Abstract
Background
Transfusion complicates a significant proportion of births in the United States, and Black women have greater prevalence of transfusion at delivery than White women. Antepartum anaemia, a risk factor for peripartum transfusion, is more common among Black women than White women. We aimed to describe the racial distribution of antepartum anaemia in three national datasets and to evaluate the peripartum transfusion rate and characteristics of transfusion recipients, to investigate disparities in haemostatic outcomes.
Material and methods
We performed a retrospective analysis of Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network Cesarean Registry (CR), NICHD Consortium on Safe Labor Registry (CSL), and a cohort of deliveries at Universal Health Services hospitals (UHS). Univariable associations and multivariable logistic regressions were calculated between race, anaemia and transfusion. Covariates included age, parity, smoking, body mass index, type of insurance, and delivery mode.
Results
We included n=56,964 deliveries from CR (28% Black), 87,465 from UHS (12% Black), and 140,324 from CSL (24% Black). Anaemia prevalence was 8% in CR, 7% in UHS, and 13% in CSL. Anaemia was more common among Black patients (ORs 2.52, 2.61, and 1.48 respectively) and was associated with transfusion in all databases (ORs 6.46 [95% CI 5.78–7.22]; 5.79 [4.74–7.27]; 1.27 [1.18–1.37] respectively). After adjusting for covariates, Black patients had greater odds of transfusion than non-Black patients in CR (aOR 1.32 [1.16–1.50]), but not in UHS or CSL (aORs 1.19 [0.89–1.59] and 0.40 [0.36–0.44] respectively).
Discussion
In our retrospective cohort study using three US registries, we emphasized the link between anaemia and transfusion. Although anaemia was more prevalent among Black patients, the race-transfusion relationship differed between databases, indicating other unexplored factors are involved.
Key words: postpartum haemorrhage, anaemia, blood transfusion, maternal mortality, health equity
INTRODUCTION
Responsible for 10.7% of maternal deaths between 2014 and 2017, postpartum haemorrhage (PPH) is a leading cause of maternal morbidity and mortality in the United States1. Rates of peripartum blood transfusions have quadrupled in recent years, and transfusion affects approximately 1% of delivery hospitalizations2. In the US, non-Hispanic Black women consistently endure the highest burden of adverse pregnancy outcomes3. Black women are more likely to receive transfusions at delivery and have higher incidence of complications like disseminated intravascular coagulation than White women, even when controlling for comorbidities4,5. These trends are well documented, yet the precise mechanisms by which Black women experience greater morbidity and mortality have not been fully elucidated.
An elevated prevalence of antepartum anaemia among Black women may help explain their heightened rate of subsequent PPH. For pregnant and non-pregnant women alike, the prevalence of iron deficiency and anaemia tends to be higher among women of colour than among White women and has been demonstrated to be greatest among Black women6,7. Further, anaemia in pregnancy is associated with increased maternal morbidity during delivery. Women with antepartum anaemia are more likely to receive peripartum blood transfusions8,9, and antepartum anaemia has been associated with greater likelihood of caesarean delivery10.
Our objective was to describe the racial distribution of anaemia in pregnancy across three national datasets. Secondarily, we sought to determine the rate of peripartum transfusion and characteristics of those receiving transfusion in these large cohorts, to assess disparities between Black and non-Black obstetric patients.
MATERIAL AND METHODS
This protocol was not determined to meet the criteria for human subjects research and was consequently granted a waiver from further institutional review board evaluation on April 21, 2020 by the George Washington University Office of Human Research.
We conducted a retrospective cohort analysis of three de-identified registries composed of medical record data collected for individual deliveries. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network Cesarean Registry (CR) was an observational study of women who underwent caesarean section (primary or repeat) or trial of labour after caesarean section (TOLAC) between January 1999 and July 2002 at thirteen hospitals across the United States, and aimed to describe characteristics of patients attempting TOLAC and identify factors underlying complications of caesarean delivery11. The NICHD Consortium on Safe Labor (CSL) includes delivery records from twelve sites across all nine American College of Obstetricians and Gynecologists districts between July 2002 and July 2008. CSL sought to delineate the progression of labour in a nationwide population and help pinpoint the appropriate time to perform caesarean delivery in patients with labour protraction and arrest12. CR and CSL are publicly available, and while only CSL has been validated specifically, data from both registries have been extensively evaluated12–14.
