ABSTRACT
Following COVID-19 vaccination, ipsilateral axillary and cervical lymphadenopathy may occur, called vaccine-related hypermetabolic lymphadenopathy, which is considered reactive lymphadenopathy. We report here a case of Kikuchi-Fujimoto disease, which occurred three months after vaccination with COVID-19 vaccine. The patient had cervical and axillary lymph node enlargement and a short-term fever that resolved spontaneously after the first and second vaccines. On the 90th day after the first vaccination, the patient developed a high fever and pathologically diagnosed necrotizing lymphadenitis in the axilla, which was diagnosed as Kikuchi-Fujimoto disease. Gallium scintigraphy showed localized swelling and strong uptake in the ipsilateral axilla. It implies the possibility of Kikuchi-Fujimoto Disease in axillary drainage lymph nodes in association with COVID-19 vaccine. Although only a few cases have been reported so far, this case is novel because of its later onset and diagnosis based on pathological and gallium scintigraphy imaging findings.
KEYWORDS: Kikuchi-Fujimoto disease, COVID-19, mRNA vaccine, COVID-19 vaccine, necrotizing histiocytic lymphadenitis, lymphadenopathy, fever of unknown origin
Introduction
Vaccination is a powerful strategy for COVID-19 prevention. Local injection site pain, fever, chills, myalgia, headache, and fatigue commonly occur after vaccination, which improve within a few days. Enlarged ipsilateral axillary and cervical lymph nodes have been reported as a side effect of vaccination. Recent analyses have shown that they occur immediately after the COVID-19 vaccine, up to an average of 10 days, and within two months at most. These side effects are diagnosed by positron emission tomography-computed tomography or ultrasound.1,2
Kikuchi-Fujimoto disease (KFD) is an inflammatory disease of unknown cause with spontaneous remission in one to three months, characterized by painful lymphadenopathy and fever, accompanied by leukopenia, thrombocytopenia and liver dysfunction.3 Lymphadenopathy is mainly in the cervical region, and is diagnosed by pathological examination of lymph node biopsies, which reveals partial necrosis and infiltration of histiocytes, T lymphocytes, and other cells in the cortex and paracortex of the lymph nodes. Three case reports of KFD in axillary lymph nodes associated with COVID-19 vaccine have been published, but the frequency and course of the disease are still unclear.4–6
We report a case of KFD that occurred 90 days after the first COVID-19 vaccination. Unlike usual KFD, this case was characterized by swelling confined to the axillary lymph node on the vaccination side only. Post-vaccination lymph node reactions may persist until as late as in this case. Physicians treating patients with long-lasting high fever, even as late as three to four months after vaccination, should be alert to the findings of KFD, including enlarged lymph nodes, lymphocyte counts and liver dysfunction.
Patient presentation
A 27-year-old Japanese woman with no prior history received her first COVID-19 vaccine on 17 September 2021 (Day 1). She noticed swelling in the left submandibular region the day after vaccination, but was given antimicrobials, which produced an improvement within a few days. She then received a second COVID-19 vaccine on 8 October 2021 (Day 22). On both occasions, the patient received the Pfizer-BioNTech COVID-19 mRNA vaccine. She noticed a fever of 38.3º C and axillary swelling the next day, which spontaneously improved after three days.
Later, on 15 December 2021 (Day 90), she developed a fever of 39.0°C and became aware of discomfort in the axillary lymph nodes. As the fever persisted despite antimicrobial therapy, she visited our hospital on 17 January 2022 (Day 123). Her maximum temperature was 39.3°C Atypical lymphocytes, leukopenia (white blood cells, 2200/µL; lymphocytes, 35.7%) and mild hepatic dysfunction were detected (aspartate transaminase, 36 U/L; alanine transaminase, 35 U/L; lactate dehydrogenase, 459 U/L). A computed tomography scan of her chest on admission (Day 123) showed multiple enlarged lymph nodes (max, 35 × 32 x 15 mm) localized on the vaccination side (left side) (Figure 1a). Gallium scintigraphy on Day 4 of hospitalization (Day 126) showed high uptake in multiple lymph nodes in the left axilla (Figure 1b–c). There was no uptake except in the ipsilateral axillary lymph node. Abdominal ultrasound showed hepatomegaly and splenomegaly. Polymerase chain reaction testing of nasal swabs for COVID-19 was negative. Blood tests for cytomegalovirus immunoglobulin (Ig) G and IgM, Epstein Barr virus IgG and IgM, antinuclear antibodies, autoantibodies for autoimmune disease, quantiFERON TB-GOLD for Mycobacterium tuberculosis, and Bartonella DNA were all negative.
Figure 1.
