Abstract
Background and Aims
Patients with inflammatory bowel disease (IBD) are at increased risk of type 2 diabetes (T2D), but the underlying mechanisms remain elusive. We aimed to determine the impact of small and large bowel resections on the risk of developing T2D in patients with IBD.
Methods
We conducted a nationwide, prospective study of all IBD patients undergoing small bowel resection (Crohn’s disease [CD]) and large bowel resection (CD and ulcerative colitis [UC]) in Denmark (1996–2018). Each patient was matched with up to 5 patients with IBD and no history of bowel resection. We used Cox proportional hazards regression models to estimate adjusted hazard ratios (aHRs) of T2D.
Results
We included 2469 patients with CD and small bowel resection, 1361 patients with CD and large bowel resection, and 3787 patients with UC and large bowel resection. Small bowel resection in CD patients was associated with lower risk of T2D (aHR 0.65, 95% CI, 0.44–0.92), compared with matched patients with CD and no bowel resection. Large bowel resection in patients with CD or UC was associated with aHRs of 0.95 (95% CI, 0.67–1.31) and 1.25 (95% CI, 1.03–1.51), respectively, compared with matched patients with CD or UC and no bowel resection.
Conclusion
Patients with CD and small bowel resection have a lower risk of T2D, whereas patients with UC and large bowel resection have a higher risk of T2D, compared with patients with IBD and no bowel resection history. The underlying mechanisms remain to be explored.
Keywords: Crohn’s Disease, Diabetes Mellitus, Digestive System Surgical Procedures, Ulcerative colitis
Introduction
Inflammatory bowel diseases (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), are chronic immune-mediated intestinal diseases with increasing incidence worldwide.1 CD is characterized by transmural inflammation in any segment of the gastrointestinal tract, with the terminal ileum being the most commonly affected.2 In contrast, inflammation in UC is limited to the colonic mucosa.3 IBD has no cure, and most patients experience a relapsing remitting course with progressive intestinal injury.3 Complications such as strictures, fistulas, and abscesses in CD and medically refractory colitis in UC may necessitate gastrointestinal resections.2, 3, 4
Previous studies have shown that the risk of cerebrovascular and ischemic heart disease is higher in patients with IBD.5, 6, 7, 8, 9 Moreover, patients with IBD are at increased risk of type 2 diabetes (T2D).10,11 However, the mechanisms underlying these observations remain largely elusive. Previous studies have shown that traditional cardiometabolic risk factors such as body weight, hypertension, and hyperlipidemia were not increased in patients with IBD,12,13 suggesting that alternate mechanisms such as persistent systemic inflammation, aberrant gut microbiota, and altered metabolic pathways may play a role. While bariatric surgery improves glycemia,14 we have shown that colonic resections, especially of the left segment of the colon, for indications, including cancer, diverticulosis, or IBD, are associated with an increased risk of T2D.15
The aim of this study was to examine the influence of small and large bowel resections on the risk of developing T2D in patients with IBD. We conducted a nationwide, population-based, prospective study of patients with CD undergoing small bowel resection and patients with CD or UC undergoing large bowel resections.
Methods
Study Population
Based on the Danish Civil Registration System,16 we identified all individuals living in Denmark between January 1, 1996, and December 31, 2018. As the disease course and treatment vary considerably between pediatric and adult onset IBD, we included only individuals aged ≥15 years.17 Using the unique personal identification number assigned to all citizens at birth, we linked this population to the Danish National Patient Register18 and the Danish National Prescription Register.19
We generated 2 study populations: population A including patients with CD undergoing small bowel resection and population B including patients with CD or UC undergoing large bowel resection. For population A, all patients with CD diagnosed between January 1, 1977, and December 31, 2018, were eligible for inclusion (International Classification of Disease [ICD] eighth revision code 563.01-09 or ICD-10 code DK50; n = 35,026). For population B, all patients with CD or UC diagnosed between January 1, 1977, and December 31, 2018, were eligible for inclusion (ICD-8 code 563.01-09, 563.19, 569.04 or ICD-10 code DK50 or DK51; n = 92,073). For both study populations, we made the following exclusions: (1) patients with only one registration with an inpatient or outpatient contact with CD or UC unless this contact lasted 7 days or more; (2) patients with appendectomy or bariatric surgery before the date of diagnosis of CD or UC and patients with small or large bowel resections more than 1 month before date of diagnosis of CD or UC; we included patients with small or large bowel resections within 1 month of the date of CD or UC diagnosis to allow for the CD or UC diagnosis being given following pathological examination of resected specimens; (3) For population A: patients with CD and large bowel resection, appendectomy, or bariatric surgery after date of CD diagnosis and before date of small bowel resection or no small bowel resection; For population B: patients with CD or UC and small bowel resection, appendectomy, or bariatric surgery after date of CD or UC diagnosis and before date of large bowel resection or no large bowel resection; (4) patients not living in Denmark in the 1 year before bowel resection; (5) patients with history of any diabetes before their date of bowel resection (ICD-8 code 24900–25009, ICD-10 code E10-14, or ATC code A10).
