, et al., 2017). In the United States, a study using NHANS dataset showed that 6–24‐month‐olds with egg consumption had longer recumbent length (79.2 ± 0.2 vs. 78.7 ± 0.1 cm for those without egg consumption; p = 0.03), although the association with weight was not significant (Papanikolaou & Fulgoni, 3rd, 2018). However, an RCT study conducted in Malawi of 660 children aged 6–9 months found no significant effect on LAZ after early egg introduction between February and July 2018 (Stewart et al., 2019), which might be due to the higher mean baseline LAZ and higher infant intake of animal source food, especially fish, in Malawi.
Many studies have shown that egg consumption at an early age was associated with greater daily nutrient intakes, which were beneficial for physical growth and mental development (Iannotti, Lutter, Waters, et al., 2017; Papanikolaou & Fulgoni, 3rd, 2019). A modelling analysis using NHANES revealed that the addition of one or two eggs to breakfast in 1‐ to 18‐year‐old children substantially increased the levels of several dietary nutrients, including total choline, lutein + zeaxanthin, docosahexanoic acid, vitamin D, vitamin A, and pantothenic acid, in comparison to the usual breakfast (Papanikolaou & Fulgoni, 3rd, 2021). For infants, eggs provide a large proportion of several vital micronutrients' recommended dietary allowance or adequate intake (AI). For example, one 50‐g egg provides 88% of the AI of vitamin B12 for a healthy infant aged 7–12 months (Lutter et al., 2018). An RCT among North Indian children aged 6–35 months in 2011 found that vitamin B12 supplementation for 6 months could significantly improve linear growth in a subgroup of children who were stunted at baseline (Strand et al., 2015). Other nutrients in eggs that may also help explain their beneficial effect on reducing stunting are essential amino acids and cholesterol (Iannotti et al., 2014). Stunted children could potentially have a poor dietary intake of essential amino acids (Semba et al., 2016), and eggs may improve linear growth (height gain) through elevating concentrations of amino acids and total cholesterol (Nachvak et al., 2020). Earlier intake of these critical nutrients from eggs in infancy may lead to more prolonged benefits on child growth outcomes.
The effect of eggs on child growth outcomes is likely to change with child age, but only a few studies have examined the long‐term effects of egg consumption on child growth. In an observational cohort study conducted from January 2008 to February 2009, vitamin B12 status during infancy could predict linear growth at 5 years old among 500 mother–infant dyads randomly selected from Nepal (Strand et al., 2018). This indicated that early egg consumption (via vitamin B12) potentially had a long‐term effect on reducing stunting risk. However, another study found that the intervention effect of 6‐month egg consumption among infants aged 6–9 months on height growth was significant only at end of intervention (ages 12–15 months), but not significant at the follow‐up when children were 2–3 years old (Iannotti et al., 2020). This inconsistency of the long‐term effects of egg consumption on child growth might be due to inadequate sample size and relatively low prevalence of stunting.
Although egg consumption can help to increase protein, vitamin B12, and other micronutrient intakes for infants and children, child growth is multifactorial (Papanikolaou & Fulgoni, 3rd, 2018). In addition to egg consumption, other complementary food, overall diet quality, and living environment also play important role in child growth. This might explain our overall null results on short‐term or long‐term effects of egg introduction on obesity or wasting. But we observed a trend that later egg introduction during infancy might be associated with a lower risk of obesity at 12 months in females (not in males). Some biological evidence supported this sex interaction. A previous animal study showed that female rats fed with a high protein diet after weaning had increased food efficiency, body weight, and fat pads (an indicator of obesity), whereas these phenotypes were not observed in male rats (Thone‐Reineke et al., 2006). In addition, this sex difference might be related to the insulin‐like growth factor‐1 (IGF‐1) response to dietary protein intake. For example, an RCT conducted in five European countries among 1090 infants reported that female (vs. male) infants with higher protein intake had a stronger response and higher concentrations of total IGF‐1 and leptin, both of which might be associated with higher fat mass and body weight (Closa‐Monasterolo et al., 2011). In epidemiological studies, the association between age at complementary food introduction and long‐term obesity (or adiposity) was still inconclusive (English et al., 2019). Our results were consistent with some studies showing null association (Barrera et al., 2016; Kanoa et al., 2011).
