ABSTRACT
To achieve the full benefits of vaccination, it is key to understand the underlying causes of low vaccination by researching the barriers to vaccination at a local level. This systematic literature review aims to identify the reasons given by community members for the non-vaccination and under-vaccination of children and adolescents in sub-Saharan Africa. PubMed, Web of Science, PsycINFO, African Index Medicus, and African Journals Online databases were searched to identify articles published between 2010 and 2020. A total of 37 articles were included. As 17 studies did not report the reasons for non-vaccination and under-vaccination separately, we considered these two outcomes as “incomplete vaccination”. The most common reasons for incomplete vaccination were related to caregiver’s time constraints, lack of knowledge regarding vaccination, the unavailability of vaccines/personnel in healthcare facilities, missed opportunities for vaccination, caregiver’s fear of minor side effects, poor access to vaccination services, and caregiver’s vaccination beliefs.
KEYWORDS: Sub-Saharan Africa, child, adolescent, vaccination, reasons, systematic review
Introduction
Although routine childhood vaccination coverage in Africa has significantly improved since the 2000s, overall coverage rates remain below expected targets.1 According to the 2019 World Health Organization (WHO)/United Nations Children’s Fund (UNICEF) estimates, coverage with three doses of diphtheria-tetanus-pertussis vaccine (DTP3) – used as a proxy for full childhood vaccination coverage - in Africa have stagnated below 80% during the past decade. Furthermore, 9.4 million of the 19.7 million non-vaccinated and under-vaccinated (without DTP3) children worldwide live in Africa, primarily in Nigeria, the Democratic Republic of the Congo, Ethiopia, and Angola.2 As a consequence, vaccine-preventable diseases (VPDs) are a major public health threat in Africa. More than 30 million children under five suffer from VPDs each year, accounting for a third of VPDs incidence worldwide.1 Moreover, over half a million children die from VPDs annually on the continent, mainly due to pneumococcal diseases and rotavirus.1
Political commitment to improve vaccination coverage in Africa and reduce VPDs has increased in recent years. The Addis Declaration on Immunization was endorsed by African Heads of State in January 2017, and commits to advance universal access to immunization across the continent.3 In 2018, the 2030 Ambition for Immunization in Africa1 was published by the WHO African Region and aims to (i) sustain the control, elimination, or eradication of poliomyelitis, rubella, tetanus, measles, and hepatitis B; (ii) reduce mortality attributable to rotavirus, cervical cancer, pneumococcal diseases, and malaria and; (iii) empower high-risk countries to fight against meningitis, cholera, yellow fever, and typhoid. These aims will help countries reach Universal Health Coverage and Sustainable Development Goals.
To achieve the full benefits of vaccination, it is important to understand the underlying causes of low vaccination by researching the barriers to vaccination at a local level. This has been identified as a key research area in the Addis Declaration on Immunization4 and the WHO Immunization Agenda 2030.5 These findings will help shape messages and strategies to address these barriers.
Several systematic reviews have studied the barriers to complete childhood vaccination in low- and middle-countries.6–11 These studies mainly focused on the sociodemographic factors associated with incomplete vaccination. However, sociodemographic predictors of vaccination are not sufficient to explain the barriers to vaccination. Indeed, it is important to understand the reasons that drive caregivers to incompletely vaccinate children.12 Although three systematic reviews also looked at the reasons for incomplete vaccination, they did not report the reasons separately from the factors6,7,11 and only one of them was specific to sub-Saharan Africa.11
This systematic literature review aims to identify the reasons given by community members for non-vaccination, under-vaccination, and untimely vaccination of children and adolescents in sub-Saharan Africa. The findings of this review will help guide policymakers to achieve the benefits of vaccination in sub-Saharan Africa.
Methods
The protocol for this systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020178123), and the review was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.
Criteria for considering studies and quality assessment
Studies on the reasons given for non-vaccination (i.e., receiving no vaccine doses), under-vaccination (i.e., receiving at least one but not all recommended vaccine doses), and untimely vaccination (i.e., receiving the recommended vaccines but not within the recommended delays) of children and adolescents (birth to 19 years) in the general population, eligible for vaccination, and living in sub-Saharan Africa, were considered for the review. Of note, the outcome “untimely vaccination” was dropped as we found too few studies (less than five) reporting that outcome. The exposure of interest was childhood and adolescent vaccines, regardless of the vaccine delivery strategy (e.g., routine vaccination services, vaccination campaigns). Studies had to be (1) empirical; (2) observational or interventional (if reasons for non-vaccination or under-vaccination were reported); and (3) qualitative, quantitative, or mixed methods.
The level of relevance of the eligible articles was assessed by one author (LP) and reviewed by another (PPW) according to five criteria: (1) one of the study objectives was to investigate the reasons for non-vaccination and/or under-vaccination; (2) the study took place in a community setting; (3) the study linked the reasons to children’s vaccination status; (4) the results were reported in sufficient detail; (5) the findings were compared to the results of other studies. Only articles meeting at least three of these five criteria were included in the review.
Search strategy
PubMed, Web of Science, PsycINFO, African Index Medicus, and African Journals Online databases were searched on 3 March 2020 using a comprehensive search strategy. We have provided the search strategy for all databases searched (Table 1). We searched for published articles with no language and publication date restriction. However, we then decided to exclude studies published before 2010 as a systematic review by Rainey et al. had already identified the reasons for non- and under-vaccination of children and adolescents in low- and middle-income countries using studies published between 1999 and 2009.6 Authors were contacted when the full-text article could not be accessed. The reference sections of the articles assessed for eligibility were also examined to supplement the search. Searches were re-run before the final analysis on 11 December 2020. The reference management software Zotero (Center for History and New Media, George Mason University) was used to manage the literature search output and remove duplicates.
Table 1.
Key words used in the search strategy.
| Theme # | Theme | Key words (in title/abstract) |
|---|---|---|
| 1 | Reasons | Reasons OR reason OR motive OR motives OR motivation OR motivations |
| 2 | Child | Child OR children OR infant OR infants OR parents OR parents OR newborn OR newborns OR baby OR babies |
| 3 | Adolescent | Adolescents OR adolescent OR “young adult” OR “young adults” OR teenager OR teenagers OR teen OR teens |
| 4 | Vaccination | Vaccin* OR immunis* OR immuniz* |
| 5 | Under-vaccination | Complet* OR incomplete OR partial OR adherence OR adhesion OR compliance OR undervaccination OR under-vaccination OR under immunization OR underimmunization OR under-immunisation OR under-immunization OR coverage OR status OR dropout OR drop-out OR contin* OR suboptimal |
| 6 | Untimely vaccination | Delay* OR time* |
| 7 | Non-vaccination | Non-vaccin* OR non-immuniz* OR non-immunis* OR nonimmunis* OR nonimmuniz* OR nonvaccin* |
| 8 | Sub-Saharan Africa | Africa OR angola OR benin OR botswana OR “burkina faso” OR burundi OR cameroon OR “cape verde” OR “central african republic” OR chad OR comoros OR “democratic republic of the congo” OR “republic of the congo” OR djibouti OR equatorial guinea OR “guinea-bissau” OR guinea OR eritrea OR ethiopia OR gabon OR gambia OR ghana OR ivory coast OR “cote d’ivoire” OR kenya OR lesotho OR liberia OR madagascar OR malawi OR mali OR mauritania OR mauritius OR mozambique OR namibia OR niger OR nigeria OR rwanda OR “sao tome and principe” OR senegal OR seychelles OR “sierra leone” OR somalia OR “south africa” OR swaziland OR eswatini OR sudan OR south sudan OR tanzania OR togo OR uganda OR zambia OR zimbabwe |
Notes: Search 1 AND (2 OR 3) AND 4 AND (5 OR 6 OR 7) AND 8.
