Highlights
-
•
Human laboratory models of drinking behavior provide an efficient mechanistic evaluation of a medication signal on drinking.
-
•
Existing alcohol self-administration models have yet to focus on important FDA-endpoints for clinical trial investigations.
-
•
We report on a novel alcohol self-administration paradigm modeling the ability to resist drinking and heavy drinking.
-
•
Results demonstrate that our model generates heavy drinking and is sensitive to stress effects on drinking.
Keywords: Alcohol self-administration, Human laboratory paradigm, Medication screening, Stress, Ability to resist, Heavy drinking
Abstract
Background
Human laboratory analogues of drinking behavior provide an efficient, cost-effective mechanistic evaluation of a medication signal on drinking. We developed a novel alcohol self-administration paradigm which models the ability to resist drinking and heavy drinking.
Methods
We compared a de-escalating schedule of monetary reinforcement (n=16, 50% female) to no schedule (n=16, 50% female) on the ability to resist drinking (i.e., latency to start drinking) and subsequent ad-libitum alcohol consumption of preferred alcoholic beverage in participants with alcohol use disorder (AUD). Participants completed two laboratory sessions designed to model the ability to resist drinking using stress (versus neutral imagery, within-subject factor) as a prime for drinking.
Results
Participants consumed more alcohol with no schedule (74.2%) versus with the de-escalating reinforcement schedule (40.3%,). The de-escalating schedule reduced alcohol consumption by 49%. Eighty-one percent of participants drank heavily with no schedule and this was reduced with the schedule. Use of the de-escalating schedule also increased the latency to pour and sip the first drink. Participants poured and sipped alcohol faster following stress imagery (vs. neutral), had greater craving, and consumed more alcohol in the first 30 minutes.
Conclusions
Our novel alcohol self-administration model generated heavy drinking. Over 80% of participants without reinforcement consumed more than 2/3 of their preferred alcoholic beverage designed to increase blood alcohol levels to 0.12 mg% within a 2-hour window. Our model was sensitive to stress, and the de-escalating schedule highlighted stress effects on drinking. Thus, this model is ideal for a cross-over design to test medications for AUD.
1. Introduction
Alcohol consumption is the third leading cause of preventable morbidity and mortality in the U.S. (Centers for Disease Control, 2022) and losses to the economy exceed $249 billion dollars per year (Sacks et al., 2015). Alcohol use disorder (AUD) is particularly problematic for the U.S., as we exceed the global per capita alcohol consumption by 50% (Shield et al., 2013) with AUD currently affecting 33 million adults (Grant et al., 2017). Developing medications to reduce the burden of AUD continues to be a high priority. However, the process of moving a compound from discovery to Food and Drug Administration (FDA) approval takes approximately 13 years at a cost of 1.8 billion dollars (Munos, 2009; Paul et al., 2010).
Use of human laboratory analogues of drinking behavior can provide an efficient, cost-effective mechanistic evaluation of a medication signal on drinking, with the result of facilitating translational work in medication development. Existing human laboratory models have primarily focused on cue reactivity, administering fixed doses of alcohol, and alcohol self-administration (Anton et al., 2004; Mason et al., 2008; McKee et al., 2009; Monti et al., 1999; O'Malley et al., 2002; Ramchandani et al., 2006). However, currently available models have yet to focus on important FDA-endpoints for clinical trial investigations. FDA approval for AUD is contingent on the percent of participants with no heavy drinking (FDA, 2006). For a medication to be effective, ideally it would increase the ability to resist drinking but should drinking commence, the medication would limit consumption to ‘light’ drinking. To this end, we have developed a novel alcohol self-administration paradigm which models the ability to resist drinking and heavy drinking.
To model the ‘ability to resist’ drinking, we have adapted procedures from our successful smoking lapse models (McKee 2009; McKee et al., 2006, 2010, 2012, 2015). The general procedure is that smokers are first exposed to known precipitants of smoking relapse behavior (e.g., nicotine deprivation, alcohol, stress, food). Following the prime, their preferred brand of cigarettes is placed in front of them with a lighter and an ashtray. Smokers are then instructed that they have the option to initiate a tobacco self-administration session or to delay initiation for up to 50 minutes in exchange for monetary reinforcement. A fixed level of monetary reinforcement is provided for each 5-minute increment that they can resist smoking during the 50-minute delay period. This delay period models their ability to resist smoking. Once participants ‘give in’ and decide to smoke, they then participate in a tobacco self-administration session, in which they can choose to smoke their preferred brand of cigarettes. Our smoking lapse models have demonstrated predictive validity with regards to smoking cessation medication effects (McKee et al., 2012, 2015), and have been utilized widely by the research community (Falcone et al., 2016; Jones et al., 2020; Leeman et al., 2010; Kahler et al., 2012, 2014; Oberleitner et al., 2018; Otto et al., 2020; Roberts et al., 2018; Roche et al., 2014; Schlagintweit et al., 2021; Verplaetse et al., 2017a, 2017b, 2018a, 2018b; Wilson et al., 2014).
For the current study, we decided to slightly modify the reinforcement schedule to a de-escalating versus a fixed monetary reinforcement schedule. Our goal was to model the impact of reducing motivation to abstain (by reducing the monetary reinforcement), making it increasingly harder to resist drinking. Current self-administration models (e.g., O'Malley et al., 2002) provide a fixed dose of alcohol to ‘prime’ continued drinking and as a result, do not provide an opportunity to abstain completely. We also wanted to generate heavy drinking in a laboratory setting. While existing alcohol self-administration models (e.g., Anton et al. 2004; O'Malley et al., 2002) provide alcohol sufficient to increase blood alcohol levels (BALs) to 0.08 g/dL, often drinking in the placebo group is minimal, resulting in floor effects when attempting to test for medication effects (Farokhnia et al., 2017; Haass-Koffler et al., 2018; Petrakis et al., 2006; Roberts et al., 2017; Udo et al., 2013; Verplaetse et al., 2016).