Patients with CD fulfilling the inclusion criteria but not undergoing small bowel resection were matched to resected patients with CD at the date of small bowel resection. Likewise, patients with CD or UC fulfilling the inclusion criteria but not undergoing large bowel resection were matched to patients with resection and CD or UC at the date of large bowel resection. Patients were matched on age at bowel resection (±5 years), sex, type of IBD, and duration of IBD (±1 year) 1:5 with replacement. In case patients were registered with both CD and UC, they were classified per majority of the diagnoses at study entry.
Exposure: Bowel Resection
We identified small and large bowel resections based on surgery codes in the Danish National Patient Register (the Danish Surgical Procedure and Treatment Classification, 1977–1995) and the Nordic Classification of Surgical Procedures (1996–2018; Table A1).
Outcome: Type 2 Diabetes
We identified incident cases of T2D based on data from the Danish National Patient Register and the Danish National Prescription Register. Incident T2D cases were identified as patients meeting one of the following criteria: 2 inpatient or outpatient contacts with diabetes (ICD-10 E10-14), 2 purchases of glucose-lowering drugs other than insulin (ATC code A10B; insulin was not included to avoid misclassification due to type 1 diabetes), or one of each. We chose to use 2 registrations to minimize misclassification bias. To minimize immortal time bias, we used the date of the second purchase/contact as the outcome date. Using the first date as the diagnosis date could introduce immortal time bias as per the outcome definition, the individuals who develop T2D would be alive at least until their second purchase/contact, whereas this may not be the case for non-T2D individuals. Next, we further minimized misclassification bias by considering diagnoses of polycystic ovarian syndrome and gestational diabetes. Thus, metformin purchases among women aged 18–40 years did not count in the construction of the outcome, T2D, and neither did diabetes registrations and purchases of glucose-lowering drugs if they occurred in the window of 30 days before or 365 days after a registration with gestational diabetes (ICD-10 O24). Finally, patients who purchased insulin before 30 years of age or purchased insulin after 30 years of age and had more registrations with type 1 diabetes than registrations with T2D were not included as T2D cases.
Covariates
We included the following covariates: age, sex, calendar period of surgery, IBD duration, area socioeconomic index, IBD-related hospitalizations, treatment with corticosteroids, and treatment with a tumor necrosis factor inhibitor (TNFi). We obtained information on age and sex from the Danish Civil Registration System. The area-level socioeconomic index is an official summary statistics used for redistribution of fonds between Danish municipalities. Information on the remaining covariates was obtained from the Danish National Patient Register and the Danish National Prescription Register. IBD-related hospitalization was defined as hospitalization with a primary diagnosis of CD or UC or a secondary diagnosis of CD or UC combined with a primary diagnosis of abdominal pain, nausea, vomiting, diarrhea, rectal bleeding, fistula, abscess, stenosis, subileus, or ileus as previously done (Table A2).20 Treatment with TNFi was defined as a registration with procedure code BOHJ18A or a purchase of TNFi (ATC code ML04AB). Treatment with oral corticosteroids was defined as a purchase of corticosteroids (ATC code H02AB). Ileal pouch-anal anastomosis was defined as explained in detail by Barnes et al.21
Statistical Analysis
We followed patients from date of bowel resection/match date until date of T2D, date of a second bowel resection, including appendectomy and bariatric surgery, date of T1D, death, emigration, December 31, 2018, or for 10 years whichever event occurred first. Patients were censored if they had a second bowel resection, appendectomy, or bariatric surgery to avoid bias due to multiple bowel resections. We followed patients for a maximum of 10 years to avoid analyzing the risk of T2D based on low numbers of patients followed for more than 10 years and as we hypothesized that T2D caused by an intestinal resection would be diagnosed within 10 years of follow-up.
We estimated the cumulative incidence of T2D by the Kaplan-Meier method. Cox proportional hazards regression models with time since bowel resection/match date as underlying time scale was used to estimate age- and sex-adjusted hazard ratios (HRs) and multifactor-adjusted hazard ratios (aHRs) of T2D. The fully adjusted models included the covariates age, sex, IBD duration, calendar period of bowel resection, area socioeconomic index, IBD-related hospitalizations, treatment with corticosteroids, treatment with a TNFi, and IBD type when analyzing the effect of large bowel resection. Age at bowel resection, sex, IBD duration, period of bowel resection (1996–2003; 2004–2011; 2012–2018), and area socioeconomic index (quartiles) were time fixed covariates. Owing to nonlinearity of the covariate-outcome relationship, the effect of age and IBD duration at bowel resection was modeled via restricted cubic splines with 5 and 3 knots, respectively. IBD-related hospitalizations and treatment with TNFi and oral corticosteroids were included as time dependent covariates, updated on daily basis. Individuals were considered exposed for 1 year after initial exposure meaning that if they were not exposed during the following year, they again contributed unexposed time from 1 year after initial exposure. In sensitivity analyses, we introduced a 6- and 12-month lag period after bowel resection where events of T2D did not count as outcomes to ensure that the outcome (T2D) occurred after the exposure (bowel resection), thus strengthening any casual interpretations. The proportional hazards assumption was investigated graphically. Statistical analyses were undertaken using SAS version 9.4 (SAS Institute, Cary, NC).