Selection of studies
Three review authors (LP, VS, PPW) independently screened the titles and abstracts to identify potentially eligible studies. Disagreements between the authors were resolved through discussion and consensus. We obtained the full texts of all potentially eligible studies. Two authors (LP, PPW) independently screened the full texts and identified included studies, resolving discrepancies through discussion and consensus.
Data extraction
One author (LP) extracted data from the included studies using a standardized Excel form. Data were reviewed by another author (PPW). The following information was extracted from each study: authors, publication year, study setting (country, level [i.e., national, state/region, local], community/health facility-based, rural/urban), study design, study population, whether the child’s or adolescent’s vaccination status was known, data collection methods (quantitative/qualitative), outcomes measured (non-vaccination/under-vaccination) and the reasons for non-vaccination and under-vaccination.
Reasons were categorized according to a modified version of the themes in the review by Rainey and colleagues6 which were adapted from the “Classification of Factors Affecting Receipt of Vaccines” from Vaccines (5th Edition).13 Each reason was classified into one theme and was weighted equally regardless of study type or the study sample size. The eleven themes were: time constraints, lack of knowledge regarding vaccination, unavailable vaccines or personnel in healthcare facilities, missed opportunities for vaccination, beliefs about vaccination, fear of minor side effects, poor access to vaccination services, poor relationship with healthcare providers, social or cultural pressure against vaccinations, cost related to vaccination and unexplored/unexplained reasons.
The main results are reported distinguishing the results from quantitative studies and qualitative ones, where possible/relevant.
Results
Results of the search
The database search generated 538 articles. After removing duplicates, we reviewed 381 abstracts and assessed 89 full-text articles for eligibility. We assessed the relevance of 71 eligible articles of which 37 were included in the literature review. The process used for the search and selection of studies for this review are described in Figure 1. Twenty-four studies had a relevance score of three, nine had a score of four, and four had a score of five.
Figure 1.
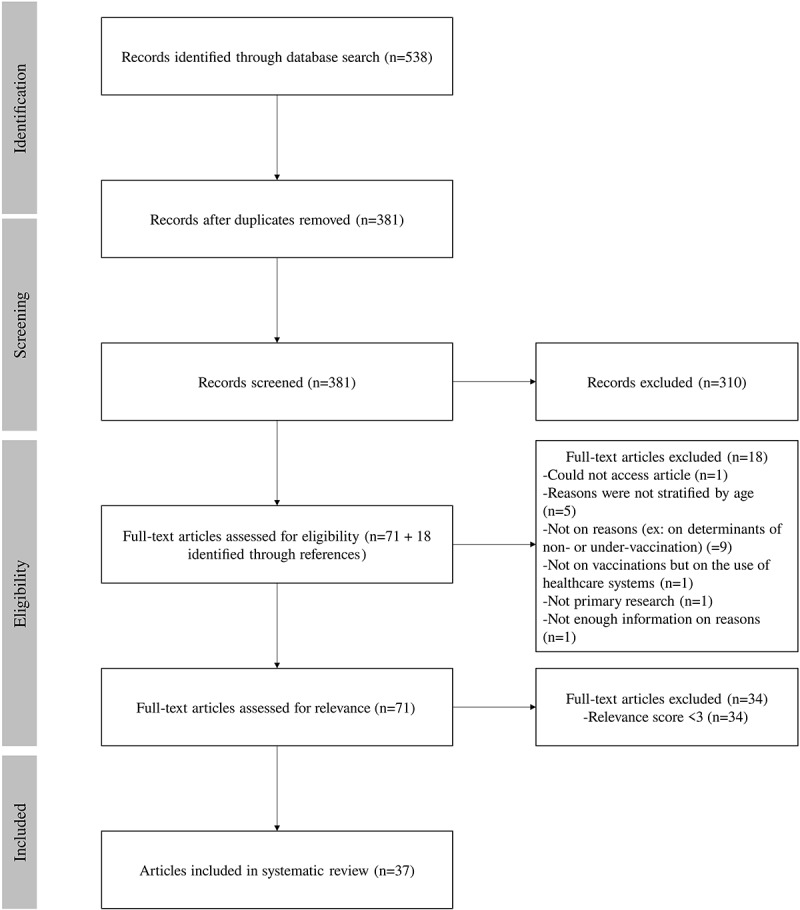
Flow diagram of literature search processes.
Description of studies
Study design and setting
Table 2 presents the description of the included studies. Studies were conducted in 11 different countries: Nigeria (12 studies), Uganda (7), Ethiopia (5), Kenya (3), Burkina Faso (2), Cameroon (2), Malawi (2), Ghana (1), Guinea-Bissau (1), South Sudan (1) and Tanzania (1). Most studies were conducted in the community setting (33) and at a local level (town/municipality/district/county) (28). Eight studies were conducted in rural areas, eight in urban areas, and 20 in both rural and urban areas (the setting was not specified in one study). All studies were cross-sectional. Reasons for non-vaccination and under-vaccination were identified using quantitative methods in 25 studies, using qualitative methods in eight studies, and four studies used both methods.
Table 2.
Study characteristics.
| First author, yearref | Study location and setting |
Methods used to study the reasons for incomplete vaccination |
Relevance score | |||||
|---|---|---|---|---|---|---|---|---|
| Country (level: national, regional/state, local*) | Healthcare facility/community (rural/urban) | Person interviewed (child/adolescent target population) | Vaccine | Child vaccination status known | Collection methods (qualitative/quantitative) | Study outcome (non-vaccination, under-vaccination, both [simultaneously or separately]) | ||
| Abdulraheem, 201114 | Nigeria (local) | Community (rural) | Mothers (0–11 months) | Routine vaccines (BCG, DTP, polio, measles, hepatitis B) | Yes | Quantitative | Under-vaccination | 4 |
| Abebe, 201915 | Ethiopia (local) | Community (both) | Mother or caregiver (12–23 months) | Measles | Quantitative: Yes Qualitative: No |
Both | Non-vaccination | 3 |
| Adamu, 201916 | Nigeria (local) | Healthcare facility (urban) | Caregivers (0–23 months) | Routine vaccines (not specified) | No | Qualitative | Both (simultaneously) | 3 |
| Atwiine, 201617 | Uganda (local) | Community (rural) | Caregivers (12–23 months) | Routine vaccines (not specified) | Yes | Quantitative | Under-vaccination | 3 |
| Babalola, 201112 | Nigeria (state) | Community (both) | Mothers (children) | Routine vaccines (not specified) | Yes | Qualitative | Both (separately) | 5 |
| Babirye, 201118 | Uganda (local) | Community (urban) | Mothers, fathers, those in charge of community mobilization for vaccination(<5 years) | Routine vaccines (not specified) | No | Qualitative | Both (simultaneously) | 3 |
| Babirye, 201419 | Uganda (local) | Community (urban) | Mothers, fathers, those in charge of community mobilisation for vaccination (<5 years) |
Routine vaccines (not specified) | No | Qualitative | Both (simultaneously) | 3 |
| Cockcroft, 201420 | Nigeria (state) | Community (both) | Mother or father (young children) | Measles | No | Qualitative | Non-vaccination | 3 |
| Holte, 201221 | Malawi (local) | Community (rural) | Main person responsible for making decisions about childhood vaccination (18–59 months old) | Routine vaccines (not specified) | Yes | Quantitative | Under-vaccination | 3 |
| Itimi, 201222 | Nigeria (local) | Community (both) | Female head of households (<2 years) | Routine vaccines (BCG, OPV, DTP) | Yes | Quantitative | Both (simultaneously) | 3 |
| Kagoné, 201823 | Burkina Faso (local) | Community (rural) | Mothers of children <3 years, godmothers, community health workers, traditional healers | Routine vaccines (BCG, OPV, pentavalent, PCV, rotavirus, yellow fever, measles) | No | Qualitative | Under-vaccination | 3 |
| Kwedi Nolna, 201824 | Cameroon (local) | Community (urban) | Parents and guardians (12–23 months) | Routine vaccines (not specified) | No | Both | Non-vaccination (quantitative) + Both (simultaneously, qualitative) |
4 |
| LaMontagne, 201125 | Uganda (local) | Community (not specified) | Any adult who could verify the girl’s vaccination status and respond accurately to survey questions (primary five class or 10 years old) | HPV | Yes | Quantitative | Both (simultaneously) | 5 |