To generate heavy drinking, we implemented two important changes. The first was to provide alcohol sufficient to increase blood alcohol levels to 0.12 g/dL which is more in line with typical BALs reached by individuals with AUD (McKee & Verplaetse, 2015). The second change was to provide subjects with their preferred beverage. Existing paradigms use a standard or limited choice liquor/mixer beverage (Anton et al. 2004; O'Malley et al., 2002). It has been our experience that these standard beverages do not align with participants’ beverage preference, contributing to limited consumption behavior. While providing subjects with their preferred beverage will result in variability with regards to alcohol concentration and volume, it will increase the ecological validity of the paradigm by providing the exteroceptive and interoceptive cues associated with their preferred beverage. Given the variability regarding alcohol concentration and volume, this paradigm requires a within-subject comparison so that each subject is their own control. As such, it is ideal for a cross-over design to test medications.
For this initial model development investigation, we compared a single de-escalating schedule to no schedule in participants with AUD. As our research group has been focused on targeting stress for medication development (Peltier et al., 2019), the first model we developed examined the impact of stress as a prime for drinking. We predicted that we would generate heavy drinking in our paradigm, and that the de-escalating schedule would reduce drinking versus not having any monetary reinforcement to resist drinking. We also predicted that our model would be sensitive to stress effects on drinking, and that stress would decrease the ability to resist drinking and increase subsequent drinking.
2. Material and Methods
2.1. Participants
Participants were enrolled in two ongoing parent studies examining the effect of stress on drinking behavior. One parent study utilized a de-escalating schedule of reinforcement and the other did not use a reinforcement schedule. Both studies had identical eligibility criteria and laboratory procedures. Participants were eligible to enroll if they were 21-65 years of age and had current (past 6-months) Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5, American Psychiatric Association, 2013) moderate to severe AUD, with reported drinking of >14 drinks per week and >4 drinks per day at least twice per week for men and >7 drinks per week and >3 drinks per day at least twice per week in women. Participants met this drinking criteria during a consecutive 30-day period within the 90 days prior to intake. Participants were excluded if they met DSM-5 criteria for other primary psychiatric and substance use disorders (excluding nicotine dependence), used illicit drugs (except occasional cannabis use), had current suicidal or homicidal ideation, were pregnant or nursing, had medical conditions that contraindicated alcohol use (e.g., liver enzymes ≥3x normal), or were likely to exhibit clinically significant alcohol withdrawal (Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised [CIWA-R] ≥ 8) (Sullivan et al., 1989). Participants were recruited from the community through television, billboard, and web-based advertisements without regard to treatment seeking status. Thirty-two participants completed the study. After completion of this laboratory study, three subjects elected to complete a treatment phase as part of a parent study. As outlined in Roberts et al. (2021), it is ethical to study self-administration behavior in treatment seeking subjects who have a goal of reducing alcohol consumption (versus total abstinence).
2.2. Design
A between-subject design was used to compare a de-escalating schedule of reinforcement (n=16, 50% female) to no schedule (n=16, 50% female) on the ability to resist drinking and subsequent ad-libitum alcohol consumption. All participants (n=32) completed two laboratory sessions designed to model the ability to resist drinking using personalized imagery (stress vs. neutral, order counterbalanced) as a within-subject factor.
2.3. Procedures
2.3.1. Intake
All participants provided written informed consent. Procedures were in accordance with the ethical standards of the Yale School of Medicine Human Investigation Committee. The Structured Clinical Interview (SCID) for DSM-5 was used to confirm diagnostic inclusion and exclusion criteria (First et al, 2016). We recorded alcohol use over the prior 90 days with the Timeline-Follow Back Interview (TLFB) (Sobell and Sobell, 1992). Participants underwent medical screening, including a physical exam, an electrocardiogram (EKG), basic blood chemistries, urine drug toxicology screen, and a blood pregnancy test for women.
2.3.2. Script development
Exposure to stress and neutral imagery used personalized guided-imagery methods (McKee et al., 2011). A stress imagery script was developed by having participants identify and describe in detail highly stressful experiences occurring within the past 6 months. Only situations rated as 8 or higher (1=’not at all stressful’ and 10=’the most stress they recently felt in their life’) were accepted as appropriate for script development. A neutral script was developed from participants’ descriptions of personal neutral-relaxing situations. Scripts were developed by a PhD-level clinician and audiotaped for presentation during the laboratory sessions. Each script was approximately 5 min in length.
2.4. Laboratory sessions
2.4.1. Baseline assessment period
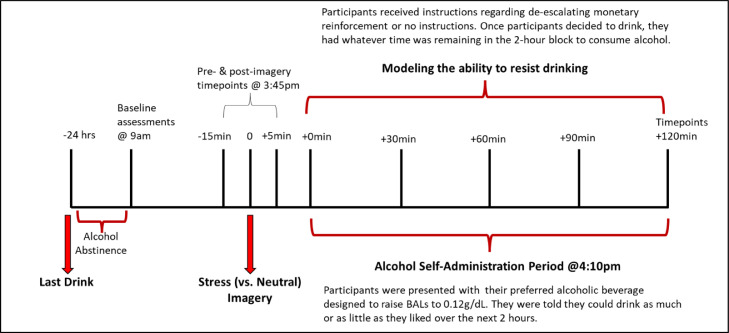
Laboratory sessions began at 9:00am at the Yale Center for Clinical Investigation, New Haven, Connecticut (see Figure 1 for study timeline). Participants were informed to not drink 24 hours prior to the laboratory session (confirmed by breath alcohol reading and self-report). Smokers were instructed to smoke as they usually do prior to the laboratory session. An IV cannula was inserted to obtain blood samples throughout the laboratory session. Baseline assessments of breath alcohol, breath CO, urine drug screens, urine pregnancy screen, and vitals were obtained. Additional measures of alcohol craving and alcohol withdrawal were obtained. Participants received a standardized lunch at 12:15pm to control for time since last food consumption. Smokers were provided with an opportunity to smoke every 2 hours up until 2:00pm to control for nicotine withdrawal. We have used similar procedures in other studies and demonstrated that smokers were not experiencing nicotine withdrawal during the alcohol self-administration session (McKee et al., 2009). Participants also practiced muscle relaxation techniques and completed imagery training during this period. From 9:00am to 2:45pm, participants were able to relax, watch TV, or read.
Figure 1.
Timeline of study procedures.