4.1. Strengths and limitations
One strength of our analysis was utilising the sizeable general population study in the United States and 6 years of follow‐up, which could help to enhance aetiological inference and generalisability. In addition, we adjusted for a comprehensive list of confounders, including sociodemographic characteristics, maternal health behaviours/status during pregnancy, child sex, the corresponding infant growth measurements at birth, and age of introduction of other complementary foods.
Our analysis had several significant limitations. First, the relatively high attrition rates at 12 months (40%) and 6 years (49%) could lead to substantial potential selection bias. Second, our definitions of obesity were only based on the sex‐ and age‐specific percentile of clinically measured BMI, which could not distinguish between fat mass and lean mass or distribution of fat mass (visceral vs. subcutaneous). Third, the timeframe for recalling infant dietary intake, including eggs in the monthly questionnaires was the past 7 days. Although the use of the past 7 days could help the mother recall accurately, it might miss the potential changes in infant dietary intake in the 3 earlier weeks of the past month. Fourth, the possibility of reverse causation (e.g., infant growth status may change parents' feeding practices) and unmeasured confounders (e.g., food allergy) might obscure our observed association between egg introduction and child growth outcomes. Fifth, IFPS II was not nationally representative with undersampling vulnerable populations, such as low‐income families, less educated mothers, unemployed, and racial minorities. IFPS II mothers were more likely to breastfeed and breastfeed longer than the general population. Lastly, IFPS II was a relatively old cohort, as it was established during 2005–2007. Children's egg consumption may change over time (Gray, 2019). For example, for US children aged 2–12 years old, egg‐source protein intake per kg of body weight increased from 0.63 g/day in 1999 to 0.69 g/day in 2010, although the percentage of egg consumers remained at 82.0% (Kim et al., 2020). However, the biological effects of egg consumption on child growth (Lutter et al., 2018) are likely to be similar over time if the nutrients in eggs remain stable (Papanikolaou & Fulgoni, 3rd, 2019). Hence, our findings still had some meaningful implications for current practices.
5. CONCLUSION
We concluded that a later age at egg introduction in US infants might delay longer‐term height growth and increase the risk of stunting at 6 years. It was also associated with a lower WHZ at 12 months among females. Our findings added scientific evidence linking infant complementary food feeding practices, particularly egg introduction, and long‐term health effects. The optimal egg introduction age may depend on the infant's nutritional status and possible growth‐related outcomes of interest. If replicated in other cohort studies, our research supports egg consumption starting in infancy as a cost‐effective nutritional strategy to promote height growth even in developed countries like the United States. Future research should improve the retention rate in the follow‐up, use higher‐quality measures of infant diet, consider the frequency of egg consumption, and examine other health outcomes such as cardio‐metabolic health.