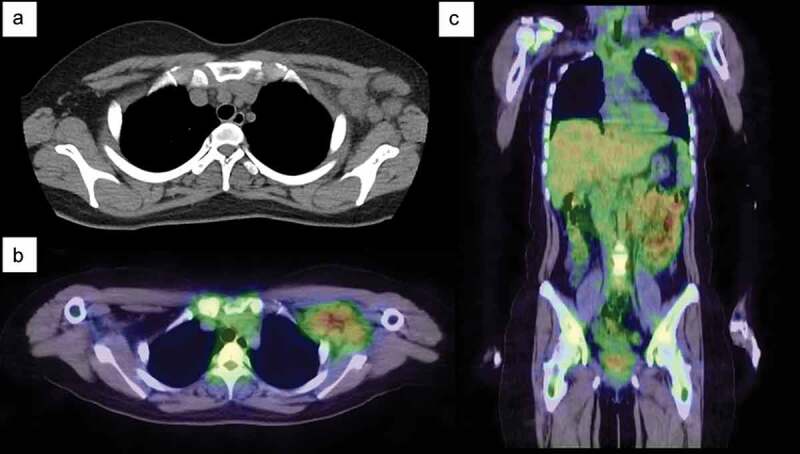
Chest computed tomography (CT) and gallium-67 single-photon emission-CT (SPECT) scintigraphy. (a) Chest CT on admission shows multiple enlarged lymph nodes in the left axilla. (b–c) Gallium-67 SPECT scintigraphy shows strong uptake in the axillary lymph nodes. No lymph node swelling or uptake is detected in other parts of the body.
A surgical biopsy of the axillary lymph node was performed on Day 130. The lymph nodes were heavily necrotic with nuclear debris (Figure 2a–c), and based on the findings of necrotizing lymphadenitis, a diagnosis of KFD was made. Bacterial and acid-fast cultures of the lymph nodes were negative. After admission, the fever resolved in 10 days (Day 133) without treatment, and the white blood cell count was at its lowest (2100/μL) on Day 130 and recovered to normal thereafter. The febrile period was 43 days.
Figure 2.
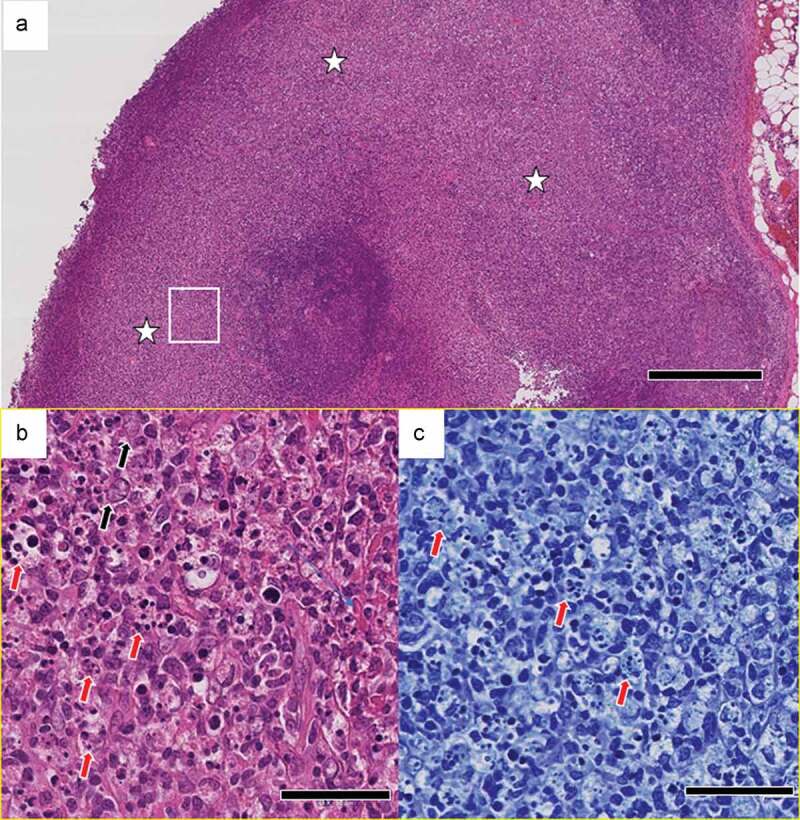
Pathological findings in the left axillary lymph node. (a) Localized, focal lesions (white stars) that obscure the lymph node structure. [hematoxylin & eosin (H&E)]. Scale bar: 500 μm. (b) Numerous nuclear debris (red arrows) and some enlarged lymphocytes (black arrows). High-magnification view of the area in the white rectangle in (a). Scale bar: 50 μm. (c) Granulocytes are not evident. Nuclear debris (red arrows) that appear to have been phagocytosed by histiocytes. High-magnification view of the area in the white rectangle in (a). [Giemsa stain.] Scale bar: 50 μm.