2.4.2. Personalized imagery procedure
At 3:35pm, participants were instructed to relax their body, clear their mind, and focus on deep breathing. At 3:40pm, participants were told “You will soon hear a situation being described to you. Your task is to close your eyes and imagine yourself in the situation being described, ‘as if’ it were happening right now. Allow yourself to become completely involved in the situation, by involving your mind and body in actually doing what is being described. Continue imagining for as long as you can.” The participant then listened to a personalized script.
2.4.3. De-escalating schedule
Prior to the 2-hour alcohol self-administration period, n=16 participants were informed that for each minute they can resist drinking, they would receive monetary compensation. The amount of money earned over the 2-hour self-administration period started at $0.24 per minute and reduced by a penny every 5 minutes. By 120 minutes, the compensation reduced to $0.00 per minute. Participants were provided this information in table format during the laboratory session (see Supplementary Table S1 for the de-escalating schedule and verbal instructions). If a participant resisted drinking for the entire 2-hour self-administration period, they earned a total of $15.00. Money was used as the alternative reinforcer to provide some incentive to resist drinking and to enhance the likelihood that the reinforcing value of alcohol would be detected (Amlung et al., 2017, MacKillop, 2016). Sixteen participants did not receive the de-escalating schedule and did not receive monetary reinforcement for resisting drinking.
2.4.4. Alcohol beverages and dose
Subjects were provided with their preferred alcohol beverage for this study, including any alcohols (e.g., beer, wine, liquor) or mixers (e.g., juice, soda). For the laboratory sessions, subjects were provided with a pre-determined amount of their preferred alcoholic beverage designed to raise BALs to 0.12 g/dL. These calculations were based on total body water and considered sex, age, height, and weight (Watson, 1989).
While providing participants with their preferred beverage may introduce variability regarding the rate of alcohol absorption, each subject completed two laboratory sessions, and each subject was their own within-subject control. Beverage choice was invariant across the two laboratory sessions.
2.4.5. Alcohol self-administration period
The alcohol self-administration period began at 4:00pm and lasted for a 2-hour period. The entire 0.12 g/dL dose of alcohol was presented to the subjects at the start of the session in an appropriate vessel (e.g., carafe for wine, beer jug for beer, etc.) and preferred drinking glass (e.g., pint glass, wine glass, tumbler, high ball).
Participants were instructed that they could consume as much as they like over the next 2 hours. For participants in the de-escalating schedule, they were further instructed that once they started drinking, they could no longer earn any additional monetary reinforcement. For example, if a subject decided to ‘give in’ and drink at the 1-hour mark, then they would have earned $11.10 (see Supplementary Table S1) and have a remaining hour to consume as much alcohol as they desired. Subjects were free to pour alcohol from the vessel to their glass as desired.
The range of assessments during this period was limited to avoid interfering with the evaluation of drinking behavior. The alcohol self-administration portion of the study ended at 6:00pm. Participants remained at the research unit overnight. At 7:00am the next morning, participants were discharged if their breath alcohol levels were below 0.02% (confirmed by two BAC readings).
2.4.6. Timing of assessments
Alcohol craving was assessed pre-imagery, post-imagery, and +30, +60, +90, and +120min during the ad-lib drinking period. Drinking topography and subjective alcohol effects were assessed at +30, +60, +90, and +120min during the ad-lib drinking period.
2.4.7. Measures
The primary measures were % alcohol consumed during the ad-lib period, time to initiate drinking, and alcohol craving. Additional measures are described below.
2.4.8. Subjective measures
Alcohol craving was assessed with the statement “I crave a drink right now” using a visual analog scale (VAS), range 1-100 (Bohn et al., 1995). The Alcohol Effects Scale (AES), a 5-item self-report questionnaire, was used to assess subjective alcohol intoxication (VAS, range 1-100) (Schuckit, 1984). Items consisted of descriptive words (e.g., high, like, intoxicated, dizzy) on effects the participant may be feeling as they are completing the questionnaire (i.e., right now). Demographic variables, the Contemplation Ladder (Beiner and Abrams, 1991), the Center for Epidemiological Studies-Depression Scale (CES-D) (Radloff, 1977), and the Childhood Trauma Questionnaire (CTQ) (Bernstein & Fink, 1998) were collected at intake. The Contemplation Ladder was used to assess readiness to abstain from alcohol (range 0 – 10). The CES-D, a 20-item self-report questionnaire, was used to assess symptoms associated with depression during the past week (range 0 – 60). The CTQ, a 28-item self-report inventory, was used to assess childhood trauma and maltreatment (e.g., physical, emotional, or sexual abuse, physical or emotional neglect; range 25 – 125).
2.4.9. Drinking topography
Drinking topography measures included % consumed first sip, latency to first pour and sip, and number of pours and sips (see Table 2). The participant kept their glass on a weighted scale to capture milliliters consumed per sip. Participant drinking behavior was videotaped and subsequently scored by two raters to capture timing and pouring and sipping of alcohol.
Table 2.
Drinking topography and alcohol effects by schedule (mean, SE or n, %)
| Deescalating schedule |
|||
|---|---|---|---|
| No | Yes | ||
| Beverage choice | |||
| Beer | 6, 38% | 6, 38% | |
| Wine | 3, 19% | 5, 31% | |
| Liquor | 7, 44% | 5, 31% | |
| Heavy drinking | 13, 81% | 5, 31%* | |
| Alcohol proof | 41.86, 7.98 | 38.5, 7.38 | |
| True alcohol % | 0.09, 0.03 | 0.12, 0.03 | |
| % consumed total | 0.74, 0.07 | 0.40, 0.07* | |
| % consumed first sip | 0.07, 0.01 | 0.05, 0.01* | |
| Latency to 1st pour (seconds)a | 226.37, 296.58 | 1551.60, 296.58* | |
| Latency to 1st sip (seconds)a | 276.63, 279.26 | 1704.03, 288.42* | |
| Total no. pours | 3.31, 0.41 | 1.83, 0.42* | |
| Total no. sips | 27.78, 2.70 | 11.50, 2.78* | |
| BrAC (+120min)b | 0.09, 0.02 | 0.05, 0.02* | |
| AES – Intoxicated | 41.22, 5.86 | 21.73, 5.86* | |
| AES – Rush | 32.11, 4.93 | 16.34, 4.93* | |
| AES – Drug-effect | 33.53, 5.72 | 15.17, 5.72* | |
| AES – Want more | 48.98, 6.60 | 26.05, 6.60* | |
| AES – Jittery | 10.34, 2.59 | 4.69, 2.59* | |
Note: aLatency to first pour and latency to first sip are from the start of the alcohol self-administration session, Alcohol Effects Scale (AES); b Breath Alcohol Concentration (BrAC); *denotes p<.05
2.4.10. Breath alcohol concentration (BrACs)
BrACs were measured at the end of the ad-lib period using an Alco-Sensor III (Intoximeters, Inc., St. Louis, M.O.), which is a precision instrument for detecting alcohol levels in exhaled breath. Breath alcohol was not assessed during the 2-hour drinking period, as recency of drinking would artificially inflate BrACs.