AUTHOR CONTRIBUTIONS
The authors’ responsibilities were as follows: Xiaozhong Wen and Baibing Mi designed the research. Xiaozhong Wen, Baibing Mi, Huimeng Liu, and Yutong Wang analysed data. Xiaozhong Wen, Baibing Mi, Huimeng Liu, and Yutong Wang wrote the paper. Xiaozhong Wen had primary responsibility for the final content. Ariana Surguy‐Bowers, Hannah Small, Todd C. Rideout, Claire E. Cameron, Huimeng Liu, and Krystal Starke provided additional interpretation of the data and revisions to the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
All study procedures were conducted in accordance with the ethical standards of the responsible committee on social and behavioural science research and with the Helsinki Declaration of 1975, as revised in 2000. This secondary data analysis project used deidentified and public‐use data from the Center for Disease Control and Prevention. It was approved by the University at Buffalo Institutional Review Board and the Human Research Ethics Committee of Xi'an Jiaotong University. Consent from participants was not needed, since it was determined as nonhuman research.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
The authors appreciate staff from the Centres for Disease Control and Prevention for reviewing and approving the analytic plan and providing access to the deidentified and public use data of Infant Feeding Practices Study II and its Year 6 Follow‐Up Study. This study was supported by the grant “Effects of infant egg consumption on child health and cognition development” through the Egg Nutrition Centre/American Egg Board (PI, Xiaozhong Wen) and by the Health Resources and Services Administration of the US Department of Health and Human Services under R40MC31880 entitled “Socioeconomic disparities in early origins of childhood obesity and body mass index trajectories” (PI, Xiaozhong Wen). Baibing Mi's effort was supported by the Chinese National Key Research and Development Programme of China (Grant Numbers: 2017YFC0907200 and 2017YFC0907201), the National Natural Science Foundation of China (Grant Number: 62141223 and 82103944), the Natural Science Basic Research Plan of the Shaanxi Province (Grant Number: 2020JQ‐090), the Fundamental Research Funds for the Central Universities (Grant Number: xzy032020033), and Xi'an Special Science and Technology Projects (Grant Number: 20200005YX005).
Mi, B. , Liu, H. , Wang, Y. , Small, H. , Surguy‐Bowers, A. , Rideout, T. C. , Cameron, C. E. , Lehman, H. K. , Starke, K. , & Wen, X. (2022). Infant age at egg introduction and malnutrition‐related child growth in the United States. Maternal & Child Nutrition, 18, e13390. 10.1111/mcn.13390
DATA AVAILABILITY STATEMENT
Data used in the manuscript, codebooks, and analytic codes will not be made available because the raw data was owned by the Center for Disease Control and Prevention (CDC). Researchers who are interested in using the data can contact the CDC directly.
REFERENCES
- Alman, K. L. , Lister, N. B. , Garnett, S. P. , Gow, M. L. , Aldwell, K. , & Jebeile, H. (2021). Dietetic management of obesity and severe obesity in children and adolescents: A scoping review of guidelines. Obesity Reviews, 22(1), e13132. 10.1111/obr.13132 [DOI] [PubMed] [Google Scholar]