We also included data extracted from the electronic medical records of women who delivered between January 2015 and June 2018 at twenty hospitals owned by Universal Health Services (UHS), a large national hospital management company in the United States. UHS data were extracted by a trained IT professional and have been included in other published analyses from our group15–17. By utilizing three datasets, we accumulated a large sample from a variety of hospital sizes and locations, in the hopes of boosting the generalizability of our findings and reducing potential bias in participant selection. Patients from these registries were eligible for inclusion in our analysis if they had complete data on race, anaemia status, and transfusion receipt, and this was how our sample size was determined.
All analyses were done in SAS version 9.4 first using listwise deletion of missing data based on the three primary variables of interest: anaemia, race and transfusion. In other words, any subject with missing data for any of these three primary variables were excluded. If a subject had missing data on a covariate (variables only used for adjustment purposes), this was coded as a categorical variable with a missing category. Then, in sensitivity analyses, we used multiple imputation, with the Markov chain Monte Carlo method with 25 iterations, and imputed all missing data in each data set separately using the mean imputed value for each subject, and redid all analyses.
We first examined univariable associations in each database between race, anaemia and transfusion. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated. We then used multivariable logistic regression to examine the association of race with transfusion, independent of anaemia, and of anaemia with transfusion, independent of race. Next, we examined the anaemia by race interaction, which tells us whether the association of anaemia with transfusion differs by race. Finally, we also included in the multivariable models additional potential covariates linked to higher risk of morbidity, including age, parity, smoking while pregnant, body mass index, type of insurance, and mode of delivery18. From these multivariable models, adjusted odds ratios (aORs) and adjusted confidence intervals (95% aCIs) were calculated. We analysed whether the race effect differed significantly across databases using a multivariable logistic regression model including main effects of race and database, as well as the database by race interaction. We also did the same with anaemia.
Following these initial analyses, we stratified patients by type of delivery and calculated adjusted odds ratios and confidence intervals for transfusion, in order to identify any potential interaction of delivery mode with the relationship between race and transfusion.
In all three datasets, patients’ racial identification was determined through medical chart review by trained research personnel. For the purpose of our analyses, a patient was considered “non-Black” if their race was reported as anything other than Black.
We defined anaemia as haematocrit <30% on admission for patients from the CR and UHS registries. Importantly, the CSL database reported only a patient’s anaemia status and did not include haemoglobin or haematocrit values. For these patients, we used their anaemia diagnosis as documented in the registry for our analysis.
RESULTS
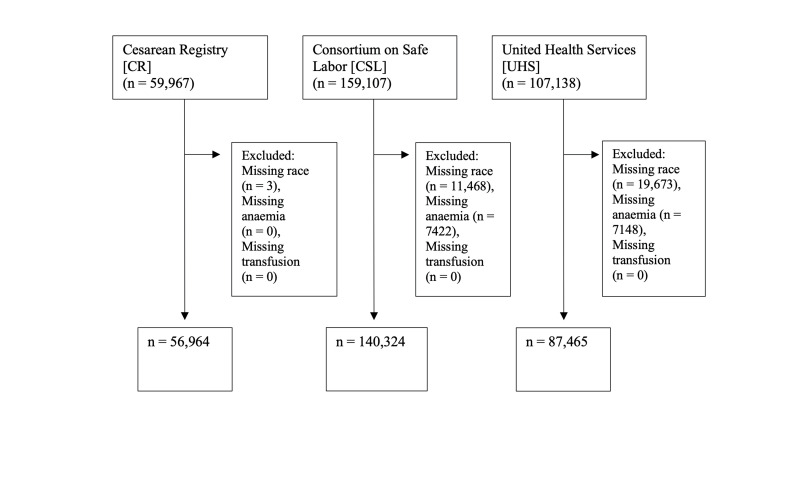
At the outset, there were 59,967 patients in CR, 107,138 in UHS, and 159,107 in CSL (Figure 1). Three patients in CR, 19,673 in UHS, and 11,468 in CSL were excluded because of missing race identification. An additional 7,148 patients in UHS and 7,422 patients in CSL were excluded because of absent anaemia status.
Figure 1.