| Mbabazi, 201326 | South Sudan (national) | Community (both) | Mothers or caretakers (12–23 months) | Routine vaccines (BCG, DTP, OPV, measles) | Yes | Quantitative | Both (simultaneously) | 4 |
| Mekonnen, 201927 | Ethiopia (local) | Community (both) | Mothers or caregivers (12–23 months) | Routine vaccines (BCG, measles, rotavirus, pentavalent, OPV, PCV) | Yes | Quantitative | Both (simultaneously) + Under-vaccination (for one sub analysis) |
3 |
| Meyer, 201528 | Burkina Faso (national) | Community (both) | PsA: Parent or head of household (2–15 years) MCV: parent or guardian (12–23 months) |
Measles | Yes | Quantitative | Non-vaccination | 3 |
| Michael, 201429 | Nigeria (local) | Community (both) | Caregiver (<5 years) | OPV | Yes | Both | Non-vaccination | 3 |
| Michael, 201430 | Nigeria (local) | Community (both) | Caregiver (<5 years) | OPV | Yes | Quantitative | Non-vaccination | 5 |
| Msyamboza, 201731 | Malawi (local) | Both (both) | School: girls 9-13 Community: parents/caregivers of adolescents (9–13 years) |
HPV | Yes | Quantitative | Both (simultaneously) | 3 |
| Murele, 201432 | Nigeria (local) | Community (both) | Parents (children) | OPV | Yes | Qualitative | Non-vaccination | 3 |
| Nabirye, 202033 | Uganda (local) | Community (both) | Adolescents 9–15 years | HPV | Yes | Quantitative | Both (simultaneously) | 3 |
| Nguefack Dongmo, 201634 | Cameroon (local) | Community (urban) | Mothers (11–48 months) | Routine vaccines (BCG, pentavalent, polio, yellow fever, measles) | Yes | Quantitative | Both (simultaneously) | 3 |
| Njeru, 201635 | Kenya (national) | Community (both) | Parents and guardians (<5 years old) | Polio | Yes | Quantitative | Non-vaccination | 3 |
| Nsubuga, 201936 | Uganda (national) | Community (both) | Caretakers (12–23 months) | Routine vaccines (BCG, OPV, PCV, measles) | Yes | Quantitative | Both (simultaneously) | 3 |
| Okenwa, 201937 | Nigeria (state) | Healthcare facility (both) | Mothers (infants) | Hepatitis B birth dose | Yes | Quantitative | Non-vaccination | 3 |
| Oladokun, 201038 | Nigeria (local) | Community (urban) | Mothers (12–23 months) | Routine vaccines (BCG, OPV, DTP, measles, hepatitis B, yellow fever) | Yes | Quantitative | Both (simultaneously) | 4 |
| Oria, 201339 | Kenya (local) | Community (both) | Questionnaire: head of households FGD : parents partially or non-vaccinated children (6m-10 years) |
Influenza | Yes | Both | Both (separately) | 3 |
| Porth, 201940 | Ethiopia (national) | Community (both) | Caregivers (12–23 months) | Routine vaccines (BCG, OPV, pentavalent, PCV, measles, rotavirus) | Yes | Quantitative | Both (simultaneously) | 4 |
| Sally, 201741 | Ghana (local) | Community (rural) | Mothers or guardians (12–23 months) | Routine vaccines (DTP, BCG, OPV, PCV, MMR) | Yes | Quantitative | Both (simultaneously) | 3 |
| Sanni, 201942 | Nigeria (local) | Community (rural) | Parents or caregivers (0–11 months) | Routine vaccines (HepB0, OPV, BCG, pentavelent, PCV, IPV, measles, yellow fever) | Yes | Quantitative | Both (simultaneously) | 4 |
| Sato, 202043 | Nigeria (national) | Community (both) | Mothers or caregivers (<2 years old) | Routines vaccines (HepB0, BCG, OPV, pentavalent or DPT, PCV, IPV, measles, yellow fever) | Yes | Quantitative | Both (separately) | 5 |
| Tefera, 201844 | Ethiopia (local) | Community (urban) | Mothers (12–23 months) | Routine vaccines (BCG, pentavalent, Hib, PCV, rotavirus, OPV, measles) | Yes | Quantitative | Both (simultaneously) | 4 |
| Thysen, 201445 | Guinea-Bissau (local) | Community (rural) | Mothers (<2 years) | BCG | Yes | Quantitative | Non-vaccination | 3 |
| Vermandere, 201446 | Kenya (local) | Community (urban) | Mothers (9–14 years) | HPV | Yes | Quantitative | Non-vaccination | 3 |
| Vonasek, 201647 | Uganda (local) | Community (rural) | Women 15–49 (<5 years) | Routine vaccines (Polio, pentavalent BCG) | No | Quantitative | Both (simultaneously) | 3 |
| Watson-Jones, 201248 | Tanzania (local) | Community (both) | Parents and children (14 years/class 6) | HPV | Yes | Quantitative | Non-vaccination | 4 |
| Zewdie, 201649 | Ethiopia (local) | Healthcare facility (both) | Mothers (6–11 months) | DTP | Yes | Qualitative | Under-vaccination | 4 |
*Town, municipality, district, county.
Note: Abbreviations: BCG = Bacillus Calmette-Guerin, DTP = diphtheria-tetanus-pertussis, HepB0 = hepatitis B birth dose vaccine, Hib = haemophilus influenzae type B, HPV = human papillomavirus vaccine, IPV = inactivated polio vaccine, MMR = measles mumps rubella, OPV = oral polio vaccine, PCV = pneumococcal conjugate vaccine.
Population
In 33 studies, caregivers, including mothers, were interviewed. The target population was children under two years old in 19 studies.
Exposure
Sixteen studies reported reasons for non-vaccination and under-vaccination with one type of vaccine (measles, polio, human papillomavirus [HPV], hepatitis B birth dose [HepB-BD], influenza, diphtheria-tetanus-pertussis [DTP], Bacillus Calmette Guerin [BCG]), and twenty-one studies reported reasons for several routine vaccines (such as the combination of BCG, polio, pentavalent/DTP, rotavirus, yellow fever, measles, etc.).
Outcomes
Five studies only identified the reasons for under-vaccination and 12 only examined the reasons for non-vaccination. Twenty studies explored the reasons for both non-vaccination and under-vaccination; three studies reported the reasons separately whilst 17 studies did not distinguish the reasons for non-vaccination and under-vaccination.
As 17 studies did not report the reasons for non-vaccination and under-vaccination separately, we considered these two outcomes as “incomplete vaccination”.43
The most frequently mentioned reasons for incomplete vaccination were related to time constraints (27 studies), lack of knowledge regarding vaccinations (26), the unavailability of vaccines/personnel in healthcare facilities (26), missed opportunities for vaccination (25), fear of minor side effects (23), poor access to vaccination services (21) and beliefs about vaccination (17).
In the narrative synthesis, reasons are presented from the most to the less frequently reported. The study findings are presented in Table S1 in the appendices.
Time constraints
In 27 studies,12,14–24,26–28,31,34,36,38–41,43,44,46,47,49 children and adolescents were incompletely vaccinated because of their caregiver’s time constraints: caregivers were unavailable, waiting times at vaccination centers were too long, and/or vaccination times were inconvenient.