Ethics
The study was approved by the Danish Data Protection Agency, and data were analyzed on a secure server at the Danish Health Data Authority. Ethical approval is not required for registry-based research in Denmark.
Results
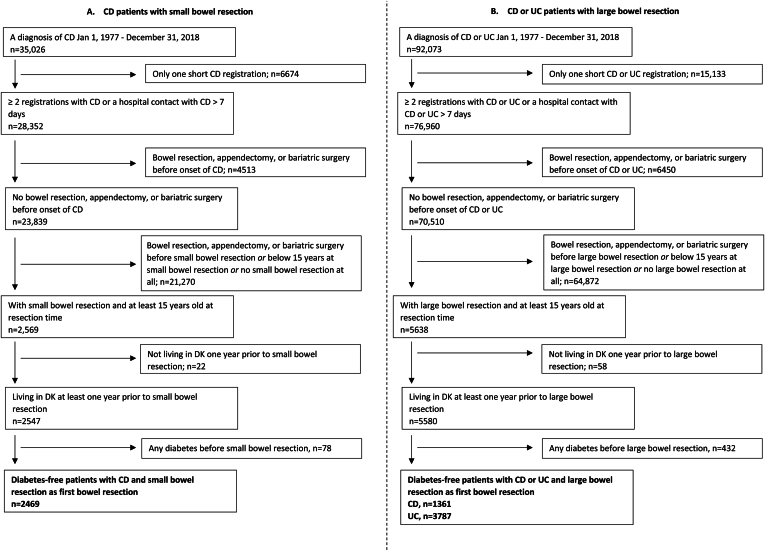
We included 2469 patients with CD and small bowel resection and 5148 patients with CD (n = 1361) or UC (n = 3787) and large bowel resection (Figure 1).
Figure 1.
Flowchart illustrating inclusion and exclusion criteria. The source population included all individuals aged ≥15 years living in Denmark between January 1, 1996, and December 31, 2018. Patients with inflammatory bowel disease (IBD) and small or large bowel resections were matched with patients with IBD fulfilling the above inclusion and exclusion criteria, but without a history of small or large bowel resection at the date of matching. Nonresected patients were matched on age at surgery, sex, type of IBD (Crohn’s disease [CD] or ulcerative colitis [UC]), and duration of IBD, 1:5 with replacement. Population A includes patients with CD undergoing small bowel resection and population B includes patients with CD or UC undergoing large bowel resection.
Small Bowel Resection in Patients With CD
We followed 2469 patients with CD undergoing small bowel resection and 12,331 matched patients with CD and no history of bowel resection for development of T2D. The mean (SD) age at small bowel resection was 39.3 (17.1) years (Table 1), and 56% were women. At the time of resection, CD patients had a median (interquartile range [IQR]) duration of CD of 1.1 (0.1–4.6) years. Ileocecal resection (ICR) was the most common procedure accounting for 79% of resections. The median (IQR) follow-up time for CD patients with small bowel resection was 7.0 (2.4–10.0) years.
Table 1.