Inclusion criteria for analysis
Our analysis included 56,964 in CR (41% primary caesarean, 51% scheduled repeat caesarean, 8% repeat caesarean after TOLAC), 87,465 in UHS (36% caesarean, 1% vaginal birth after caesarean [VBAC], 64% spontaneous vaginal), and 140,324 in CSL (65% spontaneous vaginal, 5% vaginal assisted, 3% VBAC, 17% primary caesarean, 10% repeat caesarean) (Table I). CR had the highest proportion of patients identifying as Black at 28%, versus 12% and 24% in UHS and CSL respectively. The prevalence of anaemia was highest in CSL at 13%, while it was 8% and 7% in CR and UHS respectively. CSL had the largest absolute number of transfusions at 5,506 (3.9% of deliveries), whereas CSL had 1,488 (2.6%) and UHS had 346 (0.4%).
Table I.
Patient characteristics by database
| Patient variable | UHS (n=87,465) | CSL (n=140,324) | CR (n=56,964) |
|---|---|---|---|
|
| |||
| Age | 28 ± 6 | 27 ± 6 | 28 ± 6 |
|
| |||
| Body mass index (kg/m 2 ) | 32 ± 6 | 26 ± 6 | 33 ± 7 |
|
| |||
| Race | |||
| Black | 10,218 (12%) | 33,466 (24%) | 115,845 (28%) |
| White | 45,620 (52%) | 79,934 (57%) | 22,840 (40%) |
| Hispanic | 21,074 (15%) | 15,401 (27%) | |
| Other* | 31,627 (36%) | 5,850 (4%) | 1878 (5%) |
|
| |||
| Anaemia | 5812 (7%) | 18,153 (13%) | 4,751 (8%) |
|
| |||
| Parity | |||
| 0 | 13,657 (17%) | 60,072 (43%) | 15,927 (28%) |
| 1 | 42,768 (52%) | 39,017 (28%) | 21,863 (39%) |
| 2 | 12,171 (15%) | 22,473 (16%) | 11,637 (21%) |
| 3 or more | 13,201 (16%) | 18,762 (13%) | 7,214 (13%) |
|
| |||
| Smoking while pregnant | 11,985 (14%) | 9016 (6%) | 7723 (14%) |
|
| |||
| Insurance | |||
| Private | 35,840 (41%) | 89,840 (64%) | 24,814 (44%) |
| Public | 23,663 (27%) | 47,186 (34%) | 21,455 (38%) |
| Self | 2,241 (3%) | 1,808 (1%) | 10,680 (19%) |
| Other / Unknown | 25,621 (29%) | 1,490 (1%) | 0 |
|
| |||
| Delivery mode | |||
| Vaginal | 55,677 (64%) | 90,887 (65%) | 0 |
| Vaginal assisted | 7,561 (5%) | 0 | |
| CS† | 21,197 (36%) | 23,807 (17%) | 23,315 (41%) |
| Repeat CS | 14,383 (10%) | 28,854 (51%) | |
| VBAC‡ | 591 (1%) | 3,686 (3%) | (failed) 4,795 (8%) |
|
| |||
| Transfusion | 346 (0.4%) | 5,506 (3.9%) | 1,488 (2.6%) |
Data are mean ± standard deviation or n (%).
The category "Other" includes individuals who identified as neither Black nor White.
CS: Caesarean section.
VBAC: Vaginal birth after caesarean section.
Univariable associations showed Black patients were more likely to have anaemia than non-Black patients in all three registries (CR OR 2.52 [95% CI 2.37–2.68], UHS OR 2.61 [2.45–2.79], CSL OR 1.48 [1.43–1.53]) (Table II). Anaemia was significantly associated with transfusion in all three databases, and the association was strongest in CR and UHS (ORs 6.46 [5.78–7.22] and 5.79 [4.74–7.27] respectively). Black patients were more likely to receive blood transfusions than non-Black patients in CR and UHS (ORs 1.83 [1.65–2.03] and 1.62 [1.23–2.14] respectively), though the association of race with transfusion was weaker than that of anaemia. In CSL, Black race was protective against transfusion (OR 0.44 [0.41–0.49]) (Table II).
Table II.