- Barrera, C. M. , Perrine, C. G. , Li, R. , & Scanlon, K. S. (2016). Age at introduction to solid foods and child obesity at 6 years. Childhood Obesity, 12(3), 188–192. 10.1089/chi.2016.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, J. A. , Dharmage, S. C. , Allen, K. , Koplin, J. , Garcia‐Larsen, V. , Boyle, R. , Waidyatillake, N. , & Lodge, C. J. (2019). Age at introduction to complementary solid food and food allergy and sensitization: A systematic review and meta‐analysis. Clinical and Experimental Allergy, 49(6), 754–769. 10.1111/cea.13383 [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention Division of Nutrition Physical Activity and Obesity . (2019a). A SAS program for the 2000 CDC growth charts (ages 0 to <20 years). https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- Center for Disease Control and Prevention Division of Nutrition Physical Activity and Obesity . (2019b). A SAS program for the WHO growth charts (ages 0 to <2 years). https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas-who.htm
- Cheikh Ismail, L. , Al Dhaheri, A. S. , Ibrahim, S. , Ali, H. I. , Chokor, F. , O'Neill, L. M. , Mohamad, M. N. , Kassis, A. , Ayesh, W. , Kharroubi, S. , & Hwalla, N. (2022). Nutritional status and adequacy of feeding practices in infants and toddlers 0‐23.9 months living in the United Arab Emirates (UAE): Findings from the feeding Infants and Toddlers Study (FITS) 2020. BMC Public Health, 22(1), 319. 10.1186/s12889-022-12616-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closa‐Monasterolo, R. , Ferré, N. , Luque, V. , Zaragoza‐Jordana, M. , Grote, V. , Weber, M. , Koletzko, B. , Socha, P. , Gruszfeld, D. , Janas, R. , Xhonneux, A. , Dain, E. , Scaglioni, S. , Escribano, J. , & Childhood Obesity Project Study, G . (2011). Sex differences in the endocrine system in response to protein intake early in life. American Journal of Clinical Nutrition, 94(6 Suppl), 1920s–1927s. 10.3945/ajcn.110.001123 [DOI] [PubMed] [Google Scholar]
- Drewnowski, A. (2010). The nutrient rich foods index helps to identify healthy, affordable foods. American Journal of Clinical Nutrition, 91(4), 1095S–1101S. 10.3945/ajcn.2010.28450D [DOI] [PubMed] [Google Scholar]
- English, L. K. , Obbagy, J. E. , Wong, Y. P. , Butte, N. F. , Dewey, K. G. , Fox, M. K. , Greer, F. R. , Krebs, N. F. , Scanlon, K. S. , & Stoody, E. E. (2019). Timing of introduction of complementary foods and beverages and growth, size, and body composition: A systematic review. American Journal of Clinical Nutrition, 109(Suppl_7), 935s–955s. 10.1093/ajcn/nqy267 [DOI] [PubMed] [Google Scholar]
- Fein, S. B. , Labiner‐Wolfe, J. , Shealy, K. R. , Li, R. , Chen, J. , & Grummer‐Strawn, L. M. (2008). Infant Feeding Practices Study II: Study methods. Pediatrics, 122(Suppl 2), S28–S35. 10.1542/peds.2008-1315c [DOI] [PubMed] [Google Scholar]
- Glynn, R. J. , Laird, N. M. , & Rubin, D. B. (1993). Multiple imputation in mixture models for nonignorable nonresponse with follow‐ups. Journal of the American Statistical Association, 88, 984–993. [Google Scholar]
- Gray, J. (2019). Egg consumption in pregnancy and infancy: Advice has changed. Journal of Health Visiting, 7(2), 68–77. 10.12968/johv.2019.7.2.68 [DOI] [Google Scholar]
- Grummer‐Strawn, L. M. , Li, R. , Perrine, C. G. , Scanlon, K. S. , & Fein, S. B. (2014). Infant feeding and long‐term outcomes: Results from the year 6 follow‐up of children in the Infant Feeding Practices Study II. Pediatrics, 134(Suppl 1), S1–S3. 10.1542/peds.2014-0646B [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummer‐Strawn, L. M. , Reinold, C. , & Krebs, N. F. (2010). Use of World Health Organization and CDC growth charts for children aged 0‐59 months in the United States. MMWR: Recommendations and Reports, 59(RR‐9), 1–15. [PubMed] [Google Scholar]
- Harding, J. E. , Cormack, B. E. , Alexander, T. , Alsweiler, J. M. , & Bloomfield, F. H. (2017). Advances in nutrition of the newborn infant. Lancet, 389(10079), 1660–1668. 10.1016/S0140-6736(17)30552-4 [DOI] [PubMed] [Google Scholar]