Characteristics of the Study Population at Study Entry
| Variables | Small bowel resection |
Large bowel resection |
||||
|---|---|---|---|---|---|---|
| Patients with CD |
Patients with CD |
Patients with UC |
||||
| Yes (N = 2469) | No (N = 12,331) | Yes (N = 1361) | No (N = 6763) | Yes (N = 3787) | No (N = 18,932) | |
| Matching variables | ||||||
| Age at bowel resection / match date | ||||||
| <20 y | 207 (8.4) | 995 (8.1) | 84 (6.2) | 353 (5.2) | 215 (5.7) | 838 (4.4) |
| 20–39 y | 1252 (50.7) | 6341 (51.4) | 513 (37.7) | 2644 (39.1) | 1371 (36.2) | 7088 (37.4) |
| 40–59 y | 626 (25.4) | 3096 (25.1) | 406 (29.8) | 2005 (29.6) | 1097 (29.0) | 5522 (29.2) |
| ≥60 y | 384 (15.6) | 1899 (15.4) | 358 (26.3) | 1761 (26.0) | 1104 (29.2) | 5484 (29.0) |
| Sex | ||||||
| Women | 1393 (56.4) | 6955 (56.4) | 761 (55.9) | 3782 (55.9) | 1838 (48.5) | 9188 (48.5) |
| Men | 1076 (43.6) | 5376 (43.6) | 600 (44.1) | 2981 (44.1) | 1949 (51.5) | 9744 (51.5) |
| Duration of IBD | ||||||
| <1 y | 1217 (49.3) | 5597 (45.4) | 555 (40.8) | 2577 (38.1) | 1308 (34.5) | 6120 (32.3) |
| 1–5 y | 671 (27.2) | 3830 (31.1) | 338 (24.8) | 1886 (27.9) | 1169 (30.9) | 6293 (33.2) |
| 5–10 y | 327 (13.2) | 1646 (13.3) | 239 (17.6) | 1182 (17.5) | 565 (14.9) | 2825 (14.9) |
| ≥10 y | 254 (10.3) | 1258 (10.2) | 229 (16.8) | 1118 (16.5) | 745 (19.7) | 3694 (19.5) |
| Other variables | ||||||
| Year of bowel resection/match date | ||||||
| 1996–2003 | 784 (31.8) | 3919 (31.8) | 480 (35.3) | 2381 (35.2) | 1223 (32.3) | 6115 (32.3) |
| 2004–2011 | 822 (33.3) | 4105 (33.3) | 487 (35.8) | 2420 (35.8) | 1391 (36.7) | 6952 (36.7) |
| 2012–2018 | 863 (35.0) | 4307 (34.9) | 394 (28.9) | 1962 (29.0) | 1173 (31.0) | 5865 (31.0) |
| Type of bowel resection | ||||||
| Small bowel resections | ||||||
| Ileocecal resection | 1955 (79.2) | - | - | - | - | |
| Other | 514 (20.8) | - | - | - | - | |
| Large bowel resections | ||||||
| Total colectomy | - | - | 601 (44.2) | - | 3140 (82.9) | - |
| Right-sided colectomya | - | - | 443 (32.5) | - | 254 (6.7) | - |
| Left-sided colectomyb | - | - | 256 (18.8) | - | 302 (8.0) | - |
| Other | - | - | 61 (4.5) | - | 91 (2.4) | - |
CD, Crohn’s disease; UC, ulcerative colitis.
Right-sided colectomy included right hemicolectomy and resection of colon transversum.
Left-sided colectomy included left hemicolectomy and sigmoidectomy.
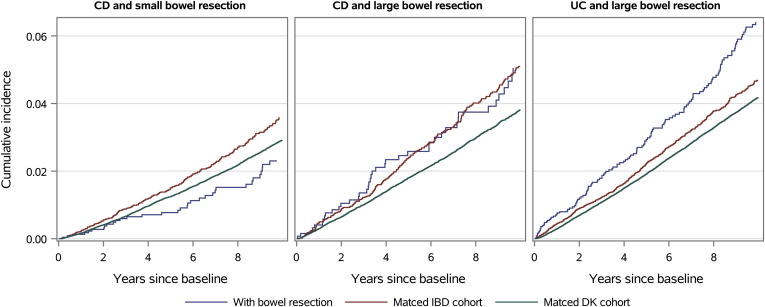
The cumulative incidence of T2D in patients with CD and small bowel resection was 2.4% (95% CI 1.7–3.4) after 10 years, whereas the corresponding number was 3.6% (95% CI, 3.2–4.1) in the matched CD population (Figure 2). On Cox regression modeling, the aHR of T2D was 0.65 (95% CI, 0.44–0.92) in CD patients with vs without small bowel resection (Table 2). Low statistical power precluded the investigation of sex-based differences.
Figure 2.
Cumulative incidence of type 2 diabetes in patients with Crohn’s disease (CD) and small bowel resection; Crohn’s disease and large bowel resection; and ulcerative colitis (UC) and large bowel resection compared with matched patients with CD or UC not undergoing resections and the general Danish background population.
Table 2.
Risk of Type 2 Diabetes in Patients With Inflammatory Bowel Disease and Bowel Resection
| Type of resection | Resection |
No resection |
HR (95% CI) | aHR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| N | Person-years | Events | N | Person-years | Events | |||
| Small bowel resection | ||||||||
| CD | 2469 | 15,299 | 34 | 12,331 | 76,164 | 263 | 0.64 (0.44–0.90) | 0.65 (0.44–0.92) |
| Large bowel resection | ||||||||
| Type of IBD | ||||||||
| CD | 1361 | 8067 | 41 | 6763 | 43,630 | 221 | 1.04 (0.74–1.44) | 0.95 (0.67–1.31) |
| UC | 3787 | 21,174 | 137 | 18,932 | 128,098 | 605 | 1.38 (1.14–1.66) | 1.25 (1.03–1.51) |
CD, Crohn’s disease; Cl, confidence limits; HR, hazard ratio; UC, ulcerative colitis.