2.5. Statistical analysis
Baseline characteristics were compared across de-escalating schedule with chi-square and t-tests. Analysis of variance (ANOVAs using general linear models [GLM]; IBM SPSS Data Editor Version 28) was used to examine within-subject effects of imagery condition (stress vs. neutral) by de-escalating schedule (yes vs. no) on time to initiate first pour, time to initiate drinking, % consumed in the first sip, and BrACs at 6pm. Separate repeated measures ANOVA was used to examine within-subject effects of imagery condition and time (+30, +60, +90, +120min) by de-escalating schedule on % total alcohol consumed. Alcohol consumption was evaluated as percentage of the beverage consumed to equate for alcohol volume across participants. Repeated measures ANOVA was also used to examine within-subject effects of imagery condition and time (pre-imagery, post-imagery; +0, +30, +60, +90, +120min) by de-escalating schedule on alcohol craving and imagery condition and time (+60 and +120min) by de-escalating schedule on alcohol intoxication. Age, race, smoking status, education, marital status, CES-D, and childhood trauma were evaluated as covariates and were retained if they were significantly associated with each outcome. Percent of alcohol contained in their preferred beverage (e.g., alcohol + mixer) and motivation to abstain from alcohol were also evaluated as a covariates across all outcomes, and were not significant, and were not retained as covariates.
3. Results
Participants did not differ on any baseline characteristic by de-escalating schedule (see Table 1). The average age was 38.44 years old (SE=2.39). Fifty percent of participants were female. Participants were primarily white (37.5%) or black (53.1%), and primarily college educated (59.4%). Participants drank 4.83 days per week (SE=0.31) and 6.64 drinks per episode (SE=0.63). Participants scored 2.69 (SE=0.48) on the Contemplation Ladder. Participants chose their preferred alcoholic beverage for the laboratory sessions. Beverage type did not differ by de-escalating schedule (37.5% beer, 25.0% wine, 37.5% liquor), nor did the percent of alcohol contained in their preferred beverage (e.g., alcohol + mixer; 10.4%; see Table 2).
Table 1.
Baseline demographics by schedule (mean, SE or n, %)
| Deescalating schedule |
|||
|---|---|---|---|
| No | Yes | ||
| Age | 37.13, 3.82 | 39.75, 2.96 | |
| Sex | |||
| Female | 8 | 8 | |
| Male | 8 | 8 | |
| Race | |||
| White | 7 | 5 | |
| Black | 7 | 10 | |
| Hispanic | 1 | 0 | |
| Other | 1 | 1 | |
| Education | |||
| HS or less | 8 | 5 | |
| College + | 8 | 11 | |
| Marital status | |||
| Married | 2 | 3 | |
| Not married | 14 | 13 | |
| Smoker | 6 | 11 | |
| AUDIT | 13.63,1.51 | 12.56, 1.40 | |
| Drinking days per week | 5.21, 0.34 | 4.45, 0.52 | |
| Drinks per drinking day | 7.15, 1.01 | 6.13, 0.77 | |
| Contemplation ladder | 2.56, 0.59 | 2.81, 0.77 | |
Note: All chi-square and t-tests for comparison were non-significant at p>0.05; Alcohol Use Disorders Identification Test (AUDIT) scores ≥ 8 are associated with harmful or hazardous drinking, contemplation ladder (range 0 - 10) assesses readiness to abstain from alcohol.
3.1. Ad-libitum drinking
A main effect of de-escalating schedule (F=10.88, p=0.003) on ad-lib drinking demonstrated that participants consumed more alcohol without the de-escalating schedule (74.2%) versus with the de-escalating schedule (40.3%), such that the de-escalating schedule reduced alcohol consumption by 49%. Further, more participants drank heavily (i.e., consumed more than 2/3 of their beverage reaching a theoretical blood alcohol content of 0.08 mg%) without the de-escalating schedule compared to with the de-escalating schedule (81% vs. 31%, respectively; see Table 2).
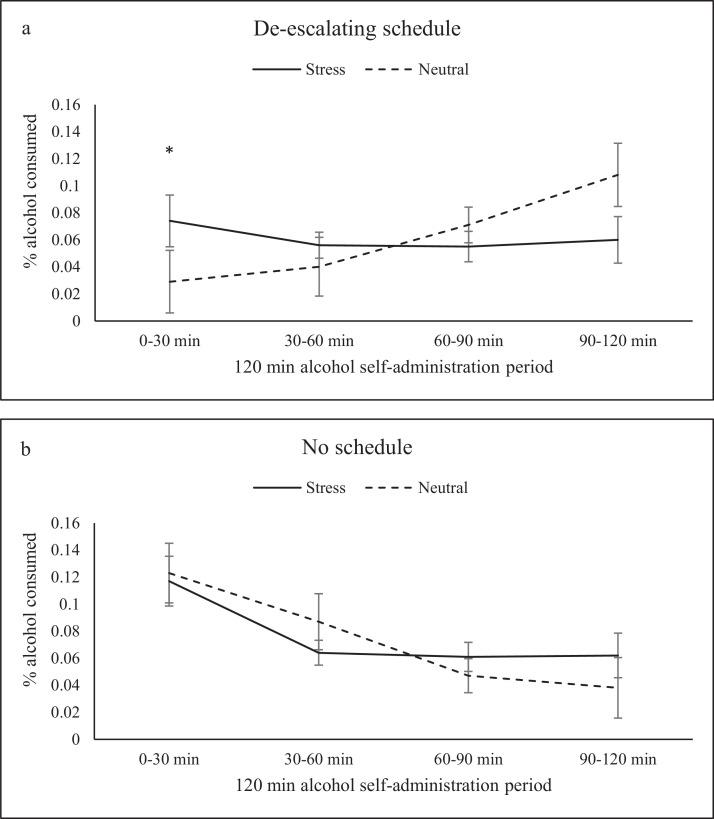
When stress was considered, a significant interaction between de-escalating schedule, imagery condition, and time (F=5.73, p=0.025) on ad-lib drinking demonstrated that with the de-escalating schedule participants consumed 40% more alcohol in the first 30 minutes of the alcohol self-administration session following stress imagery (7.4%) compared to neutral imagery (2.9%; see Figure 2a). With no schedule, participants consumed equal amounts of alcohol following stress and neutral imagery (11.7 and 12.3%, respectively; see Figure 2b). Additionally, there was a schedule x time interaction (F=5.15, p<.03). With no schedule, drinking was greatest in the first 30-min block and continued to decrease over the 2-hour period. With the de-escalating schedule, drinking was lowest in the first 30-minute block and then continued to increase over the 2-hour period (See Figure 2a & b).