Univariable associations of anaemia with transfusion, of race with transfusion, and of race with anaemia, with listwise deletion for missing data
| Database | Anaemia | n | Transfusion | OR (95% CI) | p |
|---|---|---|---|---|---|
|
| |||||
| UHS | Yes | 5,812 | 100 (1.7%) | 5.79 (4.58–7.32) | <0.0001 |
| No | 81,653 | 246 (0.3%) | |||
|
| |||||
| Black | |||||
|
| |||||
| Yes | 10,218 | 61 (0.6%) | 1.62 (1.23–2.14) | 0.001 | |
| No | 77,247 | 285 (0.4%) | |||
|
| |||||
| Black | Anaemia | ||||
|
| |||||
| Yes | 10,218 | 1,397 (13.7%) | 2.61 (2.45–2.79) | <0.0001 | |
| No | 77,247 | 4415 (5.7%) | |||
|
| |||||
| CSL | Anaemia | n | Transfusion | OR (95% CI) | p |
|
| |||||
| Yes | 18,153 | 868 (4.8%) | 1.27 (1.18–1.37) | <0.0001 | |
| No | 122,171 | 4,638 (3.8%) | |||
|
| |||||
| Black | |||||
|
| |||||
| Yes | 33,466 | 694 (2.1%) | 0.44 (0.41–0.49) | <0.0001 | |
| No | 106,858 | 4,812 (4.5%) | |||
|
| |||||
| Black | Anaemia | ||||
|
| |||||
| Yes | 33,466 | 5,524 (16.5%) | 1.48 (1.43–1.53) | <0.0001 | |
| No | 106,858 | 12,629 (11.8%) | |||
|
| |||||
| CR | Anaemia | n | Transfusion | OR (95% CI) | p |
|
| |||||
| Yes | 4,751 | 518 (10.9%) | 6.46 (5.78–7.22) | <0.0001 | |
| No | 52,213 | 970 (1.9%) | |||
|
| |||||
| Black | |||||
|
| |||||
| Yes | 15,845 | 609 (3.8%) | 1.83 (1.65–2.03) | <0.0001 | |
| No | 41,119 | 879 (2.1%) | |||
|
| |||||
| Black | Anaemia | ||||
|
| |||||
| Yes | 15,845 | 2,236 (14.1%) | 2.52 (2.37–2.68) | <0.0001 | |
| No | 41,119 | 2,515 (6.1%) | |||
Transfusion and anaemia data are n (%).
In multivariable analyses, controlling for race slightly weakened the association between anaemia and transfusion in CR and UHS (aORs 6.00 [95% aCI 5.35–6.72] and 5.61 [4.42–7.11] respectively), and strengthened it in CSL (aOR 1.34 [1.24–1.44]), though all remained significant (Table III, Model 1). Adjusting for anaemia reduced the association between Black race and transfusion in CR, and made the association non-significant in UHS. In CSL, Black race remained protective against transfusion when anaemia was adjusted for (aOR 0.44 [0.41–0.48]). The interaction of Black race and anaemia on transfusion was significant in CR and CSL (p’s <0.01), but not in UHS (Table III, Model 2). Further investigation of the race and anaemia interaction on transfusion revealed in all three datasets that the race and transfusion association was most robust in the absence of anaemia, although as before the strength of the race-transfusion relationship varied between races and among datasets (Online Supplementary Content, Table SI). Additionally, anaemia was associated with transfusion in patients of all races, but the relative strength of the anaemia-transfusion relationship for Black patients as compared to non-Black patients varied between datasets (Online Supplementary Content, Table SII). After adjusting for additional covariates, the association between anaemia and transfusion remained strong in all three datasets (CR aOR 6.00 [5.35–6.72], UHS aOR 5.61 [4.42–7.11], CSL aOR 1.34 [1.24–1.44]) (Table III, Model 3). As before, Black race was associated with transfusion in CR (aOR 1.32 [1.16–1.50]), but was protective against transfusion in CSL (aOR 0.40 [0.36–0.44]). In UHS, the association between Black race and transfusion remained non-significant. In CSL, anemia was a stronger predictor of transfusion in Black patients than non-Black patients (ORs 2.24 [1.90–2.64] and 1.19 [1.10–1.30] respectively) (Table IV). This pattern was not detected in CR or UHS, where non-Black patients had a stronger association of anemia with transfusion.
Table III.