These three reasons were reported separately in quantitative studies. Caregivers were unavailable to bring their child for vaccination because they were too busy (work, travel) or were sick.12,14,17,21,24,26,34,36,38–41,43,44,46,47 This was mentioned by more than 10% of caregivers in twelve studies.12,21,24,26,34,36,39–41,43,44,46 On the other hand, long waiting times14,22,26–28,31,38–40,44,47 and inconvenient vaccination times15,22,26,28,31,38–41,44 were less frequently mentioned (generally by less than 10% of participants).
Qualitative results showed that in most settings, women were responsible for childcare (including vaccination activities) as well as for other household and economic activities. Vaccination was therefore seen as both a time and an economic constraint.16,18–20,23,49 Often, vaccination times were not adapted to women’s working hours18 and women did not have time to wait in healthcare facilities for vaccination.19,20,23,49 One study in Nigeria found that men could not take time off work to bring their child for vaccination, due to high job insecurity in the area.18
Lack of knowledge regarding vaccination
Lack of knowledge regarding vaccines and the organization of vaccination services was a reason for incomplete vaccination in 26 studies12,14,15,20,22–29,31,33,37–44,46–49 on both specific vaccines and routine vaccines.
Lack of knowledge regarding vaccination included: not knowing about the vaccine or vaccination in general,15,27,33,39 not knowing the benefits or understanding the need for vaccination,12,23,24,26,41–43 and not knowing the vaccination schedule.12,22,24,26,33,37,38,41–43 In eight quantitative studies, more than 20% of participants said that they lacked knowledge of vaccination.12,15,24,33,37,43,47,48 In qualitative results, some caregivers believed that children only needed one vaccine dose to be immunized, or that the child did not need additional vaccines.12,24
Community members were sometimes unaware of the organization of vaccination services. For example, they did not know the place and/or time of vaccination15,23,26,28,39–44 or were unaware of the vaccination program or campaign.25,28,29,31,38 Eight studies were on vaccines delivered through vaccination campaigns: measles15,20,28 HPV25,31,46,48 polio29 flu.39 This was rarely cited in quantitative studies (mentioned by less than 10% of participants14,15,24,25,28,29,31,38–44).In two studies, parents did not differentiate mass vaccination campaigns from routine immunization and were therefore waiting for the vaccinators to come visit their homes to perform routine vaccines.12,24 Qualitative results showed that mothers who traveled frequently did not know they could receive vaccination services in facilities outside their residential area49 and that caregivers thought that children without a birth certificate could not be vaccinated in healthcare facilities.20
Unavailable vaccines/personnel at healthcare facilities
In 26 studies12,14,17–22,24,26–29,34–38,40–45,47,49 children and adolescents were incompletely vaccinated because there were no vaccines in healthcare facilities, or vaccinators were absent/unavailable.
Vaccine unavailability was an issue for at least 20% of participants in most of the quantitative studies.12,17,21,22,27,36–38,40,42 Qualitative results showed that caregivers went to vaccination services several times but there were no vaccines, which discouraged them from returning.19,20,49 In Uganda, vaccines were stored at headquarters as healthcare facilities did not have refrigerators, but it could take healthcare workers several hours to get the vaccines.19
The unavailability or absence of vaccinators was mostly reported on specific vaccines administered through campaigns. This was rarely cited as a reason in quantitative studies (reported by less than 10% of participants26,28,38,40,41,43,44) except in polio campaigns in Kenya35and Nigeria29 where respectively 44% and 25% of participants said that children were not vaccinated because vaccinators did not visit their homes.
Missed opportunities for vaccination
In 25 studies12,14,16,17,22–28,30,31,34–40,43–45,48,49 children and adolescents were incompletely vaccinated because of missed opportunities for vaccination; mostly due to wrong contraindications and the child’s absence during vaccination times. These were only mentioned in quantitative studies.
Wrong contraindications included the child being ill at the time of vaccination,14,22,24,26,28,30,31,34,36,38–40,44 caregivers believing that the child had already been vaccinated or was not at risk,12,28,30,31,43or that child was too young.12,28,30,31 These were frequently mentioned (by more than 10% of participants12,17,22,24,25,36,38–40,43).
The child’s absence at the time of vaccination was mentioned in seven studies, mainly during school or community vaccination campaigns (i.e., polio30,35 HPV31,48 flu39).
Other missed opportunities to vaccinate, which were less frequently reported, included giving birth on a day when no vaccination sessions were being held,36,37 not having a vaccination card,17 healthcare workers not checking the child’s vaccination status,16 caregivers missing the appointment date,27,49 and healthcare workers refusing to vaccinate.16,27,40
Fear of minor side effects
In 23 studies,12,15,16,18,20,22–24,26–28,31,34,38–44,46–48 children and adolescents were incompletely vaccinated because their caregivers feared vaccination minor side effects. This was mentioned in studies on HPV,31,46,48 influenza,39 measles,15,20,28 and routine vaccines.12,16,18,22–24,26,27,34,38,40–44,47 In most studies using quantitative methods, this was a reason for incomplete vaccination for at least 10% of participants.15,22,24,27,34,40,42–44,46–48 Qualitative results revealed that fever16,18and crying18,23 were the most cited side effects. Some caregivers were scared of the vaccination side effects because the vaccine was new and therefore untested before (e.g. flu vaccines),39 they had seen other children with side effects following vaccination,20 or their child already had side effects with previous vaccines.18,23
Poor access to vaccination services
Poor access to vaccination services was reported as a reason for incomplete vaccination in 21 studies12,14,15,18–20,22,24,26–28,33,37,39–41,43–47 in both rural and urban areas. The findings of quantitative and qualitative studies were similar. The distance to the vaccination point was reported as a reason for non-vaccination and under-vaccination in the majority of studies12,14,15,18,19,24,26–28,33,37,39–41,43–45 and was frequently reported by the caregivers in quantitative studies (by more than 10% of study participants14,15,24,26,27,33,40,41,43,45). Three studies mentioned transportation cost12,18,46 as a reason for incomplete vaccination.
Beliefs about vaccination
In 17 studies12,14,18,20,22–25,29–33,36,39,44,48 children and adolescents were incompletely vaccinated because of their caregiver’s beliefs about vaccination.
Participants believed that vaccines caused serious adverse events, and this was cited by at least 20% of them14,29,30,36,44,48 in most quantitative studies. In particular, participants thought that vaccines could cause infertility (e.g., in studies on HPV vaccines30,48), had heard myths and rumors about the negative consequences of vaccines on health,22,33,44 and did not trust the government.29,30 In qualitative results, participants believed that receiving too many vaccines was harmful for children12,23 and that vaccines had ingredients that caused infertility, diseases, physical disability, or death.12,18,23 For instance, in a study in northern Nigeria, caregivers believed that vaccines contained HIV or family planning.12 Some also thought that vaccinators gave expired vaccines and that the government had a hidden agenda.18,20 In one study, “paralysis of the leg” and becoming “lame” were also mentioned.18
On the other hand, some participants thought that vaccines were not effective. Five studies were on measles,20 polio,29,30 and flu39 vaccines. At least 10% of participants cited this reason in most quantitative studies.29,30,44 In qualitative results, participants believed that vaccination did not protect against diseases12,20 and thought that there was no difference between vaccinated and unvaccinated children.20
Poor relationship with healthcare providers
In 16 studies, participants mentioned the poor relationship with healthcare providers as a reason for incomplete vaccination.12,17–19,23,24,26,27,29,32,33,38,40,42,47,49
Respondents criticized healthcare workers’ attitudes (i.e., unkind, disrespectful, rude, unfriendly). In quantitative studies, this was rare (mentioned by less than 10% of participants12,24,26,29,33,38,47). In qualitative studies, participants gave specific situations in which the attitude of healthcare providers attitudes dissuaded them from using vaccination services, for example, if they had dirty baby shawls,18 had lost their child’s vaccination card, or had forgotten the previous vaccination appointment date.23,49
Participants also criticized the quality of vaccination services. In Uganda19 and Burkina Faso,23 caregivers criticized how vaccinators performed vaccination (e.g., hurting the child) and in Ethiopia, caregivers felt that healthcare providers did not give them sufficient information on vaccination.49
Social or cultural pressure against vaccination
In 13 studies,12,16,18,21,23,24,26,27,31,32,46–48 participants explained that social or cultural pressure against vaccination dissuaded them from completely vaccinating children and adolescents. Studies were conducted in nine different countries. The majority were on routine vaccines, three were on the HPV vaccine and one was on the polio vaccine. Qualitative data was the most informative.