HR: hazard ratio adjusted for age and sex.
aHR: hazard ratio adjusted for age, sex, calendar period of bowel resection, area socioeconomic index, IBD duration, IBD-related hospitalizations, treatment with corticosteroids and treatment with a tumor necrosis factor inhibitors. HRs in bold are statistically significant.
Large Bowel Resection in Patients With CD or UC
We followed 1361 patients with CD and 3787 patients with UC undergoing large bowel resection for the development of T2D. We matched them with 6763 patients with CD and 18,932 patients with UC with no history of bowel resection, respectively. The mean (SD) age at large bowel resection was 45.9 (19.3) and 47.0 (19.3) years for patients with CD and UC, respectively (Table 1), and 51% were women overall. At the time of resection, patients with CD and UC had a median (IQR) duration of IBD of 2.2 (0.1–7.3) and 2.4 (0.4–7.8) years, respectively. Total colectomy was the most common procedure accounting for 44% of the resections in patients with CD and 83% of the resections in patients with UC. The median (IQR) follow-up time for patients with CD and UC and large bowel resection was 6.4 (2.1–10.0) and 5.9 (1.3–10.0) years, respectively.
Patients with CD and large bowel resection had similar cumulative incidence of T2D as the matched CD population (Figure 2). On Cox regression modeling, the aHR of T2D was 0.95 (95% CI, 0.67–1.31) in CD patients with vs without large bowel resection (Table 2). Patients with UC and large bowel resection had a higher cumulative incidence of T2D than the matched UC population (Figure 2). The cumulative incidence of T2D in patients with UC and large bowel resection was 6.4% (95% CI, 5.4–7.6) after 10 years, whereas that in the matched UC population was 4.7% (95% CI, 4.3–5.1). On Cox regression modeling, the aHR of T2D was 1.25 (95% CI, 1.03–1.51) in UC patients with vs without large bowel resection (Table 2). The association between large bowel resection and T2D in patients with UC did not differ by sex (P for interaction = .42). When examining the risk of T2D according to the type of large bowel resection, aHRs of T2D for total colectomy, right-sided colectomy, and left-sided colectomy were 1.15 (95% CI, 0.92–1.43), 1.76 (95% CI, 0.93–2.95), and 1.54 (95% CI, 0.91–2.41), respectively, in UC patients with vs without resection. When we introduced a 6- or 12-month lag period after large bowel resection where events of T2D did not count as outcomes, the aHRs of T2D were 1.18 (95% CI, 0.96–1.43) and 1.25 (95% CI, 1.02–1.52) in UC patients with vs without large bowel resection. We also stratified patients with UC and large bowel resection according to whether they had an ileal pouch-anal anastomosis and found that the proportion of patients developing T2D was 5.1% among patients without a pouch and 1.4% among those with a pouch (P < .001). Finally, to examine the impact of UC disease status after surgery, we compared the proportion of UC patients developing T2D among those using vs not using corticosteroids, thiopurines, methotrexate, TNFi or other biologics within 1 year after large bowel resections and found no difference (P = .91).
Discussion
In a nationwide study of 2469 patients with CD and small bowel resection and 5148 patients with IBD and large bowel resection, we observed that small bowel resection was associated with a lower risk of T2D in patients with CD, whereas large bowel resection was associated with a higher risk of T2D in patients with UC.
These findings are intriguing and to our knowledge, there are no previous data reporting on the risk of T2D in association with small bowel resections. We have previously reported a higher risk of T2D after large bowel resections for colorectal cancer and other indications.15 However, within the latter subgroup, UC or CD were not studied separately. Of note, in case of segmental resection, the risk of T2D was higher in individuals who underwent left hemicolectomy and sigmoidectomy, but not proctectomy, right hemicolectomy, or transverse colon resection. In the present study, we did not note an association between segmental colectomy and T2D, although the numbers were small, limiting the statistical power to detect associations with segmental resections. Using the national health insurance database in Taiwan, Wu et al22 reported that in patients who underwent colectomy for indications other than colorectal cancer, the risk of cardiovascular disease was lower with cecectomy and right hemicolectomy and higher with left hemicolectomy. This study, which included 34 total colectomies, reported no association between total colectomy and cardiovascular disease.22 Another study based on Danish nationwide registers, which included 1530 colectomies, also reported no association between total colectomy and development of cardiovascular disease.23
CD most commonly affects the terminal ileum, and in our study, 79% of the small bowel resections in CD patients were ICRs. This surgery can lead to bile acid malabsorption and more frequent bowel movements due to loss of the ileocecal valve,24 which may lead to malabsorption of sugars and fats, and thereby a decrease in the risk of T2D. This means that after surgery, CD patients may be at risk of malabsorption due to removal of critical parts of the small intestine and/or due to persistent CD disease activity. Weight loss is strongly associated with decreased risk of T2D; reduced caloric intake and subsequent weight loss may be other potential mechanisms to mediate this association. Adipokines secreted by mesenteric fat mediate insulin resistance,25 and change in mesenteric fat due to decrease in CD-related inflammation as well as weight loss may play a role in the reduced risk of T2D. Furthermore, the terminal ileum hosts a dense population of L-cells, which secretes the incretin hormone glucagon-like peptide 1 (GLP-1), which in turn stimulates postprandial insulin secretion from the pancreas.26 It is possible the GLP-1 secretion may already be altered in the inflamed terminal ileum before surgery, increasing the risk of T2D among patients with CD, which after ICR is lower. Microbiome changes may also play a role. Certainly, the gut microbiome has been implicated in the development of T2D,27 and ICR can impact the gut microbiome.28 Finally, cecectomy with or without ascending colon resection during ICR may be relevant toward T2D risk, which would be consistent with data reported by Wu et al.22 In contrast, removal of the appendix, which has previously been linked to higher risk of T2D29 is unlikely to account for our findings of a lower risk of T2D after ICR.