Association between anaemia and transfusion adjusted for race, and between race and transfusion adjusted for anaemia
| Database | Transfusion incidence | Predictor | Model 1* | Model 2† | Model 3‡ | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Adjusted OR (95% CI) | p | Interaction p | Adjusted OR (95% CI) | p | |||
|
| |||||||
| UHS | 346 (0.4%) | Anaemia | 5.61 (4.42–7.11) | <0.0001 | 0.06 | 4.99 (3.89–6.39) | <0.0001 |
| Black | 1.26 (0.95–1.67) | 0.11 | 1.19 (0.89–1.59) | 0.24 | |||
|
| |||||||
| CSL | 5,506 (3.9%) | Anaemia | 1.34 (1.24–1.44) | <0.0001 | <0.0001 | 1.28 (1.17–1.40) | <0.0001 |
| Black | 0.44 (0.41–0.48) | <0.0001 | 0.40 (0.36–0.44) | <0.0001 | |||
|
| |||||||
| CR | 1,488 (2.6%) | Anaemia | 6.00 (5.35–6.72) | <0.0001 | 0.007 | 5.45 (4.81–6.18) | <0.0001 |
| Black | 1.43 (1.29–1.60) | <0.0001 | 1.32 (1.16–1.50) | <0.0001 | |||
Adjusted for anaemia or race.
p-value for interaction of Black race and anaemia on transfusion.
Adjusted for age, parity, insurance, smoking during pregnancy, BMI, mode of delivery.
Table IV.
Association between anemia and transfusion, stratified by race
| Database | Race | OR for transfusion for anaemic patients (95% CI) | p |
|---|---|---|---|
|
| |||
| UHS | Black | 3.60 (2.13–6.10) | <0.0001 |
| Non-Black | 6.31 (4.86–8.20) | <0.0001 | |
|
| |||
| CSL | Black | 2.24 (1.90–2.64) | <0.0001 |
| Non-Black | 1.19 (1.10–1.30) | <0.0001 | |
|
| |||
| CR | Black | 5.07 (4.29–6.00) | <0.0001 |
| Non-Black | 6.91 (5.94–8.04) | <0.0001 | |
In a logistic regression model using database and race to predict transfusion, both race (p=0.0006) and database (p<0.0001) had significant main effects, and the database by race interaction was significant (p<0.0001). In other words, across databases, with total n of 284,753, after adjusting for database as a covariate, non-Black patients had significantly higher risk of peripartum transfusion (aOR 1.41 [1.33–1.49], p<0.0001). However, the race effect differed significantly across databases, such that the higher risk for transfusion among non-Black patients only occurred in the CSL database (CSL aOR 2.23 [2.05–2.41], CR aOR 0.55 [0.49–0.61], UHS aOR 0.62 [0.47–0.81]). After adjusting for additional covariates (BMI, smoking, anemia, mode of delivery, parity, insurance), the overall pattern remained the same. The OR for non-Black patients was 1.43 ([1.33–1.54], p<0.0001) across databases, and the database effects and interaction also remained highly significant. The adjusted OR for transfusion in non-Black patients in CSL was 2.74 [2.46–3.05], while in CR it was 0.54 [0.48–0.61], and in UHS it was 0.70 [0.53–0.92]. In a model including anaemia, race, and database, anaemia had a significant independent association with transfusion (aOR 2.06 [1.94–2.18], p<0.0001) after adjusting for database and race. However, the effect of anaemia differed significantly by database (p<0.0001). Anaemia was only weakly associated with transfusion in CSL (aOR 1.31 [1.22–1.41], and was a stronger predictor in CR and UHS (aORs 7.07 [6.32–7.90] and 6.09 [4.82–7.70] respectively). When stratifying by mode of delivery, Black patients had significantly lower odds of transfusion for vaginal, vaginal-assisted, VBAC, and caesarean deliveries (primary and repeat) in CSL (Online Supplementary Content, Table SIII). UHS showed no significant association between race and transfusion for any mode of delivery. However, Black patients in CR delivering by repeat caesarean had significantly greater odds of transfusion compared to non-Black patients (aOR 1.49 [1.22–1.83]).