Figure 2.
Percent alcohol consumed by de-escalating schedule over the 120-min alcohol self-administration session: (a) with the de-escalating schedule participants consumed 40% more alcohol in the first 30 minutes of the alcohol self-administration session following stress imagery compared to neutral imagery, (b) with no schedule, participants consumed equal amounts of alcohol following stress and neutral imagery.
When examining % milliliters consumed in the first sip, a main effect of imagery condition (F=5.18, p=0.03) demonstrated that participants consumed 7.3% of their total beverage in the first sip following stress imagery compared to 4.7% of their total beverage following neutral imagery. Participants drank 50% more of their beverage in the first sip following stress vs. neutral imagery. A main effect of de-escalating schedule demonstrated that participants poured more alcohol (F=6.36, p=0.02) and sipped more alcohol (F=7.04, p=0.01) with no schedule compared to those with the de-escalating schedule. Participants who did not receive the de-escalating schedule poured and sipped nearly twice as much as those who did receive the de-escalating schedule.
3.2. Time to initiate drinking
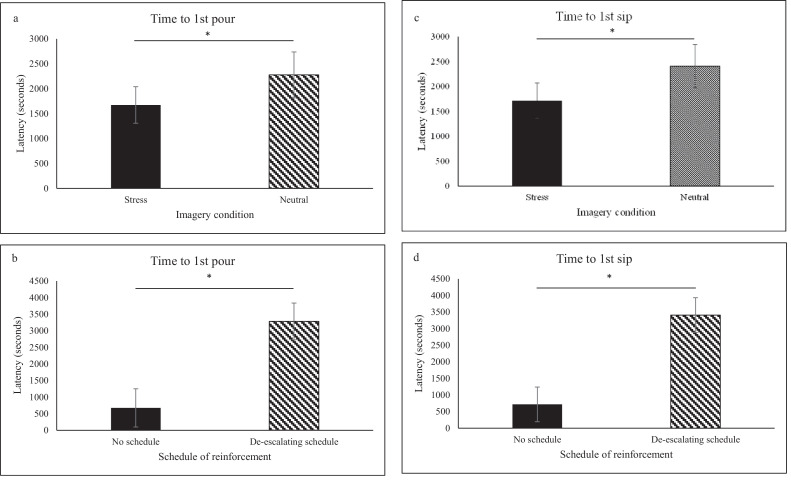
Participants initiated pouring faster following stress imagery compared to neutral imagery (F=7.32, p=0.012; see Figure 3a). There was a main effect of de-escalating schedule on time to initiate pouring (F=9.43, p=0.005) such that participants initiated pouring faster with no schedule compared to those with the de-escalating schedule (see Figure 3b).
Figure 3.
Latency (seconds) to initiate pouring and sipping by imagery condition and de-escalating schedule: (a) participants initiated pouring faster following stress imagery compared to neutral imagery, (b) participants initiated pouring faster with no schedule compared to those with the de-escalating schedule, (c) participants initiated drinking faster following stress imagery compared with the neutral imagery session, (d) participants initiated drinking faster with no schedule compared to those with the de-escalating schedule.
Further, participants initiated drinking faster following stress imagery compared with the neutral imagery session (F=8.06, p=0.009; see Figure 3c). There was a main effect of de-escalating schedule on time to initiate drinking (F=11.90, p=0.002) such that participants initiated drinking faster with no schedule compared to those with the de-escalating schedule (see Figure 3d).
3.3. Alcohol craving
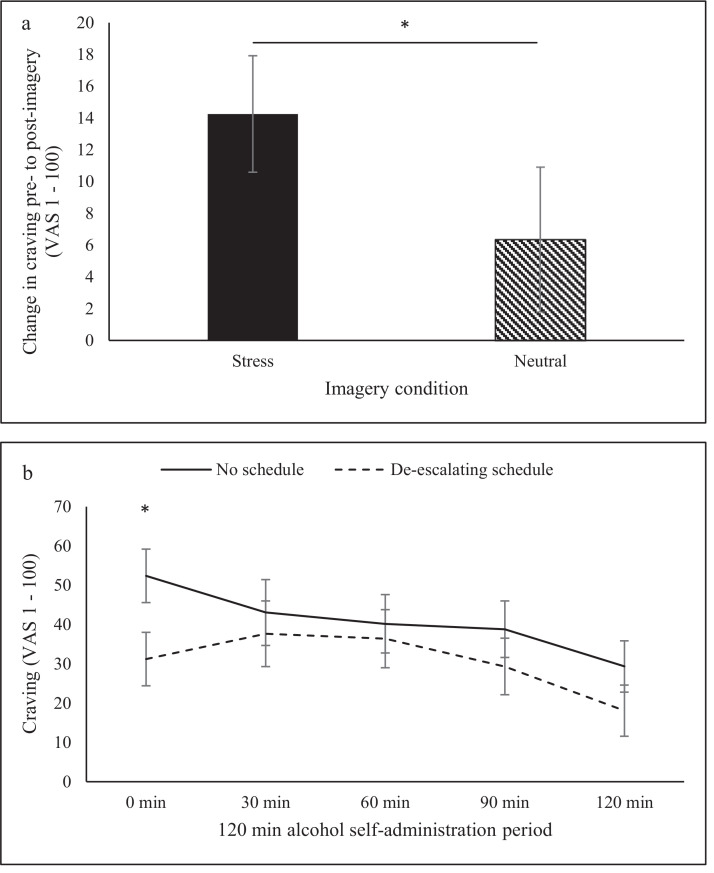
When examining craving pre- to post-imagery, a significant imagery condition by time interaction (F=4.28, p=0.05) demonstrated that craving scores increased to a greater degree following stress imagery (change pre- to post imagery mean=14.25, SE=3.67) compared to neutral imagery (change pre- to post-imagery mean=6.34, SE=4.55; See Figure 4a). When examining craving throughout the alcohol self-administration period, a significant de-escalating schedule by time effect (F=4.71, p=0.04) demonstrated higher craving rates at +0 time-point without the de-escalating schedule compared to with the de-escalating schedule (see Figure 4b).