The increased risk of T2D related to large bowel resection, specifically in UC, is even less clear. Potentially, changes in the colonic microbiome,27 short-chain fatty acid metabolism,30 and the secretion of the appetite-reducing hormone peptide YY31 may play a role. Also, changes in GLP-1 secretion from L-cells in the colon32,33 could be involved, yet the role of colonic GLP-1 is unclear. Further studies into the role of the colon in glucose homeostasis will be informative. The difference in the risk of T2D after large bowel resection in patients with CD and UC may be because of the limited number of patients with CD who underwent this surgery because in our previous study, large bowel resection was associated with T2D across all indications. Our findings could also reflect those patients with UC who, upon experiencing clinical improvement post-colectomy, may shift dietary patterns toward increased calorie consumption, putting them potentially at risk for T2D. This may also explain, at least in part, why patients with CD who underwent colon resection did not have a higher risk of T2D. Our findings also suggest a higher risk of T2D among UC patients not having a pouch compared with those having a pouch. The underlying mechanism for this difference remains unclear, but microbiome differences could be at play.
The strengths of this study include the nationwide, unselected cohort of IBD patients over a 20-year period from a country with high IBD prevalence, with patients followed for up to 10 years after bowel resection. Using matching and adjustment in statistical models, we controlled for relevant confounding variables, which included corticosteroids, TNFi, and socioeconomic status, each of which could have impacted the exposure or the outcome. Furthermore, in contrast to previous studies,15,22 this study is based on a homogenous cohort of patients with IBD, implying that confounding by indication is less likely. Inclusion of other patient groups, that is, patients with colorectal cancer, diverticulosis, or ileus, could introduce substantial heterogeneity, limiting interpretation of any findings. Our study has certain limitations as well. Although we included only patients with IBD, some heterogeneity may still exist. For example, patients with UC and total colectomy represent various stages of colectomy and pouch transition. The registers do not include data on variables such as adiposity or body mass index precluding adjustment for those potential confounders. There may be a risk of misclassification of the outcome T2D. We based the definition of T2D on 2 previously published algorithms for identifying T2D in the Danish nationwide registers.34,35 However, the algorithms were slightly different, wherefore we used a combined and modified version. Given the fact that we identified T2D based on diagnosis codes and/or medication, it is possible that we did not identify mild cases of T2D, which were not treated with glucose-lowering drugs or seen in hospitals. On the other hand, we may have included patients with temporary hyperglycemia from a severe flare treated with high doses of corticosteroids. Yet, this is likely to be nondifferential, which could lead to underestimation, but not overestimation, of the associations under study. The validity of ICD-8 and ICD-10 codes for CD and UC in the Danish National Patient Register has been demonstrated to be high.36,37 In a recent validation study, Lo et al36 found excellent sensitivity and specificity for the ICD-10 diagnoses of CD and UC in the Danish National Patient Register when using the requirement of 2 separate records. In addition to using 2 separate records, we included IBD patients with one contact lasting 7 days or more to account for patients having a long inpatient or outpatient contact. As these patients accounted for 10.5% of the included patients, substantial misclassification of CD and UC in our study is unlikely. The Danish National Patient Register does not contain detailed information on disease location and behavior, precluding analyses taking these into account.36 Finally, our study may lack adequate power to detect differences in subgroups.
In summary, we report that in patients with CD who undergo small bowel resection, the risk of T2D was lower, and in patients with UC who undergo large bowel resection, the risk of T2D was higher, compared with IBD patients who did not undergo bowel resection surgery. Especially, the higher risk of T2D in patients with UC undergoing large bowel resection has clinical implications, as this patient group is not traditionally considered a patient group at risk of metabolic diseases. The findings also set the framework for future studies exploring cardiometabolic outcomes in IBD.