- Iannotti, L. L. , Chapnick, M. , Nicholas, J. , Gallegos‐Riofrio, C. A. , Moreno, P. , Douglas, K. , Habif, D. , Cui, Y. , Stewart, C. , Lutter, C. K. , & Waters, W. F. (2020). Egg intervention effect on linear growth no longer present after two years. Maternal & Child Nutrition, 16(2), e12925. 10.1111/mcn.12925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti, L. L. , Lutter, C. K. , Bunn, D. A. , & Stewart, C. P. (2014). Eggs: The uncracked potential for improving maternal and young child nutrition among the world's poor. Nutrition Reviews, 72(6), 355–368. 10.1111/nure.12107 [DOI] [PubMed] [Google Scholar]
- Iannotti, L. L. , Lutter, C. K. , Stewart, C. P. , Gallegos Riofrío, C. A. , Malo, C. , Reinhart, G. , Palacios, A. , Karp, C. , Chapnick, M. , Cox, K. , & Waters, W. F. (2017). Eggs in early complementary feeding and child growth: A randomized controlled trial. Pediatrics, 140(1), e20163459. 10.1542/peds.2016-3459 [DOI] [PubMed] [Google Scholar]
- Iannotti, L. L. , Lutter, C. K. , Waters, W. F. , Gallegos Riofrío, C. A. , Malo, C. , Reinhart, G. , Palacios, A. , Karp, C. , Chapnick, M. , Cox, K. , Aguirre, S. , Narvaez, L. , López, F. , Sidhu, R. , Kell, P. , Jiang, X. , Fujiwara, H. , Ory, D. S. , Young, R. , & Stewart, C. P. (2017). Eggs early in complementary feeding increase choline pathway biomarkers and DHA: A randomized controlled trial in Ecuador. The American Journal of Clinical Nutrition, 106(6), 1482–1489. 10.3945/ajcn.117.160515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriart, C. , Boursaw, B. , Rodrigues, G. P. , & Handal, A. J. (2013). Obesity and malnutrition among hispanic children in the United States: Double burden on health inequities. Revista Panamericana de Salud Pública, 34(4), 235–243. [PubMed] [Google Scholar]
- Jakobsen, J. C. , Gluud, C. , Wetterslev, J. , & Winkel, P. (2017). When and how should multiple imputation be used for handling missing data in randomised clinical trials—A practical guide with flowcharts. BMC Medical Research Methodology, 17(1), 162. 10.1186/s12874-017-0442-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoa, B. J. , Zabut, B. M. , & Hamed, A. T. (2011). Nutritional status compared with nutritional history of preschool aged children in gaza strip: Cross sectional study. Pakistan Journal of Nutrition, 10(3), 282–290. 10.3923/pjn.2011.282.290 [DOI] [Google Scholar]
- Kim, H. , Caulfield, L. E. , Rebholz, C. M. , Ramsing, R. , & Nachman, K. E. (2020). Trends in types of protein in US adolescents and children: Results from The National Health and Nutrition Examination Survey 1999‐2010. PLoS One, 15(3), e0230686. 10.1371/journal.pone.0230686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley‐Evans, S. C. (2015). Nutrition in early life and the programming of adult disease: A review. Journal of Human Nutrition & Dietetics, 28(Suppl 1), 1–14. 10.1111/jhn.12212 [DOI] [PubMed] [Google Scholar]
- Lutter, C. K. , Iannotti, L. L. , & Stewart, C. P. (2018). The potential of a simple egg to improve maternal and child nutrition. Maternal & Child Nutrition, 14(Suppl 3), e12678. 10.1111/mcn.12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKune, S. L. , Stark, H. , Sapp, A. C. , Yang, Y. , Slanzi, C. M. , Moore, E. V. , Omer, A. , & Wereme N'Diaye, A. (2020). Behavior change, egg consumption, and child nutrition: A cluster randomized controlled trial. Pediatrics, 146(6), e2020007930. 10.1542/peds.2020-007930 [DOI] [PubMed] [Google Scholar]
- Miller, L. C. , Neupane, S. , Joshi, N. , & Lohani, M. (2020). MILK symposium review: Milk consumption is associated with better height and weight in rural nepali children over 60 months of age and better head circumference in children 24 to 60 months of age. Journal of Dairy Science, 103(11), 9700–9714. 10.3168/jds.2020-18289 [DOI] [PubMed] [Google Scholar]