After imputing for missing data, sensitivity analyses included 56,964 in CR, 159,107 (11.81% imputed) in CSL, and 107,138 (18.36% imputed) in UHS (Online Supplementary Content, Table SIV). Sensitivity analyses of imputed data adjusted for covariates revealed Black race was significantly more strongly associated with transfusion than non-Black race in CR (aOR1.37 [1.21–1.55]). Conversely, in CSL, Black race remained protective against transfusion (aOR 0.48 [0.44–0.52]). In UHS, Black patients tended to have greater odds of transfusion than non-Black patients but the association was ultimately nonsignificant (aOR 1.31 [1.00–1.72]).
Sensitivity analyses confirmed that Black patients in CSL had lower odds of transfusion for all modes of delivery compared to non-Black patients (Online Supplementary Content, Table SV). Notably, Black patients in CR delivering by both primary and repeat caesarean had significantly higher odds of transfusion than non-Black patients (aORs 1.27 [1.07–1.50; p=0.007] and 1.51 [1.25–1.83; p<0.0001] respectively). The effect of Black race on odds of transfusion remained non-significant in UHS when focusing on mode of delivery.
DISCUSSION
Anaemia was associated with peripartum transfusion in all three databases, reinforcing the relationship between antepartum anaemia and transfusion described elsewhere8–10. Interestingly, the strength of anaemia’s effect differed between datasets when adjusting for race and database as covariates, such that in CSL this association was substantially weaker than in CR or UHS. Across all registries, Black patients were more likely to have anaemia than non-Black patients, consistent with prior investigations, though the association of transfusion with race was weaker than that of transfusion with anaemia. In CR, Black patients had greater odds of transfusion than non-Black patients, while in CSL, non-Black patients were more likely to receive transfusions. However, anemia was a stronger predictor of transfusion for Black patients in CSL than for non-Black patients. When combining the populations of all three databases and adjusting for database as a covariate, non-Black patients had greater odds of transfusion than Black patients. However, this was likely due to the larger n of the CSL dataset (146% larger than CR, 60% larger than UHS), which caused its effect to predominate over that of the other two datasets. The interaction of Black race and anaemia on transfusion was significant in both CR and CSL, indicating that the association of transfusion and anaemia varies by race. Subsequently, we attempted to discern any interaction between delivery mode and the race-transfusion relationship by analysing outcomes by mode of delivery. However, we observed no consistent trend across datasets that clarified the patterns of transfusion receipt we had initially detected.
It is unclear why transfusion trends differ drastically between registries. This discrepancy raises questions about protocols for care provided to women experiencing significant blood loss at delivery. Without institutional standards of practice to guide clinical decision-making, patients of different racial and ethnic groups are provided different obstetric interventions, ostensibly contributing to observed disparities in pregnancy outcomes19. Black women are more likely to undergo caesarean section than women of other races, which may put them at higher risk of transfusion20–21. Patients such as Jehovah’s Witnesses may have personal reasons for refusing blood transfusions, potentially raising their risk for poor outcomes22–23. For these patients, it is vital to understand each individual’s preferences for or against certain blood products, and to discuss alternative approaches to preventing consequences of haemorrhage24.
Potential factors underlying the comparatively high burden of anaemia among Black patients in our sample relative to non-Black patients deserve additional consideration. Although certain anaemias, such as sickle cell disease, more frequently affect Black individuals, an elevated prevalence of iron deficiency anaemia has also been described among Black Americans6,7. The latter trend may be related to higher rates of food insecurity and resulting malnutrition in marginalized communities, as pregnant women with food insecurity are more likely to develop iron deficiency than those who are food secure25,26. Our analysis has several limitations. The CSL registry does not provide patients’ haemoglobin or haematocrit values, rather, it states whether or not a patient has anaemia. Without knowledge of how anaemia was defined for this cohort, we cannot rigorously compare our findings between datasets. CSL had nearly twice as many anaemic patients than the other registries, suggesting its threshold for anaemia may have differed. Further, the datasets did not enumerate causes of patients’ anaemia, preventing us from detecting associations between specific anaemia aetiologies and PPH, relationships that are clinically important to understand.
This study focused on understanding anaemia’s contribution to haemorrhage morbidity among Black mothers relative to mothers of other races, whom we called “non-Black”. Correspondingly, our results describe a trend in individuals of one race, compared to all others, a simplification that limits the generalizability of our findings. Race is an artificial designation and signifies more about one’s socio-political and environmental context than it does one’s biology. Nevertheless, it is important to disentangle mechanisms underlying the frequency of adverse outcomes affecting deliveries of Black women in the United States. In future investigations, it would be beneficial to understand the impact of antepartum anaemia on PPH in other populations with concerning incidence of maternal morbidity and mortality, such as American Indian and Alaska Native individuals.