Acknowledgments
Authors' Contributions:
Kristine H. Allin: Conceptualization: Equal, Formal analysis: Supporting, Funding acquisition: Equal, Methodology: Equal, Writing – original draft: Lead, Writing – review & editing: Equal. Manasi Agrawal: Formal analysis: Supporting, Methodology: Equal, Writing – original draft: Supporting, Writing – review & editing: Equal. Aske T. Iversen: Data curation: Lead, Formal analysis: Lead, Methodology: Equal, Writing – review & editing: Equal. Jacob Antonsen: Writing – review & editing: Equal. Marie Villumsen: Writing – review & editing: Equal. Tine Jess: Conceptualization: Equal, Formal analysis: Supporting, Funding acquisition: Equal, Methodology: Equal, Writing – review & editing: Equal. All authors approved the final version of the manuscript.
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Funding: K.H.A. was supported by the Novo Nordisk Foundation (grant number NNF16OC0022586) and the Danish Diabetes Association. T.J. was supported by the Danish National Research Foundation (grant no. DNRF148). M.A. was supported by NIH K23 Career Development Award (K23DK129762-01). These had no role in the study design or in the collection, analysis, and interpretation of data.
Ethical Statement: The corresponding author, on behalf of all authors, jointly and severally, certifies that their institution has approved the protocol for any investigation involving humans or animals and that all experimentation was conducted in conformity with ethical and humane principles of research.
Data Transparency Statement: The study is based on data from the Danish nationwide registers (https://sundhedsdatastyrelsen.dk). The register data are protected by the Danish Act on Processing of Personal Data and are accessed through application to and approval from the Danish Data Protection Agency and the Danish Health Data Authority.
Material associated with this article can be found in the online version at https://doi.org/10.1016/j.gastha.2022.06.007.
Supplementary Materials
References
- 1.Ng S.C., Shi H.Y., Hamidi N., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 2.Torres J., Mehandru S., Colombel J.F., et al. Crohn's disease. Lancet. 2017;389:1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 3.Ungaro R., Mehandru S., Allen P.B., et al. Ulcerative colitis. Lancet. 2017;389:1756–1770. doi: 10.1016/S0140-6736(16)32126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rungoe C., Langholz E., Andersson M., et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979-2011. Gut. 2014;63:1607–1616. doi: 10.1136/gutjnl-2013-305607. [DOI] [PubMed] [Google Scholar]
- 5.Rungoe C., Basit S., Ranthe M.F., et al. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut. 2013;62:689–694. doi: 10.1136/gutjnl-2012-303285. [DOI] [PubMed] [Google Scholar]
- 6.Singh S., Singh H., Loftus EV J., et al. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:382–393.e1. doi: 10.1016/j.cgh.2013.08.023. quiz e22. [DOI] [PubMed] [Google Scholar]
- 7.Aniwan S., Pardi D.S., Tremaine W.J., et al. Increased risk of acute myocardial infarction and heart failure in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16:1607–1615.e1. doi: 10.1016/j.cgh.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng W., Chen G., Cai D., et al. Inflammatory bowel disease and risk of ischemic heart disease: an updated meta-analysis of cohort studies. J Am Heart Assoc. 2017;6:e005892. doi: 10.1161/JAHA.117.005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirchgesner J., Beaugerie L., Carrat F., et al. Increased risk of acute arterial events in young patients and severely active IBD: a nationwide French cohort study. Gut. 2018;67:1261–1268. doi: 10.1136/gutjnl-2017-314015. [DOI] [PubMed] [Google Scholar]
- 10.Dregan A., Charlton J., Chowienczyk P., et al. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation. 2014;130:837–844. doi: 10.1161/CIRCULATIONAHA.114.009990. [DOI] [PubMed] [Google Scholar]
- 11.Jess T., Jensen B.W., Andersson M., et al. Inflammatory bowel diseases increase risk of type 2 diabetes in a nationwide cohort study. Clin Gastroenterol Hepatol. 2020;18:881–888.e1. doi: 10.1016/j.cgh.2019.07.052. [DOI] [PubMed] [Google Scholar]
- 12.Yarur A.J., Deshpande A.R., Pechman D.M., et al. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011;106:741–747. doi: 10.1038/ajg.2011.63. [DOI] [PubMed] [Google Scholar]
- 13.Aarestrup J., Jess T., Kobylecki C.J., et al. Cardiovascular risk profile among patients with inflammatory bowel disease: a population-based study of more than 100 000 individuals. J Crohns Colitis. 2019;13:319–323. doi: 10.1093/ecco-jcc/jjy164. [DOI] [PubMed] [Google Scholar]
- 14.Madsbad S., Holst J.J. Bariatric surgery—which procedure is the optimal choice? Lancet. 2019;393:1263–1264. doi: 10.1016/S0140-6736(19)30489-1. [DOI] [PubMed] [Google Scholar]