Figure 4.
Alcohol craving across the alcohol self-administration period: (a) craving increased to a greater degree following stress imagery compared to neutral imagery, (b) participants reported higher craving at the post-imagery time-point with no schedule compared to with the de-escalating schedule.
3.4. Subjective alcohol intoxication and BrACs
A significant main effect of de-escalating schedule on subjective alcohol effects demonstrated that participants reported greater effects of alcohol without the de-escalating schedule compared to with the de-escalating schedule (see Table 2). Participants reported greater ratings on feelings of intoxication (F=5.53, p=0.03), rush (F=5.12, p=0.03), drug-effect (F=5.16, p=0.03), and want more (F=5.75, p=0.02) with no schedule. A significant imagery condition by time effect (F=7.88, p=0.009) demonstrated that participants reported greater ratings of feeling jittery at the end of hour 1 (+60 min) of the self-administration period following stress imagery (mean=10.56, SE=2.82) compared to neutral imagery (mean=5.03, SE=1.63).
Following ad-lib drinking, a main effect of de-escalating schedule (F=4.79, p=0.04) on BrACs demonstrated that BrACs were higher at 6pm without the de-escalating schedule (mean=0.09, SE=0.02) versus with the de-escalating schedule (mean=0.05, SE=0.02).
4. Discussion
As predicted, our novel alcohol self-administration model generated heavy drinking. Over 80% of participants without reinforcement consumed more than 2/3 of their preferred alcoholic beverage designed to increase BALs to 0.12 mg% within a 2-hour window. Similar to our smoking models (McKee 2009), we observed that manipulating monetary reinforcement for resisting drinking reduced overall consumption. Participants with the de-escalating schedule consumed 40% of their beverage, versus the 74% consumed by participants without a schedule (a 49% reduction). In general, we found that those without a schedule had almost no latency to pour their first drinking or to start drinking and consumed heavily at the start of the ad-libitum period. Those with monetary reinforcement to resist drinking, delayed the start of their consumption by almost 30 minutes on average, and drank larger amounts towards the end of the ad-libitum period. Subjective alcohol effects (intoxication, drug-effect, want more) and breath alcohol levels were consistent with drinking behavior (i.e., were greater in the ‘no schedule’ condition).
We also demonstrated that this model was sensitive to stress, and that the de-escalating schedule highlighted stress effects on drinking. We found that subjects exposed to stress and the de-escalating schedule consumed more alcohol, more quickly, shortly after being exposed to the stress manipulation. In fact, subjects tended to ‘gulp’ their first sip following stress (consuming 7% of their total beverage in the first sip) versus when they experienced the neutral condition. As with our stress/smoking models (McKee et al., 2011), we demonstrated that stress had an impact on increasing alcohol craving relative to the neutral imagery condition.
An important and novel aspect of this model was to provide participants with their preferred beverages including alcohols, mixers, and temperature. The procedure was feasible, and choices ranged from peach vodka mixed with fruit punch to organic beer with cereal grains to pinot noir. Given the variability in alcohol concentration and volume, future medication studies using this paradigm will ideally implement cross-over designs where subjects can function as their own controls. Importantly, in the current study, all outcomes were independent of variations in alcohol concentration and volume.
We recruited subjects who reported a range of treatment seeking status, but none were currently in treatment. We have previously addressed the need to enroll treatment seekers in human laboratory studies for medication screening to increase consilience with clinical trials, and that it is ethical and feasible to do so (Roberts et al., 2021). In the current study, three subjects elected to complete a treatment phase following completion of the laboratory component. As outlined in our protocol, these subjects desired to reduce their alcohol consumption, and did not wish to completely quit drinking. Across our participants, the overall motivation to abstain from alcohol was low and we did not find any relationships between treatment motivation and our outcomes.
In this first study detailing the development of a novel alcohol self-administration paradigm, we demonstrated that we were able to generate heavy drinking and that the addition of a de-escalating schedule of reinforcement increased the ability to resist drinking and decreased the rate of heavy drinking. Further, the model was sensitive to stress effects, and that the impact of stress was most noticeable early on during the ad-libitum phase, highlighting the importance of assessing changes in drinking over the 2-hour period. For future medication testing, the condition with no reinforcement schedule may be appropriate when the focus is on percent of the sample engaging in heavy drinking behavior, and the medication comparison is one-tailed (i.e., hypothesize that medication will lower drinking). In this scenario, it would be desirable for the placebo condition to have high rates of heavy drinking behavior in the paradigm, and there is no concern about ceiling effects. This scenario would be most appropriate to test medications which have demonstrated some initial efficacy for reducing consumption and there is little concern that the medication may actually increase drinking.
In a scenario where you have a two-tailed medication hypothesis regarding medication (i.e., hypothesize that the medication may increase or decrease heavy drinking), it would be desirable for your placebo group to be consuming ∼50% of the alcohol and ∼50% meeting criteria for heavy drinking behavior in the paradigm. In our smoking lapse models, we have termed this happy middle ‘target model behavior’ (McKee, 2009). In the latter scenario, using the de-escalating schedule of reinforcement would achieve this goal. This scenario would be most appropriate for testing a novel medication, where you would have the ability to determine whether a medication increased or decreased drinking behavior.