In quantitative studies in Kenya46 Uganda47 Malawi31 South Sudan26 and Tanzania48 participants experienced social pressure against vaccination from community members (e.g. family, friends, parents). However, this was rare (generally cited by less than 10% of participants).
Pressure against vaccination from the husband or partner was mentioned in Nigeria,12,16 Uganda,18,47 and Kenya.46 In three quantitative studies, this was a reason to incompletely vaccinate children for more than 10% of participants.12,46,47 Qualitative results revealed that women needed their husband’s consent to immunize their child16 and feared going against their husband’s will.16,18 Partners refused to have their child vaccinated because the child would suffer afterwards or because they were not vaccinated themselves.16,18
In studies in Nigeria,12,32 Malawi,21 Burkina Faso,23 Cameroon,24 and Ethiopia,27 children were incompletely vaccinated for cultural or religious reasons. For example, in some settings, mothers needed to stay in the family compound after the child’s birth.12,23 However, in the studies that mentioned religious reasons, the religious beliefs that led to incomplete vaccination were not specified (e.g., ethical reasons, animal-derived products).
Cost related to vaccination
Vaccines were provided for free to communities within the Expanded Programme on Immunization (EPI) and during vaccination campaigns but in 10 studies, cost remained a reason for incomplete vaccination14,19,20,24,26,33,37,38,45,47 Six studies were on routine vaccines14,19,24,26,38,47 and the others were on measles,20 HPV,33 HepB0,37 and BCG.45 In most studies, the type of cost was not detailed, but in one study caregivers had to pay for syringes and vaccination cards.24 In more than half of quantitative studies, this was frequently mentioned (at least 10% of participants14,24,33,45,47). Qualitative findings revealed that some vaccinators charged for EPI services.19,20,26 In a study in Uganda, women were dissatisfied about having to pay for services that were supposed to be free (e.g., vaccination cards, syringes).19
Reasons not explored or not explained
In 22 studies12,15,20–24,30–32,35,36,40,42,45–48 the reasons for incomplete vaccination were not specified. For example, the reasons included “unspecified worries”, “disinterest”, “maternal disapproval” “lack of interest”, “lack of faith” (without specifying what was meant by “faith”), and they were frequently mentioned in quantitative studies (more than 10% of caregivers.12,15,21,22,24,26,29,31,40,42–47
Discussion
Summary of results
To the best of our knowledge, this is the first systematic review to identify the reasons expressed by community members for the incomplete vaccination of children and adolescents in sub-Saharan Africa. We found that only half of the studies distinguished between non-vaccination and under-vaccination, leading us to consider these two outcomes as “incomplete vaccination”. The most common reasons for incomplete vaccination given by community members were related to caregiver’s time constraints, lack of knowledge regarding vaccination, the unavailability of vaccines or personnel in healthcare facilities, missed opportunities for vaccination, caregiver’s fear of minor side effects, poor access to vaccination services, and caregiver’s vaccination beliefs.
Agreements and disagreements with other reviews and studies
We identified three systematic reviews on the reasons and factors of incomplete childhood vaccination in low- and middle-income countries, one of which focused on sub-Saharan Africa.6,7,11 These reviews did not study separately the reasons (expressed by individuals) and the factors (obtained through statistical analysis) for incomplete vaccination. One review reported separately the barriers for non-vaccination and under-vaccination.6 The themes we used to categorize the reasons were adapted from those used by two reviews6,7 and were slightly different from those of the review by Bangura et al.11 which divided the reasons into parenteral/caretaker barriers, health system barriers, and provider barriers, without further sub-categories.
The reasons we identified are similar to those reported in the reviews, but the relative importance of these reasons slightly differs. Bangura et al.11 found that parental/caretakers’ barriers were the main reasons/factors for incomplete vaccination, whilst Favin et al.7 found that the main reasons/barriers were related to the vaccination systems. In the review by Rainey et al.6 the main reasons/factors for under-vaccination were related to vaccination systems, and those for non-vaccination were related to parenteral attitudes and knowledge. However, in our study, both parental/caretakers’ and healthcare system barriers seem to be equally important reasons for the incomplete vaccination of children in sub-Saharan Africa.
Other studies have identified the barriers to vaccination at a global level and in other world regions. A recent overview of systematic reviews on the parent-level barriers to uptake of childhood vaccination by Faufman et al.50 found that two thirds of systematic reviews currently published on the topic are from high-income countries. Our review therefore contributes to the literature in low- and middle-income countries. Moreover, the study by Kaufman et al.50 identified six categories of barriers to vaccination (related to access, clinic or health system barriers, concerns and beliefs, health perceptions and experiences, knowledge and information, and social or family influence) and found that less than half of the reviews reported barriers from all six themes. However, in our review we have identified reasons from all six categories. Moreover, some of the barriers most frequently mentioned in the overview were also reported in several of the studies we identified (e.g., time constraints, concerns about vaccine safety, lack of knowledge regarding vaccination, etc.).
Furthermore, a systematic literature review of the barriers to vaccination in Latin America and the Caribbean51 found that individual/group influences (i.e., risk/benefits perceived, knowledge/awareness, beliefs, attitudes and motivation about health and prevention, etc.) were most frequently reported, followed by contextual factors (i.e., socio/economic/religion/culture/gender, geographic barriers, communication and media environment). Our review also found that individual and group influences are key vaccination barriers.
Public health implications
The wide range of reasons identified calls for a varied approach to reach incompletely vaccinated children and achieve the full benefits of vaccination in sub-Saharan Africa.6 We identified two types of interventions that can help address most barriers to vaccination: (i) improving and reinforcing communication around vaccines and (ii) improving the organization of vaccination services. Interventions need to be locally adapted, depending on the knowledge, perceptions, and social norms around vaccination.
Improving and reinforcing communication around vaccines
Caregivers’ lack of knowledge regarding vaccines and/or the organization of vaccination services were reasons for incomplete vaccination in the majority of studies.12,14,15,20,22–29,31–33,37–44,46–49 However, good knowledge of vaccines is important for effective vaccine acceptance and utilization.11 For instance, a study in Kenya highlighted that empowering caregivers and healthcare workers with immunization information could benefit vaccination uptake.52 Therefore, health education programs targeting those responsible for vaccination are essential to strengthen their understanding of the purposes and benefits of vaccination, vaccination schedules, as well as the organization of vaccination services. These programs can be delivered in healthcare services but also community settings.7
The beliefs and perceptions of caregivers and community members about vaccination are also key for childhood vaccination. Indeed, beliefs that vaccines cause serious adverse events or are not effective,12,14,18,20,22–26,29–33,36,38,39,42–44,48 caregiver’s fear of minor side effects,12,15,16,18,20,22–24,26–28,31,34,38–44,46–48 and pressures against vaccination from partners, community members, or religious authorities12,16,18,21,23,24,26,27,31,32,46–48were frequently mentioned as reasons for incomplete vaccination.