- Nachvak, S. M. , Sadeghi, O. , Moradi, S. , Esmailzadeh, A. , & Mostafai, R. (2020). Food groups intake in relation to stunting among exceptional children. BMC Pediatrics, 20(1), 394. 10.1186/s12887-020-02291-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou, Y. , & Fulgoni, V. L., 3rd (2018). Egg consumption in infants is associated with longer recumbent length and greater intake of several nutrients essential in growth and development. Nutrients, 10(6), 719. 10.3390/nu10060719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou, Y. , & Fulgoni, V. L., 3rd (2019). Egg consumption in U.S. children is associated with greater daily nutrient intakes, including protein, lutein + zeaxanthin, choline, α‐linolenic acid, and docosahexanoic acid. Nutrients, 11(5), 1137. 10.3390/nu11051137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou, Y. , & Fulgoni, V. L., 3rd (2020). Eggs are cost‐efficient in delivering several shortfall nutrients in the American diet: A cost‐analysis in children and adults. Nutrients, 12(8), 2406. 10.3390/nu12082406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanikolaou, Y. , & Fulgoni, V. L. 3rd (2021). Increasing egg consumption at breakfast is associated with increased usual nutrient intakes: A modeling analysis using NHANES and the USDA child and adult care food program school breakfast guidelines. Nutrients, 13(4), 1379. 10.3390/nu13041379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpin, L. , Jebb, S. , Johnson, L. , Wardle, J. , & Ambrosini, G. L. (2016). Dietary protein intake is associated with body mass index and weight up to 5 y of age in a prospective cohort of twins. American Journal of Clinical Nutrition, 103(2), 389–397. 10.3945/ajcn.115.118612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado, E. L. , Maleta, K. , Caswell, B. L. , George, M. , Oakes, L. M. , DeBolt, M. C. , Bragg, M. G. , Arnold, C. D. , Iannotti, L. L. , Lutter, C. K. , & Stewart, C. P. (2020). Early child development outcomes of a randomized trial providing 1 egg per day to children age 6 to 15 months in Malawi. Journal of Nutrition, 150(7), 1933–1942. 10.1093/jn/nxaa088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinbott, A. , & Jordan, I. (2016). Determinants of child malnutrition and infant and young child feeding approaches in Cambodia. World Review of Nutrition and Dietetics, 115, 61–67. 10.1159/000444609 [DOI] [PubMed] [Google Scholar]
- Schack‐Nielsen, L. , Sorensen, T. , Mortensen, E. L. , & Michaelsen, K. F. (2010). Late introduction of complementary feeding, rather than duration of breastfeeding, may protect against adult overweight. American Journal of Clinical Nutrition, 91(3), 619–627. 10.3945/ajcn.2008.27078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba, R. D. , Shardell, M. , Sakr Ashour, F. A. , Moaddel, R. , Trehan, I. , Maleta, K. M. , Ordiz, M. I. , Kraemer, K. , Khadeer, M. A. , Ferrucci, L. , & Manary, M. J. (2016). Child stunting is associated with low circulating essential amino acids. EBioMedicine, 6, 246–252. 10.1016/j.ebiom.2016.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, C. P. , Caswell, B. , Iannotti, L. , Lutter, C. , Arnold, C. D. , Chipatala, R. , Prado, E. L. , & Maleta, K. (2019). The effect of eggs on early child growth in rural Malawi: The Mazira Project randomized controlled trial. American Journal of Clinical Nutrition, 110(4), 1026–1033. 10.1093/ajcn/nqz163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand, T. A. , Taneja, S. , Kumar, T. , Manger, M. S. , Refsum, H. , Yajnik, C. S. , & Bhandari, N. (2015). Vitamin B‐12, folic acid, and growth in 6‐ to 30‐month‐old children: A randomized controlled trial. Pediatrics, 135(4), e918–e926. 10.1542/peds.2014-1848 [DOI] [PubMed] [Google Scholar]