We performed imputations to account for missing race, anaemia, and transfusion data within each registry. The UHS and CSL datasets required the most imputation, while CR needed very few. In both UHS and CSL, more non-Black patients had missing data than Black patients. The practice of multiple imputations relies on the assumption that missing data points occur at random. This assumption may not always be true, and consequently this is another limitation of our analysis. We have no reason to believe that race or haematocrit (or anaemia status in CSL) was systematically documented less frequently for a particular group within our sample, but if so, this would reduce the validity of our approach. Nevertheless, it is reassuring that sensitivity analyses with imputed data yielded similar trends as did the analyses restricted to subjects with complete data. Because the registries utilized demographic data extracted from patient medical records, it is not known whether patients’ racial and ethnic identification was obtained via self-report or through another method. Data gleaned from patient self-report would certainly be of stronger quality.
CONCLUSIONS
In this study, we emphasized the association between antepartum anaemia and peripartum transfusion and described the effects of race on this relationship in three large datasets. By adjusting for anaemia diagnosis upon admission, Black-White disparity in severe maternal morbidity has been shown to markedly diminish27. This highlights a valuable opportunity to reduce morbidity among vulnerable women. Successfully treating antepartum anaemia has the potential to mitigate risk of transfusion and haemorrhage in those who are most susceptible.
Supplementary Information
ACKNOWLEDGMENTS
Findings were presented in virtual poster format at the International Society on Thrombosis and Haemostasis Congress in Philadelphia, PA, USA July 2021.
Footnotes
AUTHORSHIP CONTRIBUTIONS
ED helped with the study design and writing of the manuscript. RA helped with the study design, analysis, and review of the manuscript. HA helped with the study concept, study design and writing of the manuscript.
The Authors declare no conflicts of interest.
FUNDING AND RESOURCES
This project was supported by National Heart Lung Blood Institute grant K23HL141640 to HA
REFERENCES
- 1.Centers for Disease Control and Prevention. Pregnancy Mortality Surveillance System. Centers for Disease Control and Prevention website; [Accessed on 2/01/2021.]. Available at: https://www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Freproductivehealth%2Fmaternalinfanthealth%2Fpregnancy-mortality-surveillance-system.htm#causes. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Rates in Severe Morbidity Indicators per 10,000 Delivery Hospitalizations 1993–2014. [Accessed on 2/01/2021.]. Available at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/smm/rates-severe-morbidity-indicator.htm.
- 3.Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008–2010. Am J Obstet Gynecol. 2014;210:435.e1–435.e8. doi: 10.1016/j.ajog.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Admon LK, Winkelman TNA, Zivin K, et al. Racial and ethnic disparities in the incidence of severe maternal morbidity in the United States, 2012–2015. Obstet Gynecol. 2018;132:1158–66. doi: 10.1097/AOG.0000000000002937. [DOI] [PubMed] [Google Scholar]
- 5.Gyamfi-Bannerman C, Srinivas SK, Wright JD, et al. Postpartum hemorrhage outcomes and race. Am J Obstet Gynecol. 2018;219:185.e1–185.e10. doi: 10.1016/j.ajog.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 6.Le CHH. The prevalence of anemia and moderate-severe anemia in the US population (NHANES 2003–2012) PLoS ONE. 2016;11:e0166635. doi: 10.1371/journal.pone.0166635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta PM, Hamner HC, Suchdev PS, et al. Iron status of toddlers, nonpregnant females, and pregnant females in the United States. Am J Clin Nutr. 2017;106:1640–6. doi: 10.3945/ajcn.117.155978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beckert RH, Baer RJ, Anderson JG, et al. Maternal anemia and pregnancy outcomes: a population-based study. J Perinatol. 2019;39:911–9. doi: 10.1038/s41372-019-0375-0. [DOI] [PubMed] [Google Scholar]
- 9.Petty K, Waters JH, Sakamoto SB, Yazer MH. Antenatal anemia increases the risk of receiving postpartum red blood cell transfusions although the overall risk of transfusion is low. Transfusion. 2018;58:360–5. doi: 10.1111/trf.14434. [DOI] [PubMed] [Google Scholar]
- 10.Smith C, Teng F, Branch E, et al. Maternal and perinatal morbidity and mortality associated with anemia in pregnancy. Obstet Gynecol. 2019;134:1234–44. doi: 10.1097/AOG.0000000000003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landon MB, Hauth JC, Leveno KJ, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. N Engl J Med. 2004;351:2581–9. doi: 10.1056/NEJMoa040405. [DOI] [PubMed] [Google Scholar]
- 12.National Institute of Health (NHI) EB Research: Consortium on Safe Labor (CSL) [Accessed on 16/02/2021.]. Available at: https://www.nichd.nih.gov/about/org/diphr/officebranch/eb/safe-labor.