To address these issues, understanding the concerns and points of view of communities regarding vaccination and involving the entire community in the response (including men, religious leaders, and community leaders) are important. Indeed, although women were generally responsible for child vaccination, the community had a strong influence on the final decision in several settings. Some interventions include informing community members about the risks and benefits of vaccination and how to handle common side effects,7 improving counseling at vaccination sites,7 and developing approaches that acknowledge parental concerns and try to address their misconception.11
Finally, to address missed opportunities for vaccination12,14,16,17,22,24–28,30,31,34–40,43–45,48,49and poor relationships with healthcare providers12,17–19,23,24,26,27,29,32,33,38,40,42,47,49 improved communication within healthcare services is necessary. For instance, interventions include improving the treatment of staff and providing supportive supervision, providing training on vaccination, and ensuring that all health staff knows and accept the national contraindications policy.7
Improving the organization of vaccination services
Poor or insufficient organization of vaccination services is an important reason for incomplete vaccination of children and adolescents in sub-Saharan Africa. Indeed, caregiver’s time constraints and/or long waiting times at vaccination services12,14–24,26–28,31,34,36,38–41,43,44,46,47,49 the unavailability of vaccines and/or personnel at vaccination sites12,14,17–22,24,26–29,34–38,40–45,47,49 and missed opportunities for vaccination12,14,16,17,22,24–28,30,31,34–39,40,43–45,48,49 were common in the studies identified.
To reduce waiting times at vaccination services, vaccination sessions could be regularly organized at times where most caregivers are available and caregivers could also be encouraged, where possible, to come throughout the vaccination hours to avoid long waiting times.7 If needed, employers could be encouraged to exceptionally allow caregivers to attend vaccination services during working hours.7
Furthermore, skills and performance in forecasting stocks of vaccines, supplies, and equipment, as well as in their storage should be improved.7 For instance, each facility director should be responsible for ensuring that vaccination services are available when they should be, that staff is present, and that services are still available when staff is away.7
Poor access to vaccination services was mentioned in several studies12,14,15,18–20,22,24,26–28,33,37,39–41,43–47and could be reduced by identifying the largest pockets of incompletely vaccinated children and increasing outreach activities in these areas.
Further research
Although vaccines provided through the EPI and campaigns were free for communities, the cost related to vaccination remained a barrier in several studies,14,19,20,24,26,33,37,38,45,47 suggesting that caregivers have to pay for extra costs at vaccination sites or that they are unaware that vaccines are free. However, only a few studies gave details on the types of costs (e.g., syringes, vaccination cards). Therefore, further research on the type of vaccination costs incurred by households is needed to then determine how these barriers can be overcome (e.g., through improved communication on free EPI vaccines).
Second, reasons for incomplete vaccination should be more fully investigated in quantitative studies to understand the barriers to vaccination. Indeed, in more than half the studies12,20–24,30–32,34–36,40,42,45–48 reasons for incomplete vaccination were not further explored (e.g., “unspecified worries”, “disapproval”), whereas these were frequently mentioned.
Furthermore, identifying the reasons for incomplete vaccination of children and adolescents during the COVID-19 pandemic (from the point of view of community members) could show whether the are barriers to vaccination are different before and during/after the pandemic. The COVID-19 pandemic has disrupted routine childhood vaccination uptake worldwide53 but when the literature search for this systematic review was conducted in March and December 2020, no study on the reasons of incomplete vaccination of children and adolescents during the pandemic in sub-Saharan Africa had been published.
Finally, several studies have investigated the willingness of adults to have their own child vaccinated for COVID-19. For instance, a study conducted mid-2021 in Burkina Faso, Guinea, Mali, Senegal and Sierra Leone found that 36% of parents would refuse/probably refuse to have their child vaccinated for COVID-19.54 Given that few countries in sub-Saharan Africa have included children and adolescents in their COVID-19 vaccination strategy (11 African countries in January 2022),55 we have not identified studies on the reasons for non-vaccination and/or under-vaccination of children and adolescents in settings where the vaccine is available to that age group.
Methodological issues in the studies
The main limitation of the studies included is that more than half of them do not differentiate non-vaccination from under-vaccination, whereas the underlying reasons in each situation generally differ. For example, two studies in Nigeria12,43 found that the reasons for non-vaccination were related to demand factors (e.g., beliefs, lack of faith in vaccination, ignorance of vaccination), whilst supply factors, misunderstanding of the full vaccination schedule, and physical inconvenience were important for under-vaccination. Similarly, a systematic review highlighted that most reasons for non-vaccination were linked to demand-side factors (parenteral attitudes and knowledge), and those for under-vaccination were linked to immunization systems.6 The strategies to address non-vaccination and under-vaccination therefore differ.6,12 For instance, reasons for non-vaccination that relate to an individual’s beliefs about vaccination may require an understanding of the underlying causes of non-vaccination and locally adapted communication strategies. To reduce under-vaccination, it may be necessary to provide the correct information on vaccine schedule and timing and organize vaccination systems more effectively.
Other limitations include potential recall bias of the reasons for incomplete vaccination in older children and selection bias as participants were purposefully selected in some studies.
Limitations of this systematic review
Our study has several limitations. First, we may have been unable to locate all relevant articles published during the specified review period, and some subjective judgment may have been employed in selecting relevant articles for a full review. However, subjectivity was minimized by using a set of pre-defined inclusion criteria. Furthermore, we did not search for gray literature, whereas information from varied sources can be used to better understand reasons for incomplete vaccination and enhance the development of effective strategies to address these barriers.
Second, the categories used to differentiate the reasons were functional and based on previous literature reviews. We tried to have as many relevant categories as possible, but these categories may not fully capture the multi-causality of incomplete vaccination. The classification was not always straightforward as some reasons could cross categories, but these were discussed in pair.
Third, because the review focused on qualitative reasons linked to incomplete vaccination from different study designs, sample sizes, and methodologies (e.g., focus group discussions, open-ended questions, structured or semi-structured questionnaires), we did not assess the reasons identified through various methodologies and each reason was abstracted and weighted equally. This might have increased the relative importance of reasons that did not have a strong impact on vaccination status. The frequency of each reason was an attempt to see the relative importance of each reason but should be interpreted with caution due to the diverse nature of the studies included.
Finally, the reasons were not specific to the type of vaccine or the timing. Furthermore, the studies were only conducted in 12 countries and cannot be representative of a region or even a country.
Conclusion
This systematic review highlights the multiplicity of reasons given by community members for the incomplete vaccination of children and adolescents in sub-Saharan Africa, showing the diverse range of barriers that need to be considered when attempting to understand reasons for non- and/or under-vaccination. The findings suggest that to achieve the full benefits of vaccination, strengthening communication around vaccination and improving the organization of vaccination services are essential. Future studies investigating the barriers to vaccination should aim to distinguish between non-vaccination and under-vaccination. Of note, understanding the reasons for non-vaccination or under-vaccination from the perspective of other stakeholders (e.g., healthcare professionals, governments, institutions) is also necessary to have a global picture of the underlying causes of low vaccination in a given setting.
Supplementary Material
Funding Statement
This systematic literature review is part of the ECOVACSEN project, which has received support from the French National Research Agency, as part of the 2020 generic call for projects [ANR-20-CE36-0005-01].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2076524.