- 15.Jensen A.B., Sorensen T.I., Pedersen O., et al. Increase in clinically recorded type 2 diabetes after colectomy. Elife. 2018;7 doi: 10.7554/eLife.37420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt M., Pedersen L., Sorensen H.T. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 17.Duricova D., Burisch J., Jess T., et al. Age-related differences in presentation and course of inflammatory bowel disease: an update on the population-based literature. J Crohns Colitis. 2014;8:1351–1361. doi: 10.1016/j.crohns.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M., Schmidt S.A., Sandegaard J.L., et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pottegard A., Schmidt S.A.J., Wallach-Kildemoes H., et al. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46 doi: 10.1093/ije/dyw213. 798–798f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomsen S.B., Allin K.H., Burisch J., et al. Outcome of concomitant treatment with thiopurines and allopurinol in patients with inflammatory bowel disease: a nationwide Danish cohort study. United European Gastroenterol J. 2020;8:68–76. doi: 10.1177/2050640619868387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes E.L., Allin K.H., Iversen A.T., et al. Increasing incidence of pouchitis between 1996 and 2018: a population-based Danish cohort study. Clin Gastroenterol Hepatol. 2022 doi: 10.1016/j.cgh.2022.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C.C., Hsu T.W., Yeh C.C., et al. The impact of colectomy on the risk of cardiovascular disease among patients without colorectal cancer. Sci Rep. 2020;10:2925. doi: 10.1038/s41598-020-59640-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen A.B., Ajslev T.A., Brunak S., et al. Long-term risk of cardiovascular and cerebrovascular disease after removal of the colonic microbiota by colectomy: a cohort study based on the Danish National Patient Register from 1996 to 2014. BMJ Open. 2015;5:e008702. doi: 10.1136/bmjopen-2015-008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camilleri M. Advances in understanding of bile acid diarrhea. Expert Rev Gastroenterol Hepatol. 2014;8:49–61. doi: 10.1586/17474124.2014.851599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabe K., Lehrke M., Parhofer K.G., et al. Adipokines and insulin resistance. Mol Med. 2008;14:741–751. doi: 10.2119/2008-00058.Rabe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holst J.J., Gribble F., Horowitz M., et al. Roles of the gut in glucose homeostasis. Diabetes Care. 2016;39:884–892. doi: 10.2337/dc16-0351. [DOI] [PubMed] [Google Scholar]
- 27.Allin K.H., Tremaroli V., Caesar R., et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia. 2018;61:810–820. doi: 10.1007/s00125-018-4550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mondot S., Lepage P., Seksik P., et al. Structural robustness of the gut mucosal microbiota is associated with Crohn's disease remission after surgery. Gut. 2016;65:954–962. doi: 10.1136/gutjnl-2015-309184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei P.L., Tsai M.C., Hung S.H., et al. Risk of new-onset type II diabetes after appendicectomy. Br J Surg. 2015;102:1267–1271. doi: 10.1002/bjs.9875. [DOI] [PubMed] [Google Scholar]
- 30.Allin K.H., Nielsen T., Pedersen O. Mechanisms in endocrinology: gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2015;172:167. doi: 10.1530/EJE-14-0874. [DOI] [PubMed] [Google Scholar]
- 31.Wynne K., Bloom S.R. The role of oxyntomodulin and peptide tyrosine-tyrosine (PYY) in appetite control. Nat Clin Pract Endocrinol Metab. 2006;2:612–620. doi: 10.1038/ncpendmet0318. [DOI] [PubMed] [Google Scholar]
- 32.Eissele R., Goke R., Willemer S., et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22:283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 33.Gunawardene A.R., Corfe B.M., Staton C.A. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92:219–231. doi: 10.1111/j.1365-2613.2011.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Danish Health Data Authority . The Danish Health Data Authority; Copenhagen, Denmark: 2019. Algoritmer for udvalgte kroniske sygdomme og svære psykiske lidelser. [Google Scholar]
- 35.Carstensen B., Ronn P.F., Jorgensen M.E. Prevalence, incidence and mortality of type 1 and type 2 diabetes in Denmark 1996-2016. BMJ Open Diabetes Res Care. 2020;8:e001071. doi: 10.1136/bmjdrc-2019-001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo B., Vind I., Vester-Andersen M.K., et al. Validation of ulcerative colitis and Crohn's disease and their phenotypes in the Danish National Patient Registry using a population-based cohort. Scand J Gastroenterol. 2020;55:1171–1175. doi: 10.1080/00365521.2020.1807598. [DOI] [PubMed] [Google Scholar]
- 37.Fonager K., Sorensen H.T., Rasmussen S.N., et al. Assessment of the diagnoses of Crohn's disease and ulcerative colitis in a Danish hospital information system. Scand J Gastroenterol. 1996;31:154–159. doi: 10.3109/00365529609031980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.