Discussion
Benign lymphomatosis in the ipsilateral axillary or supraclavicular lymph node has been recently reported after COVID-19 vaccine.7,8 It usually begins the day after vaccination, and the pathology is reactive lymphadenitis, with scant symptoms, such as fever. It is called vaccine-related hypermetabolic lymphadenopathy (VRHL) and improves within about 10 days, but reports of lymph node evaluation in vaccinated patients by positron emission tomography-computed tomography have shown that it can be present for as long as 10 weeks.1
We identified KFD associated with COVID-19 vaccine. The patient experienced transient, presumed VRHL symptoms after vaccination, followed by high fever and enlarged lymph nodes due to KFD after a 60-day period.
KFD is a rare cause of lymphadenopathy. It presents with painful lymphadenopathy and is characterized by liver dysfunction, leukopenia, and atypical lymphocytes, accompanied by fever. KFD is known to resolve spontaneously and has a favorable course, but it is often difficult to diagnose because of the need to differentiate between collagenous, malignant, and infectious diseases. KFD after vaccination is very rare, and there have been reports of KFD caused by human papillomavirus vaccine and Japanese encephalitis vaccine.9
Three case reports were recently published on KFD after COVID-19 vaccination.4–6According to these reports, KFD after COVID-19 vaccination, unlike VRHL, presented with systemic symptoms, such as fever, and did not occur immediately after the last vaccination, but after 10, 17, 21, and 35 days in these cases.
Our case is characterized by delayed onset, 90 days after the first vaccine and 68 days after the second vaccine. Interestingly, each of the two vaccinations resulted in a short period of lymphadenopathy immediately after each vaccination, which resolved spontaneously. KFD then began after an interval of about 60 days. KFD after COVID-19 has also been reported.10–14 Several of the patients in these reports developed KFD two to three months after COVID-19 infection, supporting the association of our case of KFD with the COVID-19 vaccine.11,12
The possibility that the present case is not related to the vaccine cannot be ruled out. However, cervical lymph nodes are usually swollen in KFD, with a frequency of 56%-98%.15 On the other hand, KFD confined to the axillary lymph nodes has been reported only rarely.16 Considering this case together with other COVID-19 vaccine-related lymphadenitis in recent years, it is reasonable to speculate that this case is another side effect of the COVID-19 vaccine. Symptomatic treatment with antipyretic analgesics is usually sufficient for KFD, but steroids may be used in severe or recurrent cases. In our case, as in previous reports, treatment with acetaminophen and non-steroidal anti-inflammatory drugs was effective and no further treatment was necessary.
The cause of KFD is unknown, although two main hypotheses have been proposed.17 The first is primarily a viral infection theory. The clinical presentation of KFD resembles that of viral infections, with histopathologic features that include immune cell proliferation, nuclear necrosis in the paracortex, T-cell-dominant lymphomegaly, and atypical lymphocytes in the peripheral blood. Therefore, many viruses, such as Epstein-Barr virus, herpes simplex virus, varicella-zoster virus, and human herpesvirus 6, 7, and 8, have been proposed to be associated with KFD. However, no study has clearly demonstrated a causal relationship between viruses and KFD. Therefore, another hypothesis, autoimmunity, is often presumed to be related to KFD. The cytoplasm of lymphocytes and histiocytes seen in KFD has a tubular reticular structure, similar to that seen in autoimmune diseases, such as systemic lupus erythematosus. It is suggested that patients with a genetic component, especially HLA-DPA1 and HLA-DPB1 (more frequent in Asians), develop an immune response that is mainly composed of T cells, especially cytotoxic T cells.
mRNA vaccines induce a rapid and localized infiltration of neutrophils, monocytes, and dendritic cells at the site of administration and in the draining lymph node promptly after vaccination.18 COVID-19 mRNA vaccine rapidly induces CD8+ T cells as well as antibody production, contributing to vaccine efficacy.19 Although no basic analysis was performed in this case, it is possible that the mRNA vaccine induced a CD8+ T cell-related immune response in the draining lymph node, resulting in the development of KFD localized to the ipsilateral axillary lymph node. However, a causal relationship between KFD and COVID-19 vaccine has never been proven, and further pathophysiological analysis is warranted.
In conclusion, it has been reported rarely that KFD may develop in association with COVID-19 vaccine and may have a later onset than VRHL. However, our case was even later than previously reported, about three months after the start of vaccination. Physicians should be aware of axillary lymph node findings, liver function, and lymphocyte counts in patients with long-lasting fever, even at three months post-vaccination. The response should be the same as for usual KFD. Further accumulation of knowledge about this condition is needed.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
No potential conflicts of interest was reported by the author(s).