A limitation of the current paper is the lack of a within-subject comparison for the de-escalating schedule. For this initial model development paper, we utilized subjects from two ongoing studies with identical methods, but one with a de-escalating schedule and one without. We are continuing work to refine the parameters of this model (e.g., testing other reinforcement schedules), investigate additional primes of drinking (e.g., context effects), and to use the models to study individual difference variables (e.g., sex differences, psychiatric co-morbidities). We are currently using the stress-prime version of this model to test noradrenergic medications (NCT03764098), which are hypothesized to target stress-reactivity. As with our smoking models, we have begun to disseminate the procedure to other research groups (e.g., IV alcohol paradigm). While this line of work is still at a very nascent stage, we are hopeful that this novel paradigm will facilitate translational work in alcohol medication development.
Funding
Support provided by the Office of Research on Women's Health and NIAAA grants U54AA027989 (SAM), P01AA027473 (SAM), and R01AA022285 (SAM); and K01AA025670 (TLV). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author disclosures
None to report.
Contributors
SAM conceptualized the project. SAM and TLV contributed to data collection, analysis, drafting and editing the manuscript.
Declaration of Competing Interest
Nothing to declare.
Acknowledgements
None to report.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dadr.2022.100085.
Appendix. Supplementary materials
References
- American Psychiatric Association . 5th ed. 2013. Diagnostic and statistical manual of mental disorders. [DOI] [Google Scholar]
- Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J. Steep delay discounting and addictive behavior: A meta-analysis of continuous associations. Addiction. 2017 Jan;112(1):51–62. doi: 10.1111/add.13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Duraxo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology (Berl) 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P, Fink L. The Psychological Corporation; San Antonio, TX: 1998. Childhood Trauma Questionnaire: A retrospective self-report manual. [Google Scholar]
- Biener L, Abrams DB. The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin. Exp. Res. 1995;19(3):600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Alcohol and public health: alcohol-related disease impact (ARDI) In: Annual average for United States 2011–2015 alcohol-attributable deaths due to excessive alcohol use, all ages. 2022 https://nccd.cdc.gov/DPH_ARDI/Default/Default.aspx [Google Scholar]
- Falcone M, Bernardo L, Ashare RL, Hamilton R, Faseyitan O, McKee SA, Loughead J, Lerman C. Transcranial Direct Current Brain Stimulation Increases Ability to Resist Smoking. Brain Stimul. 2016 Mar-Apr;9(2):191–196. doi: 10.1016/j.brs.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhnia M, Schwandt ML, Lee MR, Bollinger JW, Farinelli LA, Amodio JP, Lionetti TA, Spero DE, Leggio L. Biobehavioral effects of baclofen in anxious alcohol-dependent individuals: a randomized, double-blind, placebo-controlled, laboratory study. Translational Psychiatry. 2017;7:e1108. doi: 10.1038/tp.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Williams J.B.W., Karg R.S., Spitzer R.L. User's guide for the SCID-5-CV Structured Clinical Interview for DSM-5® disorders: Clinical version. 2016 [Google Scholar]
- Food and Drug Administration . US Department of Health and Human Services; Rockville, MD: 2006. Medical Review of Vivitrol; pp. 21–897. [Google Scholar]
- Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74(9):911–923. doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Goodyear K, Zywiak WH, Leggio L, Kenna GA, Swift RM. Comparing and combining topiramate and aripiprazole on alcohol-related outcomes in a human laboratory study. Alcohol Alcohol. 2018;53(3):268–276. doi: 10.1093/alcalc/agx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Babalonis S, Marcus R, Vince B, Kelsh D, Lofwall MR, Fraser H, Paterson B, Martinez S, Martinez DM, Nunes EV, Walsh SL, Comer SD. A randomized, double-blind, placebo-controlled study of the kappa opioid receptor antagonist, CERC-501, in a human laboratory model of smoking behavior. Addict. Biol. 2020 Jul;25(4):e12799. doi: 10.1111/adb.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, Spillane NS, Leventhal AM, McKee SA, Tidey JW, McGeary JE, Knopik VS, Rohsenow DJ. Sex differences in stimulus expectancy and pharmacologic effects of a moderate dose of alcohol on smoking lapse risk in a laboratory analogue study. Psychopharmacology (Berl) 2012 Jul;222(1):71–80. doi: 10.1007/s00213-011-2624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Metrik J, Spillane NS, Day A, Leventhal AM, McKee SA, Tidey JW, McGeary JE, Knopik VS, Rohsenow DJ. Acute effects of low and high dose alcohol on smoking lapse behavior in a laboratory analogue task. Psychopharmacology (Berl) 2014 Dec;231(24):4649–4657. doi: 10.1007/s00213-014-3613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, O'Malley SS, White MA, McKee SA. Nicotine and food deprivation decrease the ability to resist smoking. Psychopharmacology (Berl) 2010 Sep;212(1):25–32. doi: 10.1007/s00213-010-1902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J. The behavioral economics and neuroeconomics of alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2016 Apr;40(4):672–685. doi: 10.1111/acer.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Light JM, Escher T, Drobes DJ. Effect of positive and negative affective stimuli and beverage cues on measures of craving in non-treatment-seeking alcoholics. Psychopharmacology (Berl) 2008;200:141–150. doi: 10.1007/s00213-008-1192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O'Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology (Berl) 2006 Dec;189(2):201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol. Psychiatry. 2009;66(2):185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict. Biol. 2009 Jan;14(1):99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J. Psychopharmacol. 2011;25(4):490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob. Res. 2012 Nov;14(11):1362–1371. doi: 10.1093/ntr/nts090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Potenza MN, Kober H, Sofuoglu M, Arnsten AF, Picciotto MR, Weinberger AH, Ashare R, Sinha R. A translational investigation targeting stress-reactivity and prefrontal cognitive control with guanfacine for smoking cessation. J. Psychopharmacol. 2015 Mar;29(3):300–311. doi: 10.1177/0269881114562091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Verplaetse TL. Symposium talk presented at the annual meeting of the Research Society on Alcoholism. San Francisco CA. 2015. To drink or not to drink. Modeling the ability to resist in the human laboratory. [Google Scholar]