- 13.Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326.e1–326.e10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eunice Kennedy Shriver - National Institute of Child Health and Human Development. Maternal-Fetal Medicine Units Network. Research Projects. [Accessed on 16/02/2021.]. Available at: https://mfmunetwork.bsc.gwu.edu/PublicBSC/MFMU/MFMUPublic/research-projects/
- 15.Ahmadzia HK, Hynds EB, Amdur RL, et al. National trends intranexamic acid use in the peripartum period, 2015–2019. J Thromb Thrombolysis. 2020;50:746–52. doi: 10.1007/s11239-020-02141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bade NA, Kazma JM, Amdur RL, et al. Blood type association with bleeding outcomes at delivery in a large multi-center study. J Thromb Thrombolysis. 2020;50:439–45. doi: 10.1007/s11239-019-02023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadzia HK, Phillips JM, Kleiman R, et al. Hemorrhage risk assessment on admission: utility for prediction of maternal morbidity. Am J Perinatol. 2021;38:1126–33. doi: 10.1055/s-0040-1710501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rukuni R, Bhattacharya S, Murphy MF, et al. Maternal and neonatal outcomes of antenatal anemia in a Scottish population: A retrospective cohort study. Acta Obstet Gynecol Scand. 2016;95:555–4. doi: 10.1111/aogs.12862. [DOI] [PubMed] [Google Scholar]
- 19.Grobman WA, Bailit JL, Rice MM, et al. Racial and ethnic disparities in maternal morbidity and obstetric care. Obstet Gynecol. 2015;125:1460–7. doi: 10.1097/AOG.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stark EL, Grobman WA, Miller ES. The association between maternal race and ethnicity and risk factors for primary cesarean delivery in nulliparous women. Am J Perinatol. 2021;38:350–6. doi: 10.1055/s-0039-1697587. [DOI] [PubMed] [Google Scholar]
- 21.Valdes EG. Examining cesarean delivery rates by race: a population-based analysis using the Robson ten-group classification system. J Racial Ethn Health Disparities. 2021;8:844–51. doi: 10.1007/s40615-020-00842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singla AK, Lapinski RH, Berkowitz RL, Saphier CJ. Are women who are Jehovah’s Witnesseses at risk of maternal death? Am J Obstet Gynecol. 2001;185:893–5. doi: 10.1067/mob.2001.117357. [DOI] [PubMed] [Google Scholar]
- 23.van Wolfswinkel ME, Zwart JJ, Schutte JM, et al. Maternal mortality and serious maternal morbidity in Jehovah’s witnesses in the Netherlands. BJOG. 2009;116:1103–10. doi: 10.1111/j.1471-0528.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 24.Husarova V, Donnelly G, Doolan A, et al. Preferences of Jehovah’s Witnesses regarding haematological supports in an obstetric setting: experience of a single university teaching hospital. Int J Obstet Anesth. 2016;25:53–7. doi: 10.1016/j.ijoa.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Park CY, Eicher-Miller HA. iron deficiency is associated with food insecurity in pregnant females in the United States: National Health and Nutrition Examination Survey 1999–2010. J Acad Nutr Diet. 2014;114:1967–73. doi: 10.1016/j.jand.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 26.Odoms-Young A, Bruce MA. Examining the impact of structural racism on food insecurity. Fam Community Health. 2018;41(Suppl 2):S3–S6. doi: 10.1097/FCH.0000000000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard SA, Main EK, Scott KA, et al. Racial and ethnic disparities in severe maternal morbidity prevalence and trends. Ann Epidemiol . 2019;33:30–6. doi: 10.1016/j.annepidem.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.