References
- 1.El-Sayed MS, Wechie GN, Low CS, Adesanya O, Rao N, Leung VJ.. The incidence and duration of COVID-19 vaccine-related reactive lymphadenopathy on 18F-FDG PET-CT. Clin Med (Lond). 2021;21:e633–4. doi: 10.7861/clinmed.2021-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park JY, Lee JY, Yi SY. Axillary lymphadenopathy on ultrasound after COVID-19 vaccination and its influencing factors: a single-center study. J Clin Med. 2022;11(1):238. doi: 10.3390/jcm11010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry AM, Choi SM. Kikuchi-Fujimoto disease: a review. Arch Pathol Lab Med. 2018;142:1341–46. doi: 10.5858/arpa.2018-0219-RA. [DOI] [PubMed] [Google Scholar]
- 4.Soub HA, Ibrahim W, Maslamani MA, Ali GA, Ummer W, Abu-Dayeh A. Kikuchi-Fujimoto disease following SARS CoV2 vaccination: case report. Idcases. 2021;25:e01253. doi: 10.1016/j.idcr.2021.e01253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan HM, Hue SS, Wee A, See KC. Kikuchi-Fujimoto disease post COVID-19 vaccination: case report and review of literature. Vaccines (Basel). 2021;9(11):1251. doi: 10.3390/vaccines9111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caocci G, Fanni D, Porru M, Greco M, Nemolato S, Firinu D, Faa G, Scuteri A, La Nasa G. Kikuchi-Fujimoto disease associated with hemophagocytic lymphohistiocytosis following the BNT162b2 mRNA COVID-19 vaccination. Haematologica; 2021; Dec 30. Advance online publication. doi: 10.3324/haematol.2021.280239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tu W, Gierada DS, Joe BN. COVID-19 vaccination-related lymphadenopathy: what to be aware of. Radiol Imaging Cancer. 2021;3:e210038. doi: 10.1148/rycan.2021210038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam DL, Flanagan MR. Axillary lymphadenopathy after COVID-19 vaccination in a woman with breast cancer. Jama. 2022;327:175–76. doi: 10.1001/jama.2021.20010. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe T, Hashidate H, Hirayama Y, Iinuma Y. Kikuchi-Fujimoto disease following vaccination against human papilloma virus infection and Japanese encephalitis. Eur J Pediatr. 2012;171:1409–11. doi: 10.1007/s00431-012-1729-1. [DOI] [PubMed] [Google Scholar]
- 10.Masiak A, Lass A, Kowalski J, Hajduk A, Zdrojewski Z. Self-Limiting COVID-19-associated Kikuchi-Fujimoto disease with heart involvement: case-based review. Rheumatol Int. 2022;42:341–48. doi: 10.1007/s00296-021-05088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Ghadeer HA, AlKadhem SM, AlMajed MS, AlAmer HM, AlHabeeb JA, Alomran SH, AlMajed AS. Kikuchi-Fujimoto disease following COVID-19. Cureus. 2022;14:e21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stimson L, Stitson R, Bahhadi-Hardo M, Renaudon-Smith E. COVID-19 associated Kikuchi-Fujimoto disease. Br J Haematol. 2021;192:e124–e126. doi: 10.1111/bjh.17292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Racette SD, Alexiev BA, Angarone MP, Bhasin A, Lima K, Jennings LJ, Balasubramanian S, Matsuoka AJ. Kikuchi-Fujimoto disease presenting in a patient with SARS-CoV-2: a case report. BMC Infect Dis. 2021;21:740. doi: 10.1186/s12879-021-06048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaseb K, Nameh Goshay Fard N, Rezaei N, Sadeghian S, Sadeghian S. COVID-19 in a case with Kikuchi-Fujimoto disease. Clin Case Rep. 2021;9:1279–82. doi: 10.1002/ccr3.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen LC, Wang CJ, Chang YC, Shie SS, Lin TY, Hsieh YC, Huang KYA, Kuo CY, Chiu CH, Huang YC, et al. Distribution of lymphadenopathy in patients with Kikuchi disease. J Microbiol Immunol Infect. 2021;54:299–304. doi: 10.1016/j.jmii.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Al-Maghrabi J, Kanaan H. Histiocytic necrotising lymphadenitis (Kikuchi-Fujimoto disease) in Saudi Arabia: clinicopathology and immunohistochemistry. Ann Saudi Med. 2005;25:319–23. doi: 10.5144/0256-4947.2005.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masab M, Surmachevska N, Farooq H. Kikuchi disease. Treasure Island (FL): StatPearls Publishing LLC; 2022. [PubMed] [Google Scholar]
- 18.Liang F, Lindgren G, Lin A, Thompson EA, Ols S, Röhss J, John S, Hassett K, Yuzhakov O, Bahl K, et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol Ther. 2017;25:2635–47. doi: 10.1016/j.ymthe.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–99. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]