- Monti PM, Rohsenow DJ, Hutchison KE, Swift RM, Mueller TI, ColbySM Brown RA, Gulliver SB, Gordon A, Abrams DB. Naltrexone’seffect on cue-elicited craving among alcoholics in treatment. Alcohol Clin. Exp. Res. 1999;23:1386–1394. [PubMed] [Google Scholar]
- Munos B. Lessons from 60 years of pharmaceutical innovation. Nat. Rev. 2009;8:959–968. doi: 10.1038/nrd2961. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Oberleitner LM, Moore KE, Verplaetse T, Roberts W, McKee SA. Developing a laboratory model of smoking lapse targeting stress and brief nicotine deprivation. Exp. Clin. Psychopharmacol. 2018 Jun;26(3):244–250. doi: 10.1037/pha0000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Zvolensky MJ, Rosenfield D, Hoyt DL, Witkiewitz K, McKee SA, Bickel WK, Smits JAJ. A randomized controlled trial protocol for engaging distress tolerance and working memory to aid smoking cessation in low socioeconomic status (SES) adults. Health Psychol. 2020 Sep;39(9):815–825. doi: 10.1037/hea0000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat. Rev. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- Peltier MR, Verplaetse TL, Mineur YS, Petrakis IL, Cosgrove KP, Picciotto MR, McKee SA. Sex differences in stress-related alcohol use. Neurobiol. Stress. 2019 Feb 8;10 doi: 10.1016/j.ynstr.2019.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Buonopane A, O’Malley S, Cermik O, Trevisan L, Boutros NN, Limoncelli D, Krystal J. The effect of tryptophan depletion on alcohol self-administration in non-treatment-seeking alcoholic individuals. Alcohol Clin. Exp. Res. 2006;26(7):969–975. doi: 10.1097/01.ALC.0000021338.38350.95. [DOI] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: A self report depression scale for research in the general population. Appl. Psychol. Measure. 1977;1:385–401. [Google Scholar]
- Ramchandani VA, O’Connor S, Neumark Y, Zimmermann US, Morzorati SL, de Wit H. The alcohol clamp: applications, challenges, and new directions – an RSA 2004 Symposium summary. Alcohol Clin. Exp. Res. 2006;30:155–164. doi: 10.1111/j.1530-0277.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- Roberts W, Verplaetse TL, Moore KE, Oberleitner LM, McKee SA. A preliminary investigation into the effects of doxazosin on cognitive functioning in tobacco-deprived and -satiated smokers. Hum. Psychopharmacol. 2018 May;33(3):e2660. doi: 10.1002/hup.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W., Verplaetse T.L., Moore K., Oberleitner L., Picciotto M.R., McKee S.A. Effects of varenicline on alcohol self-administration and craving in drinkers with depressive symptoms. J. Psychopharmacol. 2017;31(7):906–914. doi: 10.1177/0269881117699618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W, Verplaetse TL, Ramchandani VA, McKee SA. A Critical Review of Alcohol Administration Guidelines in Laboratory Medication Screening Research: Is It Time to Include Treatment Seekers? Alcohol Clin. Exp. Res. 2021;45(1):15–24. doi: 10.1111/acer.14514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, Bujarski S, Moallem NR, Guzman I, Shapiro JR, Ray LA. Predictors of smoking lapse in a human laboratory paradigm. Psychopharmacology (Berl) 2014 Jul;231(14):2889–2897. doi: 10.1007/s00213-014-3465-x. Epub 2014 Feb 6. [DOI] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 national and state costs of excessive alcohol consumption. Am. J. Prev. Med. 2015;49(5):e73–e79. doi: 10.1016/j.amepre.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Schlagintweit HE, Tyndale RF, Hendershot CS. Acute effects of a very low nicotine content cigarette on laboratory smoking lapse: Impacts of nicotine metabolism and nicotine dependence. Addict. Biol. 2021 May;26(3):e12930. doi: 10.1111/adb.12930. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch. Gen. Psychiatry. 1984;41(9):879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Shield KD, Rylett M, Gmel G, Gmel G, Kehoe-Chan TA, Rehm J. Global alcohol exposure estimates by country, territory and region for 2005—a contribution to the comparative risk assessment for the 2010 global burden of disease study. Addiction. 2013;108(5):912–922. doi: 10.1111/add.12112. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Springer; 1992. Timeline follow-back. Measuring alcohol consumption; pp. 41–72. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br. J. Addict. 1989;84(11):1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Udo T, Harrison ELR, Shi J, Tetrault J, McKee SA. A preliminary study on the effect of combined nicotine replacement therapy on alcohol responses and alcohol self-administration. Am. J. Addict. 2013;22:590–597. doi: 10.1111/j.1521-0391.2013.12014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee SA. Effect of Lowering the Dose of Varenicline on Alcohol Self-administration in Drinkers with Alcohol Use Disorders. J. Addict. Med. 2016 May-Jun;10(3):166–173. doi: 10.1097/ADM.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Smith PH, Smith KM, Oberleitner LM, McKee SA. Guanfacine alters the effect of stress and smoking on heart rate variability in regular daily smokers. Psychopharmacology (Berl) 2017 Mar;234(5):805–813. doi: 10.1007/s00213-016-4517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Weinberger AH, Oberleitner LM, Smith KM, Pittman BP, Shi JM, Tetrault JM, Lavery ME, Picciotto MR, McKee SA. Effect of doxazosin on stress reactivity and the ability to resist smoking. J. Psychopharmacol. 2017 Jul;31(7):830–840. doi: 10.1177/0269881117699603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verplaetse TL, Weinberger AH, Ashare RL, Pittman BP, Shi JM, Tetrault JM, Lavery M, McKee SA. Pilot investigation of the effect of carvedilol on stress-precipitated smoking-lapse behavior. J. Psychopharmacol. 2018 Sep;32(9):1003–1009. doi: 10.1177/0269881118767647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. In: Crow KE, Batt Rd (eds) Human Metabolism of Alcohol, Vol I, Boca Raton, FL, CRC Press, p 41-56, 1989.
- Wilson SJ, Delgado MR, McKee SA, Grigson PS, MacLean RR, Nichols TT, Henry SL. Weak ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cogn. Affect. Behav. Neurosci. 2014 Dec;14(4):1196–1207. doi: 10.3758/s13415-014-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.