References
- 1.WHO Regional Office for Africa . 2018. Business case for WHO immunization activities on the African continent, 2018-2030 https://www.afro.who.int/sites/default/files/2018-05/WHO_Bcase_Brochure_2018_05_11_FINAL%20VERSION_ISBN_WEB_0.pdf. Brazzaville: 978-929023411-1 Accessed 25 May 2021 [Google Scholar]
- 2.WHO, UNICEF . Progress and challenges with achieving Universal Immunization Coverage: 2019 WHO/UNICEF estimates of national immunization coverage [Internet]; 2020. [accessed 2021 May 25]. https://cdn.who.int/media/docs/default-source/immunization/coverage/who-immuniz.pdf?sfvrsn=72fd7237_2&download=true
- 3.Addis Declaration on Immunization [Internet] . Ministerial conference on immunization in Africa [accessed 2021 May 27]. http://immunizationinafrica2016.org/ministerial-declaration-english
- 4.WHO Regional Office for Africa . Roadmap for implementing the Addis declaration on immunization: advocacy, action, and accountability [Internet]; 2017. https://www.afro.who.int/sites/default/files/2017-09/ADI%20Roadmap%20-%20English.pdf
- 5.WHO . Immunization agenda 2030: a global strategy to leave no one behind [Internet]; 2020. https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/ia2030 [DOI] [PubMed]
- 6.Rainey JJ, Watkins M, Ryman TK, Sandhu P, Bo A, Banerjee K.. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999–2009. Vaccine. 2011;29(46):8215–12. doi: 10.1016/j.vaccine.2011.08.096. [DOI] [PubMed] [Google Scholar]
- 7.Favin M, Steinglass R, Fields R, Banerjee K, Sawhney M. Why children are not vaccinated: a review of the grey literature. Int Health. 2012;4(4):229–38. doi: 10.1016/j.inhe.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Sridhar S, Maleq N, Guillermet E, Colombini A, Gessner BD. A systematic literature review of missed opportunities for immunization in low- and middle-income countries. Vaccine. 2014;32(51):6870–79. doi: 10.1016/j.vaccine.2014.10.063. [DOI] [PubMed] [Google Scholar]
- 9.Tauil MDC, Sato APS, Waldman EA. Factors associated with incomplete or delayed vaccination across countries: a systematic review. Vaccine. 2016;34(24):2635–43. doi: 10.1016/j.vaccine.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Phillips DE, Dieleman JL, Lim SS, Shearer J. Determinants of effective vaccine coverage in low and middle-income countries: a systematic review and interpretive synthesis. BMC Health Serv Res. 2017;17:681. doi: 10.1186/s12913-017-2626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangura JB, Xiao S, Qiu D, Ouyang F, Chen L. Barriers to childhood immunization in sub-Saharan Africa: a systematic review. BMC Public Health. 2020;20(1):1108. doi: 10.1186/s12889-020-09169-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babalola S. Maternal reasons for non-immunisation and partial immunisation in northern Nigeria. J Paediatr Child Health. 2011;47 5:276–81. doi: 10.1111/j.1440-1754.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- 13.Hadler SC, Hadler VD, Okwo-Bele JM, Cutts FT. Vaccines Plotkin, SA, Orenstein, WA, Offit, PA. Fifth Edition (W.B. Saunders; ). 2008. Chapter 70 - Immunization in developing countries;1541–72 doi: 10.1016/B978-1-4160-3611-1.50074-X. [DOI] [Google Scholar]
- 14.Abdulraheem IS, Onajole AT, Jimoh AAG, Oladipo AR. Reasons for incomplete vaccination and factors for missed opportunities among rural Nigerian children. J Public Health Epidemiol. 2011;3 4 :194–203. [Google Scholar]
- 15.Abebe AM, Mengistu T, Mekuria AD. In, BMC Res Notes. 2019. Measles case, immunization coverage and its determinant factors among 12–23 month children, in Bassona Worena Woreda, Amhara Region, Ethiopia, 2018. Vol. 12. p. 71. doi: 10.1186/s13104-019-4104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adamu AA, Uthman OA, Gadanya MA, Cooper S, Wiysonge CS. Using the theoretical domains framework to explore reasons for missed opportunities for vaccination among children in Kano, Nigeria: a qualitative study in the pre-implementation phase of a collaborative quality improvement project. Expert Rev Vaccines. 2019;18 8:847–57. doi: 10.1080/14760584.2019.1643720. [DOI] [PubMed] [Google Scholar]
- 17.Atwiine B, Rukundo A, Elias B, MacDonald NE. Reasons for non-timely completion of the routine infant immunization schedule by children in rural South West Uganda. Can J Public Health. 2016;106 8 :e564. doi: 10.17269/CJPH.106.5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babirye JN, Rutebemberwa E, Kiguli J, Wamani H, Nuwaha F, Engebretsen IM. More support for mothers: a qualitative study on factors affecting immunisation behaviour in Kampala, Uganda. BMC Public Health. 2011;11(1):723. doi: 10.1186/1471-2458-11-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babirye JN, Engebretsen IM, Rutebemberwa E, Kiguli J, Nuwaha F. Urban settings do not ensure access to services: findings from the immunisation programme in Kampala Uganda. BMC Health Serv Res. 2014;14:111. doi: 10.1186/1472-6963-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cockcroft A, Usman MU, Nyamucherera OF, Emori H, Duke B, Umar NA, Andersson N. Why children are not vaccinated against measles: a cross-sectional study in two Nigerian States. Arch Public Health. 2014;72 1:48 doi: 10.1186/2049-3258-72-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holte JH, Mæstad O, Jani JV. The decision to vaccinate a child: an economic perspective from southern Malawi. Soc Sci Med. 2012;75 2:384–91. doi: 10.1016/j.socscimed.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Itimi K, Dienye PO, Ordinioha B. Community participation and childhood immunization coverage: a comparative study of rural and urban communities of Bayelsa State, south-south Nigeria. Niger Med J. 2012;53 1:21–25. doi: 10.4103/0300-1652.99826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kagone M, Ye M, Nebie E, Sie A, Muller O, Beiersmann C. Community perception regarding childhood vaccinations and its implications for effectiveness: a qualitative study in rural Burkina Faso. BMC Public Health. 2018;18(1):324. doi: 10.1186/s12889-018-5244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwedi Nolna S, Bonono C-R, Nsangou Moncher M, Bindé T, Nolna D, Ongolo Zogo P. Factors influencing the performance of routine immunization in urban areas: a comparative case study of two cities in Cameroon: Douala and Yaoundé. Vaccine. 2018;36(49):7549–55. doi: 10.1016/j.vaccine.2018.10.048. [DOI] [PubMed] [Google Scholar]
- 25.LaMontagne DS, Barge S, Le NT, Mugisha E, Penny ME, Gandhi S, Janmohamed A, Kumakech E, Mosqueira NR, Nguyen NQ, et al. Human papillomavirus vaccine delivery strategies that achieved high coverage in low- and middle-income countries. Bull World Health Organ. 2011;89 11:821–30B. doi: 10.2471/BLT.11.089862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mbabazi W, Lako AK, Ngemera D, Laku R, Yehia M, Nshakira N. Maiden immunization coverage survey in the republic of South Sudan: a cross-sectional study providing baselines for future performance measurement. Pan Afr Med J. 2013;16:110. doi: 10.11604/pamj.2013.16.110.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mekonnen AG, Bayleyegn AD, Ayele ET. Immunization coverage of 12-23 months old children and its associated factors in Minjar-Shenkora district, Ethiopia: a community-based study. BMC Pediatr. 2019;19:198. doi: 10.1186/s12887-019-1575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer SA, Kambou JL, Cohn A, Goodson JL, Flannery B, Medah I, Messonnier N, Novak R, Diomande F, Djingarey MH, et al. Serogroup A meningococcal conjugate (PsA-TT) vaccine coverage and measles vaccine coverage in Burkina Faso–implications for introduction of PsA-TT into the Expanded Programme on Immunization. Vaccine. 2015;33:1492–98. doi: 10.1016/j.vaccine.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michael CA, Ashenafi S, Ogbuanu IU, Ohuabunwo C, Sule A, Corkum M, Mackay S, Storms AD, Achari P, Biya O, et al. An evaluation of community perspectives and contributing factors to missed children during an oral polio vaccination campaign–Katsina State, Nigeria. J Infect Dis. 2014;210(Suppl 1):S131–135. doi: 10.1093/infdis/jiu288. [DOI] [PubMed] [Google Scholar]