- Strand, T. A. , Ulak, M. , Kvestad, I. , Henjum, S. , Ulvik, A. , Shrestha, M. , Thorne‐Lyman, A. L. , Ueland, P. M. , Shrestha, P. S. , & Chandyo, R. K. (2018). Maternal and infant vitamin B12 status during infancy predict linear growth at 5 years. Pediatric Research, 84(5), 611–618. 10.1038/s41390-018-0072-2 [DOI] [PubMed] [Google Scholar]
- Textor, J. , Hardt, J. , & Knüppel, S. (2011). DAGitty: A graphical tool for analyzing causal diagrams. Epidemiology, 22(5), 745. 10.1097/EDE.0b013e318225c2be [DOI] [PubMed] [Google Scholar]
- Textor, J. , van der Zander, B. , Gilthorpe, M. S. , Liśkiewicz, M. , & Ellison, G. T. (2017). Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. International Journal of Epidemiology, 45(6), 1887–1894. 10.1093/ije/dyw341 [DOI] [PubMed] [Google Scholar]
- Thone‐Reineke, C. , Kalk, P. , Dorn, M. , Klaus, S. , Simon, K. , Pfab, T. , Godes, M. , Persson, P. , Unger, T. , & Hocher, B. (2006). High‐protein nutrition during pregnancy and lactation programs blood pressure, food efficiency, and body weight of the offspring in a sex‐dependent manner. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 291(4), R1025–R1030. 10.1152/ajpregu.00898.2005 [DOI] [PubMed] [Google Scholar]
- UNICEF . (2021). Malnutrition in children. https://data.unicef.org/topic/nutrition/malnutrition/
- van Buuren, S. , Boshuizen, H. C. , & Knook, D. L. (1999). Multiple imputation of missing blood pressure covariates in survival analysis. Statistics in Medicine, 18(6), 681–694. [DOI] [PubMed] [Google Scholar]
- Wang, S. , Mei, Y. , Ma, Z. H. , Zhao, W. H. , Tang, X. J. , Pang, X. H. , Zhang, Q. , Li, R. L. , Wang, Y. Y. , & Xu, T. (2021). The patterns of complementary feeding and growth among 12 to 23 Month‐Old children in China. Biomedical and Environmental Sciences, 34(11), 847–858. 10.3967/bes2021.118 [DOI] [PubMed] [Google Scholar]
- Weigle, D. S. , Breen, P. A. , Matthys, C. C. , Callahan, H. S. , Meeuws, K. E. , Burden, V. R. , & Purnell, J. Q. (2005). A high‐protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. American Journal of Clinical Nutrition, 82(1), 41–48. 10.1093/ajcn.82.1.41 [DOI] [PubMed] [Google Scholar]
- Wen, X. , Kong, K. L. , Eiden, R. D. , Sharma, N. N. , & Xie, C. (2014). Sociodemographic differences and infant dietary patterns. Pediatrics, 134(5), e1387–e1398. 10.1542/peds.2014-1045 [DOI] [PubMed] [Google Scholar]
- Westerterp‐Plantenga, M. S. , Lemmens, S. G. , & Westerterp, K. R. (2012). Dietary protein ‐ its role in satiety, energetics, weight loss and health. British Journal of Nutrition, 108(Suppl 2), S105–S112. 10.1017/S0007114512002589 [DOI] [PubMed] [Google Scholar]
- White, I. R. , Royston, P. , & Wood, A. M. (2011). Multiple imputation using chained equations: Issues and guidance for practice. Statistics in Medicine, 30(4), 377–399. 10.1002/sim.4067 [DOI] [PubMed] [Google Scholar]
- WHO multicenter growth reference study group . (2006). WHO child growth standards based on length/height, weight and age. Acta Paediatrica Supplement, 450, 76–85. [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2019). Nutrition Landscape Information System (NLiS) country profile indicators: Interpretation guide (2nd ed.). [Google Scholar]
- World Health Organization . (2020a). Malnutrition. https://www.who.int/news-room/q-a-detail/malnutrition
- World Health Organization . (2020b). Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- World Health Organization . (2021). Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
Data used in the manuscript, codebooks, and analytic codes will not be made available because the raw data was owned by the Center for Disease Control and Prevention (CDC). Researchers who are interested in using the data can contact the CDC directly.