- 30.Michael CA, Ogbuanu IU, Storms AD, Ohuabunwo CJ, Corkum M, Ashenafi S, Achari P, Biya O, Nguku P, Mahoney F, et al. An assessment of the reasons for oral poliovirus vaccine refusals in Northern Nigeria. J Infect Dis. 2014;210(suppl 1):S125–30. doi: 10.1093/infdis/jiu436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Msyamboza KP, Mwagomba BM, Valle M, Chiumia H, Phiri T. Implementation of a human papillomavirus vaccination demonstration project in Malawi: successes and challenges. BMC Public Health. 2017;17(1):599. doi: 10.1186/s12889-017-4526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murele B, Vaz R, Gasasira A, Mkanda P, Erbeto T, Okeibunor J. Vaccine perception among acceptors and non-acceptors in Sokoto State, Nigeria. Vaccine. 2014;32(26):3323–27. doi: 10.1016/j.vaccine.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 33.Nabirye J, Okwi LA, Nuwematsiko R, Kiwanuka G, Muneza F, Kamya C, Babirye JN. Health system factors influencing uptake of Human Papilloma Virus (HPV) vaccine among adolescent girls 9-15 years in Mbale District, Uganda. BMC Public Health. 2020;20(1):171. doi: 10.1186/s12889-020-8302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguefack Dongmo F, Tassadong C, Dongmo R, Tatah S, Fodoung Wamba DS, Chiabi A, Kago I, Kobela M. World J. of Vaccines. 2016. Factors influencing routine vaccination of children of mothers live-stock retailers in the markets of Yaoundé;06:23–33. doi: 10.4236/wjv.2016.62004 [DOI] [Google Scholar]
- 35.Njeru I, Ajack Y, Muitherero C, Onyango D, Musyoka J, Onuekusi I, Kioko J, Muraguri N, Davis R. Did the call for boycott by the Catholic bishops affect the polio vaccination coverage in Kenya in 2015? a cross-sectional study. Pan Afr Med J. 2016;24:120. doi: 10.11604/pamj.2016.24.120.8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nsubuga F, Kabwama, SN, Ampeire, I, Luzze, H, Gerald, P, Bulage, L, Toliva, OB. Comparing static and outreach immunization strategies and associated factors in Uganda, Nov-Dec 2016. Pan Afr Med J 32 123. 2019. doi: 10.11604/pamj.2019.32.123.16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okenwa UJ, Dairo MD, Uba B, Ajumobi O. Maternal reasons for non-receipt of valid Hepatitis B birth dose among mother-infant pairs attending routine immunization clinics, South-east, Nigeria. Vaccine. 2019;37(46):6894–99. doi: 10.1016/j.vaccine.2019.09.056. [DOI] [PubMed] [Google Scholar]
- 38.Oladokun RE, Adedokun BO, Lawoyin TO. Children not receiving adequate immunization in Ibadan, Nigeria: what reasons and beliefs do their mothers have? Niger J Clin Pract. 2010;13 2:173–78. [PubMed] [Google Scholar]
- 39.Oria PA, Arunga G, Lebo E, Wong JM, Emukule G, Muthoka P, Otieno N, Mutonga D, Breiman RF, Katz MA. Assessing parents’ knowledge and attitudes towards seasonal influenza vaccination of children before and after a seasonal influenza vaccination effectiveness study in low-income urban and rural Kenya, 2010-2011. BMC Public Health. 2013;13:391. doi: 10.1186/1471-2458-13-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porth JM, Wagner AL, Teklie H, Abeje Y, Moges B, Boulton ML. Vaccine non-receipt and refusal in Ethiopia: the expanded program on immunization coverage survey, 2012. Vaccine. 2019;37(15):2106–21. doi: 10.1016/j.vaccine.2019.02.045. [DOI] [PubMed] [Google Scholar]
- 41.Sally ET, Kenu E. Evaluation of access and utilization of EPI services amongst children 12-23 months in Kwahu Afram Plains, Eastern region, Ghana. Pan Afr Med J. 2017;28:238. doi: 10.11604/pamj.2017.28.238.11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanni TA, Olasehinde OK, Adeniyi MA, Ipinnimo TM. Uptake of immunization and associated factors among 0-11 months infants in a rural community of Ekiti State. Nig J Med 28 4 440–450. 2019. [Google Scholar]
- 43.Sato R. Differential determinants and reasons for the non- and partial vaccination of children among Nigerian caregivers. Vaccine. 2020;38(1):63–69. doi: 10.1016/j.vaccine.2019.09.097. [DOI] [PubMed] [Google Scholar]
- 44.Tefera YA, Wagner AL, Mekonen EB, Carlson BF, Boulton ML. Predictors and barriers to full vaccination among children in Ethiopia. Vaccines. 2018;6(2). doi: 10.3390/vaccines6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thysen SM, Byberg S, Pedersen M, Rodrigues A, Ravn H, Martins C, Benn CS, Aaby P, Fisker AB. BCG coverage and barriers to BCG vaccination in Guinea-Bissau: an observational study. BMC Public Health. 2014;14(1 1037). doi: 10.1186/1471-2458-14-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vermandere H, Naanyu V, Mabeya H, Vanden Broeck D, Michielsen K, Degomme O. Determinants of acceptance and subsequent uptake of the HPV vaccine in a Cohort in Eldoret, Kenya. PLoS ONE. 2014;9 10:e109353. doi: 10.1371/journal.pone.0109353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vonasek BJ, Bajunirwe F, Jacobson LE, Twesigye L, Dahm J, Grant MJ, Sethi AK, Conway JH. Do maternal knowledge and attitudes towards childhood immunizations in rural Uganda correlate with complete childhood vaccination? PLoS ONE. 2016;11 2:e0150131. doi: 10.1371/journal.pone.0150131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Watson-Jones D, Tomlin K, Remes P, Baisley K, Ponsiano R, Soteli S, de Sanjosé S, Changalucha J, Kapiga S, Hayes RJ, et al. Reasons for receiving or not receiving HPV vaccination in primary schoolgirls in Tanzania: a case control study. PLoS ONE. 2012;7(10):e45231. doi: 10.1371/journal.pone.0045231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zewdie A, Letebo M, Mekonnen T. Reasons for defaulting from childhood immunization program: a qualitative study from Hadiya zone, Southern Ethiopia. BMC Public Health. 2016;16(1):1240. doi: 10.1186/s12889-016-3904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaufman J, Tuckerman J, Bonner C, Durrheim DN, Costa D, Trevena L, Thomas S, Danchin M. Parent-Level barriers to uptake of childhood vaccination: a global overview of systematic reviews. BMJ Glob Health. 2021;6 9:e006860. doi: 10.1136/bmjgh-2021-006860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guzman-Holst A, DeAntonio R, Prado-Cohrs D, Juliao P. Barriers to vaccination in Latin America: a systematic literature review. Vaccine. 2020;38(3):470–81. doi: 10.1016/j.vaccine.2019.10.088. [DOI] [PubMed] [Google Scholar]
- 52.Li AJ, Tabu C, Shendale S, Sergon K, Okoth PO, Mugoya IK, Machekanyanga Z, Onuekwusi IU, Sanderson C, Ogbuanu IU. Assessment of missed opportunities for vaccination in Kenyan health facilities, 2016. PLoS ONE. 2020;15(8):e0237913. doi: 10.1371/journal.pone.0237913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shet A, Carr K, Danovaro-Holliday MC, Sodha SV, Prosperi C, Wunderlich J, Wonodi C, Reynolds HW, Mirza I, Gacic-Dobo M, et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: evidence of disruption and recovery from 170 countries and territories. Lancet Glob Health. 2022;10 2:e186–94 doi: 10.1016/S2214-109X(21)00512-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faye SLB, Krumkamp R, Doumbia S, Tounkara M, Strauss R, Ouedraogo HG, Sagna T, Barry AM, Mbawah AK, Doumbia CO, et al. Factors influencing hesitancy towards adult and child COVID-19 vaccines in rural and urban West Africa: a cross-sectional study. BMJ Open. 2022;12(4):e059138. doi: 10.1136/bmjopen-2021-059138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sam-Agudu NA, Quakyi NK, Masekela R, Zumla A, Nachega JB. Children and adolescents in African countries should also be vaccinated for COVID-19. BMJ Glob Health. 2022;7 2:e008315. doi: 10.1136/bmjgh-2021-008315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


